Abstract
Cognitive impairment, particularly in the domain of cognitive control, is characteristic of schizophrenia (SZ) spectrum and bipolar disorders (BD). The longitudinal trajectory of these impairments, however, remains unclear. Indeed, some studies have observed degeneration and others stability or even improvement over time in these illnesses. Here we examined the longitudinal stability of the AX-Continuous Performance Task (AX-CPT), a cognitive control task, in 52 patients with recent onset SZ (< 2 years from first study measurement), 20 patients with recent onset BD Type I with psychotic features, and 70 healthy control subjects. Subjects performed the AX-CPT at two time points separated by an average of 365 days (range 270–620). Previously identified deficits in cognitive control were replicated in both patient groups. No effects of time or interactions between time and diagnosis were observed. Intraclass correlation coefficients also suggested AX-CPT performance was stable across time for all diagnostic groups. Although performance was stable on average, a positive association was noted between change in cognitive control and change in disorganization symptom severity across patient groups. In conclusion, the present findings suggest that deficits in cognitive control are present in both disorders and stable over the early course of psychotic illness. No evidence was observed for progression or deterioration of cognitive control or differential recovery in SZ compared to BD.
Keywords: AX-CPT, Bipolar Disorder, Schizophrenia
Introduction
A major aspect of the traditional “Kraepelinian dichotomy” of schizophrenia (SZ) and bipolar disorder (BD) is that only SZ was theorized to be degenerative (hence the name “dementia praecox”) (Kraepelin, 1899). The degree to which this hypothesis is supported by the existing literature is debatable. Early research into cognitive deficits in SZ supported a degenerative view of the illness (e.g. Bleuler (1972)), a finding later supported by structural imaging studies showing accelerated gray matter loss (reviewed by Lieberman (1999)). Many recent studies, however, suggest that cognitive deficits are relatively stable in SZ for at least the first few years after the first episode of illness (e.g. Hoff, Svetina, Shields, Stewart, and DeLisi (2005), Nopoulos, Flashman, Flaum, Arndt, and Andreasen (1994); recently reviewed by Heilbronner, Samara, Leucht, Falkai, and Schulze (2016)). The few studies conducted in first-episode BD show mixed results. A 2017 meta-analysis found only 3 longitudinal studies of cognition with minimum average follow-up duration of 1 year in first-episode BD patients (Bora & Ozerdem, 2017); these studies reported either stability or improvement. In contrast, an early study by Quackenbush, Kutcher, Robertson, Boulos, and Chaban (1996) observed deterioration in academic performance at 4-year follow-up following the first manic episode.
Research into the course of cognitive deficits across and between psychotic disorders is necessary to characterize the natural progression of potential cognitive biomarkers, determine whether the disorders are neurodegenerative or neurodevelopmental, and help optimize these biomarkers for treatment development. For example, if a marker worsens with age (i.e., is neurodegenerative), an effective treatment should lead to normalization with age. On the other hand, effective treatment of a stable (i.e., neurodevelopmental) marker should improve it regardless of age. One potential cognitive biomarker for psychotic illness is cognitive control, a prefrontal cortex-dependent process that refers to the capacity to formulate, adapt, and maintain an overarching goal (Lesh, Niendam, Minzenberg, & Carter, 2011). This essential ability may also facilitate a myriad of other cognitive processes resulting in an overall higher level of cognitive function (Lesh, et al., 2011). Importantly, disrupted cognitive control is consistently observed in both SZ and BD and is associated with the conceptual disorganization (Liddle, 1987) and global functioning deficits characteristic of the disorders (Lesh, et al., 2011). The stability of cognitive control deficits in SZ and BD, however, is relatively understudied.
One established paradigm for examining cognitive control is the Expectancy AX-Continuous Performance Task (AX-CPT). This powerful task confers a number of advantages compared to other tasks, including high construct validity, translational potential, known ties to functional neuronal circuits, ease of administration in a laboratory setting, and good test-retest reliability (Carter, Minzenberg, West, & Macdonald, 2012; Strauss et al., 2014). Unlike other tests of executive function, the AX-CPT allows the experimenter to parse out specific aspects of cognitive control, such as response inhibition and goal maintenance (Lopez-Garcia et al., 2016). For these reasons, the AX-CPT was recently selected for biomarker development by the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia initiative (Carter, et al., 2012). Clinically, deficits in AX-CPT performance have been consistently observed in SZ (e.g. Lesh et al. (2013), Barch, Carter, MacDonald, Braver, and Cohen (2003)). A previous study in a small sample has also observed intermediate cognitive control performance (between HC and SZ) on the AX-CPT in BD (Brambilla et al., 2007). Interestingly, impaired goal maintenance during the AX-CPT has also been associated with severity of disorganization symptoms, explicitly tying the cognitive control aspect of the task to goal-directedness, thinking, and planning ability (Yoon et al., 2012). One previous longitudinal study found no effect of time in first-episode SZ patients on the AX-CPT over a one-year period (Richard, Carter, Cohen, and Cho (2013)); cognitive control stability in BD, however, to our knowledge has not been examined with the AX-CPT.
To that end, the primary goal of this study was to determine if previously identified deficits in AX-CPT goal maintenance (d-prime context) in early psychotic illness are increased, decreased, or remain stable over a one-year period. Based on the aforementioned literature suggesting that cognitive deficits are relatively stable in early psychosis and in accordance with a non-degenerative view of illness, we hypothesized stable cognitive control deficits in SZ and BD patients. More specifically, we hypothesized 1) no significant effect of time on d-prime context (the primary measure of cognitive control), 2) no significant time X diagnosis interactions on d-prime context, and 3) sufficiently high (> 0.60) intraclass correlation coefficients (ICC’s) for all subject groups on d-prime context with no additional evidence for time effects (i.e. non-significant paired t-tests between time points and overlapping effect sizes (vs. controls) at each time point). Additional behavioral measures (e.g. accuracy for each trial type) were analyzed in an exploratory fashion.
Materials and Methods
Subjects
193 subjects (ages 15–32) participated in this study — 80 patients with either SZ (n = 65), schizophreniform (n = 2) or schizoaffective (n = 13) disorder, 27 patients with BD Type I with psychotic features, and 86 healthy control subjects. Participants provided written informed consent and were compensated for participation. The study complied with APA ethical standards and was approved by the UC Davis Institutional Review Board (approval number 226043-32, project title “Understanding Early Psychosis”).
All patients were recruited as outpatients from the University of California, Davis (UCD) Early Diagnosis and Preventive Treatment (of Psychosis) (EDAPT) research clinic, with the onset of psychosis occurring within 2 years of study. Psychopathology and Axis I disorders were assessed with the Structured Clinical Interview for DSM-IV-TR (SCID) (First, Spitzer, Gibbon, & Williams, 2002). Patients were excluded for a diagnosis of major medical or neurological illness, head trauma (loss of consciousness > 10 minutes), substance abuse in the previous 3 months (as well as a positive urinalysis), and Weschler Abbreviated Scale of Intelligence (WASI) (Weschler, 1999) 2-subtest IQ estimate < 70. Control subjects were excluded for all of the above as well as a history of Axis I mental illness or first-degree family history of psychosis.
In early psychosis participants, symptoms were assessed using the Brief Psychiatric Rating Scale (BPRS) (Ventura et al., 1993), and the Scales for Assessment of Negative Symptoms (SANS) (Andreasen, 1984a) and Positive Symptoms (SAPS) (Andreasen, 1984b). Consistent with prior work (Barch, et al., 2003), three core symptom dimensions were calculated: Poverty, Disorganization, and Reality Distortion. “Poverty” combined emotional withdrawal, motor retardation, and blunted affect from the BPRS with anhedonia/asociality, avolition/apathy, alogia, and affective flattening from the SANS. “Disorganization” combined conceptual disorganization, mannerisms and posturing, and disorientation scores from the BPRS, the attention score from the SANS, as well as positive formal thought disorder and bizarre behavior scores from the SAPS. “Reality distortion” combined grandiosity, suspiciousness, hallucinations, and unusual thought content from the BPRS with hallucinations and delusions from the SAPS. Participants also completed the Young Mania Rating Scale (YMRS) (Young, Biggs, Ziegler, & Meyer, 2000); YMRS scores were calculated without the Content subscale to avoid crossover with the SAPS/Reality Distortion symptoms.
Study Design
This was a longitudinal study that involved an initial baseline visit followed by a follow-up visit. The follow-up visit was designed to occur approximately one year following the baseline visit, although due to scheduling constraints was allowed to vary over a period of ~9–20 months (see Results). Subjects performed the AX-CPT at both visits. Clinical ratings were obtained within one month from each visit. Subjects that did not complete both visits were excluded from analysis (see Results for completion rate).
Description of the AX-CPT
Briefly, participants are presented with a series of cues and probes and are instructed to make a target response (pressing a button with the index finger) to the probe letter “X” only if it was preceded by the cue letter “A” (Supplementary Figure 1). All cues and nontarget probes require nontarget responses (pressing a button with the middle finger). Target sequence trials (i.e. “AX” trials) are frequent (60–70% occurrence) and set up a prepotent tendency to make a target response when the probe letter X occurs. As a result, a nontarget sequence trial in which any non-A cue (collectively called “B” cues) is presented and followed by a probe letter X (i.e. “BX” trials) requires the most cognitive control. AY and BY trials are included to test for generalized deficits and represent an internal control. The task was presented using EPrime2 software (Psychology Software Tools, Inc.).
Subject data were collected using two task protocol versions over a 14-year period. Parameters for each protocol (AX-1 and AX-2) are provided in Supplementary Table 1. Protocols were not mixed within participants, i.e. subjects who underwent the AX-1 version of the task at baseline completed the same version at follow-up.
The behavioral outcome measure of interest in this study is d-prime context. D-prime context captures the goal maintenance aspect of cognitive control and context processing during the task and is calculated as a function of AX hits minus BX false alarms (Cohen, Barch, Carter, & Servan-Schreiber, 1999).
Individual subject data were only included in analyses if results suggested the subject understood the AX-CPT (specifically, accuracy greater than 44% on AX trials, 0% on AY trials, 0% on BX trials, and 50% on BY trials at both baseline and follow-up) (Henderson et al., 2012)
Longitudinal Analysis
The primary goal of this study was to determine if previously identified deficits in goal maintenance (d-prime context) in psychotic illness are increased, decreased, or remain stable over time. Exploratory analyses were also conducted for auxiliary behavioral measures (i.e. accuracy and reaction time).
To assess stability, we conducted two complementary sets of analyses. The first (primary) set used repeated measures ANOVA’s with diagnosis (healthy vs. SZ vs. BD) as a between-subjects factor and time as a within-subjects factor. The secondary set of analyses incorporated a series of tests including comparison of effect sizes, paired t-tests, and intraclass correlation coefficients (ICC’s). For these two sets of analyses, only complete cases were included, i.e., subjects with missing follow-up data were excluded. Additional details of these analyses are provided in Supplementary Methods.
Results
Demographic and Clinical
Between-group demographic and clinical comparisons, information regarding subject attrition, and longitudinal analysis of clinical measures are presented in Supplementary Results, Table 1, and Table 2. 70 controls, 52 SZ patients, and 20 BD patients were included in the final sample.
Table 1.
Demographic information for participants, excluding subjects lost to follow-up and/or did not meet AX-CPT performance criteria at both time points (see Methods). Information taken from baseline unless otherwise specified. Numbers in parentheses represent the standard deviation.
| HC | SZ | BD | F or χ2 (p) | |
|---|---|---|---|---|
| Age | 20.29 (3.28) | 20.67 (3.37) | 21.29 (3.27) | 0.75 (0.47) |
| Gender (M/F) | 44/26 | 40/12 | 10/10 | 5.37 (0.068) |
| Handedness (L/R) | 5/65 | 5/47 | 3/17 | 1.18 (0.56) |
| Education Level (Years) | 13.50 (2.64) | 12.67 (1.95) | 12.95 (1.70) | 2.00 (0.14) |
| Parental Education Level (Years) | 14.77 (2.33) | 14.48 (3.10) | 14.45 (2.28) | 0.23 (0.80) |
| IQ (WASI-2) | 116.53 (10.97) | 104.54 (14.28) | 105.75 (10.76) | 16.10 (<0.001) |
| AX-1 Participants | 40 | 21 | 12 | 4.04 (0.13) |
| AX-2 Participants | 30 | 31 | 8 | |
| Days between Baseline and Follow-Up | 370.17 (63.17) | 356.31 (57.93) | 371.60 (41.22) | 0.97 (0.38) |
Abbreviations: AX-1 = AX-CPT Protocol 1, AX-2 = AX-CPT Protocol 2, BD = Bipolar Disorder, HC = Healthy Controls, SZ = Schizophrenia, WASI-2 = Weschler Abbreviated Scale of Intelligence, 2nd Edition.
Table 2.
Raw patient clinical information, excluding subjects lost to follow up or who did not meet AX-CPT performance criteria (see Methods). Includes baseline and one-year follow-up data. Numbers in parentheses represent the standard deviation. Patients not taking antipsychotics were not counted in dose calculation. Paired comparisons of antipsychotic type were conducted using a Wilcoxon test; other comparisons were conducted using paired t-tests.
| SZ | BD | |||||
|---|---|---|---|---|---|---|
| Baseline | 1 Year | t(p) or Z(p) | Baseline | 1 Year | t(p) or Z (p) | |
| Antipsychotic(s) Taken | 0.83 (0.41) | 1.63 (0.10) | ||||
| Typical | 0 | 1 | 0 | 1 | ||
| Atypical | 45 | 43 | 17 | 12 | ||
| Both | 3 | 1 | 1 | 0 | ||
| None | 4 | 7 | 2 | 7 | ||
| CPZ Equivalent Dose (Mg/Day) | 265.96 (207.61) | 263.03 (183.06) | −0.18 (0.86) | 248.43 (181.09) | 242.07 (317.17) | −1.05 (0.31) |
| YMRS Total (Excluding Content Score) | 5.49 (4.48) | 3.71 (3.96) | −2.85 (0.007) | 6.15 (7.98) | 5.41 (7.31) | −0.66 (0.52) |
| Poverty Symptoms | 14.83 (5.92) | 12.83 (5.94) | −2.24 (0.03) | 11.80 (5.44) | 8.93 (3.75) | −2.42 (0.03) |
| Disorganization Symptoms | 6.94 (3.59) | 5.67 (2.52) | −2.59 (0.013) | 5.25 (2.07) | 5.93 (3.86) | 0.21 (0.83) |
| Reality Distortion Symptoms | 15.29 (7.42) | 9.52 (5.99) | −5.10 (<0.001) | 9.45 (5.81) | 7.07 (6.31) | −1.74 (0.10) |
Abbreviations: BD = Bipolar Disorder, CPZ = Chlorpromazine, SZ = Schizophrenia, YMRS = Young Mania Rating Scale.
Primary Stability Analyses: Repeated Measures ANOVAs
Raw behavioral data (overall and segregated by AX protocol version) and behavioral comparisons between protocols are presented in Supplementary Tables 3a–d. Although the primary measure of interest (d-prime context) was not significantly different between the two protocols (AX-1 and AX-2), differences were observed in other metrics (Supplementary Table 3d). Behavioral data was therefore adjusted for protocol effects (in addition to age and gender) as described in Supplementary Methods. These adjusted z-scores are presented in Table 3 and Figure 1. Importantly, no group difference was observed in the ratio of participants who performed AX-1 vs. AX-2 (Table 1). All subjects also performed the same protocol at baseline and follow-up to minimize protocol effects on stability measures.
Table 3.
Longitudinal analysis of AX-CPT data.
| Dx | Age, Gender, and Task-Adjusted Z Scores, Mean (SD) | Effect Size (Hedges Bias Corrected Cohen’s d) vs. Baseline HC {95% CI} | Within-Dx Follow-Up vs. Baseline Comparison and Stability | ||||||
|---|---|---|---|---|---|---|---|---|---|
| D-Prime Context | |||||||||
| Baseline | Follow-Up | Baseline | Follow-Up | Pair Difference {95% CI} | t | p | ICC {95% CI} | p | |
| HC | 0.00 (1.00) | −0.08 (1.01) | n/a | −0.08 {−0.41 − 0.25} | −0.08 {−0.30 – 0.14} | −0.76 | 0.45 | 0.74 {0.57 – 0.84} | <0.001 |
| SZ | −0.93 (1.23) | −0.86 (1.29) | −0.84 {1.21 − −0.46} | −0.75 {−1.13 − −0.38} | 0.07 {−0.26 – 0.40} | 0.43 | 0.67 | 0.73 {0.52 – 0.84} | <0.001 |
| BD | −0.86 (1.18) | −0.75 (1.06) | −0.82 {−1.33 − −0.31} | −0.73 {−1.24 − −0.23} | 0.11 {−0.40 – 0.62} | 0.46 | 0.65 | 0.70 {0.24 – 0.88} | 0.007 |
| AX Accuracy | |||||||||
| Baseline | Follow-Up | Baseline | Follow-Up | Pair Difference {95% CI} | t | p | ICC {95% CI} | p | |
| HC | 0.00 (1.00) | −0.21 (1.27) | n/a | −0.18 {−0.51 − 0.15} | −0.21 {−0.53 – 0.10} | −1.35 | 0.18 | 0.50 {0.19 – 0.69} | 0.002 |
| SZ | −1.26 (1.46) | −0.91 (1.49) | −1.03 {−1.41 − −0.65} | −0.73 {−1.10 − −0.36} | 0.35 {−0.06 – 0.75} | 1.71 | 0.09 | 0.67 {0.42 – 0.81} | <0.001 |
| BD | −0.89 (1.30) | −1.04 (1.23) | −0.82 {−1.33 − −0.31} | −0.98 {−1.50 − −0.46} | −0.15 {−0.73 – 0.42} | −0.57 | 0.58 | 0.70 {0.22 – 0.88} | 0.007 |
| AY Accuracy | |||||||||
| Baseline | Follow-Up | Baseline | Follow-Up | Pair Difference {95% CI} | t | p | ICC {95% CI} | p | |
| HC | 0.00 (1.00) | −0.06 (0.99) | n/a | −0.06 {−0.39 − 0.27} | −0.06 {−0.31 – 0.19} | −0.46 | 0.65 | 0.62 {0.39 – 0.77} | <0.001 |
| SZ | −0.40 (1.13) | −0.27 (1.08) | −0.38 {−0.74 − −0.01} | −0.26 {−0.62 − 0.10} | 0.13 {−0.19 – 0.44} | 0.79 | 0.43 | 0.65 {0.39 – 0.80} | <0.001 |
| BD | 0.03 (1.31) | −0.09 (1.10) | 0.03 {−0.47 − 0.52} | −0.09 {−0.58 − 0.41} | −0.12 {−0.81 – 0.57} | −0.37 | 0.72 | 0.43 {−0.50 – 0.78} | 0.12 |
| BX Accuracy | |||||||||
| Baseline | Follow-Up | Baseline | Follow-Up | Pair Difference {95% CI} | t | p | ICC {95% CI} | p | |
| HC | 0.00 (1.00) | −0.07 (0.86) | n/a | −0.07 {−0.41 − 0.26} | −0.07 {−0.33 – 0.19} | −0.50 | 0.62 | 0.47 {0.14 – 0.67} | 0.005 |
| SZ | −0.55 (1.06) | −0.65 (1.11) | −0.53 {−0.90 − −0.17} | −0.62 {−0.98 − −0.25} | −0.10 {−0.41 – 0.22} | −0.62 | 0.54 | 0.64 {0.37 – 0.79} | <0.001 |
| BD | −0.75 (1.08) | −0.27 (1.09) | −0.73 {−1.24 − −0.22} | −0.26 {−0.76 − 0.24} | 0.48 {−0.03 – 1.00} | 1.95 | 0.07 | 0.62 {0.10 – 0.85} | 0.013 |
| BY Accuracy | |||||||||
| Baseline | Follow-Up | Baseline | Follow-Up | Pair Difference {95% CI} | t | p | ICC {95% CI} | p | |
| HC | 0.00 (1.00) | −0.06 (1.00) | n/a | −0.06 {−0.39 − 0.27} | −0.06 {−0.41 – 0.29} | −0.34 | 0.74 | * | * |
| SZ | −0.30 (1.50) | −0.54 (1.65) | −0.24 {−0.60 − 0.12} | −0.41 {−0.77 − −0.05} | −0.24 {−0.72 – 0.23} | −1.04 | 0.30 | 0.60 {0.30 – 0.77} | 0.001 |
| BD | −0.93 (1.82) | −0.09 (0.96) | −0.75 {−1.26 − −0.24} | −0.09 {−0.59 − 0.41} | 0.84 {−0.21 – 1.89} | 1.68 | 0.11 | * | * |
| AX Reaction Time | |||||||||
| Baseline | Follow-Up | Baseline | Follow-Up | Pair Difference {95% CI} | t | p | ICC {95% CI} | p | |
| HC | 0.00 (1.00) | 0.05 (1.13) | n/a | 0.05 {−0.38 − 0.28} | 0.05 {−0.28 – 0.17} | 0.48 | 0.63 | 0.75 {0.60 – 0.84} | <0.001 |
| SZ | 0.70 (1.31) | 0.82 (1.31) | 0.61 {0.24 − 0.98} | 0.71 {0.34 − 1.08} | 0.12 {−0.21 – 0.45} | 0.73 | 0.47 | 0.75 {0.57 – 0.86} | <0.001 |
| BD | 0.56 (1.35) | 0.44 (1.37) | 0.51 {0.01 − 1.01} | 0.40 {−0.10 − 0.90} | −0.12 {−0.85 – 0.62} | −0.33 | 0.75 | 0.51 {−0.28 – 0.81} | 0.071 |
| AY Reaction Time | |||||||||
| Baseline | Follow-Up | Baseline | Follow-Up | Pair Difference {95% CI} | t | p | ICC {95% CI} | p | |
| HC | 0.00 (1.00) | 0.02 (0.98) | n/a | 0.02 {−0.31 − 0.35} | 0.02 {−0.24 – 0.28} | 0.15 | 0.88 | 0.56 {0.29 – 0.73} | <0.001 |
| SZ | 0.49 (0.99) | 0.52 (1.10) | 0.49 {0.12 − 0.85} | 0.50 {0.13 − 0.86} | 0.03 {−0.23 – 0.30} | 0.24 | 0.81 | 0.74 {0.55 – 0.85} | <0.001 |
| BD | 0.56 (0.97) | 0.41 (1.33) | 0.56 {0.06 − 1.06} | 0.38 {−0.12 − 0.88} | −0.15 {−0.65 – 0.34} | −0.64 | 0.53 | 0.74 {0.35 – 0.90} | 0.003 |
| BX Reaction Time | |||||||||
| Baseline | Follow-Up | Baseline | Follow-Up | Pair Difference {95% CI} | t | p | ICC {95% CI} | p | |
| HC | 0.00 (1.00) | −0.08 (0.92) | n/a | −0.08 {−0.41 − 0.25} | −0.08 {−0.31 – 0.15} | −0.67 | 0.51 | 0.67 {0.47 – 0.80} | <0.001 |
| SZ | 0.51 (1.09) | 0.66 (1.18) | 0.49 {0.12 − 0.85} | 0.61 {0.24 − 0.97} | 0.15 {−0.14 – 0.44} | 1.04 | 0.30 | 0.73 {0.53 – 0.84} | <0.001 |
| BD | 0.55 (0.95) | 0.42 (1.03) | 0.55 {0.05 − 1.05} | 0.41 {−0.09 − 0.91} | −0.13 {−0.68 – 0.43} | −0.48 | 0.64 | 0.45 {−0.44 – 0.78} | 0.11 |
| BY Reaction Time | |||||||||
| Baseline | Follow-Up | Baseline | Follow-Up | Pair Difference {95% CI} | t | p | ICC | p | |
| HC | 0.00 (1.00) | 0.06 (0.95) | n/a | 0.06 {−0.27 − 0.39} | 0.06 {−0.28 – 0.17} | 0.52 | 0.61 | 0.70 {0.51 – 0.81} | <0.001 |
| SZ | 0.80 (1.06) | 0.78 (1.32) | 0.77 {0.40 − 1.15} | 0.68 {0.31 − 1.04} | −0.02 {−0.25 – 0.29} | −0.15 | 0.88 | 0.81 {0.67 – 0.89} | <0.001 |
| BD | 0.73 (1.06) | 0.58 (1.26) | 0.71 {0.21 − 1.22} | 0.54 {0.04 − 1.04} | −0.15 {−0.37 – 0.67} | −0.60 | 0.55 | 0.71 {0.25 – 0.88} | 0.006 |
Abbreviations: BD = Bipolar Disorder, CI = confidence interval, HC = Healthy Control, ICC = intraclass correlation coefficient, SD = standard deviation, SZ = Schizophrenia. *Negative correlation (violates reliability assumptions).
Figure 1.
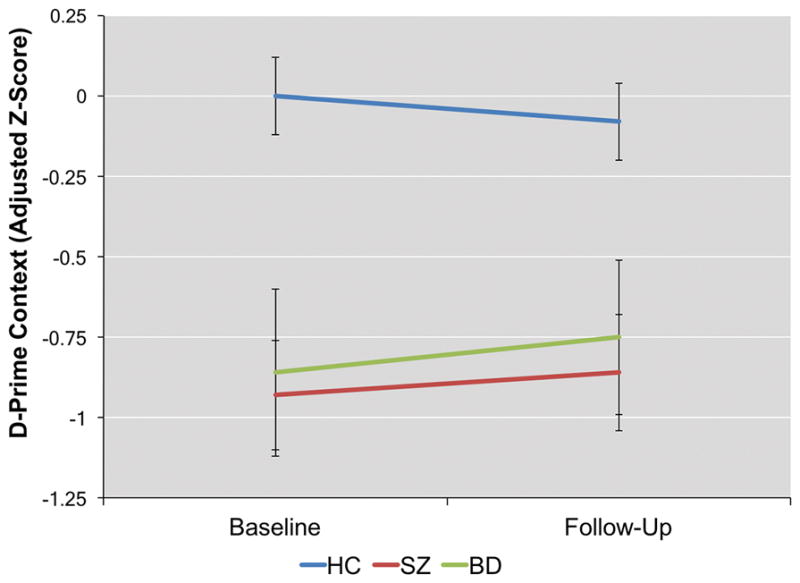
Adjusted d-prime context (z-scores) at baseline and follow-up for each diagnostic group. Error bars represent the standard error.
A main effect of diagnosis was observed for adjusted d-prime context (F(2,139) = 12.32, p < 0.001). No main effect of time or time X diagnosis interaction was observed. Post-hoc tests showed deficits in SZ patients relative to controls (p < 0.001) and BD patients relative to controls (p = 0.009) but no difference between BD and SZ patients.
ANOVA results for auxiliary behavioral measures are presented in Supplementary Results. With the exception of AY accuracy in controls (ρ = −0.42, p < 0.001), no associations were observed between time to follow and change (over time) in any behavioral measure for any group.
An important consideration in this analysis is the degree to which the two task versions (AX-1 and AX-2) measure the same cognitive control construct. A limited number of subjects (7 controls and 13 patients) performed both task versions. Using this data, moderately high consistency was observed for the majority of behavioral measures, including the index of cognitive control (d-prime context) (Supplementary Table 4).
Secondary Stability Analyses
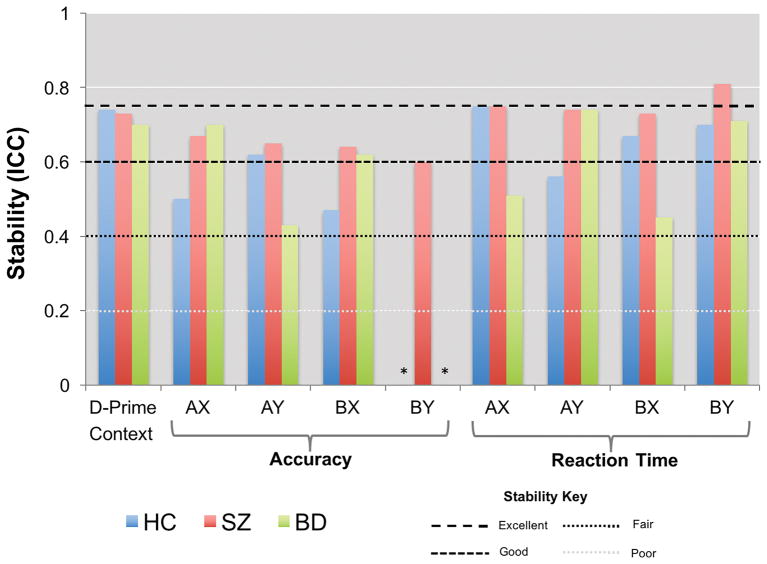
Results from the secondary analyses are presented in Table 3 and Figure 2. AX-CPT behavioral measures generally showed good stability for all three groups, including d-prime context. Neither patient nor control groups showed evidence for degeneration of AX-CPT processes between baseline and follow-up, as demonstrated by 1) effect sizes that stayed within the same range (relative to control baseline data) for both SZ and BD patient groups across time, 2) lack of significant effects of time within groups (paired t-tests) on any behavioral performance measure, and 3) ICC’s of 0.40 or greater on all measures except for BY accuracy (Table 3). Raw data (Supplementary Table 3a) suggested, furthermore, that any instability in BY accuracy occurred in a narrow window potentially driven by ceiling effects. No associations were observed between the number of days to follow-up and the effect of time on d-prime context, AX/BX/BY accuracy, or AX/AY/BX/BY reaction time. A negative association was noted between the number of days to follow up and the effect of time on AY accuracy (Spearman’s ρ = −0.29, p < 0.001). Controlling for number of days to follow up, however, did not appreciably alter AY accuracy stability values for any group.
Figure 2.
Stability (Intraclass Correlation Coefficients (ICCs)) for all AX-CPT performance measures for each group. *ICC was unavailable as a negative average covariance was observed for this measure for this group.
To examine the possibility that our performance criteria (see Methods) influenced stability, we reanalyzed the data using the entire dataset. Stability was not appreciably altered (Supplementary Table 5).
Longitudinal Clinical Correlates
Across patient groups, those subjects for whom d-prime context worsened over time also showed more severe disorganization symptoms at follow-up (ρ = −0.36, p = 0.004, Supplementary Figure 2). A trend in the same direction was also observed for poverty symptoms (ρ = −0.24, p = 0.061). No relationship was observed with reality distortion symptoms.
At baseline, patients that showed more severe disorganization symptoms also had lower d-prime context (ρ = −0.35, p = 0.002). No other clinical correlations were observed at either baseline or follow-up.
Medication Effects
No significant differences were noted between time points for antipsychotic type or dose for either SZ or BD patients. Change in antipsychotic medication dose (chlorpromazine equivalent) or type (e.g. from unmedicated to medicated with an atypical antipsychotic) was not associated with change in any behavioral metric.
Discussion
The goal of this study was to examine the stability of cognitive control deficits in early SZ and BD patients over (approximately) a one-year period. Consistent with our hypothesis, significant but stable deficits in context processing during the AX-CPT was observed in both SZ and BD. Specifically, we observed significant effects of diagnosis but no effects of time or time X diagnosis interactions on the goal maintenance aspect of cognitive control (d-prime context). Auxiliary behavioral measures (accuracy and reaction time) also did not show significant changes between baseline and follow-up for the majority of trial types. Secondary measures (e.g. ICCs) also suggested good stability between time points for almost all AX-CPT performance metrics (including d-prime context) for all three diagnostic groups. We also noted a significant relationship between disorganization symptoms over time and d-prime context over time across patient groups.
These findings are consistent with previous studies in recent onset SZ demonstrating executive function deficits are stable in the early stages of illness (e.g. Hoff, et al., 2005; Nopoulos, et al., 1994; Richard, et al., 2013). In BD, in which relatively little longitudinal research has been conducted, findings are more mixed. Quackenbush, et al. (1996) found significant deterioration in academic performance 4 years after illness onset in adolescents with BD, although baseline was measured premorbidly in this sample and it was unclear when the deterioration occurred. In contrast, in a sample of BD Type I patients Torres et al. (2014) reported improvement between baseline and 1-year follow-up using a battery of executive function tests (including Trail Making Test B, Stroop task, and analogues of the Wisconsin Card Sorting and Tower of London tasks). More aligned with our findings, in a mixed sample (Type I and II) Lera-Miguel, Andres-Perpina, Fatjo-Vilas, Fananas, and Lazaro (2015) found no significant difference between baseline and two-year follow-up in adolescent first-episode BD patients on the Wisconsin Card Sorting and Stroop tasks. Also in a mixed sample, Lee et al. (2015) found no difference between baseline and follow-up (mean follow-up time 20.6 months) on a set-shifting task in a relatively large sample (n = 61 BD patients). Taken together, the present study along with the majority of previous analyses support a neurodevelopmental view of early psychosis (Rapoport, Giedd, & Gogtay, 2012) for both SZ and BD, in which an insult occurs prior to illness onset and then remains stable for at least the first few years of disease. Nonetheless, these results do not necessarily imply that cognitive control remains stable throughout illness. Related to this point, a recent study found that symptoms and global functioning significantly declined 5 to 8 years after psychiatric illness onset (Kotov et al., 2017). As it is unclear if decline in mental illness is primarily caused by illness or secondary effects (e.g. reduced ability to make healthy lifestyle choices due to low income or relative inaccessibility of resources (Brown, Birtwistle, Roe, & Thompson, 1999)) the level of progressive deterioration in cognitive control processes requires further study over longer time periods than the relatively short period (1–2 years) examined in this study. Future studies may also examine the stability of other cognitive constructs (e.g. attention, speed of processing). Related to this point, previous work has observed that deficits in cognitive control may be domain-generalizable in that they may impact performance across multiple cognitive domains (Niendam et al., 2012; Reilly et al., 2017; Sheffield et al., 2014). In regard to biomarker development, the results of this study also suggest that an effective treatment should be able to improve AX-CPT performance closer to control levels. As our sample was restricted to BP Type I (with psychotic features) patients, future studies will be needed to determine if cognitive control deficits are equally stable in other BP populations.
The finding that AX-CPT psychometrics were stable for both diagnostic groups supports the use of cognitive control as a cognitive endophenotype. Remarkably, the observed long-term ICC’s for d-prime context were in the range of the ICC’s observed for short-term stability (on the order of weeks) (Strauss, et al., 2014). Related to this point, unaffected first-degree relatives of SZ patients as well as subjects at high risk for the illness show deficits in d-prime context during the AX-CPT, suggesting these deficits have a trait-dependent genetic basis (Niendam et al., 2014; Richard, et al., 2013). Indeed, cognitive control deficits in general are highly heritable (Glahn, Knowles, & Pearlson, 2016). Possibly at odds with this view, however, are findings from this study and others that show associations between context processing on the AX task and disorganization symptoms (Yoon, et al., 2012), suggesting that this deficit may also have state-dependent qualities. To reconcile these opposing viewpoints, it may be helpful to consider that disorganization symptoms and context processing might be tapping into shared neurocognitive processes. As noted by Andreasen et al. (1994) disorganization is characterized in part by behavior that “simply lacks goal-directedness.” Early familial studies in SZ found strong correlations between the severity of disorganization symptoms across probands and their unaffected relatives, suggesting that these symptoms may also share a genetic component (Rietkerk et al., 2008; Wickham et al., 2001). The finding that d-prime context changes correlate with disorganization changes, therefore, may be a reflection of shared endophenotypic processes rather than a state-dependent characteristic.
In this study, we did not observe any relationships between medication and AX-CPT performance over time. Although prior work by our lab has observed significantly better AX task performance in SZ patients treated with atypical antipsychotics compared to unmedicated patients (Lesh et al., 2015), this previous study was cross-sectional and therefore did not examine within-subject effects. Medication dose is typically altered according to the needs of the patient (e.g. ability to ameliorate positive symptoms while minimizing side effects), and therefore one would not necessarily predict that a higher antipsychotic dose would be associated with improved AX performance. The present study also naturalistically followed patients over time and was therefore not designed to control for variations in treatment type, dose, duration, or compliance. Future longitudinal studies may incorporate prospective designs (in the manner of clinical trials) to more closely examine the chronic effect of antipsychotics on the observed behavioral measures.
As with any longitudinal study, the role of practice effects in explaining the observed results should be considered. We believe it unlikely that practice effects explain our findings for the following reasons. First, effects of time were not observed on any measure in healthy control subjects, and no time X diagnosis interactions were observed as well. Second, the length of time between baseline and follow-up made practice effects less likely. Finally, previous longitudinal work by Strauss, et al. (2014) over a much shorter time frame (2 weeks) only observed practice effects on the AX-CPT in patients that were much older than those in this study.
A potential limitation of this study is that our strategy of censoring subjects that were either lost to follow-up or did not show adequate understanding of the task at both time-points may have biased the analysis. Although we cannot completely rule out this possibility (as doing so would require missing data to be filled in), diagnosis did not affect the likelihood that a subject with eligible baseline data would be lost to follow-up or have data censored at follow-up. Thus, it is unlikely that diagnostic bias had a significant effect on between-group analyses. Furthermore, no differences were observed in baseline performance metrics between subjects lost to follow-up and subjects with follow-up data that met criterion, suggesting that excluding subjects lost to follow-up did not bias the sample towards high performers. Finally, with the exception of WASI score, no demographic or clinical differences were observed between patients included in the sample and patients excluded due to loss of follow-up or not meeting performance criteria, suggesting that stability results were not influenced by a bias in baseline positive or negative symptom severity. The possibility that intelligence (e.g. WASI score) influences stability may be investigated in future studies.
An additional potential concern is that the dataset in these analyses combined measures from two different task protocols. An important question, therefore, is if these protocols (due to their differences) index the same cognitive control construct. Although we attempted to account for this potential confound by statistically adjusting for protocol effect, to further address this concern we examined inter-task reliability (ICC) in a limited number of subjects who performed both tasks. ICC’s calculated using this data were in the fair to good range, including d-prime context (ICC = 0.65; Supplementary Table 4). Furthermore, previous cross-sectional studies in SZ have reported significant deficits in d-prime context in SZ regardless of task parameters (e.g. Barch, et al. (2003); Cohen, et al. (1999); Henderson, et al. (2012); Strauss, et al. (2014)). D-prime context also did not differ between tasks in the present study. AY trial accuracy, on the other hand, did differ somewhat between tasks. Given that the high proportion of target AX trials drives the tendency to make “Go” responses to probes following “A” cues, however, lower accuracy on AY trials for the AX-1 version of the task may be due to the higher ratio of AX to AY trial types. AY trials are also relatively sparse in the AX-CPT, potentially reducing their reliability. Furthermore, accuracy on AY trials was not included in the equation for d-prime context. Thus, although performance may have differed somewhat between the tasks, we consider it unlikely that different cognitive control constructs were examined for each task. Nonetheless, given the small sample size (particularly in the BP group) the results of this study require replication in a larger sample in which only one task version is used.
In conclusion, our findings suggest that cognitive control is relatively stable in the early course of SZ and BD in agreement with the neurodevelopmental hypothesis of cognitive deficits for these illnesses. Our findings also support the continued use of the AX-CPT paradigm to examine endophenotypic biomarkers of cognitive control in psychosis.
Supplementary Material
General Scientific Summary.
This study suggests that deficits in cognitive control (a critical cognitive process that is involved with formulating, adapting, and maintaining overarching goals) are relatively stable in patients with recent onset schizophrenia and bipolar disorder.
Acknowledgments
Funding
This work was supported by the National Institutes of Mental Health (grant number 5R01MH059883 to C.S.C., fellowship number F32-MH114325 to J.S.). This project was also supported by the Building Interdisciplinary Research Careers in Women’s Health award (K12-HD051958 to L.M.T.) and the National Institute of Child Health and Human Development (NICHD), Office of Research on Women’s Health, Office of Dietary Supplements, and National Institute of Aging.
We would like to thank Amber Howell, Andrew Westphal, Julian Williams, Shaun Rafael, Natalie Hutchison, Benjamin Geib, Madison Titone, Taylor Salo, Estera (Neli) Mihov, Erika Steinbauer, Markie Benavidez, and Davis Ewbank for their assistance with data collection and processing. We also thank the study participants and their families.
A portion of these data was presented at the 2013 International Congress on Schizophrenia Research meeting.
Footnotes
The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- Andreasen NC. Scale for the assessment of negative symptoms (SANS) Iowa City, IA: Department of Psychiatry, College of Medicine, The University of Iowa; 1984a. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: Department of Psychiatry, College of Medicine, the University of Iowa; 1984b. [Google Scholar]
- Andreasen NC, Nopoulos P, Schultz S, Miller D, Gupta S, Swayze V, Flaum M. Positive and negative symptoms of schizophrenia: past, present, and future. Acta Psychiatr Scand Suppl. 1994;384:51–59. doi: 10.1111/j.1600-0447.1994.tb05891.x. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, MacDonald AW, 3rd, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol. 2003;112(1):132–143. [PubMed] [Google Scholar]
- Bleuler M. The Schizophrenic Disorders: Long-Term Patient and Family Studies. New Haven, CT: Yale University Press; 1972. [Google Scholar]
- Bora E, Ozerdem A. Meta-analysis of longitudinal studies of cognition in bipolar disorder: comparison with healthy controls and schizophrenia. Psychol Med. 2017:1–14. doi: 10.1017/S0033291717001490. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Macdonald AW, 3rd, Sassi RB, Johnson MK, Mallinger AG, Carter CS, Soares JC. Context processing performance in bipolar disorder patients. Bipolar Disord. 2007;9(3):230–237. doi: 10.1111/j.1399-5618.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- Brown S, Birtwistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999;29(3):697–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- Carter CS, Minzenberg M, West R, Macdonald A., 3rd CNTRICS imaging biomarker selections: Executive control paradigms. Schizophr Bull. 2012;38(1):34–42. doi: 10.1093/schbul/sbr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6(4):284–290. [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108(1):120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Glahn DC, Knowles EE, Pearlson GD. Genetics of cognitive control: Implications for Nimh’s research domain criteria initiative. Am J Med Genet B Neuropsychiatr Genet. 2016;171B(1):111–120. doi: 10.1002/ajmg.b.32345. [DOI] [PubMed] [Google Scholar]
- Hedges L. Distribution Theory for Glass’s Estimator of Effect Size and Related Estimators. Journal of Educational Statistics. 1981;6(2):107–128. [Google Scholar]
- Heilbronner U, Samara M, Leucht S, Falkai P, Schulze TG. The Longitudinal Course of Schizophrenia Across the Lifespan: Clinical, Cognitive, and Neurobiological Aspects. Harv Rev Psychiatry. 2016;24(2):118–128. doi: 10.1097/HRP.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DC, Poppe AB, Barch DM, Carter CS, Gold JM, Ragland JD, … MacDonald AW., 3rd Optimization of a Goal Maintenance Task for Use in Clinical Applications. Schizophr Bull. 2012;38(1):104–113. doi: 10.1093/schbul/sbr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res. 2005;78(1):27–34. doi: 10.1016/j.schres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Kim HY. Statistical notes for clinical researchers: Evaluation of measurement error 1: using intraclass correlation coefficients. Restor Dent Endod. 2013;38(2):98–102. doi: 10.5395/rde.2013.38.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Fochtmann L, Li K, Tanenberg-Karant M, Constantino EA, Rubinstein J, … Bromet EJ. Declining Clinical Course of Psychotic Disorders Over the Two Decades Following First Hospitalization: Evidence From the Suffolk County Mental Health Project. Am J Psychiatry. 2017;174(11):1064–1074. doi: 10.1176/appi.ajp.2017.16101191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Psychiatrie. Ein Lehrbuch für Studirende und Aertze. Leipzig: Barth; 1899. [Google Scholar]
- Lee RS, Hermens DF, Naismith SL, Lagopoulos J, Jones A, Scott J, … Hickie IB. Neuropsychological and functional outcomes in recent-onset major depression, bipolar disorder and schizophrenia-spectrum disorders: a longitudinal cohort study. Transl Psychiatry. 2015;5:e555. doi: 10.1038/tp.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lera-Miguel S, Andres-Perpina S, Fatjo-Vilas M, Fananas L, Lazaro L. Two-year follow-up of treated adolescents with early-onset bipolar disorder: Changes in neurocognition. J Affect Disord. 2015;172:48–54. doi: 10.1016/j.jad.2014.09.041. [DOI] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36(1):316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Tanase C, Geib BR, Niendam TA, Yoon JH, Minzenberg MJ, … Carter CS. A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry. 2015;72(3):226–234. doi: 10.1001/jamapsychiatry.2014.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Westphal AJ, Niendam TA, Yoon JH, Minzenberg MJ, Ragland JD, … Carter CS. Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. Neuroimage Clin. 2013;2:590–599. doi: 10.1016/j.nicl.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF. The symptoms of chronic schizophrenia. A re-examination of the positive-negative dichotomy. Br J Psychiatry. 1987;151:145–151. doi: 10.1192/bjp.151.2.145. [DOI] [PubMed] [Google Scholar]
- Lieberman JA. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry. 1999;46(6):729–739. doi: 10.1016/s0006-3223(99)00147-x. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia P, Lesh TA, Salo T, Barch DM, MacDonald AW, 3rd, Gold JM, … Carter CS. The neural circuitry supporting goal maintenance during cognitive control: a comparison of expectancy AX-CPT and dot probe expectancy paradigms. Cogn Affect Behav Neurosci. 2016;16(1):164–175. doi: 10.3758/s13415-015-0384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Lesh TA, Yoon J, Westphal AJ, Hutchison N, Daniel Ragland J, … Carter CS. Impaired context processing as a potential marker of psychosis risk state. Psychiatry Res. 2014;221(1):13–20. doi: 10.1016/j.pscychresns.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Flashman L, Flaum M, Arndt S, Andreasen N. Stability of cognitive functioning early in the course of schizophrenia. Schizophr Res. 1994;14(1):29–37. doi: 10.1016/0920-9964(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Quackenbush D, Kutcher S, Robertson HA, Boulos C, Chaban P. Premorbid and postmorbid school functioning in bipolar adolescents: description and suggested academic interventions. Can J Psychiatry. 1996;41(1):16–22. doi: 10.1177/070674379604100106. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17(12):1228–1238. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JL, Hill SK, Gold JM, Keefe RS, Clementz BA, Gershon E, … Sweeney JA. Impaired Context Processing is Attributable to Global Neuropsychological Impairment in Schizophrenia and Psychotic Bipolar Disorder. Schizophr Bull. 2017;43(2):397–406. doi: 10.1093/schbul/sbw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard AE, Carter CS, Cohen JD, Cho RY. Persistence, diagnostic specificity and genetic liability for context-processing deficits in schizophrenia. Schizophr Res. 2013;147(1):75–80. doi: 10.1016/j.schres.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietkerk T, Boks MP, Sommer IE, Liddle PF, Ophoff RA, Kahn RS. The genetics of symptom dimensions of schizophrenia: review and meta-analysis. Schizophr Res. 2008;102(1–3):197–205. doi: 10.1016/j.schres.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Ross RG, Wagner B, Heinlein S, Zerbe GO. The stability of inhibitory and working memory deficits in children and adolescents who are children of parents with schizophrenia. Schizophr Bull. 2008;34(1):47–51. doi: 10.1093/schbul/sbm104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Gold JM, Strauss ME, Carter CS, MacDonald AW, 3rd, Ragland JD, … Barch DM. Common and specific cognitive deficits in schizophrenia: relationships to function. Cogn Affect Behav Neurosci. 2014;14(1):161–174. doi: 10.3758/s13415-013-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss ME, McLouth CJ, Barch DM, Carter CS, Gold JM, Luck SJ, … Silverstein SM. Temporal stability and moderating effects of age and sex on CNTRaCS task performance. Schizophr Bull. 2014;40(4):835–844. doi: 10.1093/schbul/sbt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres IJ, Kozicky J, Popuri S, Bond DJ, Honer WG, Lam RW, Yatham LN. 12-month longitudinal cognitive functioning in patients recently diagnosed with bipolar disorder. Bipolar Disord. 2014;16(2):159–171. doi: 10.1111/bdi.12154. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Manual for the expanded brief psychiatric rating scale. International Journal of Methods in Psychiatric Research. 1993;3(3):227–244. [Google Scholar]
- Weschler D. Weschler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Wickham H, Walsh C, Asherson P, Taylor C, Sigmundson T, Gill M, … Sham P. Familiality of symptom dimensions in schizophrenia. Schizophr Res. 2001;47(2–3):223–232. doi: 10.1016/s0920-9964(00)00098-0. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Nguyen DV, McVay LM, Deramo P, Minzenberg MJ, Ragland JD, … Carter CS. Automated classification of fMRI during cognitive control identifies more severely disorganized subjects with schizophrenia. Schizophr Res. 2012;135(1–3):28–33. doi: 10.1016/j.schres.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. Handbook of Psychiatric Measures. Washington, D.C: American Psychiatric Association; 2000. Young Mania Rating Scale; pp. 540–542. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.