Abstract
Individuals with cerebral palsy (CP) exhibit neuromuscular complications and low physical activity levels. Adults with CP exhibit a high prevalence of chronic diseases, which is associated with musculoskeletal deficits. Children with CP have poor musculoskeletal accretion accompanied by excess bone marrow fat, which may lead to weaker bones. Mechanistic studies to determine the role of bone marrow fat on skeletal growth and maintenance, and how it relates to systemic energy metabolism among individuals with CP, are lacking. In this review, we highlight the skeletal status in children with CP and analyze the existing literature on the interactions among bone marrow fat, skeletal health, and cardiometabolic disease risk in the general population. Clinically vital questions are proposed, including: (1) Is the bone marrow fat in children with CP metabolically distinct from typically developing children in terms of its lipid and inflammatory composition? (2) Does the bone marrow fat suppress skeletal acquisition? (3) Or, does it accelerate chronic disease development in children with CP? (4) If so, what are the mechanisms? In conclusion, while inadequate mechanical loading may initiate poor skeletal development, subsequent expansion of bone marrow fat may further impede skeletal acquisition and increase cardiometabolic disease risk in those with CP.
Keywords: Cerebral palsy, bone marrow fat, skeletal development, cardiometabolic disease risk
Introduction
Cerebral palsy (CP) is the most common motor disability in childhood affecting 2 to 3.6 per thousand live births.1–4 It results from damage to or malformation of the infant brain leading to varying degrees of neuromuscular dysfunction and low levels of physical activity.5,6 The life expectancy for those with CP has increased over the past four decades, creating a growing adult patient population with a high prevalence and accelerated development of chronic diseases, such as cardiometabolic diseases (e.g., hypertension, hyperlipidemia).7–12 Importantly, the high cardiometabolic multimorbidity prevalence (i.e., having ≥2 chronic diseases) in middle-aged adults with CP is associated with musculoskeletal deficits,11 and adults with CP are known to have significantly diminished musculoskeletal densities.13
The health of the adult musculoskeletal system is largely determined by mechanical and physiological factors experienced throughout childhood.14,15 Children with CP never reach optimum functional capacity and are predisposed to decline as they transition into- and throughout- their adults years.16 This is largely due to neuromuscular inefficiency17–19 and underdeveloped and weak skeletal muscles that are highly infiltrated with fat.5,6 Moreover, these problems vary based on the severity of the condition, ranging from mild (ambulatory) to severe (nonambulatory) functional restriction.20 This results in a spectrum of inadequate mechanical loading from muscle contraction pulling on bone and ground reaction forces experienced during ambulatory activities (e.g., walking, running, jumping). Since childhood is a critical stage of skeletal modeling coupled with remodeling, low levels of stress on the skeleton would suppress skeletal acquisition and organization leading to lower peak bone mass attainment and weaker bones. Suppressed skeletal acquisition may be further influenced by puberty. Children with CP have stunted pubertal growth,21 which is an essential window for optimizing skeletal development. Taken together, these complications may help to explain why children and adolescents with CP experience a high rate of low-energy fractures22,23- a problem which varies widely based on the severity of the condition, and which is amplified in young adults with CP.24
Another potential contributor to low bone strength in CP is bone marrow fat, which is negatively correlated with mechanical loading25 and skeletal architecture and composition26–33 in adults. Bone marrow fat may not simply be a filler following bone atrophy, but rather an active tissue regulating the bone and marrow microenvironment.34 Bone marrow fat is subject to metabolic alterations depending on factors that impact the skeleton, including age, sex, disease status, radiation exposure, nutrition, hormonal factors, and inflammation. Bone marrow fat is also associated with markers of poor cardiometabolic health in adults.35–37 Therefore, bone marrow fat may be a key regulator of skeletal homeostasis and may contribute to systemic energy metabolism. Other than a few anatomical studies, very little is known about bone marrow fat dynamics and its relation to skeletal and overall metabolic health during human growth and development, especially in children with physical disabilities. To date, only one study has investigated bone marrow fat in children with CP and found it to be higher compared to sex-, age-, and race-matched controls.5
A better understanding of the interactions among bone marrow fat, skeletal development, and systemic energy metabolism is essential to maximize skeletal development, and offset the accelerated age-dependent chronic disease trajectory in those with CP.11 Therefore, we provide a thorough review of the existing literature related to the bone marrow fat regulation of skeletal metabolism and cardiometabolic disease risk in individuals with CP and other populations. Importantly, while this review is focused on those with CP, the questions and ideas proposed here are relevant to other populations with physical disabilities or who are physically inactive.
Skeletal Status in Children with CP
Skeletal deficits in children with CP
Children with CP have a high incidence of low-energy fracture, with a fracture distribution of roughly 80% occurring in the lower extremities.22 This is in contrast to typically developing children, wherein roughly 80% of fractures occur in the upper extremities.38 The high susceptibility and unique distribution of fractures in children with CP may be explained by a weak and poorly developed skeleton, which becomes progressively worse throughout growth and development,39 especially of the lower extremities.39–41 Using magnetic resonance imaging, Modlesky and colleagues41 showed that nonambulatory children with CP exhibit a severely underdeveloped mid-femur size as indicated by 51 – 55% lower total, cortical, and bone marrow cavity volumes, up to 48% thinner cortical walls, and up to 71% lower estimates of bone strength compared to typically developing children. While the femur is the most commonly fractured bone, the distal femur is the most commonly fractured site in children with CP.22 This can be explained by a markedly underdeveloped trabecular bone microarchitecture at the distal femur compared to typically developing children,40,42 with the deficits being more pronounced with greater distance away from the growth plate within the metaphysis.40 Bone mineral density, the most commonly used surrogate of bone strength, has also been found to be significantly lower in children with CP.39,43
Bone marrow fat in children with CP
The lone study to report on bone marrow fat in those with CP showed that ambulatory children with CP, compared to sex-, age-, and race-matched typically developing children, had underdeveloped cortical bone architecture of the middle-third tibia and estimates of bone strength of nearly 33% lower. The bone deficits in children with CP were accompanied by a higher concentration of fat in the bone marrow, which was evident even after statistically controlling for tibia length, and with no group differences in bone marrow volume. Furthermore, the children with CP had a thinner cortical wall in the medial aspect of the bone.5 In typically developing children, bone and bone marrow fat mass both increase during growth. While ambulatory children with CP develop bone marrow volumes similar to that of typically developing children, they experience inadequate bone accrual and develop higher fat content within the bone marrow.5 In contrast, nonambulatory children with more severe forms of CP have been shown to have lower bone marrow volumes compared to typically developing children.41 While unsynchronized and inadequate bone development observed in children with CP may be the result of many factors (e.g., nutrition, hormonal, or side effects of medication), partial or complete skeletal unloading is largely to blame in the early years of life. However, the skeleton adapts to chronic disuse in ways that may be deleterious to skeletal acquisition, growth, and energy metabolism, including greater fat partitioning towards skeletal depots.5 While the mechanisms in children with CP are unknown, excess bone marrow fat is associated with an imbalance in regulatory pathways leading to skeletal pathophysiology,44 a process that may be independent of mechanical loading. However, even compared to children with less severe forms of CP, children with CP who are not independently ambulant or with severe forms of CP are at a high risk for developing low areal bone mineral density.45
Given the skeletal and bone marrow fat profiles in children with CP, clinically important questions, pertaining to physical medicine and rehabilitation, include: (1) Is the elevated bone marrow fat in children with CP metabolically distinct from typically developing children in terms of its lipid and inflammatory composition? (2) Does the elevated bone marrow fat suppress skeletal acquisition? (3) Or, is it deleterious to chronic disease development in children with CP? (4) If so, what are the mechanisms?
There is a lack of mechanistic studies of bone marrow fat, skeletal acquisition, and cardiometabolic disease risk in children with CP and other child-onset disabilities. Therefore, the remainder of this review will utilize investigations of adults with skeletal disease and animal models.
Bone Marrow Fat
Bone marrow occupies the majority of the marrow cavity followed by trabecular bone. Bone marrow exists predominantly as hematopoietic tissue with prenatal conversion or replacement by fat,46,47 which progressively fills the majority of the bone marrow cavity by the 3rd decade of life from a distal to proximal direction.48,49 Adipocytes are largely made up of lipid droplets which contain fatty acids and their metabolites that facilitate cellular metabolism to support physiological homeostasis and promote anabolic processes, like growth. In adults, there is a relationship between the amount of bone marrow fat in the axial (lumbar vertebrae ~54%) and appendicular (femur/tibia ~85%) sites.36 However, while total body, visceral, and subcutaneous fat depots tend to correlate with one another and with body size, the degree of bone marrow fat tends to be independent of body size and other fat depots.31,50–52 Although, not all studies agree.53,54 For example, patients with anorexia nervosa exhibit the opposite trend as they have low total body fat stores but high bone marrow fat.55 Taken together, bone marrow fat is a unique depot, and the extent to which it contributes to or is present in concert with musculoskeletal pathophysiology is still to be determined.
The link between bone and bone marrow fat
Seminal studies, as early as the 1970s, have shown an inverse relationship between trabecular bone and bone marrow fat in humans and animals,26–28 with decreases in bone and increases in bone marrow fat with age.56 This inverse relationship is consistent in the diaphysis in healthy young31–33 and older32 adults. Bone marrow-derived mesenchymal stem cells are pluripotent and can differentiate into an array of lineages.57 Within the bone marrow cavity, these progenitor cells have a bi-potential differentiation fate of an osteogenic or adipogenic program.58 Although filling of the bone marrow cavity by adipocytes was once considered a passive and inert process,26–28,59 it is now recognized that fat tissue has autocrine, paracrine, and classic endocrine effects. While little is known about how bone marrow fat contributes to metabolic and endocrine effects on organs, high levels of bone marrow fat are associated with markers of poor bone health54,56,60 and fracture.56,60
In healthy adults, bone marrow fat expansion could be due to partitioning of mesenchymal stem cell metabolism towards adipogenesis, resulting in a greater number of bone marrow adipocytes at the expense of osteoblasts, and therefore, reduced bone-forming potential. If this conjecture is true, then in a local region, a one-unit increase in bone marrow fat would coincide with a proportionate unit loss of bone-forming potential (i.e., osteoblasts), leading to a predictable inverse relationship between bone and bone marrow fat, which has been observed in healthy adults.31–33,61
In persons with skeletal disease (e.g., osteoporosis, idiopathic low bone mineral density), the mesenchymal stem cell lineage commitment may not be as simple as a switch from an osteogenic to an adipogenic program.61,62 Cohen et al.61 reported that premenopausal women with idiopathic osteoporosis or low bone mineral density (grouped as skeletal disease) had significantly compromised trabecular bone microarchitecture compared to controls. The skeletal disease group also had higher bone marrow adipocyte size, volume, and number compared to controls. Furthermore, there were inverse relationships between adipocyte volume with trabecular bone microarchitecture and bone formation rate in the controls, but no significant relationships were observed in the skeletal disease group. These data suggest a disconnect between the extent of bone marrow fat relative to bone metabolic deficits in skeletal disease. This may reflect a difference in mesenchymal stem cell metabolism and post-differentiation modifications causing enlargement of the adipocytes within the bone marrow microenvironment, thus masking the bone-fat relationship in those with skeletal disease.
Importantly, excess accrual of fat in the bone marrow occurs at the expense of more than just bone. Bone marrow is highly complex and composed of many different cellular lineages. In addition to bone and fat, bone marrow also contains hematopoietic tissue. Justesen et al.63 reported that iliac crest bone biopsies from men and women with osteoporosis present with a greater proportion of bone marrow fat volume concurrent with less bone and hematopoietic tissue volume. This suggests that bone marrow fat accumulates at the expense of bone and hematopoietic tissue. (Although hematopoietic tissue plays a pivotal role in skeletal metabolism, the focus of this review is on the direct correlates of bone marrow fat and bone, and will no longer discuss hematopoietic tissue. However, for additional information, see34,64,65.)
These anatomical studies have led to investigations attempting to unravel the mechanisms relating bone marrow fat to bone.
Bone marrow fat and its interaction with the microenvironment
Bone turnover during skeletal modeling and remodeling is governed by bone-forming osteoblasts and bone-resorbing osteoclasts, which are derived from mesenchymal and hematopoietic stem cells, respectively, within the bone marrow. These cells regulate one another in various stages of cellular development via direct and indirect regulatory factors.66–68 With regard to osteoblast regulation of osteoclasts, one important pathway involves the early and late osteoblast expression and release of receptor activator of NfκB ligand (RANKL) and osteoprotegerin (OPG), respectively. Through competitive binding to the same osteoclast progenitor RANK receptors, RANKL induces formation and maturation of osteoclasts whereas OPG inhibits these actions.
Recent evidence demonstrates the potential role of human bone marrow adipocytes in regulating osteoclastogenesis via osteoclast regulatory molecules.69,70 In vitro, mesenchymal-derived marrow adipocytes have revealed expression of RANKL and OPG that are capable of supporting osteoclast-like cell formation.71 Similarly, isolated bone marrow from osteoporotic women, a condition associated with high bone marrow fat,26 expresses higher markers of bone resorption compared to controls.44 In addition to competing with osteoblasts at the level of their shared stem cell,58 bone marrow fat impedes mature osteoblast function and mineralization by releasing lipid-specific factors72 and induces adipocyte characteristics in osteoblasts, resulting in a reduction of mature osteoblast secreted markers.73 This finding has led to the conclusion that osteoblasts can transdifferentiate into adipocytes,73–77 which would make this mechanism a prime target for interventions intended to increase bone mass. However, other evidence suggests that instead of osteoblasts transdifferentiating into adipocytes, osteoblasts have the capacity to take on bone marrow adipocyte characteristics, such as lipid accumulation and expressing adipocyte markers.73,78 These data suggest that the function of bone marrow fat tissue may not be exclusive to bone marrow adipocyte activity, as lipids can infiltrate and influence early and mature osteoblasts and osteoclasts.
Another candidate molecule to explore in those with CP is sclerostin, which is higher in nonambulatory than ambulatory adults with CP.20 Sclerostin is an osteocyte-derived molecule that blocks bone formation via the Wnt/β-catenin pathway and stimulates adipogenesis79 from bone marrow-derived mesenchymal stem cells.80 Mechanical loading downregulates sclerostin allowing for a cascade of events that activate Wnt signaling, leading to osteoblastogensis and bone-formation.81 On the other hand, while mechanical unloading induces adipogenesis at the expense of osteogenesis, hindlimb unloaded mice lacking sclerostin (Sost−/− on C57BL/6 background) do not exhibit bone loss,82 while administration of sclerostin antibodies rescues the unloading-induced bone loss in a dose-dependent manner.79
Taken together, these data show that bone marrow fat can have a negative association with the neighboring bone tissue. Adipocyte extracellular vesicles containing adipogenic factors are released onto specialized bone cells,83 altering their differentiation potential, function, and survival. It is debated whether bone marrow adipocytes are characteristically similar to white adipocytes, which store lipids and have a low mitochondrial density, or brown adipocytes, which dissipate energy and are rich in mitochondria. However, existing evidence suggests bone marrow fat has a unique combination of characteristics of both types of fat tissue,84,85 which differentially associate with metabolic regulation.86–89 The paracrine mechanisms of bone marrow fat-releasing factors on bone include the secretion of fatty acids, fatty acid metabolites, inflammatory cytokines, and adipokines. Different diseases and stages of development likely elicit unique secretome profiles from bone marrow adipocytes, with varying degrees of similarity to other fat depots (i.e., white, beige, or brown). Therefore, identifying specific patient-population bone marrow fat secretome profiles would provide a deeper understanding of its metabolic implications, especially in children with CP who exhibit suppressed skeletal acquisition throughout growth.39
Bone marrow lipid composition
Bone marrow fat is transcriptionally and characteristically different from other adipose tissues49,90,91 and across different skeletal sites.49,92 Lecka-Czernik and Stechschulte92 show that in the proximal tibia, fat is interspersed among trabecular bone, while the distal portion of the tibia is associated with higher fat and a dense, ring-like aggregation of fat within the endosteal surface. This finding was supported by a study demonstrating that postnatal development of the mouse tibia coincided with bone marrow fat expansion in the distal portion shortly after birth, which morphologically resembled white fat.49 With aging, bone marrow fat expansion continues to the middle and proximal portion of the tibia, but the cells are morphologically distinct from the distal tibia, i.e., small, single adipocytes spread amongst hematopoietic tissue. It is therefore likely that bone marrow fat has unique functions based on the localization and lipid composition of the depot, which are derived from different, but unknown origins.49,93
The skeleton is highly involved in nutrient uptake and clearance, especially of fatty acids,94 which are stored mostly in bone marrow, and to a lesser extent in mineralized tissue independent of cell membrane constituents.95 Bone marrow adipocytes are a depot for lipids containing predominantly triacylglycerol and trace amounts of cholesterol and phospholipids. Although there is conflicting evidence as to the fatty acid profile in human bone marrow,91,96 studies have reported an increasing unsaturated index (proportion of unsaturated to saturated fatty acids) from skeletal sites in the direction of the proximal femur to distal tibia.49,91 This is driven by a conversion of the saturated fatty acids, palmitic and stearic acid, to their monounsaturated derivatives.49
The unsaturated index has been suggested as a biomarker for osteoporosis,97 and is lower in the lumbar spine in postmenopausal women with osteopenia, osteoporosis,98 and fragility fractures99 compared to controls. On the other hand, Miranda et al.96 reported a higher unsaturated index of the iliac crest in osteoporotic women with vs. without fracture, and found no differences in overall fatty acid composition from bone marrow supernatant fluid among controls, osteopenic, and osteoporotic women. Griffith et al.91 reported a similar finding of no group difference in saturated vs. unsaturated fatty acid composition in a similar cohort of men and women, but found a lower monounsaturated fatty acid index in osteoporotic vs. osteopenic and control men and women in a mixed sample of bone marrow from lumbar, hip, and knee skeletal sites.
The conflicting evidence of the bone marrow fatty acid profiles and their relation to skeletal health in humans91,96–99 may be due to variations of fatty acid composition between and within axial and appendicular skeletal sites.49 For example, an in vivo imaging study found a negative and positive association between unsaturated index and total lipid content from femoral neck and calcaneus bone marrow fat, respectively, in postmenopausal women.97 Therefore, cohort studies examining the fatty acid profiles of different skeletal sites in typically developing children and healthy to osteoporotic men and women are needed to better understand site-specific variations of bone marrow fatty acid composition and how it relates to skeletal development and health.
Anatomical studies evaluating the degree of bone marrow fat in relation to skeletal health do not capture the physiological role of bone marrow fat on skeletal metabolism. The four most highly expressed fatty acids from bone marrow fat are palmitic, stearic, linoleic, and oleic acids.91,96 Palmitic and stearic acids are saturated fatty acids that are lipotoxic to bone,100,101 and are known to impair osteoblast function.102,103 However, in their monounsaturated forms, palmitoleic and oleic acids, respectively, are characterized as fat tissue-derived hormones that inhibit osteoclast formation and function,104 promote mesenchymal stem cell and osteoblast function,101 and have positive systemic metabolic homeostatic properties.105 Moreover, oleic acid-fortified milk consumption is associated with improved skeletal metabolism in adults.106,107 Linoleic acid is an unsaturated fatty acid that augments osteoblast differentiation, function, mineralization, and survival,103,108 and inhibits osteoclast differentiation and function.109 Therefore, the volume of bone marrow fat does not necessarily equate to adverse metabolic actions on the bone. The distal tibia has higher bone marrow fat than proximal skeletal sites49,92 concurrent with a higher unsaturated index,49,91 which may be due to lower proportions of anti-osteogenic saturated fatty acids and higher proportions of their osteogenic monounsaturated forms.49 Moreover, palmitoleic acid has been shown to be positively associated with bone mineral density in adults.91 Collectively, these results may indicate that bone marrow lipid composition may be more important than overall quantity in relation to skeletal health.
In addition to bone marrow lipids, locally produced lipid metabolites (e.g., prostaglandins) have extensive biological roles in bone cell functions on many levels, including regulation of numerous bone-specific signaling pathways. While the topic of lipid intermediates and metabolites on bone health is out of the scope of the current review, a very comprehensive review by During et al.110 outlines many facets of localized lipid action in relation to skeletal physiology. Future research is certainly warranted to better understand the role of lipids and their metabolites as early regulators of skeletal health in those with CP.
What remains to be fully understood is how the bone marrow fatty acid composition varies throughout the lifespan and interacts with certain disease states, such as CP. Further, whether the elevated bone marrow fat in children with CP is reflective of a fatty acid profile exhibiting a lower unsaturated index, and what specific fatty acids or lipid-producing metabolites are altered, requires further studies.
Bone marrow fat and chronic inflammation
In addition to the direct effects of altered bone marrow fatty acid composition impacting skeletal physiology, indirect effects may include excess production of inflammatory cytokines creating a deleterious lipotoxic environment.
Inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α), are rich in fat tissue and are associated with metabolic dysregulation111–114 and bone resorption by acting through the RANKL/RANK/OPG pathway.115–117 Halade et al.117,118 demonstrated that the femur and tibia of mice fed a high fat diet exhibited deteriorated trabecular bone microarchitecture, elevated bone marrow fat, elevated RANKL (osteoclast inducer) expression in the bone, and suppressed OPG (osteoclast inhibitor) within the bone marrow. These mice also had an increased gene expression of inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, downregulation of Runx2 (a primary osteogenic transcription factor), and upregulation of PPARγ (a primary adiogenic transcription factor) from the whole femur bone and bone marrow from the femur and tibia. However, the effect of inflammation on bone deterioration may have been mediated by systemic inflammation in addition to bone and bone marrow inflammation. Nevertheless, other studies have reported that altered bone marrow fat composition is associated with excess inflammatory cytokine release causing endoplasmic reticulum stress-induced apoptosis and impaired osteoblast function.100,101 The deleterious effect of elevated inflammation caused by altered bone marrow fatty acid composition can be reversed by intervening with unsaturated fatty acids,101,107,119–121 with further benefits of lower osteoclast activation107,119–121 resulting in higher bone mineral density.119,120 This notion is supported by a study that acquired bone marrow from osteoporotic women and found higher markers for bone resorption, IL-6, and TNF-α compared to controls.44 These data suggest a possible link between high levels or altered bone marrow fat composition, inflammation, and poor bone health.
Systemic inflammation among individuals with CP may be related to prolonged neurological injury processes.122 However, little is known about the inflammatory milieu after infancy,123,124 leading to speculative models of peripheral inflammatory response due to secondary pathologies associated with CP.10 It is well known that chronic inflammation precipitates cardiometabolic diseases as well as premature mortality in the general population. It is therefore of interest in determining if those with CP have bone marrow inflammation and the extent that bone marrow inflammation contributes to skeletal metabolism and systemic inflammation. Knowing this information may help to unravel tissue-specific contributions of inflammatory-related mechanisms leading to the accelerated musculoskeletal loss and cardiometabolic disease risk in adults with CP.
Cardiometabolic Disease
Adults with CP are at a heightened risk for multiple cardiometabolic diseases,12 which is associated with musculoskeletal deficits.11 Most studies investigating body composition in children with CP have found no difference in total body fat compared to controls.125–127 Using magnetic resonance imaging, we have reported no difference in total or subcutaneous fat volume of the lower extremities, but elevated skeletal muscle and bone marrow fat in children with CP,5,6 and elevated visceral fat in adults with CP13 compared to matched controls. Future research is needed to parse and quantify the distinct functional implications of these elevated fat depots on skeletal metabolism and cardiometabolic disease risk in those with CP. For example, although vertebral bone marrow and visceral fat correlate in premenopausal obese women, bone marrow fat was independently associated with a lower marker of systemic energy metabolism.53
Bone marrow fat and cardiometabolic disease in adults
Bone marrow fat contributes to approximately 10–15% of total fat, but is the major source of systemic adiponectin,128 which is an adipokine and biomarker of insulin resistance and cardiovascular disease.129 Paradoxically, obesity and insulin-resistance is coincident with lower adiponectin levels.130,131 The discordant bone marrow fat-adiponectin axis is not fully understood. Excess bone marrow fat may lead to adipocyte dysfunction and alter adiponectin metabolism,132 leading to glucose dysregulation. Indeed, bone marrow fat content has been shown to be inversely related to bone marrow glucose uptake35 and positively related to glycated hemoglobin levels37 and serum lipid measures.36 Studies in postmenopausal women with and with type 2 diabetes reported no difference in vertebral bone marrow fat between groups,37,99 but when the diabetic group was stratified by 7% glycated hemoglobin, a cut off value for diabetes, the group >7% had greater vertebral bone marrow fat than the group <7%.37 Moreover, diabetic women had a lower bone marrow unsaturated index,99 which further emphasizes the importance of evaluating bone marrow lipid composition rather than just quantity.
Glucose dysregulation predisposes cardiovascular disease-related mortality.133 Studies clinically investigating the interaction among bone marrow fat, glucose dysregulation, and cardiovascular disease are limited and tend to have small sample sizes or use animal models. Small animals have a lower proportion of bone marrow fat compared to humans134 and diabetes models are often studied untreated, which does not necessarily represent human physiology. Therefore, questions remain as to the role of bone marrow fat in systemic energy metabolism. In those with CP, a more comprehensive understanding of the role of bone marrow fat, in conjunction with other fat depots, in relation to cardiometabolic disease is needed.
Proposed mechanisms of bone marrow fat regulation of skeletal acquisition in children with CP
Children with CP have poor development of musculoskeletal mass, strength, and function, which is associated with greater fat infiltration.5,6 Inadequate mechanical loading likely precipitates poor skeletal development, but the subsequent expansion of bone marrow fat may further impede skeletal acquisition in an independent manner. To date, no studies have examined bone marrow fat dynamics in terms of lipid composition or inflammation and how it interacts with skeletal metabolism in those with CP. Further, elevated or altered bone marrow fat composition may potentially initiate or exacerbate the accelerated chronic disease trajectory observed in adults with CP,7–12 which is interrelated with musculoskeletal deficits.11
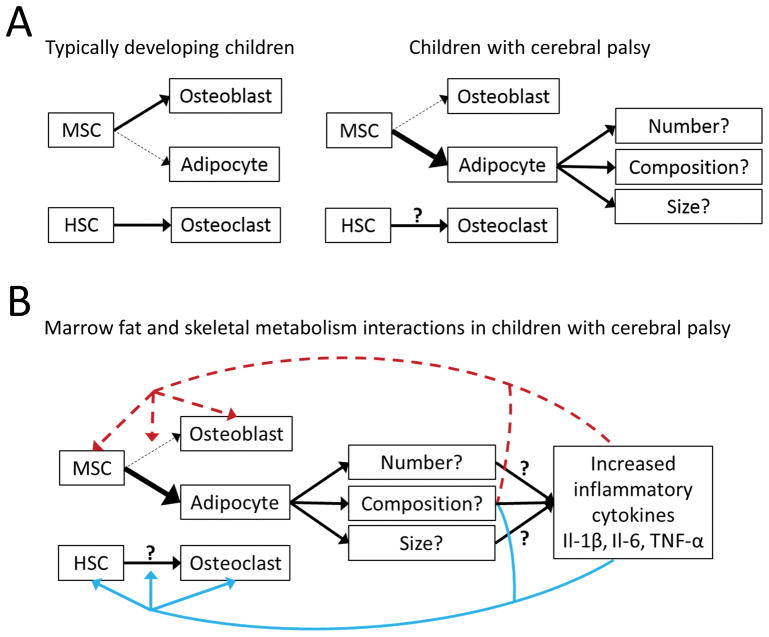
In light of the little that is actually known, clinically important questions are as follows: (1) Is the elevated bone marrow fat in children with CP metabolically distinct from typically developing children in terms of its lipid and inflammatory composition? (2) Does the elevated bone marrow fat suppress skeletal acquisition? (3) Or, is it deleterious to chronic disease development in children with CP? (4) If so, what are the mechanisms? Knowing this information will help guide further research into target-specific interventions. For example, if the elevated bone marrow fat in children with CP is not deleterious to the skeleton, then research focus needs to shift towards promoting osteogenic-interventions to ameliorate skeletal acquisition. On the other hand, if the elevated bone marrow fat is a negative regulator of the skeleton, future efforts must identify the mechanisms by which bone marrow fat is impeding skeletal development in CP. Figure 1 highlights a working model for potential mechanisms between bone marrow fat and skeletal metabolism in children with CP, based on the literature. It is unknown if the elevated bone marrow fat is due to a greater number or size of adipocytes or to a change in lipid composition, each potentially having a unique effect on local and systemic energy metabolism. It is also unknown if hematopoietic stem cell metabolism is altered in children with CP.
Figure 1.
(A) Simple diagram showing mesenchymal stem cell (MSC) and hematopoietic stem cell (HSC) differentiation. In typically developing children, MSCs favor osteogenic differentiation due to adequate loading, nutrition, hormonal milieu, and growth. In children with cerebral palsy, MSCs likely favor adipogenic differentiation due to multiple and complex factors. (B) Diagram showing potential mechanisms of elevated bone marrow fat on skeletal metabolism in children with cerebral palsy. Dashed red line indicates a suppressive role. Solid blue line indicates a stimulating role.
Conclusion
Given the inadequate musculoskeletal development in children with CP, and the high prevalence of cardiometabolic disease and multimorbidity in adults with CP,7–12 which is associated with musculoskeletal deficits,11 it is important to elucidate the extent to which bone marrow profiles in those with CP are related to skeletal acquisition, local and systemic energy metabolism, and cardiometabolic disease risk. This is especially true for children, because identifying and treating bone marrow alterations in childhood will have a significant impact on mitigating the development and/or exacerbation of chronic diseases in adults with CP. Necessary considerations for future research will need to consider the severity of disability for the skeletal pathophysiology profile and the stage of life, because a developing skeleton (childhood and adolescence) has a different metabolic demand, supply, and response to stimuli than does a developed skeleton (adulthood).
Acknowledgments
Daniel G. Whitney is supported by the University of Michigan Advanced Rehabilitation Research Training Program in Community Living and Participation from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) (90AR5020-0200). Mark D. Peterson is funded by the National Institutes of Health (NIH) (1KO1 HD074706) and NIDILRR (90IF0102-01). Christopher M. Modlesky is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH (R01 HD090126).
We thank Rachael V. Torres, University of Delaware, for critically reviewing the later versions of this manuscript.
Footnotes
All authors declare no conflict of interest.
There has been no previous presentation of this work.
Conflict of interest
All authors declare no conflict of interest.
References
- 1.Arneson CL, Durkin MS, Benedict RE, et al. Prevalence of cerebral palsy: Autism and Developmental Disabilities Monitoring Network, three sites, United States, 2004. Disability and health journal. 2009;2(1):45–48. doi: 10.1016/j.dhjo.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121(3):547–554. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 3.Kirby RS, Wingate MS, Van Naarden Braun K, et al. Prevalence and functioning of children with cerebral palsy in four areas of the United States in 2006: a report from the Autism and Developmental Disabilities Monitoring Network. Res Dev Disabil. 2011;32(2):462–469. doi: 10.1016/j.ridd.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Paneth N, Hong T, Korzeniewski S. The descriptive epidemiology of cerebral palsy. Clin Perinatol. 2006;33(2):251–267. doi: 10.1016/j.clp.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Whitney DG, Singh H, Miller F, et al. Cortical bone deficit and fat infiltration of bone marrow and skeletal muscle in ambulatory children with mild spastic cerebral palsy. Bone. 2017;94:90–97. doi: 10.1016/j.bone.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson DL, Miller F, Subramanian P, Modlesky CM. Adipose tissue infiltration of skeletal muscle in children with cerebral palsy. The Journal of pediatrics. 2009;154(5):715–720. doi: 10.1016/j.jpeds.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strauss D, Cable W, Shavelle R. Causes of excess mortality in cerebral palsy. Developmental medicine and child neurology. 1999;41(9):580–585. doi: 10.1017/s001216229900122x. [DOI] [PubMed] [Google Scholar]
- 8.Henderson RC, Lin PP, Greene WB. Bone-mineral density in children and adolescents who have spastic cerebral palsy. The Journal of bone and joint surgery American volume. 1995;77(11):1671–1681. doi: 10.2106/00004623-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Sheridan KJ. Osteoporosis in adults with cerebral palsy. Developmental medicine and child neurology. 2009;51(Suppl 4):38–51. doi: 10.1111/j.1469-8749.2009.03432.x. [DOI] [PubMed] [Google Scholar]
- 10.Peterson MD, Gordon PM, Hurvitz EA, Burant CF. Secondary muscle pathology and metabolic dysregulation in adults with cerebral palsy. American journal of physiology Endocrinology and metabolism. 2012;303(9):E1085–1093. doi: 10.1152/ajpendo.00338.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cremer N, Hurvitz EA, Peterson MD. Multimorbidity in Middle-Aged Adults with Cerebral Palsy. The American journal of medicine. 2017 doi: 10.1016/j.amjmed.2016.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson MD, Ryan JM, Hurvitz EA, Mahmoudi E. Chronic Conditions in Adults With Cerebral Palsy. JAMA. 2015;314(21):2303–2305. doi: 10.1001/jama.2015.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson MD, Zhang P, Haapala HJ, Wang SC, Hurvitz EA. Greater Adipose Tissue Distribution and Diminished Spinal Musculoskeletal Density in Adults With Cerebral Palsy. Archives of physical medicine and rehabilitation. 2015;96(10):1828–1833. doi: 10.1016/j.apmr.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kannus P, Haapasalo H, Sankelo M, et al. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123(1):27–31. doi: 10.7326/0003-4819-123-1-199507010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Modlesky CM, Lewis RD. Does exercise during growth have a long-term effect on bone health? Exercise and sport sciences reviews. 2002;30(4):171–176. doi: 10.1097/00003677-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Morgan P, McGinley J. Gait function and decline in adults with cerebral palsy: a systematic review. Disability and rehabilitation. 2014;36(1):1–9. doi: 10.3109/09638288.2013.775359. [DOI] [PubMed] [Google Scholar]
- 17.Stackhouse SK, Binder-Macleod SA, Lee SC. Voluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsy. Muscle & nerve. 2005;31(5):594–601. doi: 10.1002/mus.20302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiley ME, Damiano DL. Lower-extremity strength profiles in spastic cerebral palsy. Developmental medicine and child neurology. 1998;40(2):100–107. doi: 10.1111/j.1469-8749.1998.tb15369.x. [DOI] [PubMed] [Google Scholar]
- 19.Rose J, McGill KC. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Developmental medicine and child neurology. 2005;47(5):329–336. doi: 10.1017/s0012162205000629. [DOI] [PubMed] [Google Scholar]
- 20.Shin YK, Yoon YK, Chung KB, Rhee Y, Cho SR. Patients with non-ambulatory cerebral palsy have higher sclerostin levels and lower bone mineral density than patients with ambulatory cerebral palsy. Bone. 2017;103:302–307. doi: 10.1016/j.bone.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Kuperminc MN, Gurka MJ, Houlihan CM, et al. Puberty, statural growth, and growth hormone release in children with cerebral palsy. J Pediatr Rehabil Med. 2009;2(2):131–141. doi: 10.3233/PRM-2009-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Presedo A, Dabney KW, Miller F. Fractures in patients with cerebral palsy. Journal of pediatric orthopedics. 2007;27(2):147–153. doi: 10.1097/BPO.0b013e3180317403. [DOI] [PubMed] [Google Scholar]
- 23.Uddenfeldt Wort U, Nordmark E, Wagner P, Duppe H, Westbom L. Fractures in children with cerebral palsy: a total population study. Dev Med Child Neurol. 2013;55(9):821–826. doi: 10.1111/dmcn.12178. [DOI] [PubMed] [Google Scholar]
- 24.Trinh A, Wong P, Fahey MC, et al. Musculoskeletal and Endocrine Health in Adults With Cerebral Palsy: New Opportunities for Intervention. J Clin Endocrinol Metab. 2016;101(3):1190–1197. doi: 10.1210/jc.2015-3888. [DOI] [PubMed] [Google Scholar]
- 25.Rantalainen T, Nikander R, Heinonen A, Cervinka T, Sievanen H, Daly RM. Differential effects of exercise on tibial shaft marrow density in young female athletes. The Journal of clinical endocrinology and metabolism. 2013;98(5):2037–2044. doi: 10.1210/jc.2012-3748. [DOI] [PubMed] [Google Scholar]
- 26.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clinical orthopaedics and related research. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Minaire P, Edouard C, Arlot M, Meunier PJ. Marrow changes in paraplegic patients. Calcified tissue international. 1984;36(3):338–340. doi: 10.1007/BF02405340. [DOI] [PubMed] [Google Scholar]
- 28.Martin RB, Zissimos SL. Relationships between marrow fat and bone turnover in ovariectomized and intact rats. Bone. 1991;12(2):123–131. doi: 10.1016/8756-3282(91)90011-7. [DOI] [PubMed] [Google Scholar]
- 29.Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC. Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. The Journal of clinical investigation. 1996;97(7):1732–1740. doi: 10.1172/JCI118600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. Journal of cell science. 1992;102( Pt 2):341–351. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 31.Di Iorgi N, Mo AO, Grimm K, Wren TA, Dorey F, Gilsanz V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. The Journal of clinical endocrinology and metabolism. 2010;95(6):2977–2982. doi: 10.1210/jc.2009-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. The Journal of clinical endocrinology and metabolism. 2011;96(3):782–786. doi: 10.1210/jc.2010-1922. [DOI] [PubMed] [Google Scholar]
- 33.Shen W, Velasquez G, Chen J, et al. Comparison of the relationship between bone marrow adipose tissue and volumetric bone mineral density in children and adults. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2014;17(1):163–169. doi: 10.1016/j.jocd.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huovinen V, Saunavaara V, Kiviranta R, et al. Vertebral bone marrow glucose uptake is inversely associated with bone marrow fat in diabetic and healthy pigs: [(18)F]FDG-PET and MRI study. Bone. 2014;61:33–38. doi: 10.1016/j.bone.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 36.Slade JM, Coe LM, Meyer RA, McCabe LR. Human bone marrow adiposity is linked with serum lipid levels not T1-diabetes. Journal of diabetes and its complications. 2012;26(1):1–9. doi: 10.1016/j.jdiacomp.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Baum T, Yap SP, Karampinos DC, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? Journal of magnetic resonance imaging : JMRI. 2012;35(1):117–124. doi: 10.1002/jmri.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furlano RI, Bloechliger M, Jick H, Meier CR. Bone fractures in children with autistic spectrum disorder. Journal of developmental and behavioral pediatrics : JDBP. 2014;35(6):353–359. doi: 10.1097/DBP.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 39.Henderson RC, Lark RK, Gurka MJ, et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110(1 Pt 1):e5. doi: 10.1542/peds.110.1.e5. [DOI] [PubMed] [Google Scholar]
- 40.Modlesky CM, Whitney DG, Singh H, Barbe MF, Kirby JT, Miller F. Underdevelopment of trabecular bone microarchitecture in the distal femur of nonambulatory children with cerebral palsy becomes more pronounced with distance from the growth plate. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2015;26(2):505–512. doi: 10.1007/s00198-014-2873-4. [DOI] [PubMed] [Google Scholar]
- 41.Modlesky CM, Kanoff SA, Johnson DL, Subramanian P, Miller F. Evaluation of the femoral midshaft in children with cerebral palsy using magnetic resonance imaging. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20(4):609–615. doi: 10.1007/s00198-008-0718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modlesky CM, Subramanian P, Miller F. Underdeveloped trabecular bone microarchitecture is detected in children with cerebral palsy using high-resolution magnetic resonance imaging. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19(2):169–176. doi: 10.1007/s00198-007-0433-x. [DOI] [PubMed] [Google Scholar]
- 43.Ihkkan DY, Yalcin E. Changes in skeletal maturation and mineralization in children with cerebral palsy and evaluation of related factors. Journal of child neurology. 2001;16(6):425–430. doi: 10.1177/088307380101600608. [DOI] [PubMed] [Google Scholar]
- 44.Pino AM, Rios S, Astudillo P, et al. Concentration of adipogenic and proinflammatory cytokines in the bone marrow supernatant fluid of osteoporotic women. J Bone Miner Res. 2010;25(3):492–498. doi: 10.1359/jbmr.090802. [DOI] [PubMed] [Google Scholar]
- 45.Nazif H, Shatla R, Elsayed R, et al. Bone mineral density and insulin-like growth factor-1 in children with spastic cerebral palsy. Childs Nerv Syst. 2017;33(4):625–630. doi: 10.1007/s00381-017-3346-9. [DOI] [PubMed] [Google Scholar]
- 46.Emery JL, Follett GF. Regression of Bone-Marrow Haemopoiesis from the Terminal Digits in the Foetus and Infant. Br J Haematol. 1964;10:485–489. doi: 10.1111/j.1365-2141.1964.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 47.Hudson G. Bone-Marrow Volume in the Human Foetus and Newborn. Br J Haematol. 1965;11:446–452. doi: 10.1111/j.1365-2141.1965.tb06607.x. [DOI] [PubMed] [Google Scholar]
- 48.Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology. 1990;175(1):219–223. doi: 10.1148/radiology.175.1.2315484. [DOI] [PubMed] [Google Scholar]
- 49.Scheller EL, Doucette CR, Learman BS, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6:7808. doi: 10.1038/ncomms8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Iorgi N, Mittelman SD, Gilsanz V. Differential effect of marrow adiposity and visceral and subcutaneous fat on cardiovascular risk in young, healthy adults. Int J Obes (Lond) 2008;32(12):1854–1860. doi: 10.1038/ijo.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab. 2008;93(6):2281–2286. doi: 10.1210/jc.2007-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18(5):641–647. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bredella MA, Torriani M, Ghomi RH, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19(1):49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen W, Chen J, Gantz M, et al. Ethnic and sex differences in bone marrow adipose tissue and bone mineral density relationship. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23(9):2293–2301. doi: 10.1007/s00198-011-1873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bredella MA, Fazeli PK, Miller KK, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG. Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology. 2000;217(2):527–538. doi: 10.1148/radiology.217.2.r00nv20527. [DOI] [PubMed] [Google Scholar]
- 57.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 58.David V, Martin A, Lafage-Proust MH, et al. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148(5):2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 59.Gimble JM. The function of adipocytes in the bone marrow stroma. The New biologist. 1990;2(4):304–312. [PubMed] [Google Scholar]
- 60.Schwartz AV, Sigurdsson S, Hue TF, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. The Journal of clinical endocrinology and metabolism. 2013;98(6):2294–2300. doi: 10.1210/jc.2012-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen A, Dempster DW, Stein EM, et al. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. The Journal of clinical endocrinology and metabolism. 2012;97(8):2782–2791. doi: 10.1210/jc.2012-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19(9):1323–1330. doi: 10.1007/s00198-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 64.Fazeli PK, Horowitz MC, MacDougald OA, et al. Marrow fat and bone--new perspectives. J Clin Endocrinol Metab. 2013;98(3):935–945. doi: 10.1210/jc.2012-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou BO, Yu H, Yue R, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19(8):891–903. doi: 10.1038/ncb3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16(9):1575–1582. doi: 10.1359/jbmr.2001.16.9.1575. [DOI] [PubMed] [Google Scholar]
- 67.Andersen TL, Sondergaard TE, Skorzynska KE, et al. A physical mechanism for coupling bone resorption and formation in adult human bone. The American journal of pathology. 2009;174(1):239–247. doi: 10.2353/ajpath.2009.080627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tamma R, Zallone A. Osteoblast and osteoclast crosstalks: from OAF to Ephrin. Inflammation & allergy drug targets. 2012;11(3):196–200. doi: 10.2174/187152812800392670. [DOI] [PubMed] [Google Scholar]
- 69.Goto H, Osaki M, Fukushima T, et al. Human bone marrow adipocytes support dexamethasone-induced osteoclast differentiation and function through RANKL expression. Biomedical research. 2011;32(1):37–44. doi: 10.2220/biomedres.32.37. [DOI] [PubMed] [Google Scholar]
- 70.Hozumi A, Osaki M, Goto H, Sakamoto K, Inokuchi S, Shindo H. Bone marrow adipocytes support dexamethasone-induced osteoclast differentiation. Biochemical and biophysical research communications. 2009;382(4):780–784. doi: 10.1016/j.bbrc.2009.03.111. [DOI] [PubMed] [Google Scholar]
- 71.Holt V, Caplan AI, Haynesworth SE. Identification of a subpopulation of marrow MSC-derived medullary adipocytes that express osteoclast-regulating molecules: marrow adipocytes express osteoclast mediators. PloS one. 2014;9(10):e108920. doi: 10.1371/journal.pone.0108920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang D, Haile A, Jones LC. Dexamethasone-induced lipolysis increases the adverse effect of adipocytes on osteoblasts using cells derived from human mesenchymal stem cells. Bone. 2013;53(2):520–530. doi: 10.1016/j.bone.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 73.Clabaut A, Delplace S, Chauveau C, Hardouin P, Broux O. Human osteoblasts derived from mesenchymal stem cells express adipogenic markers upon coculture with bone marrow adipocytes. Differentiation. 2010;80(1):40–45. doi: 10.1016/j.diff.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y, Strecker S, Wang L, et al. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One. 2013;8(8):e71318. doi: 10.1371/journal.pone.0071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song L, Liu M, Ono N, Bringhurst FR, Kronenberg HM, Guo J. Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res. 2012;27(11):2344–2358. doi: 10.1002/jbmr.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PLoS One. 2014;9(1):e85161. doi: 10.1371/journal.pone.0085161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2(1):35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 78.McGee-Lawrence ME, Carpio LR, Schulze RJ, et al. Hdac3 Deficiency Increases Marrow Adiposity and Induces Lipid Storage and Glucocorticoid Metabolism in Osteochondroprogenitor Cells. J Bone Miner Res. 2016;31(1):116–128. doi: 10.1002/jbmr.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tian X, Jee WS, Li X, Paszty C, Ke HZ. Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone. 2011;48(2):197–201. doi: 10.1016/j.bone.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 80.Fairfield H, Falank C, Harris E, et al. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol. 2017 doi: 10.1002/jcp.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tu X, Rhee Y, Condon KW, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50(1):209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin C, Jiang X, Dai Z, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24(10):1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 83.Martin PJ, Haren N, Ghali O, et al. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs) BMC Cell Biol. 2015;16:10. doi: 10.1186/s12860-015-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50(2):546–552. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2012;50(2):534–539. doi: 10.1016/j.bone.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kontani Y, Wang Y, Kimura K, et al. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell. 2005;4(3):147–155. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 87.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11(4):268–272. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Enerback S. Human brown adipose tissue. Cell Metab. 2010;11(4):248–252. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 89.Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes. 2015;64(7):2361–2368. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poloni A, Maurizi G, Serrani F, et al. Molecular and functional characterization of human bone marrow adipocytes. Exp Hematol. 2013;41(6):558–566 e552. doi: 10.1016/j.exphem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 91.Griffith JF, Yeung DK, Ahuja AT, et al. A study of bone marrow and subcutaneous fatty acid composition in subjects of varying bone mineral density. Bone. 2009;44(6):1092–1096. doi: 10.1016/j.bone.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 92.Lecka-Czernik B, Stechschulte LA. Bone and fat: a relationship of different shades. Arch Biochem Biophys. 2014;561:124–129. doi: 10.1016/j.abb.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 93.Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015;3(2):141–147. doi: 10.1016/S2213-8587(14)70007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bartelt A, Koehne T, Todter K, et al. Quantification of Bone Fatty Acid Metabolism and Its Regulation by Adipocyte Lipoprotein Lipase. Int J Mol Sci. 2017;18(6) doi: 10.3390/ijms18061264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prout RE, Odutuga AA, Tring FC. Lipid analysis of rat enamel and dentine. Arch Oral Biol. 1973;18(3):373–380. doi: 10.1016/0003-9969(73)90161-1. [DOI] [PubMed] [Google Scholar]
- 96.Miranda M, Pino AM, Fuenzalida K, Rosen CJ, Seitz G, Rodriguez JP. Characterization of Fatty Acid Composition in Bone Marrow Fluid From Postmenopausal Women: Modification After Hip Fracture. J Cell Biochem. 2016;117(10):2370–2376. doi: 10.1002/jcb.25534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Di Pietro G, Capuani S, Manenti G, et al. Bone Marrow Lipid Profiles from Peripheral Skeleton as Potential Biomarkers for Osteoporosis: A 1H-MR Spectroscopy Study. Acad Radiol. 2016;23(3):273–283. doi: 10.1016/j.acra.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 98.Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005;22(2):279–285. doi: 10.1002/jmri.20367. [DOI] [PubMed] [Google Scholar]
- 99.Patsch JM, Li X, Baum T, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28(8):1721–1728. doi: 10.1002/jbmr.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alsahli A, Kiefhaber K, Gold T, et al. Palmitic Acid Reduces Circulating Bone Formation Markers in Obese Animals and Impairs Osteoblast Activity via C16-Ceramide Accumulation. Calcif Tissue Int. 2016;98(5):511–519. doi: 10.1007/s00223-015-0097-z. [DOI] [PubMed] [Google Scholar]
- 101.Gillet C, Spruyt D, Rigutto S, et al. Oleate Abrogates Palmitate-Induced Lipotoxicity and Proinflammatory Response in Human Bone Marrow-Derived Mesenchymal Stem Cells and Osteoblastic Cells. Endocrinology. 2015;156(11):4081–4093. doi: 10.1210/en.2015-1303. [DOI] [PubMed] [Google Scholar]
- 102.Gunaratnam K, Vidal C, Gimble JM, Duque G. Mechanisms of palmitate-induced lipotoxicity in human osteoblasts. Endocrinology. 2014;155(1):108–116. doi: 10.1210/en.2013-1712. [DOI] [PubMed] [Google Scholar]
- 103.Elbaz A, Wu X, Rivas D, Gimble JM, Duque G. Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J Cell Mol Med. 2010;14(4):982–991. doi: 10.1111/j.1582-4934.2009.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Heerden B, Kasonga A, Kruger MC, Coetzee M. Palmitoleic Acid Inhibits RANKL-Induced Osteoclastogenesis and Bone Resorption by Suppressing NF-kappaB and MAPK Signalling Pathways. Nutrients. 2017;9(5) doi: 10.3390/nu9050441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134(6):933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martin-Bautista E, Munoz-Torres M, Fonolla J, Quesada M, Poyatos A, Lopez-Huertas E. Improvement of bone formation biomarkers after 1-year consumption with milk fortified with eicosapentaenoic acid, docosahexaenoic acid, oleic acid, and selected vitamins. Nutr Res. 2010;30(5):320–326. doi: 10.1016/j.nutres.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Fonolla-Joya J, Reyes-Garcia R, Garcia-Martin A, Lopez-Huertas E, Munoz-Torres M. Daily Intake of Milk Enriched with n-3 Fatty Acids, Oleic Acid, and Calcium Improves Metabolic and Bone Biomarkers in Postmenopausal Women. J Am Coll Nutr. 2016;35(6):529–536. doi: 10.1080/07315724.2014.1003114. [DOI] [PubMed] [Google Scholar]
- 108.Platt ID, El-Sohemy A. Regulation of osteoblast and adipocyte differentiation from human mesenchymal stem cells by conjugated linoleic acid. J Nutr Biochem. 2009;20(12):956–964. doi: 10.1016/j.jnutbio.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 109.Rahman MM, Halade GV, Williams PJ, Fernandes G. t10c12-CLA maintains higher bone mineral density during aging by modulating osteoclastogenesis and bone marrow adiposity. J Cell Physiol. 2011;226(9):2406–2414. doi: 10.1002/jcp.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.During A, Penel G, Hardouin P. Understanding the local actions of lipids in bone physiology. Prog Lipid Res. 2015;59:126–146. doi: 10.1016/j.plipres.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 111.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunological reviews. 2012;249(1):218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell metabolism. 2012;15(1):10–18. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 113.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiological reviews. 2013;93(1):359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 114.Jansen HJ, van Essen P, Koenen T, et al. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology. 2012;153(12):5866–5874. doi: 10.1210/en.2012-1625. [DOI] [PubMed] [Google Scholar]
- 115.Cao JJ. Effects of obesity on bone metabolism. Journal of orthopaedic surgery and research. 2011;6:30. doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 117.Halade GV, El Jamali A, Williams PJ, Fajardo RJ, Fernandes G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Experimental gerontology. 2011;46(1):43–52. doi: 10.1016/j.exger.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. 2010;21(12):1162–1169. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bhattacharya A, Rahman M, Sun D, Fernandes G. Effect of fish oil on bone mineral density in aging C57BL/6 female mice. J Nutr Biochem. 2007;18(6):372–379. doi: 10.1016/j.jnutbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 120.Rahman MM, Bhattacharya A, Banu J, Kang JX, Fernandes G. Endogenous n-3 fatty acids protect ovariectomy induced bone loss by attenuating osteoclastogenesis. J Cell Mol Med. 2009;13(8B):1833–1844. doi: 10.1111/j.1582-4934.2008.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yuan J, Akiyama M, Nakahama K, Sato T, Uematsu H, Morita I. The effects of polyunsaturated fatty acids and their metabolites on osteoclastogenesis in vitro. Prostaglandins Other Lipid Mediat. 2010;92(1–4):85–90. doi: 10.1016/j.prostaglandins.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 122.Fleiss B, Gressens P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurol. 2012;11(6):556–566. doi: 10.1016/S1474-4422(12)70058-3. [DOI] [PubMed] [Google Scholar]
- 123.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F153–161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rezaie P, Dean A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology. 2002;22(3):106–132. doi: 10.1046/j.1440-1789.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- 125.Walker JL, Bell KL, Stevenson RD, Weir KA, Boyd RN, Davies PS. Differences in body composition according to functional ability in preschool-aged children with cerebral palsy. Clinical nutrition. 2015;34(1):140–145. doi: 10.1016/j.clnu.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 126.Bell KL, Davies PS. Energy expenditure and physical activity of ambulatory children with cerebral palsy and of typically developing children. The American journal of clinical nutrition. 2010;92(2):313–319. doi: 10.3945/ajcn.2010.29388. [DOI] [PubMed] [Google Scholar]
- 127.Stallings VA, Zemel BS, Davies JC, Cronk CE, Charney EB. Energy expenditure of children and adolescents with severe disabilities: a cerebral palsy model. The American journal of clinical nutrition. 1996;64(4):627–634. doi: 10.1093/ajcn/64.4.627. [DOI] [PubMed] [Google Scholar]
- 128.Cawthorn WP, Scheller EL, Learman BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20(2):368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2(3):133–141. doi: 10.1016/j.molmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de Araujo IM, Salmon CE, Nahas AK, Nogueira-Barbosa MH, Elias J, Jr, de Paula FJ. Marrow adipose tissue spectrum in obesity and type 2 diabetes mellitus. Eur J Endocrinol. 2017;176(1):21–30. doi: 10.1530/EJE-16-0448. [DOI] [PubMed] [Google Scholar]
- 131.Kern P, Di Gregorio G, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: Relationship to obesity, insulin resistance and TNF alpha expression. Diabetes. 2003;52:A329–A329. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 132.Scheller EL, Burr AA, MacDougald OA, Cawthorn WP. Inside out: Bone marrow adipose tissue as a source of circulating adiponectin. Adipocyte. 2016;5(3):251–269. doi: 10.1080/21623945.2016.1149269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PloS one. 2012;7(12):e52036. doi: 10.1371/journal.pone.0052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA. Marrow Adipose Tissue: Trimming the Fat. Trends Endocrinol Metab. 2016;27(6):392–403. doi: 10.1016/j.tem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]