Abstract
Objective
People living with HIV (PLWH) are more likely to report sleep difficulties and cognitive deficits. While cognitive impairment associated with sleep problems have been found in healthy and medical populations, less is known about the effects of poor sleep health (SH) on cognition among PLWH. This study examined differences in cognitive performance among participants classified based upon their HIV status and reported SH.
Methods
One hundred and sixteen (N = 116) adults recruited from the Greater Los Angeles community were administered a comprehensive cognitive test battery and completed a questionnaire about SH. Participants were classified into the following HIV/SH groups: [HIV+/good sleep health (SH+; n = 34); HIV−/SH+ (n = 32); HIV−/poor sleep health (SH−; n = 18) and HIV+/SH− (n = 32)].
Results
For both HIV+ and HIV− individuals, poor SH was associated with lower cognitive performance, with the domains of learning and memory driving the overall relationship. The HIV+/SH− group had poorer scores in domains of learning and memory compared to the SH+ groups. Additionally, the HIV−/SH− group demonstrated poorer learning compared to the HIV−/SH+ group.
Conclusions
Our findings suggest that sleep problems within medical populations are relevant to cognitive functioning, highlighting the clinical and scientific importance of monitoring sleep health and cognition to help identify individuals at greatest risk of poor health outcomes. Longitudinal investigations utilizing both objective and subjective measures of sleep are needed to determine the robustness of the current findings and the enduring effects of poor SH in the context of chronic disease.
Keywords: HIV, sleep health, cognition, learning, memory, chronic disease
The adverse effects of poor sleep have been well-documented in healthy populations, with studies associating it with greater likelihood of developing cognitive impairment (Fortier-Brochu, Beaulieu-Bonneau, Ivers, & Morin, 2012; Joo, Kim, Suh, & Hong, 2014) and dementia in older adults (Chen, Lee, Sun, Oyang, & Fuh, 2012; Cipriani, Lucetti, Danti, & Nuti, 2015; Cricco, Simonsick, & Foley, 2001; Foley et al., 2001). While some studies have associated deficits in attention (Chee, Tan, Parimal, & Zagorodnov, 2010) and executive function (Fernandez-Mendoza et al., 2010; Shekleton et al., 2014) with poor sleep, a growing body of literature also demonstrates the adverse effects of poor sleep on memory performance (Backhaus et al., 2006; Griessenberger et al., 2013; Joo et al., 2014; Walker & Stickgold, 2004). In their meta-analysis of the neuropsychological difference between primary insomniacs and normal sleepers, Fortier-Brochu et al. (2012) found cognitive performance impairments in insomniacs with evidence of deficits in episodic memory, problem solving, and the retention and manipulation of working memory. Though studies suggest that attention directly affects memory encoding (Chun & Turk-Browne, 2007), Fortier-Brochu and colleagues found no significant group differences in certain attention domains (e.g., alertness, complex reaction time, speed of information processing, selective attention, sustained attention/vigilance).
People living with HIV (PLWH) show a high prevalence of sleep problems (Lee et al., 2009; Rubinstein & Selwyn, 1998) with estimates ranging from 56% – 73% of PLWH reporting poor sleep compared to 10% – 30% of those in the general population (National Sleep Foundation, 2005). Sleep health (SH) is a broad term that refers to a multidimensional pattern of sleep-wakefulness that promotes well-being when adapted to individual, social, and environmental demands (Buysse, 2014). Good sleep health is characterized by subjective satisfaction, adequate duration, high efficiency, appropriate timing, and sustained alertness during waking hours. In comparison to healthy individuals, PLWH report worse SH that manifests in symptoms such as daytime sleepiness, fatigue and overall poor sleep quality (Darko, McCutchan, Kripke, Gillin, & Golshan, 1992; Darko et al., 1995; Low, Preud’ homme, Goforth, Omonuwa, & Krystal, 2011; Norman et al., 1988; Wibbeler, Reichelt, Husstedt, & Evers, 2012). Though problems with SH are commonly reported among PLWH, it is unclear whether poor sleep is a sequelae of disease pathology (White et al., 1995) or a side effect of antiretroviral (ARV) medication (Jena, Sachdeva, Sharma, & Wanchu, 2009; Kenedi & Goforth, 2011; Simen et al., 2015).
PLWH also show a high prevalence of cognitive impairment (Heaton et al., 2011; Sanmarti et al., 2014). However, despite the known relationship between sleep problems and cognitive impairment, the contribution of poor sleep to impairment in cognition among PLWH remains a relatively unexplored area of research. Gamaldo and colleagues (2013) investigated the associations between objective and subjective measures of sleep quality and cognitive functioning within a sample of PLWH, and found both subjective and objective indices of sleep continuity and quality to have a significant relationship with cognitive performance in HIV-positive (HIV+) individuals. In another study, Byun, Gay, and Lee (2016) found poorer self-reported sleep quality to be associated with poorer self-reported cognitive functions in a sample of adults living with HIV. Although the relationship between HIV and cognitive impairment has been well-documented—with HIV+ individuals showcasing poorer overall cognitive functioning in areas such as attention, memory, concentration, psychomotor and executive functioning (Becker, Thames, Woo, Castellon, & Hinkin, 2011; Heaton et al., 2011)—no study to our knowledge has included a HIV-negative (HIV−) control group to compare the adverse effects poor SH on neurocognitive function among individuals with and without HIV-infection.
The purpose of the current study was to characterize HIV+ and HIV− individuals based upon their self-reported SH and to examine differences in cognitive performance. We hypothesized that HIV+ individuals with poor SH would perform significantly worse on cognitive performance compared with the other groups.
Method
Participants
The University of California, Los Angeles Institutional Review Board approved study procedures and participants signed informed consent forms before participation. One hundred and sixteen HIV+ (n = 66) and HIV− (n = 50) participants were recruited from HIV clinics and the local community through local advertisements. Questionnaires and screeners about medical, neurological and psychiatric history were used to screen for potential confounds (see Thames et al., 2017). Briefly, participants were screened for neurological, psychiatric, and medical confounds using the Structured Clinical Interview (SCID) for DSM-IV (First, Spitzer, Gibbon, & Williams, 1995), Mini-mental Status Exam (Folstein, Folstein, & McHugh, 1975), and questionnaires about neurological and medical history. Participants were excluded if they reported a history of head injury with a loss of consciousness (> 30 min); neurological insult (e.g., seizures); HIV-associated CNS opportunistic infections (e.g., CNS lymphoma); met diagnostic criteria for alcohol or substance dependence within the past year, and/or substance abuse within the past year (except alcohol and marijuana), current or past diagnosis of psychotic-spectrum disorder (per SCID); or MRI contraindications.
Neurocognitive functioning, sleep health, and immune status
Participants were administered a brief cognitive test battery used in prior studies (Thames et al., 2016; 2017). Briefly, the battery included tests of premorbid intellectual ability (Wide Range Achievement Test – 4th edition [WRAT-4; Wilkinson & Robertson, 2004]), and cognitive tests classified into the domains of verbal fluency (Controlled Oral Word Association Test [Letter and Category Fluency]), executive functioning (Trail Making Test – Part B; Wechsler Adult Intelligence Scale – IV [WAIS-IV; Wechsler, 2008] Letter-Number Sequencing subtest; Stroop Test [Interference condition; Golden & Freshwater, 1978]), information processing speed (Trail Making Test – Part A; Stroop Test [Color and Word conditions; Reitan, 1958]; WAIS-IV Digit Symbol and Symbol Search subtests), learning (Brief Visual Memory Test-Revised [BVMT-R; Benedict, 1997]; Hopkins Verbal Learning Test-Revised [HVLT-R; Brandt & Benedict, 2001] Immediate subtest), and memory (Brief Visual Memory Test-Revised [BVMT-R]; Hopkins Verbal Learning Test-Revised [HVLT-R] Delayed subtest). All raw scores were converted to standardized t scores using published normative procedures and demographically corrected norms when available (Heaton et al., 2004). We also obtained a global cognitive score by averaging the domain t scores, which is considered a standard approach to neurocognitive data interpretation (Heaton, Grant & Matthews, 1991; Miller and Rohling, 2001).
Sleep health was assessed using the SATED scale (Buysse, 2014), which measures the quality of five dimensions of sleep within the last 30 days (i.e., Satisfaction with sleep; Alertness during waking hours; Timing of sleep; Sleep Efficiency; and Sleep Duration), along with a question addressing consistent sleep-wake cycles during the past month. A total score (i.e., “Overall Sleep Health”) is computed, which ranges from 0 – 12, with higher scores representing better SH [HIV/SH group range = 2–12]. A subset of the participants (N = 67; HIV+ = 36, HIV− = 31) also completed the Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), a psychometrically validated 19-item retrospective assessment of sleep quality over a 1-month period, with a global score ranging from 0 to 21 (higher scores indicate poorer sleep quality). Note, this subset was reflective of the overall sample, with no significant difference on demographic variables (p’s > .05); however, consistent with the overall sample of N=116, the HIV+/SH− group demonstrated greater depression. PSQI global score was used to determine the ideal cutoff for the SATED scale for the purposes of group classification, as described in detail below. The AIDS Clinical Trials Group (ACTG; Chesney et al., 2000) Adherence Baseline Questionnaire was also administered and current depression was assessed by the Beck Depression Inventory-2, with the sleep item excluded from the total score [BDI-II; Beck, Steer, & Brown, 1996]). Participants underwent blood draw to confirm HIV status. Additional laboratory testing of CD4 and HIV viral load was conducted for the HIV+ participants.
Statistical Analyses
A cut-off score of half a standard deviation (SD) below the mean on the SATED was used to classify participants into distinct HIV/SH groups: HIV+/SH+ (n = 34), HIV−/SH+ (n = 32), HIV−/SH− (n = 18), and HIV+/SH− (n = 32). This cut-point was determined based upon its strongest predictive relationship to the PSQI. First, we correlated the PSQI score with the SATED total score. Correlations between the total score from the SATED and the PSQI were statistically significant (r = −.472; p < .001). Next, using an established cut-off score of 8 for the PSQI (Carpenter & Andrykowski, 1998; Fichtenberg, Putnam, Mann, Zafonte, & Millard, 2001) we categorized participants as poor sleepers [0] or good sleepers [1]. Then, discriminant functions analysis using the leave-one-out (LOO) jackknife classification was used to determine which SATED score best classified participants into sleep problem categories on the PSQI. The cutoff score of 6 (i.e., 0.5 SD below mean) best classified group membership. The SATED cutoff score correctly classified self-reported sleep problems in 77% of cases on the PSQI. Additionally, results from a receiver operation characteristic (ROC) curve analysis demonstrated adequate sensitivity and specificity of the SATED scale, reflected by an area under curve of .80 (p < .001; CI: .69, .90).
Independent samples T-tests, one-way analysis of variance (ANOVA) and Chi-square analyses were conducted to examine demographic and clinical characteristics between groups. We used ANOVA and multivariate ANOVA (MANOVA) to examine differences between HIV/SH groups on global neurocognitive functioning and individual cognitive domains.
Results
Demographic Comparison
Of note, for demographic comparisons described below, SH was treated as a continuous variable and log-transformed due to a skewed distribution. Irrespective of SH status, HIV groups did not significantly differ in age, education, estimated premorbid IQ, and race/ethnicity (p’s > 0.10). However, there were significant differences in gender [χ2 (2) = 7.19, p = .03] with significantly greater proportion of males and transgendered (male-to-female) participants in the HIV+ group compared to HIV-controls. Gender was not significantly related to SH and cognitive outcome variables of interest, and gender did not significantly differ between the HIV/SH groups (all p’s > .10). Therefore, we did not include gender as a covariate in statistical analyses. Independent samples t-test demonstrated a significant difference between the HIV status groups on SH [t (114) = −2.22, p = 0.03], current depression [t (114) = 2.10, p = .04] and past (i.e., >12 months since study participation) marijuana [χ2 (1) = 6.34, p = .01] and cocaine use [χ2 (1) = 6.43, p = .01], with the HIV+ group reporting poorer SH, greater depression, and a greater proportion reporting past marijuana and cocaine use compared to HIV− controls. There were also statistically significant racial differences in SH [F (1, 114) = 9.35, p = .003] with African Americans reporting lower SH scores than European Americans. Depression symptoms (i.e., total BDI-II with sleep item removed) were significantly associated with SH, with greater depression related to worse SH; however, they were not significantly associated with cognitive measures. Depression was significantly different between the HIV/SH groups (F (3, 112) = 3.13, p = .03), with the HIV+ poor SH group reporting greater current depression compared to the HIV− good SH group only. No other HIV/SH group differences were found. Partial correlations, with depression as a covariate, demonstrated comparable associations between sleep and cognition to correlations that did not include depression as a covariate. Furthermore, analyses with and without depression as a covariate resulted in similar findings, as such, the results from analyses without depression as a covariate are reported. Although the HIV+ group reported greater past use of cocaine and marijuana than the HIV− group, there were no differences in current use (p’s > .10). Moreover, past marijuana and cocaine use was not significantly related to SH or cognition (p’s > .05). Additionally, there were no differences in past use of drugs between HIV+ individuals who reported poor SH and HIV+ individuals who reported better SH. Hence, these were not included as covariates in our analyses. Table 1 reports the demographic comparison between the HIV/SH groups and descriptive statistics for significant outcomes of interest (reported below).
Table 1.
Participant demographics and group statistics
| HIV+/SH+ Mean/% (SD) (n = 34) (a) |
HIV−/SH+ Mean/% (SD) (n = 32) (b) |
HIV−/SH Mean/% (SD) (n = 18) (c) |
HIV+/SH Mean/% (SD) (n = 32) (d) |
Effect Size | p-value | LSD Post-hoc Comparisons | |
|---|---|---|---|---|---|---|---|
| Demographic & Clinical Characteristics | |||||||
| Age (years) | 57.91 (6.42) | 53.63 (12.06) | 53.83 (10.03) | 56.16 (7.55) | η2 = 0.04 | .22 | |
| Education (years) | 13.74 (2.12) | 14.44 (2.44) | 13.67 (1.72) | 13.72 (2.35) | η2 = 0.02 | .48 | |
| Gender (% male) | 79% | 66% | 61% | 81% | φ = 0.25 | .32 | |
| Trans (Male-to-Female) | 3% | - | - | 3% | - | - | |
| Race/Ethnicity (%) | φ = 0.22 | .13 | |||||
| NH-AA-Black | 56% | 50% | 83% | 56% | |||
| NH-White | 44% | 50% | 17% | 44% | |||
| SATED | 9.06 (1.65) | 9.34 (1.49) | 5.28 (0.83) | 4.75 (1.24) | η2 = 0.70 | <.001** | c < a, b; d < a, b |
| BDI-II | 5.82 (6.15) | 3.88 (4.60) | 6.33 (7.51) | 9.34 (9.86) | η2 = 0.08 | .03* | d < a, b |
| WRAT (pre-morbid IQ) | 95.44 (13.54) | 100.72 (13.72) | 97.00 (10.70) | 102.34 (17.34) | η2 = 0.04 | .21 | |
| Length of HIV | 19.18 (9.53) | - | - | 19.28 (8.78) | d = 0.01 | .96 | |
| Current ARV use | 100% | - | - | 100% | - | - | |
| ARV use (years) | 15.28 (8.72) | - | - | 16.48 (6.82) | d = −0.15 | .55 | |
| ACTG Medication Adherence | 3.21 (4.92) | - | - | 4.82 (6.41) | d = −.28 | .27 | |
| Sleep apnea (%) | 6% | 3% | 0 | 6% | φ = 0.11 | .70 | |
| Immune Functioning | |||||||
| Nadir CD4 | 245.30 (154.97) | - | - | 226.70 (213.34) | d = −0.10 | .72 | |
| CD4 count | 691.74 (272.16) | - | - | 654.13 (321.76) | d = −0.13 | .61 | |
| Viral load (log) | 1.52 (0.60) | - | - | 1.77 (1.16) | d = 0.27 | .28 | |
| Current Drug Use | |||||||
| Alcohol | 6% | 6% | 6% | 3% | φ = 0.06 | .95 | |
| Marijuana | 9% | 6% | 11% | 3% | φ = 0.11 | .71 | |
| Past Drug Use | |||||||
| Alcohol | 30% | 13% | 11% | 29% | φ = 0.21 | .16 | |
| Marijuana | 12% | 0 | 6% | 16% | φ = 0.23 | .12 | |
| Cocaine | 42% | 13% | 0 | 19% | φ = 0.37 | .002* | |
| Opiates | 6% | 3% | 0 | 3% | φ = 0.11 | .69 | |
| Cognitive Functioninga | |||||||
| Global NP | 44.95 (5.61) | 46.94 (6.18) | 44.67 (5.94) | 43.98 (5.93) | η2 = 0.04 | .24 | |
| Executive | 48.92 (5.78) | 50.14 (5.50) | 51.57 (5.53) | 47.77 (6.54) | η2 = 0.05 | .15 | |
| Processing Speed | 44.23 (7.53) | 46.72 (8.07) | 45.11 (8.46) | 44.64 (8.24) | η2 = 0.02 | .62 | |
| Learning | 39.96 (10.91) | 42.23 (10.42) | 35.94 (9.50) | 34.95 (8.07) | η2 = 0.09 | .02* | d < a, b; c < b |
| Delayed Memory | 40.71 (10.99) | 43.94 (11.49) | 38.79 (10.88) | 35.27 (10.38) | η2 = 0.09 | .02* | d < a, b |
| % Retentionb | 82% | 87% | 81% | 79% | η2 = 0.03 | .21 | |
| Fluency | 47.74 (8.18) | 50.55 (8.32) | 49.47 (7.44) | 52.06 (8.59) | η2 = 0.04 | .19 | |
p < 0.05
p < 0.001
All cognitive domains report results using T-scores
Average percent retained from highest learning trial score on BVMT and HVLT
Within the HIV+ group, there was no relationship between SH and nadir CD4 [t (52) = − .37, p = .72], current CD4 [t (62) = −.41, p = .68] and log-transformed viral load [t (61) = −1.45, p = .15]. All HIV+ participants were on a stable regimen of cART and were clinically stable based upon current plasma CD4 count, and 79.4% had undetectable viral load (i.e., < 20 copies/mL).
HIV/SH status and neurocognitive performance
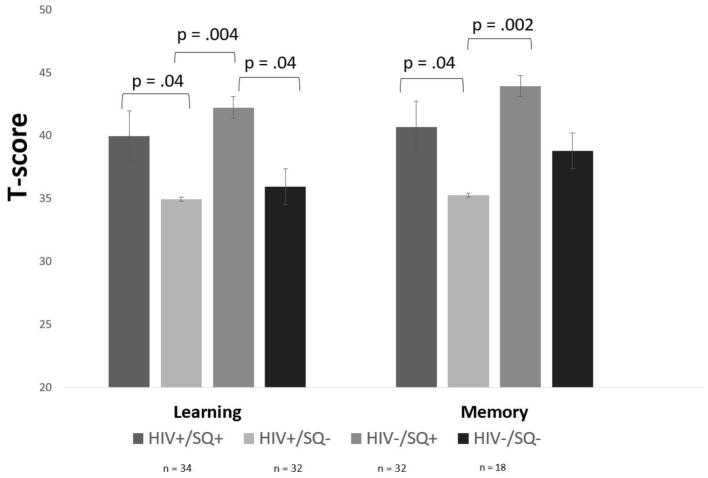
Correlations between cognitive performance, depression, and SH are provided in Table 2. There was no significant difference between the HIV/SH groups on global neurocognitive performance [F (3, 112) = 1.44, p = .24; see Table 1 for statistics]. MANOVA demonstrated significant differences among the groups on individual cognitive domains [F (3, 112) = 1.95, p = .02, Λ = .77, η2= .08]. LSD post-hoc analyses demonstrated that the effects were in the domains of learning [F (3, 112) = 3.55, p = .02, η2= .09] and delayed memory [F (3, 112) = 3.48, p = .02, η2= .09], such that HIV+ individuals with poor SH had lower scores in these domains as compared to HIV+ individuals with good SH and the HIV−/SH+ group. Further, we examined the percent of information retained after a long-delay from the participants’ highest learning trial score (see table 1). Although we did not find statistically significant differences, there was a trend towards lower retention scores among the HIV+/SH− group. HIV-controls with poor sleep health had lower learning scores compared to HIV-controls with good sleep health (see Figure 1). No other significant differences in cognitive performance were found.
Table 2.
SATED scale, Pittsburgh Sleep Quality Index (PSQI), Depression (as assessed by BDI-II) and Cognitive Domains (T-scores): Correlations and Descriptive Statistics (N = 116)
| SATED | PSQI | Depression | Fluency | Executive Function | Processing Speed | Learning | Memory | Global | |
|---|---|---|---|---|---|---|---|---|---|
| SATED | |||||||||
| Pearson Correlation | - | .472** | −.28** | −.15 | .13 | .15 | .36** | .37** | .23* |
| Mean | 7.36 | 7.52 | 6.34 | 49.98 | 49.33 | 45.17 | 38.60 | 39.81 | 45.19 |
| SD | 2.53 | 4.46 | 7.44 | 8.30 | 5.95 | 7.98 | 10.17 | 11.30 | 5.94 |
p < .05;
p < .01
Figure 1.
HIV status and SQ effects on neurocognitive performance. Note: poor SQ (SQ−); good SQ (SQ+).
Discussion
The primary interest of the current study was to characterize HIV+ and HIV− individuals based upon self-reported SH and to examine differences in cognitive performance. Considering the findings from most investigations of sleep effects have been comprised of medically healthy individuals, and problems with sleep health/quality are disproportionately high in the HIV-infected population, it is important to examine whether previous findings generalize to chronic disease populations.
The results demonstrate poor sleep to be associated with lower global cognitive performance across both HIV groups, with poorer performance on learning and memory domains driving this relationship. As expected, the HIV+ participants reported poorer SH compared to controls. Analyses investigating differences between classified groups on individual cognitive domains revealed that the HIV+/SH− group had lower learning and memory scores as compared to the HIV+/SH+ and HIV−/SH+ groups. Furthermore, the HIV−/SH− group had poorer learning performance compared to the HIV−/SH+ group. Together these results support our hypothesis of an adverse effect of poor SH in HIV+ individuals. There were no statistically significant differences between the HIV+/SH+ group and the HIV− groups, suggesting that poor SH may contribute to cognitive problems in HIV, particularly in the domains of learning and memory, or that HIV+ individuals with worse cognitive functioning experience worse sleep health. Moreover, there were no statistically significant differences between the HIV+/SH− group and the HIV−/SH− group and the cognitive differences within the learning domain between SH groups was greater for the HIV-controls as compared to the HIV+ groups, underscoring the adverse impact of poor sleep on cognitive performance, regardless of HIV-status. Although not statistically significant, HIV+ individuals with poor sleep health had lower percent retention than HIV+ individuals with good sleep health and HIV-controls with good sleep health, indicating difficulty retaining previously encoded information over time. These findings are consistent with previous studies associating poor sleep with deficits in learning and memory (Byun et al., 2016; Fortier-Brochu et al., 2012; Joo et al., 2014), as well as imaging studies implicating abnormalities in fronto-temporal regions sub-serving these cognitive functions (Altena, Vrenken, Van Der Werf, van den Heuvel, & Van Someren, 2010; Joo et al., 2014; Joo et al., 2013; Neylan et al., 2010; Riemann et al., 2007). Our findings suggest that sleep problems within medical populations are relevant to cognitive functioning, although the direction of this relationship remains unclear.
Although not a focus of the current study, mechanisms underlying changes in cognitive performance should be considered. Considering that both sleep problems and HIV status are associated with the increase of proinflammatory cytokines [e.g., interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α)] (Breen et al., 1990; Heffner et al., 2012; Irwin, 2015; Seay et al., 2013; Wirth et al., 2015), a possible mechanism underlying the additive effect of poor SH and HIV could be explained through increases in inflammatory responses which ultimately disrupt neural systems, such as the dopaminergic system (González et al., 2012; Felger et al., 2012). Studies in healthy and medical populations have found dopaminergic system dysfunction to adversely affect cognitive performance in domains such as learning, processing speed, and memory (Bäckman et al., 2011; Chang et al., 2008; Kumar, Ownby, Waldrop-Valverde, Fernandez, & Kumar, 2011). Future studies should examine the role of inflammatory response to cognitive problems among HIV+ individuals with poor sleep health.
Considering the etiology of sleep problems in HIV-infection remains up for debate (White et al., 1995; Simen et al., 2015), it is possible that ARV medication use may have potentially contributed to the increased sleep problems. However, the high levels of medication adherence reported by our HIV+ sample (regardless of SH status), the lack of significant difference in SH between the HIV+ and HIV− good SH groups, and the lack of significant difference between the HIV+ good and poor SH groups in their use of ARV medications reduces the concern that our findings were a result of ARV side effects. Nevertheless, future studies should investigate the contribution of ARV medication use on sleep-associated cognitive impairments.
There are limitations to the current study that must be acknowledged. The sample size is relatively small, and the use of self-report may not provide an accurate representation of objective sleep health. While some studies in healthy populations have shown a significant correlation between variables of objective and subjective measures of sleep (Landry, Best, & Liu-Ambrose, 2015), others have found no association (Grandner, Kripke, Yoon, & Youngstedt, 2006). Within the HIV literature, however, self-reports of prolonged sleep latency and decreased sleep efficiency by asymptomatic individuals have been corroborated by polysomnographic results (e.g., Norman et al., 1992; Wiegand et al., 1991). Furthermore, subjective indices of sleep have been shown to have a significant relationship with cognitive impairment in HIV+ individuals (Gamaldo et al., 2013), thus, bolstering the use of self-report to examine SH. Although the HIV+ group reported greater past use of cocaine and marijuana than the HIV-group, there were no differences in current use, which we would expect to have had a greater impact on our results. Nevertheless, there were no differences in past use of drugs between HIV+ individuals who reported poor SH and HIV+ individuals who reported better SH and past cocaine/marijuana use was not significantly related to SH or cognition. Therefore, we are less concerned that greater past use of these drugs in the HIV+ group impacted our study results.
Although the HIV/SH groups did not significantly differ in terms of race/ethnicity, the largely homogenous representation of African-Americans in the HIV−/SH− did raise concerns about the potential impact on study findings. Indeed, African-Americans/Blacks are nearly twice as likely to report short sleep (Durrence & Lichstein, 2006; Stamatakis, Kaplan, & Roberts, 2007; Krueger & Friedman, 2009). A recent meta-analysis found that Blacks were more likely to be short sleepers than were Whites, with larger effect sizes in studies using objective as compared to self-reported measures of sleep duration (Ruiter, DeCoster, Jacobs, & Lichstein, 2011). However, considering that the HIV+/SH− group (which had approximately equal proportions of Whites and Blacks) was found to demonstrate the lowest performance on measures of learning and memory, and that the proportion of HIV+ Blacks and Whites in the sample were approximately equal, we are less concerned that this is driven by group differences in the proportion of African Americans/Blacks. We did not gather information regarding distress due to sleeping patterns, which could potentially be a mediator to the cognitive deficits associated with poor SH. PSQI data was not available for all study participants and characterization of participants into HIV/SH groups resulted in small cell sizes, which significantly impacted statistical power. Hence, our use of the SATED scale. Though the psychometric properties of the SATED scale have yet to be determined, the scale is brief, incorporates specific quantitative criteria to assess key dimensions of sleep that have been consistently associated with health outcomes, and addresses positive dimensions of sleep-wakefulness (Buysse, 2014). Additionally, the attributes comprising this sleep health measure are not specific to any sleep disorder; therefore, they are applicable to—and can be measured across—all populations, with and without sleep disorders. We recognize that our cut-off score was validated using a subset of the sample, and therefore needs replication. Nevertheless, the SATED cutoff score that we used demonstrated adequate discriminative concurrent criterion validity, with a sensitivity of 77% and specificity of 70%. Further, while our study utilizes a subjective measure of SH in a cross-sectional design, future studies would benefit from the concurrent use of an objective measure of SH in a longitudinal setting to determine long-lasting effects of poor SH in the context of chronic disease. Finally, we did not determine whether participants were suffering from acute or chronic sleep disturbance; however, we identified 4% of total participants with sleep apnea through a self-report measure. As such, the differential effects of sleep disorder classifications, using standardized diagnostic procedures, should be considered in future investigations.
This is the first study, to our knowledge, to investigate the complex relationship between HIV status and poor SH on neurocognitive outcomes, with a specific focus on characterizing sleep health in HIV+ individuals. The study findings coincide with prior investigations of cognitive impairment associated with sleep problems. Further, our findings suggest a strong link between SH and cognition among HIV+ individuals, and further studies are needed to determine if poor SH increases vulnerability to cognitive decline among individuals with HIV. Our findings also highlight the clinical and scientific importance of monitoring sleep health and cognition to help identify individuals at greatest risk of poor health outcomes. In addition to classical assessment and evaluation methods (i.e., objective and subjective sleep measures), our findings suggest a need for clinicians to routinely inquire about cognitive functioning when determining a presence of sleep problems and implementing treatment options. Interventions such as sleep-aide medication and cognitive-behavioral therapies have continuously proven advantageous in the treatment of sleep-related problems. Thus, recognition of the impact of poor sleep health as well as adopting interventions could influence clinical outcomes, medication adherence, and the overall quality of life and well-being for PLWH. The current study underscores the utility of sleep health indices as potential tools in identifying problems with sleep and associated risk for development and progression of future HIV-associated neurocognitive disorder.
Acknowledgments
Acknowledgements/Study Funding
This work was supported by NIH/NIMH (K23 MH095661; PI: A. D. Thames; NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881).
Footnotes
Disclosure of Interest
Authors report no conflict of interests or financial disclosures.
References
- Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biological Psychiatry. 2010;67(2):182–185. doi: 10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: Influence of sleep architecture and nocturnal cortisol release. Biological Psychiatry. 2006;60(12):1324–1330. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Karlsson S, Fischer H, Karlsson P, Brehmer Y, Rieckmann A, … Nyberg L. Dopamine D 1 receptors and age differences in brain activation during working memory. Neurobiology of Aging. 2011;32(10):1849–1856. doi: 10.1016/j.neurobiolaging.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Vol. 1. San Antonio: Psychological Corporation; 1996. p. 82. [Google Scholar]
- Becker BW, Thames AD, Woo E, Castellon SA, Hinkin CH. Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS and Behavior. 2011;15(8):1888. doi: 10.1007/s10461-011-9924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R. Brief Visuospatial Memory Test — Revised professional manual. Psychological Assessment Resources, Inc; Odessa, FL: 1997. [Google Scholar]
- Brandt J, Benedict RH. Hopkins Verbal Learning Test — Revised. Psychological Assessment Resources, Inc; Lutz, FL: 2001. [Google Scholar]
- Breen EC, Rezai AR, Nakajima K, Hirano T, Beall GN, Mitsuyasu RT, … Martínez-Maza O. Elevated levels of interleukin 6 (IL-6) are associated with human immunodeficiency virus (HIV) infection. Journal of Immunology. 1990;144:480–484. [PubMed] [Google Scholar]
- Buysse DJ. Sleep health: can we define it? Does it matter. Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Byun E, Gay CL, Lee KA. Sleep, fatigue, and problems with cognitive function in adults living with HIV. The Journal of the Association of Nurses in AIDS Care. 2016;27(1):5–16. doi: 10.1016/j.jana.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh sleep quality index. Journal of Psychosomatic Research. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage. 2008;42(2):869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Tan JC, Parimal S, Zagorodnov V. Sleep deprivation and its effects on object-selective attention. NeuroImage. 2010;49(2):1903–1910. doi: 10.1016/j.neuroimage.2009.08.067. [DOI] [PubMed] [Google Scholar]
- Chen PL, Lee WJ, Sun WZ, Oyang YJ, Fuh JL. Risk of dementia in patients with insomnia and long-term use of hypnotics: A population-based retrospective cohort study. PloS one. 2012;7(11):e49113. doi: 10.1371/journal.pone.0049113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of The Outcomes Committee of The Adult Aids Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Chun M, Turk-Browne NB. Interactions between attention and memory. Current Opinion in Neurobiology. 2007;17(2):177–184. doi: 10.1016/j.conb.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Cipriani G, Lucetti C, Danti S, Nuti A. Sleep disturbances and dementia. Psychogeriatrics. 2015;15(1):65–74. doi: 10.1111/psyg.12069. [DOI] [PubMed] [Google Scholar]
- Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. Journal of the American Geriatrics Society. 2001;49(9):1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- Darko DF, McCutchan JA, Kripke DF, Gillin JC, Golshan S. Fatigue, sleep disturbance, disability, and indices of progression of HIV infection. American Journal of Psychiatry. 1992;149:514–520. doi: 10.1176/ajp.149.4.514. [DOI] [PubMed] [Google Scholar]
- Darko DF, Miller JC, Gallen C, White J, Koziol J, Brown SJ, … Munnell DT. Sleep electroencephalogram delta-frequency amplitude, night plasma levels of tumor necrosis factor alpha, and human immunodeficiency virus infection. Proceedings of the National Academy of Sciences. 1995;92(26):12080–12084. doi: 10.1073/pnas.92.26.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrence HH, Lichstein KL. The sleep of African Americans: A comparative review. Behavioral Sleep Medicine. 2006;4(1):29–44. doi: 10.1207/s15402010bsm0401_3. [DOI] [PubMed] [Google Scholar]
- Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: The subcortical source of inflammatory malaise. Frontiers in Neuroendocrinology. 2012;33(3):315–327. doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Calhoun S, Bixler EO, Pejovic S, Karataraki M, Liao D, … Vgontzas AN. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: A general population study. Sleep. 2010;33(4):459–465. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtenberg NL, Putnam SH, Mann NR, Zafonte RD, Millard AE. Insomnia screening in postacute traumatic brain injury: utility and validity of the Pittsburgh Sleep Quality Index. American Journal of Physical Medicine & Rehabilitation. 2001;80(5):339–345. doi: 10.1097/00002060-200105000-00003. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for Axis-I DSM-IV Disorders — Patient edition (SCID-I/P) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Foley D, Monjan A, Masaki K, Havlik R, White L, Launer L. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older japanese-american men. Journal of the American Geriatrics Society. 2001;49(12):1628–1632. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state:” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fortier-Brochu É, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Medicine Reviews. 2012;16(1):83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Gamaldo CE, Gamaldo A, Creighton J, Salas RE, Selnes OA, David PM, Smith MT. Evaluating sleep and cognition in HIV. Journal of Acquired Immune Deficiency Syndromes. 2013;63(5):609–616. doi: 10.1097/QAI.0b013e31829d63ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ, Freshwater SM. Stroop color and word test. Stoelting Company; Chicago, IL: 1978. [Google Scholar]
- González S, Moreno-Delgado D, Moreno E, Pérez-Capote K, Franco R, Mallol J, … Ferré S. Circadian-related heteromerization of adrenergic and dopamine D4 receptors modulates melatonin synthesis and release in the pineal gland. PLoS Biology. 2012;10(6):e1001347. doi: 10.1371/journal.pbio.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Kripke DF, Yoon IY, Youngstedt SD. Criterion validity of the Pittsburgh Sleep Quality Index: Investigation in a non-clinical sample. Sleep and Biological Rhythms. 2006;4(2):129–136. doi: 10.1111/j.1479-8425.2006.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griessenberger H, Heib DPJ, Lechinger J, Luketina N, Petzka M, Moeckel T, … Schabus M. Susceptibility to declarative memory interference is pronounced in primary insomnia. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0057394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, … Collier AC. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan battery: Demographic corrections, research findings, and clinical applications; with a supplement for the Wechsler Adult Intelligence Scale-Revised (WAIS-R) Psychological Assessment Resources; 1991. [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- Heffner KL, Ng HM, Suhr JA, France CR, Marshall GD, Pigeon WR, Moynihan JA. Sleep disturbance and older adults’ inflammatory responses to acute stress. The American Journal of Geriatric Psychiatry. 2012;20(9):744–752. doi: 10.1097/JGP.0b013e31824361de. doi: org/10.1097/JGP.0b013e31824361de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR. Why sleep is important for health: A psychoneuroimmunology perspective. Annual Review of Psychology. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena A, Sachdeva RK, Sharma A, Wanchu A. Adverse drug reactions to nonnucleoside reverse transcriptase inhibitor-based antiretroviral regimen: A 24-week prospective study. Journal of the International Association of Physicians in AIDS Care. 2009;8(5):318–322. doi: 10.1177/1545109709343967. [DOI] [PubMed] [Google Scholar]
- Joo EY, Kim H, Suh S, Hong SB. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep. 2014;37(7):1189–98. doi: 10.5665/sleep.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo EY, Noh HJ, Kim JS, Koo DL, Kim D, Hwang KJ, … Hong SB. Brain gray matter deficits in patients with chronic primary insomnia. Sleep. 2013;36(7):999–1007. doi: 10.5665/sleep.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenedi CA, Goforth HW. A systematic review of the psychiatric side-effects of efavirenz. AIDS and Behavior. 2011;15(8):1803–1818. doi: 10.1007/s10461-011-9939-5. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;169:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger PM, Friedman EM. Sleep duration in the United States: A cross-sectional population-based study. American Journal of Epidemiology. 2009;169(9):1052–1063. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. Journal of Neurovirology. 2011;17(1):26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Best JR, Liu-Ambrose T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Frontiers in Aging Neuroscience. 2015;7:166. doi: 10.3389/fnagi.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA, Gay C, Portillo CJ, Coggins T, Davis H, Pullinger CR, Aouizerat BE. Symptom experience in HIV-infected adults: a function of demographic and clinical characteristics. Journal of Pain and Symptom Management. 2009;38(6):882–893. doi: 10.1016/j.jpainsymman.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low Y, Preud’homme X, Goforth HW, Omonuwa T, Krystal AD. The association of fatigue with depression and insomnia in HIV-seropositive patients: A pilot study. Sleep. 2011;34(12):1723–1726. doi: 10.5665/sleep.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, Rohling ML. A statistical interpretive method for neuropsychological test data. Neuropsychology Review. 2001;11:143–169. doi: 10.1023/A:1016602708066. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. Adult sleep habits and lifestyle: Summary of findings. 2005 Retrieved from: http://www.sleepfoundation.org/sites/default/files/2005_summary_of_findings.pdf.
- Neylan TC, Mueller SG, Wang Z, Metzler TJ, Lenoci M, Truran D, … Schuff N. Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biological Psychiatry. 2010;68(5):494–496. doi: 10.1016/j.biopsych.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman SE, Chediak AD, Freeman C, Kiel M, Mendez A, Duncan R, … Nolan B. Sleep disturbances in men with asymptomatic human immunodeficiency (HIV) infection. Sleep. 1992;15(2):150–155. doi: 10.1093/sleep/15.2.150. [DOI] [PubMed] [Google Scholar]
- Norman SE, Resnick L, Cohn MA, Duara R, Herbst J, Berger JR. Sleep disturbances in HIV-seropositive patients. JAMA. 1988;260(7):922–922. [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8(3):271–276. [Google Scholar]
- Riemann D, Voderholzer U, Spiegelhalder K, Hornyak M, Buysse DJ, Nissen C, … Feige B. Chronic insomnia and MRI-measured hippocampal volumes: A pilot study. Sleep. 2007;30(8):955–958. doi: 10.1093/sleep/30.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, Selwyn PA. High prevalence of insomnia in an outpatient population with HIV infection. Journal of Acquired Immune Deficiency Syndromes. 1998;19(3):260–265. doi: 10.1097/00042560-199811010-00008. [DOI] [PubMed] [Google Scholar]
- Ruiter ME, DeCoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep Medicine. 2011;12(3):209–214. doi: 10.1016/j.sleep.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Sanmarti M, Ibáñez L, Huertas S, Badenes D, Dalmau D, Slevin M, … Jaen A. HIV-associated neurocognitive disorders. Journal of Molecular Psychiatry. 2014;2(1):2. doi: 10.1186/2049-9256-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seay JS, McIntosh R, Fekete EM, Fletcher MA, Kumar M, Schneiderman N, Antoni MH. Self-reported sleep disturbance is associated with lower CD4 count and 24-hour urinary dopamine levels in ethnic minority women living with HIV. Psychoneuroendocrinology. 2013;38(11) doi: 10.1016/j.psyneuen.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekleton JA, Flynn-Evans E, Miller B, Epstein LJ, Kirsch D, Brogna LA, … Rajaratnam SMW. Neurobehavioral performance impairment in insomnia: Relationships with self-reported sleep and daytime functioning. Sleep. 2014;37(1):107–116. doi: 10.5665/sleep.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen AA, Ma J, Svetnik V, Mayleben D, Maynard J, Roth A, … Fraser I. Efavirenz modulation of sleep spindles and sleep spectral profile. Journal of Sleep Research. 2015;24(1):66–73. doi: 10.1111/jsr.12196. [DOI] [PubMed] [Google Scholar]
- Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: Population prevalence and growing disparities during 34 years of follow-up. Annals of Epidemiology. 2007;17(12):948–955. doi: 10.1016/j.annepidem.2007.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Kuhn TP, Williamson TJ, Jones JD, Mahmood Z, Hammond A. Marijuana effects on changes in brain structure and cognitive function among HIV+ and HIV− adults. Drug and Alcohol Dependence. 2017;170:120–127. doi: 10.1016/j.drugalcdep.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Mahmood Z, Burggren AC, Karimian A, Kuhn TP. Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care. 2016;28(5):628–632. doi: 10.1080/09540121.2015.1124983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44(1):121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 4. Pearson; San Antonio, TX: 2008. [Google Scholar]
- White JL, Darko DF, Brown SJ, Miller JC, Hayduk R, Kelly T, Mitler MM. Early central nervous system response to HIV infection: sleep distortion and cognitive-motor decrements. AIDS. 1995;9(9):1043–50. doi: 10.1097/00002030-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Wibbeler T, Reichelt D, Husstedt IW, Evers S. Sleepiness and sleep quality in patients with HIV infection. Journal of Psychosomatic Research. 2012;72(6):439–442. doi: 10.1016/j.jpsychores.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Wiegand M, Möller AA, Schreiber W, Krieg JC, Fuchs D, Wachter H, Holsboer F. Nocturnal sleep EEG in patients with HIV infection. European Archives of Psychiatry and Clinical Neuroscience. 1991;240(3):153–158. doi: 10.1007/BF02190756. [DOI] [PubMed] [Google Scholar]
- Wilkinson G, Robertson G. Wide Range Achievement Test Fourth Edition (WRAT–4) professional manual. Psychological Assessment Resources, Inc; Lutz, FL: 2004. [Google Scholar]
- Wirth MD, Jaggers JR, Dudgeon WD, Hébert JR, Youngstedt SD, Blair SN, Hand GA. Association of markers of inflammation with sleep and physical activity among people living with HIV or AIDS. AIDS and Behavior. 2015;19(6):1098–1107. doi: 10.1007/s10461-014-0949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]