Abstract
Objective:
Caregivers of youth with heavy prenatal alcohol exposure report impaired communication, which can significantly impact quality of life. Using data collected as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), we examined whether cognitive variables predict communication ability of youth with histories of heavy prenatal alcohol exposure.
Method:
Subjects (ages 10–16) comprised two groups: adolescents with heavy prenatal alcohol exposure (AE) and non-exposed controls (CON). Selected measures of executive function (NEPSY, D-KEFS), working memory (CANTAB), and language were tested in the child, while parents completed communication ratings (VABS-II). Separate multiple regression analyses determined which cognitive domains predicted communication ability. A final, global model of communication comprised the three cognitive models.
Results:
Spatial Working Memory and Inhibition significantly contributed to communication ability across groups. Twenty Questions performance related to communication ability in the CON group only while Word Generation performance related to communication ability in the AE group only. Effects remained significant in the global model, with the exception of Spatial Working Memory.
Conclusions:
Both groups displayed a relation between communication and Spatial Working Memory and Inhibition. Stronger communication ability related to stronger verbal fluency in the AE group and Twenty Questions performance in the CON group. These findings suggest that alcohol-exposed adolescents may rely more heavily on learned verbal storage or fluency for daily communication while non-exposed adolescents may rely more heavily on abstract thinking and verbal efficiency. Interventions aimed at aspects of executive function may be most effective at improving communication ability of these individuals.
Keywords: Fetal alcohol syndrome (FAS), fetal alcohol spectrum disorders (FASD), neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE), prenatal alcohol exposure, neurobehavioral profile, communication
Introduction
Prenatal alcohol exposure can result in a wide variety of detrimental effects on the fetus that confer risk for later impairments (Mattson et al., 2011; Riley et al., 2011). Fetal alcohol spectrum disorders (FASD) encompass the range of physical, cognitive, and behavioral deficits due to prenatal alcohol exposure. Recent estimates suggest a prevalence of ~2–5% among school-age children (May et al., 2014; May et al., 2015; May et al., 2018), identifying FASD as a serious public health concern.
Extensive research has investigated the areas of neurobehavioral functioning impacted by prenatal alcohol exposure (Mattson & Riley, 1998; Mattson et al., 2011; Riley et al., 2011). One such area is communication (Crocker et al., 2009; LaDue et al., 1992; Mattson et al., 2011; Streissguth et al., 1991), including both functional and social communication, which comprises one’s ability to exchange information as well as connect with others. Young children with FASD display greater impairment in communication ability as reported by parents and caregivers than typically developing controls (e.g., trouble maintaining conversation, answering questions, staying on topic). Further, children with prenatal alcohol exposure show deficits on measures of receptive and expressive language (Akbarian, 1992; Carney & Chermak, 1991; Church & Kaltenbach, 1997; Church et al., 1997; Gentry et al., 1998; McGee et al., 2009; Wyper & Rasmussen, 2011), which can impact communication skills. Importantly, communication abilities among individuals with FASD are worse at older ages than younger ages, suggesting that these individuals experience an arrest in development rather than a delay (Crocker et al., 2009) though longitudinal studies are still needed to clarify this trajectory. The clinical impact of impaired communication ability is clear, as evidenced by the link between communication disorders, emotional disorders, and behavioral disorders in children and adolescents (Prizant et al., 1990). These communication deficits can result in diminished quality of life as impaired communication puts children at risk for learning and psychiatric disorders (Prizant et al., 1990), both of which are elevated among youth with FASD (Fryer et al., 2007).
In other neurodevelopmental disorders (e.g., autism spectrum disorders, specific language impairment), cognitive variables such as executive function, working memory, and language contribute to communication ability (Finneran et al., 2009; Im-Bolter et al., 2006; McEvoy et al., 1993; Spaulding et al., 2008). For example, among children with specific language impairment (SLI), deficits on measures of working memory have been shown to relate to language abilities (Weismer et al., 1999). Aspects of executive function, such as inhibition, are likewise impaired, which contributes to language difficulties among children with SLI (Im-Bolter et al., 2006). Similar to SLI, particular aspects of language are difficult for individuals with FASD (e.g., speech discrimination, comprehension; Akbarian, 1992; McGee et al., 2009), making these individuals more vulnerable to impairment (Akbarian, 1992). As such, individuals with FASD often struggle to meet the demands of social and functional communication, which require intact social cognition, executive functioning, and language skills (Coggins et al., 2007). However, the relationship between cognitive variables and communication ability has not been explored in individuals with prenatal alcohol exposure.
While evidence exists for functional communication (i.e., practical communication in order to get one’s needs met) deficits among youth with heavy prenatal alcohol exposure, as emphasized above, the cognitive bases for these deficits have not been explored. Some studies highlight a link between certain cognitive skills, such as executive function, and social skills (Schonfeld et al., 2006), but these connections have not been made with communication ability explicitly among youth with prenatal alcohol exposure. Theoretical frameworks for communication ability suggest social cognition, language, and executive function skills contribute to social and functional communication ability (Coggins et al., 2007); thus, we might expect that these same variables relate to communication ability among individuals with FASD.
The majority of studies investigating communication ability in prenatal alcohol exposure have focused on young children or only individuals with fetal alcohol syndrome (FAS) (Akbarian, 1992; Carney & Chermak, 1991; Church & Kaltenbach, 1997; Church et al., 1997; Coggins et al., 2007; Gentry et al., 1998; McGee et al., 2009; Wyper & Rasmussen, 2011). However, adolescence is a time during which greater difficulties can emerge due to decreased adult supervision, increased peer pressure, and greater requirement of independent functioning (Streissguth, 1986). Further investigation is likewise needed to determine communication ability among all individuals with FASD, not just those diagnosed with FAS. In doing so, the profile of abilities across the full spectrum of alcohol exposure will be strengthened. Thus, exploration of these abilities among adolescents with prenatal alcohol exposure is of utmost importance.
Investigation into the cognitive bases of communication ability among youth with histories of heavy prenatal alcohol exposure will help clarify the cognitive mechanisms that contribute to communication deficits and identify potential routes for intervention to improve communication ability. We aimed to explore whether cognitive variables could significantly predict communication ability among adolescents with heavy prenatal alcohol exposure. We hypothesized that: (1) cognitive variables (working memory, executive function, language) shown to be predictive in other neurodevelopmental disorders would significantly predict communication ability in alcohol-exposed youth; and (2) these cognitive variables would show differential relationships with communication ability between alcohol-exposed youth and typically-developing controls.
Method
General Methods
As part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders, Phase Three (CIFASD III) multisite study, subjects (N = 302) aged 10–16 (M = 13.28) completed a comprehensive battery including neuropsychological and behavioral measures. CIFASD methodology has been described elsewhere (Mattson et al., 2010). Children were tested at four sites: (1) Center for Behavioral Teratology at San Diego State University, (2) University of Minnesota FASD Program, (3) Marcus Institute at Emory University, and (4) Children’s Hospital at the University of Southern California. Subjects were recruited through community and clinical referral, including schools, professional referrals, advertisements, and community outreach. Within one session, caregivers completed questionnaires and an interview while subjects were administered a standardized, three-hour neuropsychological battery. Informed consent and assent were obtained from all caregivers and subjects prior to participation. Financial incentive was provided to caregivers and subjects. The Institutional Review Board at each site approved study procedures.
Subjects
Adolescent (age 10–16 years) subjects comprised two groups: prenatal alcohol exposure (AE; n = 142) and typically-developing controls (CON; n = 160). Subjects in the AE group had confirmed histories of heavy prenatal alcohol exposure, defined as a pattern of heavy or binge drinking at any point in pregnancy evidenced by maternal consumption of >13 drinks per week or >4 drinks per occasion on average (Jones et al., 2006; Mattson et al., 2010). In cases where direct maternal report was not available, a review of medical, social services, or court records was required. In these cases, subjects were included in the AE group if there was documentation of alcohol abuse or dependence in the biological mother or if exposure was suspected and the child met criteria for FAS. Specific information regarding maternal drinking patterns was not available for all subjects; within the AE group, 30% of subjects were direct report (i.e., biologic mother) and 70% of subjects were collateral report. Control subjects were recruited from the same communities as the AE group. Subjects were excluded from the CON group if prenatal alcohol exposure was more than minimal. Minimal exposure is defined as no more than 1 drink per week on average and never more than 2 drinks per occasion or if exposure was suspected or unknown. Confirmation of alcohol exposure histories occurred by direct report for 94% of control subjects, and the remaining 6% of subjects’ histories were verified via collateral report. Subjects were also excluded from the CON group based on parent report (on study intake questionnaire) of clinically significant behavioral problems or previous clinical diagnoses (e.g., attention-deficit/hyperactivity disorder [ADHD]) at the time of initial study enrollment. Additional exclusion criteria for both groups included: primary language other than English, being adopted from abroad within two years of participation or after the age of 5, history of significant head injury and/or loss of consciousness greater than 30 minutes (no subjects had loss of consciousness greater than 5 minutes), or presence of a severe mental, psychiatric, or physical disability that precluded participation in the study (e.g., autism spectrum disorder, active mania or psychosis, blindness). An estimate of general intellectual ability was obtained using the General Conceptual Ability (GCA) index score from the Differential Ability Scales – Second Edition (DAS-II; Elliott, 2007) and presence of symptoms associated with psychiatric conditions was determined using the Computerized Diagnostic Interview Schedule for Children Version IV (C-DISC-4.0; Shaffer et al., 2000), which was conducted in person while subjects completed testing. The rates of symptoms associated with psychiatric and behavioral conditions within the AE group are presented in Supplementary Table 1. Finally, all subjects were examined for the presence of FAS based upon CIFASD criteria (Jones et al., 2006; Mattson et al., 2010).
Measures
Measures from the CIFASD III test battery were selected to assess the relation between communication ability and performance in three cognitive domains: working memory, executive function, and language. The larger CIFASD neuropsychological test battery included the DAS-II, the California Verbal Learning Test-Children’s Version (CVLT-C), and selected subtests from the following tests: CANTAB, NEPSY-II, and Delis-Kaplan Executive Function System (D-KEFS). Initially, measures were chosen from this battery based on a theoretical relation with communication ability. Preliminary correlation analyses were used to test these relationships and selected measures were included in analyses if they showed a significant (p < .05) and strong correlation with VABS-II Communication (see Table 1). Measures included in final analyses are described below.
Table 1.
Correlation results for included measures.
| Measure | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. VABS-II | -- | |||||
| 2. SWM | .370* | -- | ||||
| 3. TQ | .302* | .378* | -- | |||
| 4. INH | .408* | .447* | .313* | -- | ||
| 5. WG | .178* | .340* | .175* | .168* | -- | |
| 6. SN | .194* | .166* | −.007 | .166* | .075 | -- |
Note:
p<.001 level. Correlation analyses determined whether chosen variables significantly correlated with communication ability. Communication standard scores from the Vineland Adaptive Behavior Scales – Second Edition (VABS-II) served as the outcome variable of interest. Spatial Working Memory (SWM) was measured by Total Errors z-score from the CANTAB; Twenty Questions (TQ) was measured by Initial Abstraction scaled score from the Delis-Kaplan Executive Function System (D-KEFS); Inhibition (INH) was measured by Total Errors scaled score from the NEPSY-II; Word Generation (WG) was measured by Semantic and Initial Combined scaled score from the NEPSY-II; Speeded Naming (SN) was measured by Combined scaled score from the NEPSY-II.
Vineland Adaptive Behavior Scales – Second Edition, Questionnaire (VABS-II; Sparrow et al., 2005).
The VABS-II is a norm-referenced caregiver-report questionnaire that provides information regarding subjects’ ability on three domains of adaptive functioning: Socialization, Communication, and Daily Living Skills. VABS-II Communication domain standard scores served as the dependent variable in all analyses. The Communication domain assesses the individual’s ability to communicate his or her needs and understand others (e.g., ‘Easily moves from one topic to another in conversation’; ‘Answers or tries to answer with words when asked a question’; ‘Stays on topic in conversations; does not go off on tangents’; ‘Has conversations that last greater than 10 minutes’). Lower scores indicate weaker performance.
CANTAB Spatial Working Memory (Cambridge Cognition Limited, 2006).
The CANTAB is a computer-administered battery of neuropsychological tests. The Spatial Working Memory subtest assesses subjects’ visuospatial working memory ability. This measure requires the subject to locate a blue token hidden inside colored boxes on the screen. Subjects are instructed not to return to a box previously found to have a blue token hidden inside and thus must remember which boxes have revealed a blue token while locating the remaining blue tokens. Though Spatial Working Memory is a nonverbal measure, it may also tap into verbal skills as subjects may use verbal encoding strategies when completing the task. Total Errors z-score was included in analyses, with lower scores indicating weaker performance.
Delis-Kaplan Executive Function System Twenty Questions (D-KEFS; Delis et al., 2001).
The D-KEFS is a well-known collection of tests that assess executive function ability. The Initial Abstraction score from Twenty Questions was selected due to its ability to measure higher-level executive functioning skills. The Twenty Questions subtest is a measure of abstract reasoning, planning, and problem solving, all of which are higher-level components of executive function ability. The subject is required to ask yes/no questions to identify the object chosen by the examiner with the goal of asking the fewest questions possible to identify the object. The Initial Abstraction scaled score was included in analyses. This score measures the number of items eliminated with the first question with lower scores indicating weaker performance. Initial Abstraction captures the subject’s ability to employ efficient, verbally mediated strategies to quickly solve a problem, skills that are likely implicated in functional and social communication.
NEPSY-II Inhibition, Word Generation, and Speeded Naming (Korkman et al., 2007).
The NEPSY-II is a battery of subtests that measure a wide array of neuropsychological constructs. Three measures were included in analyses to assess inhibition and language abilities: Inhibition Total Errors, Word Generation Initial and Semantic Combined score, and Speeded Naming Combined score. Inhibition was selected as a measure of inhibitory control. Word Generation and Speeded Naming were included as measures of executive function-based language skills and speed of verbal information processing. The Inhibition subtest has three parts: (1) Naming, which requires the subject to name objects (e.g., square or circle) as fast as possible, (2) Inhibition, which requires the subject to name the other object (i.e., square for circle), and (3) Switching, which requires the subject to name the objects under certain conditions (i.e., correct name when object is black, opposite name when object is white). The Total Errors score represents all errors across the three conditions. Word Generation requires the subject to name as many words that fall within a certain category (Semantic) or start with a certain letter (Initial) in 60 seconds. The Initial and Semantic Combined score represents all correctly named words. The Speeded Naming Combined score measures how quickly the subject can read letters and numbers printed on a page. All NEPSY-II scores are scaled scores (M = 10; SD = 3) with lower scores indicating weaker performance.
Statistical Analyses
SPSS statistical software v.23 was used for all analyses. Subjects with missing data were excluded from the corresponding analyses. Demographic data were analyzed using either Pearson’s chi-square (categorical data) or univariate analysis of variance (ANOVA; continuous data) techniques. Group differences in communication were tested using independent-samples t-tests with VABS-II Communication measuring communication ability. Finally, the relation between the three cognitive domains and communication ability were tested using stepwise multiple regression analyses. Continuous predictor variables were mean-centered prior to running multiple regression analyses and interaction terms were created between each predictor variable and Group to formally test for group differences.
A stepwise, sequential process of conducting regression analyses was employed as follows. We first determined cognitive variables that significantly related to communication ability through correlation analyses. Three domain-specific models of communication ability (working memory, executive function, language) were independently tested in separate domain-based regression analyses to determine those variables that significantly predicted communication. Within each model, variables were initially tested separately to determine those that displayed a main or interaction effect with communication ability. Higher order terms were initially tested with non-significant terms being removed sequentially. Significant effects were combined into an overall domain-specific model; final models comprised those variables that had a significant main or interaction effect with Communication and accounted for the most variability in Communication scores as measured by R2. Subsequently, each final domain model was included in the global model. Throughout this process, non-significant variables were removed to identify the strongest and most reliable variables available within our dataset that contribute to communication and to maintain parsimony. An alpha level of p < .05 was used to determine statistical significance.
Evaluation of Covariates
Age and sex were investigated as potential covariates due to their theoretical relationship with communication ability. Interactions between covariates (age, sex) and Group were created to assess homogeneity of regression assumptions for univariate analysis of covariance (ANCOVA) analyses. An alpha level of p < .05 was used to determine appropriateness as a covariate. Results showed no significant interactions between Group and sex (p = .925) or Group and age (p = .823) on the dependent variable (VABS-II Communication). However, neither sex (p = .338) nor age (p = .768) showed a significant relationship with the dependent variable. Site, race, and ethnicity (i.e., Hispanic or Latino, Not Hispanic or Latino, Unknown) were also investigated as potential covariates due to possible differences across sites. Results showed no significant relationships between the dependent variable (VABS-II Communication) and site (p = .106), race (p = .253), or ethnicity (p = .140). Thus, no covariates were included in subsequent analyses.
Results
Demographic Data
Groups were matched on all demographic variables except GCA (p = .001) and number of subjects meeting ADHD criteria (p < .001). Specifically, the AE group (M = 87.9, SD = 13.43) had significantly lower GCA scores than the CON group (M = 102.6, SD = 16.40) and the AE group (n = 96, 67.6%) had a significantly higher number of subjects meeting research criteria for ADHD than the CON group (n = 4, 2.5%). Based on the C-DISC-4.0, these 4 children in our control group were at-risk for a clinical diagnosis of ADHD. However, parents of these subjects denied any behavioral or clinical concerns upon study enrollment (an exclusion criterion), suggesting these symptoms have not reached the level at which parents would seek professional help. These subjects were retained in analyses. Demographic data are presented in Table 2.
Table 2.
Demographic data for alcohol-exposed (AE) and control (CON) groups.
| Demographic Variable | AE (n=142) |
CON (n=160) |
|---|---|---|
| Sex [n (% Female)] | 68 (47.9) | 78 (48.8) |
| Age [Mean (SD)] | 12.9 (2.07) | 13.5 (2.13) |
| Race [n (% White)] | 83 (58.5) | 97 (60.6) |
| Ethnicity [n (% Hispanic)] | 29 (20.4) | 39 (24.4) |
| Handedness [n (% Right)] | 129 (90.8) | 141 (88.1) |
| GCA [Mean (SD)]* | 87.9 (13.43) | 102.6 (16.40) |
| ADHD [n (%)]* | 96 (67.6) | 4 (2.5) |
| FAS [n (%)]* | 15 (10.6) | 0 (0.0) |
| Parental Education [% High school diploma or less] | 15.5 | 19.6 |
| CIFASD Site [n (%)] | ||
| Atlanta | 29 (20.4) | 38 (23.8) |
| Los Angeles | 23 (16.2) | 31 (19.4) |
| Minnesota | 46 (32.4) | 39 (24.4) |
| San Diego | 44 (31.0) | 52 (32.5) |
Note:
p<.05 level. General Conceptual Ability (GCA), an estimate of general intellectual ability, was measured using the Differential Ability Scales – Second Edition (DAS-II).
Neurobehavioral Data
There was a significant difference in Communication scores for the AE (M = 76.57, SD = 10.69) and CON (M = 102.36, SD = 16.39) groups (t(285) = −15.60, p < .001). Group performance significantly differed (ps < .05) on all neuropsychological variables (see Table 3). Results from regression analyses are described below for each model. See Tables 4–7 for all final regression results and Figure 3 for final models.
Table 3.
Group performance on neuropsychological variables.
| Neuropsychological Variable [Mean (SD)] |
AE (n=142) |
CON (n=160) |
|---|---|---|
| VABS-II Communication** | 76.6 (10.69) | 102.4 (16.39) |
| CANTAB Spatial Working Memory** | 0.1 (0.79) | 0.7 (0.77) |
| NEPSY-II Inhibition** | 5.5 (3.94) | 8.1 (3.73) |
| D-KEFS Twenty Questions** | 8.5 (3.12) | 9.9 (3.49) |
| NEPSY-II Word Generation* | 7.3 (3.00) | 8.3 (3.04) |
| NEPSY-II Speeded Naming* | 8.2 (2.78) | 9.0 (2.41) |
Note: Groups significantly (ps < .05*, ps < .001**) differed on all measures. Groups comprised alcohol-exposed (AE) or typically-developing control (CON) subjects. Communication was measured by the Communication standard score from the Vineland Adaptive Behavior Scales – Second Edition (VABS-II); Spatial Working Memory was measured by Total Errors z-score from the CANTAB; Inhibition was measured by Total Errors scaled score from the NEPSY-II; Twenty Questions was measured by Initial Abstraction scaled score from the Delis-Kaplan Executive Function System (D-KEFS); Word Generation was measured by Semantic and Initial Combined scaled score from the NEPSY-II; Speeded Naming was measured by Combined scaled score from the NEPSY-II.
Table 4.
Stepwise multiple regression results for Working Memory model.
| Variables | SWM β (SE) |
Group x SWM β (SE) |
Final Model β (SE)a |
|---|---|---|---|
| Group | 24.136** (1.798) | 24.133** (1.803) | 24.136** (1.798) |
| SWM | 2.173** (1.049) | 2.220 (1.510) | 2.173** (1.049) |
| Group x SWM | −0.091 (2.104) | ||
| Constant | 77.384** (1.251) | 77.401** (1.317) | 77.384** (1.251) |
| R2 | .468** | .468** | .468** |
Note:
p<.08,
p<.05. Results presented are from stepwise multiple regression analyses investigating the relation between working memory variables and communication ability. Higher order terms (i.e., interactions) were evaluated first, with non-significant terms removed to maintain parsimony. Group included alcohol-exposed (AE) and typically-developing control (CON) subjects. Spatial Working Memory (SWM) was measured by Total Errors z-score from the CANTAB. The dependent variable, communication, was measured by the Vineland Adaptive Behavior Scales – Second Edition (VABS-II) Communication domain standard score.
The final model consisted only of SWM main effect.
Table 7.
Multiple regression results for final global model.
| R2 | Constant (SE) | β | SE | p | |
|---|---|---|---|---|---|
| Global | 0.527 |
78.127 (1.210) |
< .001 | ||
| Group | 23.038 | 1.699 | < .001 | ||
| Inhibition | 0.770 | 0.219 | .001 | ||
| Twenty Questions | 0 | 0.385 | 1.0 | ||
| Group x Twenty Questions | 1.177 | 0.495 | .018 | ||
| Word Generation | 0.692 | 0.399 | .083 | ||
| Group x Word Generation | −1.134 | 0.539 | .036 |
Note: Results presented are from multiple regression analyses investigating the relation between cognitive domain variables and communication ability. Group included alcohol-exposed (AE) and typically-developing control (CON) subjects. Inhibition was measured by Total Errors scaled score from the NEPSY-II; Twenty Questions was measured by Initial Abstraction scaled score from the Delis-Kaplan Executive Function System (D-KEFS); Word Generation was measured by Semantic and Initial Combined scaled score from the NEPSY-II. The dependent variable, communication, was measured by the Vineland Adaptive Behavior Scales – Second Edition (VABS-II) Communication domain standard score.
Figure 3.
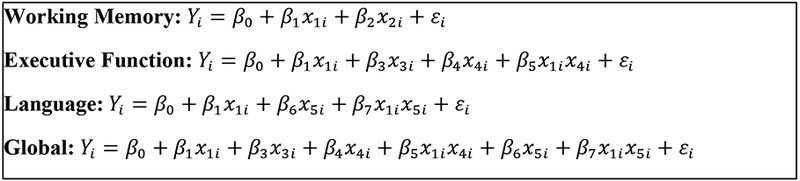
Final models for each domain and final global model from regression analyses.
Note: Y: Vineland Adaptive Behavior Scales-II Communication
X1: Group
X2: Spatial Working Memory
X3: Inhibition Total Errors
X4: Twenty Questions
X5: Word Generation
Working Memory
Results from the working memory regressions are presented in Table 4. Spatial Working Memory significantly predicted communication across group. No interaction effect was noted (p = .965); however, Spatial Working Memory showed a significant main effect (p = .039). As a result, the final working memory model comprised only a main effect of Spatial Working Memory (F(2, 284) = 123.901; p < .001; R2 = .468).
Executive Function
Results from the executive function regressions are presented in Table 5. Inhibition significantly predicted communication ability irrespective of group. No interaction effect was noted for Inhibition (p = .816); however, Inhibition showed a significant main effect (p < .001). The interaction effect of Twenty Questions Initial Abstraction Score was marginally significant (p = .074). Specifically, the relation between Twenty Questions and Communication was significant for the CON group, but not the AE group (see Figure 1). Both the main effect of Inhibition Total Errors (p < .001) and the interaction effect of Twenty Questions Initial Abstraction (p = .042) remained significant when combined into the final executive function model (F(4, 278) = 73.763; p < .001; R2 = .720).
Table 5.
Stepwise multiple regression results for Executive Function model.
| Variables | INI β (SE) |
Group x INI β (SE) |
20Q β (SE) |
Group x 20Q β (SE) |
Final Model β (SE)a |
|---|---|---|---|---|---|
| Group | 23.583** (1.698) | 23.595** (1.702) | 24.323** (1.647) | 24.474** (1.643) | 23.017** (1.688) |
| INI | 0.947** (0.209) | 0.897** (0.299) | 0.820** (0.218) | ||
| 20Q | 1.002** (0.242) | 0.494 (0.372) | 0.070 (0.383) | ||
| Group x INI | 0.098 (0.419) | ||||
| Group x 20Q | 0.875* (0.488) | 1.001** (0.490) | |||
| Constant | 78.016** (1.204) | 77.947** (1.242) | 77.452** (1.190) | 77.087** (1.202) | 77.991** (1.206) |
| R2 | .504** | .504** | .488** | .493** | .720** |
Note:
p<.08,
p<.05. Results presented are from stepwise multiple regression analyses investigating the relation between executive function variables and communication ability. Higher order terms (i.e., interactions) were evaluated first, with non-significant terms removed to maintain parsimony. Group included alcohol-exposed (AE) and typically-developing control (CON) subjects. Inhibition (INI) was measured by Total Errors scaled score from the NEPSY-II; Twenty Questions (20Q) was measured by Initial Abstraction scaled score from the Delis-Kaplan Executive Function System (D-KEFS). The dependent variable, communication, was measured by the Vineland Adaptive Behavior Scales – Second Edition (VABS-II) Communication domain standard score.
The final model consisted of INI main effect and Group x 20Q interaction effect.
Figure 1.
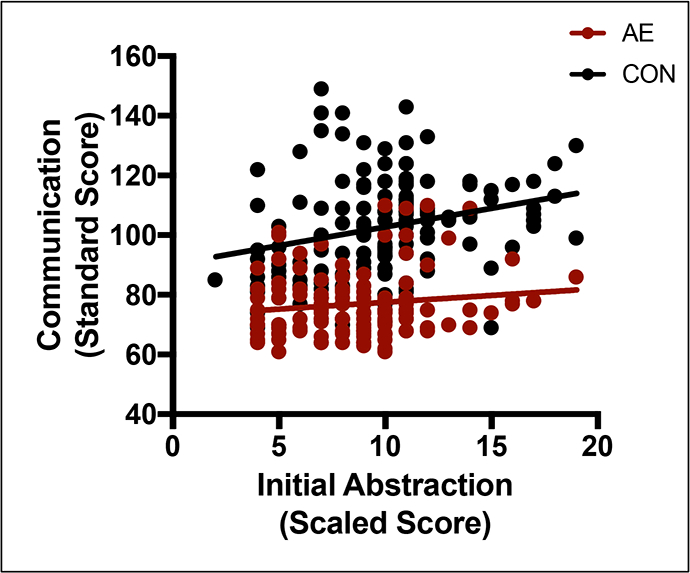
Relation between Twenty Questions Initial Abstraction score and Communication by group. In the control (CON) group only, as Initial Abstraction scores increase, predicted Communication scores also increase. Variables were not significantly related in the alcohol-exposed (AE) group. The regression line shows predicted Communication scores based on our model.
Language
Results from the language regressions are presented in Table 6. No significant interaction (p = .092) or main effect (p = .085) was noted for Speeded Naming. Word Generation displayed a significant interaction effect (p = .040). Specifically, the relation between Word Generation and Communication was significant in the AE group, but not the CON group (see Figure 2). Thus, the final language model consisted of the Word Generation interaction effect (F(3,283) = 82.572; p < .001; R2 = .469).
Table 6.
Stepwise multiple regression results for Language model.
| Variables | WG β (SE) |
Group x WG β (SE) |
SN β (SE) |
Group x SN β (SE) |
Final Model β (SE)a |
|---|---|---|---|---|---|
| Group | 25.361** (1.690) | 25.349** (1.680) | 25.249** (1.684) | 25.341** (1.680) | 25.349** (1.680) |
| WG | 0.373 (0.275) | 0.979** (0.401) | 0.979** (0.401) | ||
| SN | 0.525 (0.303) | 0.945** (0.391) | |||
| Group x WG | −1.131** (0.548) | −1.131** (0.548) | |||
| Group x SN | −1.042 (0.616) | ||||
| Constant | 76.743** (1.218) | 77.040** (1.220) | 76.859** (1.216) | 77.062** (1.218) | 77.040** (1.220) |
| R2 | .461** | .469** | .464** | .469** | .469** |
Note:
p<.08,
p<.05. Results presented are from stepwise multiple regression analyses investigating the relation between language variables and communication ability. Higher order terms (i.e., interactions) were evaluated first, with non-significant terms removed to maintain parsimony. Group included alcohol-exposed (AE) and typically-developing control (CON) subjects. Word Generation (WG) was measured by Semantic and Initial Combined scaled score from the NEPSY-II; Speeded Naming (SN) was measured by SN Combined scaled score from the NEPSY-II. The dependent variable, communication, was measured by the Vineland Adaptive Behavior Scales – Second Edition (VABS-II) Communication domain standard score.
The final model consisted of Group x WG interaction effect.
Figure 2.
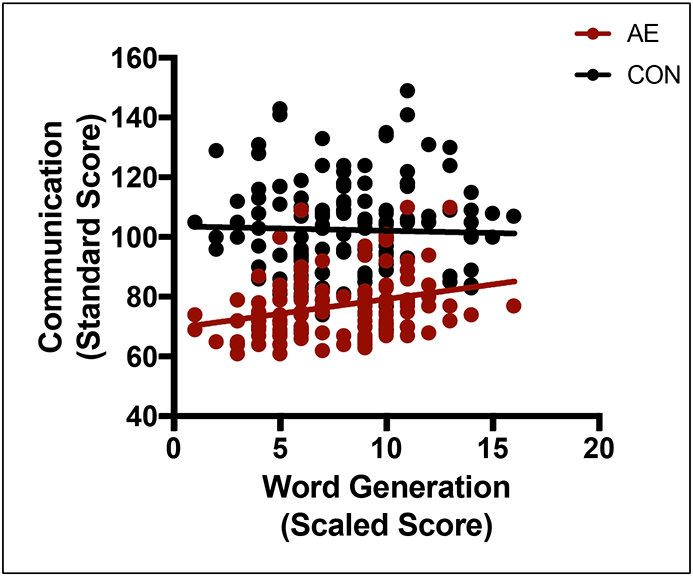
Relation between Word Generation score and Communication by group. In the alcohol-exposed (AE) group only, as Word Generation scores increase, predicted Communication scores also increase. Variables were not significantly related in the control (CON) group. The regression line shows predicted Communication scores based on our model.
Global Model
All significant variables from the three domain-specific models (Spatial Working Memory main effect, Inhibition main effect, Twenty Questions interaction effect, Word Generation interaction effect) were combined into a global model. When all variables were combined into the global model, the main effect of Spatial Working Memory (p = .581) was no longer significant, and as such was removed from the model. Results from the final global model regressions are presented in Table 7. The main effect of Inhibition (p = .001), and the interaction effects of Twenty Questions (p = .018) and Word Generation (p = .036) remained significant in the global model (F(6, 276) = 50.111; p < .001; R2 = .527).
Post Hoc Analyses
To investigate whether the presence of ADHD significantly contributed to our findings, we conducted sub-group analyses examining the impact of ADHD to each domain specific model within the AE group only. Within the working memory model, the relationship between Spatial Working Memory and Communication was not significant (p = .080) when accounting for ADHD and the main effect of ADHD was not significant (p = .188). Within the executive function model, the relationship between Twenty Questions Total Initial Abstraction and Communication was not significant (p = .138) and the effect of Inhibition Total Errors was significant (p = .012) when controlling for ADHD, and the effect of ADHD was not significant (p = .216). Finally, within the language model, the relationship between Word Generation and Communication was significant even after accounting for ADHD (p = .017), and the effect of ADHD was not significant (p = .211). Thus, with the exception of Spatial Working Memory, our results were unchanged when accounting for the presence of ADHD symptomology within the AE group.
Discussion
We sought to determine whether cognitive variables (working memory, executive function, language) could significantly predict practical day-to-day communication ability among adolescents with heavy prenatal alcohol exposure. Our findings indicate that measures of executive function and language differentially relate to communication ability as reported by parents and caregivers across groups. The ability to inhibit one response in favor of another under certain conditions, as measured by Inhibition, related to communication ability across groups. Specifically, greater scores on Inhibition related to better Communication scores across groups. However, verbal fluency, or the ability to produce words associated with a certain category and within a given set of rules as measured by Word Generation, was significantly related with communication in only alcohol-exposed subjects. That is, among alcohol-exposed adolescents, greater scores on Word Generation related to better Communication scores. This relationship was not significant within the control subjects. On the other hand, the ability to verbalize a question that efficiently solves a problem as measured by Twenty Questions Initial Abstraction, was significantly related with communication in controls. That is, among control adolescents, greater scores on Twenty Questions Initial Abstraction related to better Communication scores. This relationship was not significant within the alcohol-exposed subjects. While Spatial Working Memory was associated with communication ability across groups, this effect was not significant above and beyond other measures of executive function and language, possibly due to the visual component of this measure. In addition, the effect of Spatial Working Memory was no longer significant when accounting for ADHD symptomology, suggesting it may be more sensitive to attention difficulties. Our findings are consistent for both males and females, as observed relationships did not differ based on sex. Similarly, age did not differentially impact our findings (Panczakiewicz et al., 2016).
Our results suggest differences in the relationship between aspects of executive function and communication ability between alcohol-exposed youth and typically developing controls. Specifically, verbal reasoning efficiency (i.e., the ability to integrate multiple sources of information to produce substantive and informed speech) may more strongly relate to communication ability in the relatively higher-level daily communications of non-exposed youth. Although not included in the main analyses, exploratory analyses showed that category fluency was driving the relationship between Word Generation and Communication, indicating that a poorer verbal knowledge store or inability to retrieve the appropriate word from this store may more strongly relate to communication ability in the relatively lower-level communications of alcohol-exposed youth. It is possible that low overall word knowledge may be contributing to these findings; however, follow-up analyses showed that DAS-II Verbal Ability (a measure of verbal knowledge) was not correlated with Communication within the AE group (p = .544). Furthermore, Twenty Questions Initial Abstraction was related to communication ability within the control group only suggesting that abstract reasoning skills play an important role in these individuals’ everyday communication though these skills may not be as well-developed among youth with prenatal alcohol exposure. Our findings are consistent with previous studies in that alcohol-exposed youth show greater impairment on measures of language production (for review, see Mattson et al., 2011) and that verbal measures may be particularly sensitive to alcohol-exposure and provide a promising avenue for targeted interventions (Glass et al., 2013; Mattson et al., 1998; Mattson et al., 2013). Likewise, as in SLI, our results show that particular aspects of language are more difficult and complex, requiring more widespread recruitment of resources, and as such are more vulnerable to impairment in FASD (Akbarian, 1992). As such, the current study adds to the extant literature examining cognitive bases of communication impairment among neurodevelopmental disorders, specifically within prenatal alcohol exposure.
Numerous studies have shown that youth with heavy prenatal alcohol exposure demonstrate impaired executive function (Glass et al., 2013; Kodituwakku, 2007; Kodituwakku & Kodituwakku, 2014; Mattson et al., 2011; Mattson et al., 2013). Neuroimaging studies have also shown changes in brain structures important for language and executive function ability among youth with prenatal alcohol exposure (for review see Riley, McGee, & Sowell, 2004) and future exploration of neural correlates of communication ability among this population is warranted. However, the association between executive functioning deficits and communication difficulty in the FASD population is not well delineated. Because aspects of executive function (e.g., the ability to inhibit, attend to certain stimuli selectively, plan) are essential to complicated processes such as communication (Singer & Bashir, 1999), it is reasonable to expect that impairments in executive function would translate to impaired communication. Indeed, a connection between executive function deficits and communication impairment has been shown in other neurodevelopmental disorders (Finneran et al., 2009; Im-Bolter et al., 2006; McEvoy et al., 1993; Spaulding et al., 2008), with greater impairment in executive functioning ability predicting greater impairment in communication ability. Further, aspects of executive functioning (i.e., regulating interference, selecting an appropriate word over alternatives, inhibiting production of inappropriate words) are implicated in communication among typically-developing children (Ye & Zhou, 2009). Thus, our findings are consistent with the extant literature examining the role of executive function in language and communication among neurodevelopmental disorders as well as typically-developing populations.
Clinical interventions targeted at executive function may help improve communication deficits observed among those with heavy prenatal alcohol exposure. Interventions aimed at improving executive function ability among children with acquired traumatic brain injury have focused on training attention and providing instruction on metacognitive tasks, an aspect of executive function (Treble-Barna et al., 2015). Within the FASD population, interventions have focused on teaching metacognitive skills (Coles et al., 2015), improving self-regulation and attention (Kerns et al., 2010; Nash et al., 2015; Wells et al., 2012), social skills (Keil et al., 2010; O’Connor et al., 2006; O’Connor et al., 2012; Timler et al., 2005), and certain academic skills (Adnams et al., 2007; Kable et al., 2015). Smaller pilot studies have shown promise in improving communication ability with cognitive control studies (Paley & O’Connor, 2009). Thus, the field would benefit from development of additional interventions targeted at improving executive function related communication skills to help alleviate functional impairment associated with communication deficits within FASD. Based on our findings, interventions aimed at cognitive control and/or self-regulation may be most relevant to the deficits we found. Improved cognitive control may translate to better word retrieval (i.e., skills measured by Word Generation), which could improve communication ability of adolescents with FASD. Likewise, improved self-regulation may translate to better inhibitory control (i.e., skills measured by Inhibition), which could also improve communication ability. By identifying the particular aspects of executive function that are implicated in communication among adolescents with FASD, we have provided more specific targets to refine existing interventions and develop targeted interventions. As similar constructs are targeted (i.e., executive function), adaptation or inclusion of verbal fluency specific skills in existing interventions may be effective in improving communication ability of individuals with FASD.
Limitations/Future Directions
While a promising start to investigating the cognitive bases of communication impairment among individuals with heavy prenatal alcohol exposure, several limitations should be noted. First, while we used standard measures of neurobehavioral function, other measures might provide additional information regarding the cognitive correlates of communication ability. For example, we were only able to include a visual measure of working memory (i.e., Spatial Working Memory), though a verbal working memory measure would likely be more appropriate. In addition, the available CIFASD test battery did not include specific measures of auditory attention, an important component of communication. Future studies should aim to expand upon measures studied here and examine other components of communication that may significantly mediate the effect of prenatal alcohol exposure on communication. Similarly, our measures may not have been pure measures of the constructs under study and there may be concern regarding collinearity between our variables as all are measuring aspects of executive function. Multiple regression can determine the unique contribution of each predictor variable while controlling for other predictors. As such, the issue of collinearity is greatly reduced, particularly with inclusion of the global model.
We also relied on parent report of communication ability rather than direct assessment of the subjects. Our results show group differences in VABS-II Communication and are consistent with previous studies, indicating that this test is sensitive to some communication deficits seen in FASD. Future studies could augment parent report measures with direct assessment of communication skills. Another concern may include validity with use of caregivers with lower levels of education. The majority of the subjects’ caregivers within our sample completed standard 4-year college or university. Within the AE group, the majority of caregivers had completed at least some college, with only 15.5% achieving high school diploma or fewer years of formal education (see Table 2). A reading level roughly equivalent to the fifth grade is required to complete the VABS-II (Sparrow et al., 2005). In follow-up analyses, caregiver education level did not significantly predict VABS-II Communication scores (p = .661). Therefore, it is unlikely that parental education levels significantly impacted our results.
It may be of concern to use measures of language in predicting communication ability, for which language is an important component. However, communication, as measured by the VABS-II, is a higher-level construct than language. Aspects of language are important for communication, although language is but one component of communication. Successful communication requires social cognition, executive function skills, and intact language skills. The current study aimed to identify those aspects of executive function, as one component of communication, that are significantly related to communication among youth with heavy prenatal alcohol exposure. We were also limited in the adolescent age range we were able to investigate (i.e., 10–16 years) based on CIFASD study design. Additional important information relevant to language development was also not available for study. Further information regarding subject developmental histories (e.g., early speech or language interventions, delayed language development) will be essential to help disentangle the impact of prenatal alcohol exposure on communication ability and contributing cognitive factors.
Additional confounds inherent to this population should also be considered. Other psychiatric disorders (e.g., ADHD) are highly prevalent among youth with prenatal alcohol exposure (Burd et al., 2003; Fryer et al., 2007; Landgren et al., 2010; O’Connor & Paley, 2009; Mattson et al., 2011; Rasmussen et al., 2010) and may have contributed to our results. Previous studies have shown that executive function deficits are not exacerbated by ADHD among youth with heavy prenatal alcohol exposure (Glass et al., 2013). Thus, the deficits in executive functioning seen in the current study are likely not impacted by co-morbid ADHD diagnosis and post hoc analyses showed our results were not accounted for by the presence of ADHD. Further, previous studies have also shown both independent and synergistic impacts of ADHD and prenatal alcohol exposure on adaptive functioning (Ware et al., 2012; Ware et al., 2014). As the goal of the current study was to examine cognitive bases of communication impairment, we first aimed to determine whether differences exist among alcohol-exposed youth. Future studies will be able to expand upon our results and investigate the specificity of these deficits further by addressing the role of ADHD directly as well as investigate how the communication dysfunction observed in FASD differs from other neurodevelopmental disorders (e.g., autism spectrum disorder). Information regarding stimulant or other medication usage was not available. Although we ask all subjects to refrain from using medication on the day of testing, possible cumulative effects due to medication use cannot be fully ruled out.
Other potential confounds include maternal smoking and use of drugs (e.g., cocaine) during pregnancy. As our study is retrospective in nature, we often do not have access to specifics regarding maternal smoking or other drug use and cannot include these variables in our analyses. However, for inclusion in our AE group, we require documentation that alcohol is the primary exposure substance. While alcohol is considered one of the most detrimental teratogens with effects above and beyond that of other drugs of abuse, we cannot rule out their effects. Additional information regarding exposure to other substances with detrimental effects on cognition would provide additional clarity regarding patterns observed in this study.
Findings may also be explained by overall performance (e.g., IQ or GCA) differences between groups, as the AE group performed below the CON group on all measures although the average GCA score for both groups fell within the average range. We did not test GCA as a covariate given the statistical and methodological limitations in doing so (Dennis et al., 2009). Alternately, we considered whether differences in GCA performance could be driving the observed relationships. If this was the case, a general blunting of performance related to decreases in GCA or IQ would result in similar findings across measures for both groups, yet we observed differential relationships among variables within each group. In a separate unpublished study, we directly examined the relation between IQ and VABS-II domain scores in AE and CON groups. In the Communication domain, there was a significant interaction between IQ and Group resulting from stronger correlations in the control group between IQ and Communication than in the alcohol-exposed group. These results suggest that IQ does not fully account for communication deficits within the AE group, though other aspects of cognitive ability (not measured here) may play an important role in mediating alcohol’s effects on communication ability.
Conclusions
Youth with heavy prenatal alcohol exposure history demonstrate significant impairment on communication measures, as reported by caregivers. In highlighting specific executive function correlates of this impairment, the current study takes the first step in helping to address communication impairment among this population. To date, no known studies have investigated cognitive correlates of higher-level communication abilities among youth with prenatal alcohol exposure and as such the current study provides additional insight into the deficits associated with prenatal alcohol exposure. Impaired communication can prevent these individuals from functioning at the level expected for their age and affect quality of life in social, academic, and occupational domains. Findings from this study suggest that clinical interventions targeted at inhibition and verbal fluency may prove to be more beneficial in improving communication ability of these individuals and, ultimately, ability to function independently.
Supplementary Material
Acknowledgments
Acknowledgements: Research described in this paper was supported by NIAAA grant number U01 AA014834. Additional support was provided by U24 AA014811, U24 AA014815, K99/R00 AA022661 and F31 AA025256. The authors thank the families who graciously participate in our studies. The authors have no financial or other conflicts of interest.
All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org.
References
- Adnams CM, Sorour P, Kalberg WO, Kodituwakku P, Perold MD, Kotze A, … May PA (2007). Language and literacy outcomes from a pilot intervention study for children with fetal alcohol spectrum disorders in South Africa. Alcohol, 41, 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian GG (1992). Communication effects of prenatal alcohol exposure. Journal of Communication Disorders, 25, 221–240. [DOI] [PubMed] [Google Scholar]
- Burd L, Klug MG, Martsolf JT, & Kerbeshian J (2003). Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicology Teratology, 25, 697–705. [DOI] [PubMed] [Google Scholar]
- Cambridge Cognition Limited, CC (2006). Cantabeclipse version 3.0.0: Test administration guide. Cambridge Cognition Limited: Cambridge, UK. [Google Scholar]
- Carney LJ, & Chermak GD (1991). Performance of american indian children with fetal alcohol syndrome on the test of language development. Journal of Commuication Disorders, 24, 123–134. [DOI] [PubMed] [Google Scholar]
- Church MW, Eldis F, Blakley BW, & Bawle EV (1997). Hearing, language, speech, vestibular, and dentofacial disorders in fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research, 21, 227–237. [PubMed] [Google Scholar]
- Church MW, & Kaltenbach JA (1997). Hearing, speech, language, and vestibular disorders in the fetal alcohol syndrome: A literature review. Alcoholism: Clinical and Experimental Research, 21, 495–512. [DOI] [PubMed] [Google Scholar]
- Coggins TE, Timler GR, & Olswang LB (2007) A state of double jeopardy: Impact of prenatal alcohol exposure and adverse environments on the social communicative abilities of school-age children with fetal alcohol spectrum disorder. Language, Speech, and Hearing Services in Schools, 38, 117–127. [DOI] [PubMed] [Google Scholar]
- Coles CD, Kable JA, Taddeo E, & Strickland DC (2015). A metacognitive strategy for reducing disruptive behavior in children with fetal alcohol spectrum disorders: GoFAR pilot. Alcoholism: Clinical and Experimental Research, 39, 2224–2233. [DOI] [PubMed] [Google Scholar]
- Crocker N, Vaurio L, Riley EP, Mattson SN (2009). Comparison of adaptive behavior in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research, 33, 2015–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). The Delis-Kaplan Executive Function System: Examiner’s Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA & Fletcher JM (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychology Society, 15, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD (2007). Differential Ability Scales – Second Edition (DAS-II). San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Finneran DA, Francis AL, &Leonard LB (2009). Sustained attention in children with specific language impairment (SLI). Journal of Speech, Language, and Hearing Research, 52, 915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, & Mattson SN (2007). Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics, 119, E733–E741. [DOI] [PubMed] [Google Scholar]
- Gentry B, Griffith L, Dancer J, Davis P, Eaton B, & Schulz E (1998). Prenatal alcohol exposure and communication, behavior, and nonverbal intelligence of 3 school-age children. Perceptual and Motor Skills, 86, 1089–1090. [DOI] [PubMed] [Google Scholar]
- Glass L, Ware AL, Crocker N, Deweese BN, Coles CD, Kable JA, … the CIFASD (2013). Neuropsychological deficits associated with heavy prenatal alcohol exposure are not exacerbated by ADHD. Neuropsychology, 27, 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im-Bolter N, Johnson J, & Pascual-Leone J (2006). Processing limitations in children with specific language impairment: The role of executive function. Child Development, 77, 1822–1841. [DOI] [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, … Chambers CD (2006). Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics, 118, E1734–E1738. [DOI] [PubMed] [Google Scholar]
- Kable JA, Taddeo E, Strickland D, & Coles CD (2015). Community translation of the Math Interactive Learning Experience program for children with FASD. Research in Developmental Disabilities, 39, 1–11. [DOI] [PubMed] [Google Scholar]
- Keil V, Paley B, Frankel F, & O’Connor MJ (2010). Impact of a social skills intervention on the hostile attributions of children with prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research, 34, 231–241. [DOI] [PubMed] [Google Scholar]
- Kodituwakku P, & Kodituwakku E (2014). Cognitive and behavioral profiles of children with fetal alcohol spectrum disorders. Current Developmental Disorders Report, 1, 149–160. [Google Scholar]
- Kodituwakku PW (2007). Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: A review. Neuroscience & Biobehavioral Reviews, 31, 192–201. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, & Kemp S (2007). NEPSY II. Clinical and Interpretive Manual. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- LaDue RA, Streissguth AP, & Randels SP (1992). Clinical considerations pertaining to adolescents and adults with fetal alcohol syndrome In Sonderegger TB (Ed.), Perinatal substance abuse: Research findings and clinical implications. Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- Landgren M, Svensson L, Strömland K, & Andersson Grönlund M (2010). Prenatal alcohol exposure and neurodevelopmental disorders in children adopted from eastern europe. Pediatrics, 125, E1178–E1185. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, & Nguyen TT (2011). Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychology Review, 21, 81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund A, … the CIFASD (2010). Collaborative initiative on fetal alcohol spectrum disorders: Methodology of clinical projects. Alcohol, 44, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, & Riley EP (1998). A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcoholism: Clinical and Experimental Research, 22, 279–294. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling LJ, Delis DC, & Jones KL (1998). Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology, 12, 146–153. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, … the CIFASD (2013). Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research, 37, 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, … Hoyme HE (2014). Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics, 134, 855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Keaster C, Bozeman R, Goodover J, Blankenship J, Kalberg WO, … Hoyme HE (2015). Prevalence and characteristics of fetal alcohol syndrome and partial fetal alcohol syndrome in a Rocky Mountain region city. Drug and Alcohol Dependence, 155, 118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Fedlmen H, Buckley D, . . . Hoyme HE (2018). Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA, 319(5), 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy RE, Rogers SJ, & Pennington BF (1993). Executive function and social communication deficits in young autistic children. Journal of Child Psychology and Psychiatry, 34, 563–578. [DOI] [PubMed] [Google Scholar]
- McGee CL, Bjorkquist OA, Riley EP, & Mattson SN (2009). Impaired language performance in young children with heavy prenatal alcohol exposure. Neurotoxicology and Teratolology, 31, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash K, Stevens S, Greenbaum R, Weiner J, Koren G, & Rovet J (2015). Improving executive functioning in children with fetal alcohol spectrum disorders. Child Neuropsychology, 21, 191–209. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Frankel F, Paley B, Schonfeld AM, Carpenter E, Laugeson EA, & Marquardt R (2006). A controlled social skills training for children with fetal alcohol spectrum disorders. Journal of Consulting and Clinical Psychology, 74, 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ, Laugeson EA, Mogil C, Lowe E, Welch-Torres K, Keil V, & Paley B (2012). Translation of an evidence-based social skills intervention for children with prenatal alcohol exposure in a community mental health setting. Alcoholism: Clinical and Experimental Research, 36, 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ, & Paley B (2009). Psychiatric conditions associated with prenatal alcohol exposure. Developmental Disabilities Research Reviews, 15, 225–234. [DOI] [PubMed] [Google Scholar]
- Paley B, & O’Connor MJ (2009). Intervention for individuals with fetal alcohol spectrum disorders: Treatment approaches and case management. Developmetnal Disabilities Research Reviews, 15, 258–267. [DOI] [PubMed] [Google Scholar]
- Panczakiewicz AL, Glass L, Coles CD, Kable JA, Sowell ER, Wozniak JR, Jones KL, … the CIFASD (2016). Neurobehavioral deficits consistent across age and sex in youth with prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research, 40, 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prizant BM, Audet LR, Burke GM, Hummel LJ, Maher SR, & Theadore G (1990). Communication disorders and emotional/behavioral disorders in children and adolescents. The Journal of Speech and Hearing Disorders, 55, 179–192. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Benz J, Pei J, Andrew G, Schuller G, Abele-Webster L, Alton C, Lord L (2010). The impact of an ADHD co-morbidity on the diagnosis of fasd. The Canadian Journal of Clinical Pharmacology, 17, E165–E176. [PubMed] [Google Scholar]
- Riley EP, Infante MA, & Warren KR (2011). Fetal alcohol spectrum disorders: An overview. Neuropsychology Review, 21, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, McGee CL, & Sowell ER (2004). Teratogenic effects of alcohol: A decade of brain imaging. American Journal of Medical Genetics, 127C, 35–41. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, & O’Connor MJ (2006). Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychology, 12, 439–452. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 28–38. [DOI] [PubMed] [Google Scholar]
- Singer BD, & Bashir AS (1999). What are executive functions and self-regulation and what do they have to do with language-learning disorders? Language, Speech, and Hearing Services in Schools, 30, 265–273. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, & Balla DA (2005). Vineland Adaptive Behavior Scales, 2nd edition: Survey Forms Manual. Circle Pines, MN: AGS Publishing. [Google Scholar]
- Spaulding TJ, Plante E, & Vance R (2008). Sustained selective attention skills of preschool children with specific language impairment: Evidence for separate attentional capacities. Journal of Speech, Language, and Hearing Research, 51, 16–34. [DOI] [PubMed] [Google Scholar]
- Streissguth AP (1986). The behavioral teratology of alcohol: Performance, behavioral, and intellectual deficits in prenatally exposed children In West JR (Ed.), Alcohol and brain development. New York, NY: Oxford. [Google Scholar]
- Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, & Smith DF (1991). Fetal alcohol syndrome in adolescents and adults. JAMA, 265, 1961–1967. [PubMed] [Google Scholar]
- Timler GR, Olswang LB, & Coggins TE (2005). “Do I know what I need to do?” A social communication intervention for children with complex clinical profiles. Language, Speech, and Hearing Services in Schools, 36, 73–85. [DOI] [PubMed] [Google Scholar]
- Treble-Barna A, Sohlber MM, Harn BE, & Wade SL (2015). Cognitive intervention for attention and executive function impairments in children with traumatic brain injury: A pilot study. The Journal of Head Trauma Rehabilitation, 6, 407–418. [DOI] [PubMed] [Google Scholar]
- Ware AL, Crocker N, O’Brien JW, Deweese BN, Roesch SC, Coles CD, … Mattson SN (2012). Executive function predicts adaptive behavior in children with histories of heavy prenatal alcohol exposure and attention-deficit/hyperactivity disorder. Alcoholism: Clinical and Experimental Research, 36, 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware AL, Glass L, Crocker N, Deweese BN, Coles CD, Kable JA, … the CIFASD (2014). Effects of prenatal alcohol exposure and attention-deficit/hyperactivity disorder on adaptive functioning. Alcoholism: Clinical and Experimental Research, 38, 1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismer SE, Evans J, & Hesketh LJ (1999). An examination of verbal working memory capacity in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 42, 1249–1260. [DOI] [PubMed] [Google Scholar]
- Wells AM, Chasnoff IJ, Schmidt CA, Telford E, & Schwartz LD (2012). Neurocognitive habilitation therapy for children with fetal alcohol spectrum disorders: An adaptation of the Alert Program. American Journal of Occupational Therapy, 66, 24–34. [DOI] [PubMed] [Google Scholar]
- Wyper KR, & Rasmussen CR (2011). Language impairments in children with fetal alcohol spectrum disorders. Journal of Population Therapeutics and Clinical Pharmacology, 18, E364–E376. [PubMed] [Google Scholar]
- Ye Z, & Zhou X (2009). Executive control in language processing. Neuroscience & Biobehavioral Reviews, 33, 1168–1177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


