Abstract
Objective
Alzheimer’s disease (AD) typically eludes clinical detection for years, if not decades. The identification of subtle cognitive decline associated with preclinical AD would not only advance understanding of the disease, but also provide clinical targets to assess preventative and early intervention treatments. Disrupted retrieval of detailed episodic autobiographical memories may be a sensitive indicator of subtle cognitive decline, because this type of memory taxes a core neural network affected by preclinical AD neuropathology.
Method
To begin to address this idea, we assessed the episodic specificity of autobiographical memories retrieved by cognitively normal middle-aged and older individuals who are carriers of the apolipoprotein E ε4 allele – a population at increased risk for subtle cognitive decline related to neuropathological risk factors for AD. We compared the ε4 carriers to non-carriers of ε4 similar in age, education, and gender.
Results
The ε4 carriers did not perform worse than the non-carriers on a comprehensive battery of neuropsychological tests. In contrast, as a group, the ε4 carriers generated autobiographical memories that were reduced in “internal” or episodic details relative to non-carriers.
Conclusions
These findings support the notion that reduced autobiographical episodic detail generation may be a marker of subtle cognitive decline associated with AD.
Keywords: Autobiographical memory, Episodic memory, Alzheimer’s disease, Aging, APOE, Preclinical
Alzheimer’s disease (AD) is an age-related degenerative condition of the brain that gradually affects memory, other aspects of cognition (e.g., decision making), emotional processing, social relationships, and ultimately one’s sense of self. The pathogenic cascade of AD is believed to begin years before clinical signs of cognitive and functional decline emerge, a period known as preclinical AD (Sperling et al., 2011). The accumulation of beta amyloid (Aβ) and tau-related neurodegeneration are hallmark neuropathological mechanisms of preclinical AD and both increase risk for conversion to mild cognitive impairment (MCI) and dementia (Jack et al., 2010; 2013). According to some conceptual models, these preclinical AD neuropathological processes initially cause subtle cognitive decline, which is mild cognitive deficiency that has not reached the severity to warrant a diagnosis of MCI (Caselli & Reiman, 2013; Edmonds et al., 2015; Han, Nguyen, Stricker, & Nation, 2017). Currently, there is not a definitive neuropsychological profile of subtle cognitive decline associated with AD, nor do we know how early in the preclinical phase subtle cognitive decline emerges and is detectable. The development of cognitive assays that are sensitive to such subtle decline has the potential to address these issues, facilitating earlier diagnosis and clinical treatment.
Long before diagnosable cognitive impairment is present, Aβ and tau-related neurodegenerative mechanisms can collectively affect much of the neural network that supports episodic memory (Chen et al., 2017; Gilboa, 2004; Monge et al., 2017; Rugg & Vilberg, 2013). For instance, the earliest stages of AD have been associated with increased accumulation of Aβ in parietal lobe regions that are anatomically and functionally connected to the medial temporal lobes (MTL) (Buckner et al., 2005; Chételat et al., 2010), with frontal and lateral temporal lobe regions also sites of Aβ accumulation (Masters et al., 2015; Price & Morris, 1999; Schmitt et al., 2000; Sperling et al., 2011). Tau-related neurodegenerative mechanisms, on the other hand, begin in the MTL (entorhinal cortex and hippocampal formation) before spreading cortically (Masters et al., 2015; Price & Morris, 1999; Schmitt et al., 2000; Sperling et al., 2011). Other disease processes not specific to Aβ or tau affect these brain regions as well (Jack et al., 2017). Therefore, given the neural targets and distributed nature of early neuropathological risk factors for AD, one potentially fruitful approach to improving sensitivity to subtle cognitive decline is to focus on episodic memory components that tax the involvement of MTL-cortical interaction.
Episodic autobiographical memory (EAM), which is memory for personal, real world events, involves multiple cognitive processes that rely on dynamic MTL-cortical interaction (McCormick et al., 2015), including scene construction (Hassabis et al., 2007) and flexible retrieval and binding of multimodal episodic details (Addis et al., 2008; Cohen & Eichenbaum, 1993). In line with this idea, functional neuroimaging studies have implicated the MTL, as well as frontal, parietal, and lateral temporal lobe regions in the retrieval of EAMs (Addis et al., 2016; Martinelli et al., 2013; Svoboda et al., 2006). Lesion studies have corroborated these neuroimaging findings and shown that EAM retrieval not only depends on the MTL (Cermak & O’Connor, 1983; Grilli & Verfaellie, 2014; Tulving, 1985) but also cortical regions of the episodic memory neural network (Berryhill et al., 2007; Bertossi et al., 2016). Critically, recent task-based fMRI findings (Chen et al., 2017; Monge et al., 2017) have shown that when directly compared to lab-based episodic memory tasks (i.e., comparing the retrieval of episodic autobiographical memories cued by visual images or words to the recollection of prior exposure to visual images and word “chains”), EAM retrieval results in greater activation in the MTL (hippocampus and parahippocampal gyri), as well as connected posterior and anterior cortical regions (i.e., posterior cingulate cortex, retrosplenial cortex, angular gyrus, medial prefrontal cortex, and anterior lateral temporal lobe). Thus, compared to other components of episodic memory, EAM may be more sensitive to subtle changes in an MTL-cortical network that if compromised, increases risk for conversion to AD-related MCI and dementia.
Identifying cognitive factors that increase risk for AD-related clinical decline faces several challenges, including low incidence of dementia until the seventh and eighth decade of life and the need for longitudinal data. However, the study of cognitively normal middle-aged and older individuals who are carriers of the ε4 allele of the apolipoprotein E (APOE) gene has proven to be a successful way to begin to address the sensitivity of different cognitive and neural risk factors for AD dementia (Caselli & Reiman, 2013). Although the ε4 allele is present in only 20 to 25 percent of the general population in most global regions, it contributes to nearly half of all cases of late-onset AD (i.e., typical onset after age 65) (Caselli & Reiman, 2013). The ε4 allele has been shown to be a promising model for tracking episodic memory decline associated with AD. Specifically, in longitudinal studies, cognitively normal ε4 carriers exhibit accelerated decline of episodic memory relative to non-carriers beginning in their mid- to late-50s, years before obvious signs of memory impairment typically emerge (Caselli et al., 2009). This appears to be related to their increased probability of having neural risk factors for AD dementia. Indeed, according to recent estimates, ε4 carriers can account for approximately half of all cognitively normal middle-aged and older adults who are positive for Aβ (Jack et al., 2017). This population commonly exhibits additional brain imaging markers suggesting that the MTL-cortical network that supports episodic memory is compromised, including smaller cortical volume (den Heijer et al., 2002), reduced regional cerebral glucose metabolism (Reiman et al., 2005), white matter integrity differences (Ryan et al., 2011), and abnormal fMRI task-based and resting state patterns of activation (Sheline et al., 2010; Trachtenberg et al., 2012) in regions of this distributed neural network. Therefore, the power to detect subtle memory disruptions that have potential as cognitive indicators of risk for clinical decline secondary to AD is increased by comparing cognitively normal middle-aged and older ε4 carriers to non-carriers.
To begin to address the possibility that EAM may be sensitive to the presence of subtle cognitive decline associated with AD, we investigated whether the episodic specificity of EAM is compromised in cognitively normal middle-aged and older ε4 carriers relative to non-carriers. To measure EAM episodic specificity, we used the Autobiographical Interview (AI) approach developed by Levine and colleagues (Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002), which involves assessing the ability to generate “internal” (i.e., episodic) details of autobiographical events from across the lifespan as opposed to details that are “external” to these events (e.g., semantic details). Prior studies using this approach have established that EAM episodic specificity, meaning the ability to populate EAMs with internal details, is disrupted in individuals with MCI and Alzheimer’s dementia relative to healthy peers (Bastin et al., 2013; Gamboz et al., 2010; Irish et al., 2011; Irish, Addis, Hodges, & Piguet, 2012; Murphy et al., 2008; Tramoni et al., 2012). Prior studies also have found that in older adult populations that include individuals with varying degrees of clinical impairment, ε4 status is sensitive to episodic memory retrieval mechanisms (El Haj et al., 2016; van der Flier, Schoonenboom, Pijnenburg, Fox, & Scheltens, 2006; van der Vlies et al., 2007), including EAM retrieval (Buckley et al., 2014a). However, whether EAM episodic specificity is reduced among ε4 carriers relative to non-carriers prior to clinical impairment is not known. We hypothesized that if EAM is sensitive to subtle cognitive decline associated with AD, cognitively normal middle-aged and older ε4 carriers would retrieve EAMs with fewer internal details relative to non-carriers.
Methods
Participants
Seemingly cognitively intact middle-aged and older adult individuals (n = 40; age range: 52–80) were recruited from an existing pool of participants with previously collected genetic information. Our goal was to enroll approximately an equal number of ε4 carriers and non-carriers in the study sample, with the two groups comparable overall on age, education, and gender. Prior to enrollment in the present study, participants were interviewed about their cognition and daily activity independence, and all reported no concerns. To screen participants for MCI, we used a recently developed conceptual framework for actuarial decision making on the basis of performance across multiple neuropsychological tests (Bondi et al., 2014; Bondi et al., 2008). In this approach, two test scores are selected from multiple cognitive domains and individuals are considered to have MCI if one of two conditions are met: 1) they perform more than one standard deviation below the age-corrected normative mean on both scores in one domain, or 2) they perform more than one standard deviation below the age-corrected normative mean on one test in three domains. Consistent with Bondi and colleagues (Bondi et al., 2014), in our application of this actuarial approach, we used Trail Making Test A and B (Reitan & Wolfson, 1993) as our two measures of speed/executive function, and we used the Boston Naming Test (BNT; Goodglass et al., 2001) and animal fluency from the Controlled Oral Word Association Test (Benton, 1969) as our measures of language function. Whereas Bondi and colleagues (Bondi et al., 2014) used two scores from the Rey Auditory Verbal Learning Test for learning and memory, we used the California Verbal Learning Test (CVLT-II; Delis, Kramer, Kaplan & Ober, 2000) long delay free recall and Rey-Osterrieth Complex Figure Test (RCFT; Rey, 1941) delay recall scores. Finally, we also added a fourth cognitive domain, namely visuospatial functioning, to increase our power to detect non-amnestic MCI. For this cognitive domain, we used Block Design from the Wechsler Adult Intelligence Scale (WAIS-IV; Weschsler, 2008) and RCFT Copy scores. We used age (and education if available) corrected norms. With this actuarial decision making approach, 4 individuals were excluded because of MCI. One additional individual was excluded based on endorsing a high number of depressive symptoms on the Center for Epidemiological Studies Depression Scale (Radloff, 1977). Therefore, 35 participants were included in the final study sample. This included 18 carriers of the ε4 allele (ε3/ε4 n = 15, ε2/ε4 n = 1, ε4/ε4 n = 2) and 17 non-carriers (ε3/ε3 n = 16, ε2/ε3 n = 1). Participants were blind to their ε4 status. As shown in Table 1, the groups were comparable on age, education, and gender, p’s > .42. All participants provided informed consent, and this study was approved by the Institutional Review Board of the University of Arizona.
Table 1.
Demographics and mean normative scores on the standard neuropsychological tests for the ε4 carriers and non-carriers. Standard deviations are presented in parentheses.
| ε4 Carriers | Non-Carriers | |
|---|---|---|
|
|
|
|
| Demographics | ||
| Age | 67.4 (5.8) | 65.7 (7.2) |
| Education | 17.3 (1.8) | 16.9 (1.8) |
| Gender | 12 female/6 male | 11 female/6 male |
| WAIS-IV | ||
| VCI | Std = 119.3 (10.2) | Std = 122.9 (11.8) |
| WMI | Std = 106.4 (9.7) | Std = 112.5 (13.5) |
| Memory | ||
| CVLT long delay free recall | Z = 0.7 (0.8) | Z = 0.5 (0.9) |
| RCFT delay recall | Z = 1.1 (0.9)* | Z = 0.0 (0.8) |
| Language | ||
| BNT total score | Z = 1.1 (0.8) | Z = 0.6 (0.9) |
| Animal fluency | Z = −0.3 (1.2) | Z = 0.0 (1.2) |
| Speed/Executive Function | ||
| Trails A | Z = 0.3 (1.2) | Z = 0.3 (1.2) |
| Trails B | Z = 0.1 (0.9) | Z = 0.3 (0.9) |
| Visuospatial processing | ||
| WAIS-IV Block Design | Z = 0.9 (1.0) | Z = 0.6 (1.0) |
| RCFT copy trial | Z = 0.4 (1.3) | Z = −0.2 (0.8) |
Note:
= difference between groups, p < .05; VCI = Verbal Comprehension Index (WAIS-IV), WMI = Working Memory Index (WAIS-IV), CVLT II= California Verbal Learning Test - II, RCFT = Rey-Osterrieth Complex Figure Test, BNT = Boston Naming Test. Norms for the WAIS are from the manual (Wechsler, 2008). Norms the CVLT-II are from the manual (Delis et al., 2000). Norms for the RCFT are from Fastenau and colleagues (Fastenau et al., 1999). Norms for animal fluency, BNT, and Trail Making Test are from Heaton and colleagues (Heaton, Miller, Taylor, & Grant, 2004).
Power analysis
We powered our study to detect a group difference in EAM internal detail generation. Given that no study has investigated the effect of ε4 status on EAM episodic specificity in cognitively normal older adults, we used studies comparing individuals with MCI to cognitively normal older adults. For 14 such studies, Cohen’s d for the reduction in internal detail generation or overall EAM success ranged from .61 to 2.90, with a mean of 1.25. We assumed an effect in the range of the mean to slightly weaker (d = 1.25 to 1.04), with the lower bound being the effect size from a study that found a relation of ε4 to EAM retrieval in a sample including individuals with MCI (Buckley et al., 2014a). On the basis of effect sizes of d = 1.25 and 1.04 and the parameters of α = .05 and power (1 – β) = .80, 12 to 16 participants per group was the estimated required sample size for a two-tailed between group comparison.
Procedures
Standard neuropsychological tests
Participants were administered a battery of standard measures of neuropsychological function, including the tests mentioned above as part of the actuarial decision making approach for MCI. Also, given that our EAM assessment can be assumed to place relatively high demands on narrative ability and working memory, consistent with prior work (Grilli & Verfaellie, 2015; Grilli et al., 2018), we administered the subtests that contribute to the Wechsler Adult Intelligence Scale (WAIS-IV; Weschsler, 2008) Verbal Comprehension Index and the Working Memory Index.
Episodic autobiographical memory test
EAM details were assessed using an adapted version of the AI (Levine et al., 2002). This adaptation used the general methods of the AI, but included six, rather than five, time periods spanning the lifespan, including childhood to adolescence (up to 17 years old), early adulthood (age 18 up to 30 years old), middle adulthood (age 30 up to 5 years ago), later adulthood (5 years ago to 1 year ago), recent (1 year ago to 1 week ago), and very recent (last week, not including the day of experiment).
In the general probe section, for each time period, participants were instructed to describe a specific event. They were told that they could choose any specific event in which they remembered being personally involved. Participants were also instructed that a specific event is an event that occurred at a particular time and place. If the participant could not think of a specific event, they were asked to use a list of events included in the AI materials to help cue a memory. They were given five minutes to freely describe each event, and if the participant finished elaboration within five minutes, the experimenter provided one general probe (i.e., “Can you tell me more? I want to know all of the details.”). If the participant did not provide a specific event, the experimenter probed for one until one was generated.
Following the general probe section, there was a specific probe section during which each memory was revisited in order to ask four specific probes: (1) “Can you think of any more visual details, such as colors and other features of objects or people?”, (2) “Can you remember any sounds/smells/tastes/temperatures?”, (3) “Can you think of any more details about the scenery, such as where objects and people were located in relation to each other?”, and (4) “Can you remember what you were thinking, or how you felt at the time, in terms of emotion?”. Each probe was only asked once per memory. These specific probes are modifications of the probes from the AI. All responses were audio recorded and transcribed by research assistants blind to group membership.
Events were scored using the internal and external detail categories of the AI protocol (Levine et al., 2002). Briefly, details were scored as internal if they described an event (event details), the place of the event (event-place details), the time of the event (event-time details), thoughts or feelings that one had during the event (thought/emotion details), and sensory-perceptual and spatial features of the event (perceptual details). Details were scored as external if they were a semantic detail, such as a personal fact or general knowledge of things, places, or time, meta-comments about the experimental task or one’s current state of mind (e.g., “I’m trying to remember…”), repetitions of previous statements, and reference to other events not related to the unique event being described.
Following established scoring procedures (Verfaellie et al., 2014), a primary scorer, who was blind to participant status, scored all of the memories. Inter-rater reliability for detail scoring both in the general probe and specific probe sections was calculated based on a random selection of four ε4 carriers and four non-carriers (approximately 25 percent of the memories in total), which were scored by a secondary rater who was also blind to participant status. For the general and specific probe sections, inter-rater reliability was excellent for total details, total internal details, and total external details (Cronbach’s α’s range = .98 to .91). Inter-rater reliability was excellent to good for each of the detail subtypes (Cronbach’s α’s range = .94 to .80) with the exception of other event details (Cronbach’s α’s = .31 for general probe and .31 for specific probe), which reflects the infrequency of generation of this detail type (< 1 per participant).
Analyses
Parametric tests were used for analyzing performance on the standard neuropsychological tests and EAM task, as initial review revealed that the data met assumptions for such analysis. For the standard neuropsychological tests, the ε4 carrier and non-carrier groups were compared with independent samples t tests (Table 1). The EAM details generated by carriers and non-carriers were submitted to mixed analysis of variance (ANOVA). First, we investigated whether ε4 status influenced the generation of internal or external details in the general probe section across the six time periods with a 2 (Group: ε4 carriers vs. non-carriers) X 2 (Detail type: internal vs. external) X 6 (Time period: time 1– time 6) ANOVA (Figure 1) and whether group differences varied by internal detail subtype with a 2 (Group: ε4 carriers vs. non-carriers) X 5 (Internal detail type: event, event-place, event-time, thought/emotion, and perceptual) ANOVA (Table 2). Next, we repeated these analyses including the additional details provided during the specific probe section (i.e., cumulative performance after all probing; Figure 2 and Table 2).
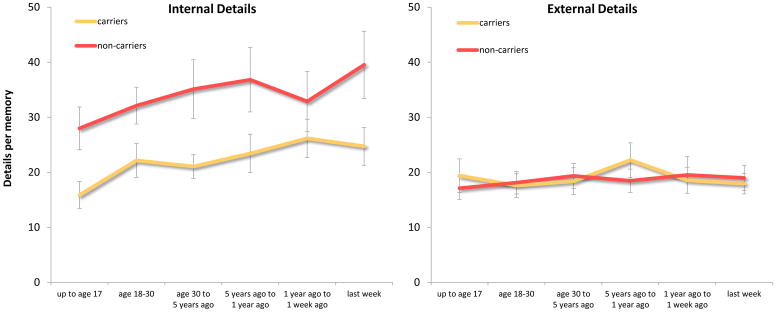
Figure 1.
Mean number of details retrieved per memory by each group before providing the specific probes. Error bars depict standard error of the mean.
Table 2.
Mean frequencies of internal detail subtype retrieval per episodic autobiographical memory. The specific probe is cumulative. Standard deviations are presented in parentheses.
| General Probe | Specific Probe | |||
|---|---|---|---|---|
|
|
|
|||
| Internal Detail Subtype | ε4 carriers | non-carriers | ε4 carriers | non-carriers |
| Event | 13.8 (5.8) | 17.8 (9.3) | 19.4 (7.1) | 26.8 (12.7) |
| Event-Place | 2.3 (1.2) | 3.96 (2.0) | 3.6 (1.1) | 5.9 (2.4) |
| Event-Time | 1.3 (0.7) | 2.1 (0.9) | 1.6 (0.9) | 2.8 (1.2) |
| Thought/Emotion | 2.0 (0.9) | 3.3 (1.7) | 4.2 (1.6) | 8.5 (3.3) |
| Perceptual | 2.9 (1.9) | 6.9 (5.7) | 10.1 (4.5) | 20.3 (12.2) |
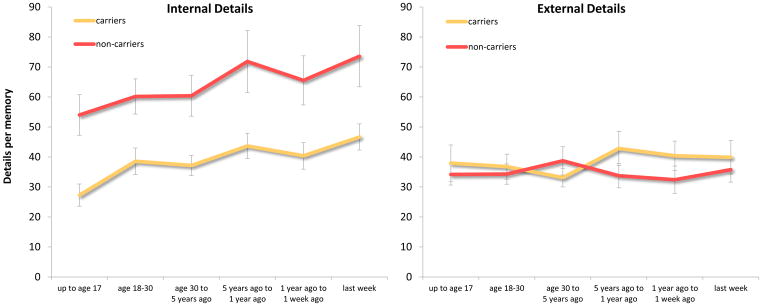
Figure 2.
Mean number of details retrieved per memory by each group after providing the specific probes (cumulative). Error bars depict standard error of the mean.
Edmonds and colleagues (Edmonds et al., 2015) recently extended the conceptual framework developed by Bondi and colleagues (Bondi et al., 2014) to operationalize subtle cognitive decline on the basis of actuarial judgment using neuropsychological data (as well as reported functional independence). Given our focus on the presence of subtle cognitive decline among cognitively normal middle-aged and older adults, we applied this actuarial approach and operational definition of Edmonds and colleagues (Edmonds et al., 2015) to our neuropsychological test scores. This approach considers two scores more than one standard deviation below the normative mean on tests from different cognitive domains as evidence of subtle cognitive decline. For our sample, we compared the proportion of carriers and non-carriers with subtle cognitive decline as defined in this way using chi square.
Results
Standard neuropsychological tests
Normative scores across the neuropsychological tests for the ε4 carrier and non-carrier groups are presented in Table 1. Because of an administrative error, two of the non-carriers did not complete the RCFT. With the exception of the RCFT delay recall score, there were not significant group differences for any of the tests (or indices), t’s ≤ 1.81, p’s ≥ .08. The ε4 carriers actually performed better than the non-carriers on the RCFT delay recall score, t (31) = 3.56, p = .001, d = 1.2. This difference appears to be driven by the ε4 carriers being slightly above the normative mean as a group, whereas the non-carriers were comparable to the normative mean on this test.
Autobiographical memory task
All participants were able to generate an EAM for each time period. For the general probe section, groups did not differ in the average number of probes given, t < 1, p = .45, the proportion of each group who received a probe did not differ for any time period, and how often participants were given probes did not vary by time period, x2’s ≤ 2.42, p’s ≥ .12.
Autobiographical memory episodic specificity: general probe
Figure 1 shows the mean number of internal details (collapsed across subtype) and external details generated per time period in the general probe section for ε4 carriers and non-carriers. The 2 (Group: ε4 carriers vs. non-carriers) X 2 (Detail type: internal vs. external) X 6 (Time period: time 1- time 6) ANOVA revealed that the non-carriers generated marginally more detailed memories relative to the ε4 carriers (main effect of group), F (1, 33) = 3.77, p = .06, d = .67, and not surprisingly internal details were generated more than external details (main effect of detail type), F (1, 33) = 17.64, p < .001, d = .73. However, there was also a significant interaction between group and detail type, F (1, 33) = 7.58, p = .01, partial η2 = .19, such that, relative to non-carriers, ε4 carriers generated fewer internal details, t (22.7) = 2.54, p = .02, d = .87, but not fewer external details, t (33) = .43, p = .67.
Total detail generation was affected by memory remoteness (main effect of time period), F (5, 165) = 3.37, p = .006, partial η2 = .09, such that memories from the earliest time period were less detailed relative to memories from two of the three most recent time periods (5 to 1 year ago and last week not including today), t’s ≥ 3.39, p’s ≤ .002, d’s ≥ .62 (corrected α level = .003). However, memory remoteness did not interact with group or detail type, nor was there a three-way interaction, F’s ≤ 1.48, p’s ≥ .20.
Given that there was a marginally significant reduction in total detail in the ε4 carriers, we also calculated internal to total detail ratios for each time period and conducted a 2 (Group: ε4 carriers vs. non-carriers) X 6 (Time period: time 1 – time 6) ANOVA. Consistent with the ANOVA on frequency of internal and external detail degeneration, non-carriers generated a higher internal to total detail ratio in comparison to ε4 carriers (main effect of group), F (1, 33) = 5.08, p = .03, d = .77. Also, there was not an effect of time period on ratio of internal details, nor was there a significant interaction between group and time period, F’s ≤ 1, p’s ≥ .65.
The 2 (Group: ε4 carriers vs. non-carriers) X 5 (Internal detail type: event, event-place, event-time, thought/emotion, and perceptual) ANOVA investigated whether the internal detail group difference varied by subtype of internal content, but the interaction was not significant, F (1.46, 48.2) = 1.88, p = .17 (see Table 2 for subtype retrieval frequencies).
Autobiographical memory episodic specificity: specific probe
As seen in Figure 2, not surprisingly there was a numerical increase in details per memory after taking into account the additional details generated in the specific probe section, but the overall pattern of results appeared to be largely the same as the general probe section. This impression was supported by the 2 (Group: ε4 carriers vs. non-carriers) X 2 (Detail type: internal vs. external) X 6 (Time period: time 1-time 6) ANOVA, as the non-carriers generated marginally more detailed memories relative to the ε4 carriers (main effect of group), F (1, 33) = 3.89, p = .06, d = .70, internal details were generated more than external details (main effect of detail type), F (1, 23) = 18.69, p < .001, d = .61, and group interacted with detail type, F (1, 33) = 17.55, p < .001, partial η2 = .35, such that relative to non-carriers, ε4 carriers generated fewer internal details, t (20.9) = 3.35, p = .003, d = 1.24, but not fewer external details during EAM retrieval, t (33) = .67, p = .51.
Similar to the general probe section, memory remoteness affected total detail generation (main effect of time period), F (5, 165) = 5.65, p < .001, partial η2 = .15. However, there also was a significant interaction between time period and detail type, F (5, 165) = 2.93, p = .02, partial η2 = .08, such that memories from the earliest time period contained fewer internal details relative to memories from the three most recent time periods, t’s ≥ 3.48, p’s ≤ .001, d’s ≥ .60, but not fewer external details, p’s ≥ .23 (corrected α level = .0017). Importantly, memory remoteness did not interact with group, and there was not a three-way interaction, F’s < 1, p’s > .46.
Consistent with the general probe section, we conducted a 2 (Group: ε4 carriers vs. non-carriers) X 6 (Time period: time 1-time 6) ANOVA on the ratio of internal to total details. In line with the ANOVA on frequency of internal and external detail degeneration, non-carriers generated a higher internal to total detail ratio in comparison to ε4 carriers (main effect of group), F (1, 33) = 14.32, p = .001, d = 1.31. Also, there was not an effect of time period on ratio of internal details, nor was there a significant interaction between group and time period, F’s ≤ 2.09, p’s ≥ .09.
In contrast to the general probe section, the 2 (Group: ε4 carriers vs. non-carriers) X 5 (Internal detail type: event, event-place, event-time, thought/emotion, and perceptual) ANOVA revealed a significant interaction, F (1.63, 53.93) = 4.73, p = .018, partial η2 = .13. Critically, the ε4 carriers generated fewer internal details than the non-carriers across all subtypes, t’s ≥ 2.12, p’s ≤ .044, d’s ≥ .75. The interaction reflects that whereas non-carriers generated thought/emotion details more than place details, t (16) = 3.17, p = .006, d = .86, ε4 carriers did not, t (17) = 1.46, p = .16. However, the former does not survive correction for multiple comparisons (α = .003).
Subtle cognitive decline using standard neuropsychological test scores
When we applied the actuarial decision making approach developed by Edmonds and colleagues (Edmonds et al., 2015), two ε4 carriers and one non-carrier met criteria for subtle cognitive decline on the basis of standard neuropsychological test scores. The proportion of carriers was not significantly different from the proportion of non-carriers, x2 = .003, p = .96. The results of all EAM analyses described above persisted when these individuals were removed.
Discussion
The present study found that cognitively normal ε4 carriers recalled autobiographical memories with fewer internal details, but not fewer external details, relative to matched non-carriers. These results, therefore, suggest that episodic specificity is not only reduced in individuals with MCI and AD dementia (Bastin et al., 2013; Gamboz et al., 2010; Irish et al., 2011; 2012; Murphy et al., 2008; Tramoni et al., 2012), but also in individuals who, although cognitively normal, are at increased risk of developing AD dementia. Given that the groups did not differ on a standard neuropsychological test score of verbal memory (i.e., CVLT-II) and the ε4 carriers actually outperformed the non-carriers on a standard neuropsychological test score of nonverbal memory (i.e., RCFT), the EAM disruption appears to be capturing a subtle episodic memory reduction not also reflected in the scores of these two commonly used neuropsychological tests. Overall, the ε4 carriers were not more likely than non-carriers to be identified as exhibiting subtle cognitive decline using an actuarial decision making approach with standard neuropsychological data (Edmonds et al., 2015). This supports the idea that EAM assessment, if adapted for and incorporated into neuropsychological evaluation, has potential to further facilitate the detection of subtle cognitive decline associated with AD.
Although we considered several possible retrieval mechanisms and qualitative features of EAM that might influence episodic specificity, none of them appeared to drive the difference between ε4 carriers and non-carriers. For instance, the episodic specificity discrepancy between ε4 carriers and non-carriers was not attenuated by drawing attention to specific internal content with additional specific probes. The findings also indicate that the reduction in episodic specificity among ε4 carriers was not affected by the remoteness of the memories. Rather, this disruption of personal memory reaches far back along the autobiographical timeline. When examined at a fine-grained level, the reduced specificity seems not to be exclusively driven by certain internal content, because ε4 carriers produced fewer internal details of all types relative to non-carriers – the spatial-temporal context, perceptual detail, and emotional content. Therefore, although these internal detail subtypes may vary in their episodic qualities, the fact that all were reduced among ε4 carriers highlights the extent to which EAM episodic specificity is compromised. Also, the fact that external detail generation did not differ between groups suggests that the internal detail deficit is unlikely a consequence of a tendency for ε4 carriers to focus on semantic content, or because of group differences in executive control (Levine, 2004; Spreng et al., 2017).
At this point we can only speculate as to the cognitive processes that may be contributing to this ε4-related memory discrepancy. On the basis of cognitive neuroscience theory of autobiographical memory, three cognitive processes are important to consider. First, the mental construction of a scene is thought to create the “stage” on which an event plays out. The ability to form a vivid scene is dependent on the MTL (Hassabis et al., 2007; Maguire & Mullally, 2013; Palombo et al., in press) and frontal and parietal lobe regions (Irish et al., 2015; Summerfield et al., 2010), likely because of shared contributions to spatial-perceptual detail retrieval and binding. Therefore, reduced EAM episodic specificity may be related to an inability to construct a vivid scene. Second, the ability to populate EAMs with internal details about the scene, people, and objects involves flexible retrieval and binding of content – a relational process that goes beyond scene construction (Roberts et al., in press; Schacter & Addis, 2007). This (re)constructive retrieval also depends on the MTL for binding (Cohen & Eichenbaum, 1993) and interaction with frontal and parietal regions for flexible retrieval of multimodal contents (McCormick et al., 2015). There is some evidence that relational processing is sensitive to a genetic variant of early onset AD (Parra et al., 2010), as well as brain amyloid deposition in older adults (Rentz et al., 2011). Therefore, a general disruption to flexible retrieval and binding may lead to a reduction in episodic specificity that is not limited to scenes. Third, narrative ability may be contributing to our results as well. Although ε4 carriers and non-carriers did not differ on standard neuropsychological indices of verbal intelligence or working memory, these aspects of cognition could be expected to account for only some variance in the ability to construct a complex narrative. Perhaps there are subtle differences in narrative ability that reduce episodic specificity. Or, multiple mechanisms may contribute to our finding. We are inclined to adopt this latter viewpoint and propose that EAM episodic specificity is more sensitive to ε4 status in cognitively normal middle-aged and older adults in comparison to other more traditional neuropsychological tests of memory, because EAM retrieval engages multiple MTL-cortically mediated cognitive mechanisms, all of which may be subtly compromised by preclinical AD. To refine this cognitive index into the most sensitive predictor it can be, it will be important to elucidate the contributions of EAM sub-components to the EAM disruption.
It is interesting to consider our results in light of emerging neuroimaging findings related to the neural bases of internal details and external details of autobiographical memory. For instance, recent research has shown that variation in volume of certain hippocampal subregions (i.e., dentate gyrus, CA2/3, and subiculum) is associated with internal detail generation (Miller et al., 2017; Palombo et al., 2018), but not external detail generation (Miller et al., 2017). Also, Hodgetts and colleagues (Hodgetts et al., 2017) showed that in young adults, internal detail generation was associated with diffusion measures of white matter integrity of the fornix, whereas semantic detail generation was related to white matter integrity of the inferior longitudinal fasciculus. There also is evidence that in young and middle-aged individuals, subjective report of one’s general tendency to utilize episodic-driven autobiographical remembering is related to MTL-posterior cortical intrinsic functional connectivity (Sheldon, Farb, Palombo, & Levine, 2016). In addition, resting state functional coupling between default mode and executive control regions in older adults has been shown to be associated with the tendency to generate external details during EAM retrieval (Spreng et al., 2017). Although we caution against strong claims about the neural bases of our ε4 deficit, these findings support the notion that reduced internal detail generation may relate to MTL integrity. Future research will need to determine the neural markers of internal detail reductions in older adults, and in particular whether this cognitive marker tracks with amyloid or tau biomarkers.
Our findings raise several additional questions that will need to be addressed in future work. For instance, prior research has shown that reduced EAM retrieval in individuals with MCI and AD is not only apparent in objective analysis of their narratives but also in their subjective report of retrieval experience (Buckley et al., 2014b; Irish et al., 2010). It will be important to determine if subjective report of EAM is a sensitive marker of subtle cognitive decline. Related to this point, although participants did not report cognitive concerns in an initial interview, it is possible that a more comprehensive screening would reveal mild concerns among the ε4 carriers. Also, it is interesting to consider whether cognitively normal ε4 carriers might demonstrate subtle deficits in cognitive functions that are believed to be supported by EAM, including the construction and maintenance of a self-concept (Addis & Tippet, 2004; El Haj, Antoine, Nandrino, & Kapogiannis, 2015). The sensitivity of EAM to ε4 status in older adults with clinical impairment relative to other measures of learning and memory remains an open question as well (El Haj et al., 2016; van der Flier et al., 2006; van der Vlies et al., 2007). We note that the present study was cross-sectional, and longitudinal research will need to determine the extent to which reduced EAM quality can predict clinical conversion to dementia. Longitudinal research also could shed light on how external detail generation during EAM retrieval might vary at distinct stages of cognitive aging. It is noteworthy that whereas cognitively normal older adults commonly generate more external details relative to young adults (Levine et al., 2002; St. Jacques & Levine, 2007), ε4 carriers did not exhibit increased retrieval of such content relative to non-carriers. Interestingly, there is mixed evidence as to whether individuals with amnestic MCI generate more external details relative to cognitively normal older adults (increased: Murphy, Troyer, Levine, & Moscovitch, 2008; Sheldon, Vandermorris, Al-Haj, Cohen, Winocur, & Moscovitch, 2015; not increased: Barnabe et al., 2012; Bastin et al., 2013), and individuals with AD dementia commonly do not show increased generation of external details (Barnabe et al., 2012; Benjamin et al., 2015; Irish et al., 2011; 2018). In comparison, focal dorsolateral prefrontal cortex lesions and frontotemporal dementia can result in elevated generation of external details (Levine, 2004; cf., Irish et al., 2011; 2018). Finally, given that our participants were highly educated, it will be important to determine the sensitivity of EAM to ε4 status and subtle cognitive decline in individuals from more diverse demographic backgrounds.
Despite these unresolved issues, the present study highlights the potential that investigating the qualitative recollection of autobiographical memory has for capturing subtle cognitive decline associated with AD. This may be a promising direction for the neuropsychological detection of preclinical AD and the development of new assessment tools.
Acknowledgments
We have no conflicts of interest to report. This work was supported by the Arizona Alzheimer’s Disease Core Center Pilot Grant Program, National Institute on Aging, P30 AG 019610 (MDG); the Arizona Alzheimer’s Consortium, Department of Health Services (MDG); The University of Arizona College of Science Dean’s Innovation and Education Fund (MDG); and the University of Arizona Research, Development, and Innovation Faculty Seed Grant Program (MDG).
References
- Addis DR, Moloney EE, Tippett LJ, Roberts PR, Hach S. Characterizing cerebellar activity during autobiographical memory retrieval: ALE and functional connectivity investigations. Neuropsychologia. 2016;90:80–93. doi: 10.1016/j.neuropsychologia.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Addis DR, Tippett L. Memory of myself: Autobiographical memory and identity in Alzheimer’s disease. Memory. 2004;12:56–74. doi: 10.1080/09658210244000423. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychol Sci. 2008;19(1):33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- Barnabe A, Whitehead V, Pilon R, Arsenault-Lapierre G, Chertkow H. Autobiographical memory in mild cognitive impairment and Alzheimer’s disease: A comparison between the Levine and Kopelman interview methodologies. Hippocampus. 2012;22:1809–1825. doi: 10.1002/hipo.22015. [DOI] [PubMed] [Google Scholar]
- Bastin C, Feyers D, Jedidi H, Ali Bahri M, Degueldre C, Lemaire C, Collette F, Salmon E. Episodic autobiographical memory in amnestic mild cognitive impairment: What are the neural correlates? Human Brain Mapping. 2013;34:1811–1825. doi: 10.1002/hbm.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin MJ, Cifelli A, Garrard P, Caine D, Jones FW. The role of working memory and verbal fluency in autobiographical memory in early Alzheimer’s disease and matched controls. Neuropsychologia. 2015;78:115–121. doi: 10.1016/j.neuropsychologia.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Benton AL. Development of a multilingual aphasia battery: Progress and problems. Journal of the Neurological Sciences. 1969;9:39–48. doi: 10.1016/0022-510x(69)90057-4. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. J Neurosci. 2007;27(52):14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertossi E, Tesini C, Cappelli A, Ciaramelli E. Ventromedial prefrontal damage causes a pervasive impairment of episodic memory and future thinking. Neuropsychologia. 2016;90:12–24. doi: 10.1016/j.neuropsychologia.2016.01.034. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, … Salmon DP. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42(1):275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Jak AJ, Delano-Wood L, Jacobson MW, Delis DC, Salmon DP. Neuropsychological contributions to the early identification of Alzheimer's disease. Neuropsychol Rev. 2008;18(1):73–90. doi: 10.1007/s11065-008-9054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RF, Saling MM, Irish M, Ames D, Rowe CC, Villemagne VL … Group A. I. B. a. L. S. o. A. A. R. Autobiographical narratives relate to Alzheimer's disease biomarkers in older adults. Int Psychogeriatr. 2014a;26(10):1737–1746. doi: 10.1017/S1041610214001136. [DOI] [PubMed] [Google Scholar]
- Buckley RF, Saling MM, Irish M, Ames D, Rowe CC, Lautenschlager NT, … Ellis KA. Personal memory function in mild cognitive impairment and subjective memory complaints: results from the Australian Imaging, Biomarkers, and Lifestyle (AIBL) Study of Ageing. J Alzheimers Dis. 2014b;40(3):551–561. doi: 10.3233/JAD-131820. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, … Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, … Reiman EM. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM. Characterizing the preclinical stages of Alzheimer's disease and the prospect of presymptomatic intervention. J Alzheimers Dis. 2013;33(Suppl 1):S405–416. doi: 10.3233/JAD-2012-129026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak LS, O'Connor M. The anterograde and retrograde retrieval ability of a patient with amnesia due to encephalitis. Neuropsychologia. 1983;21(3):213–234. doi: 10.1016/0028-3932(83)90039-8. [DOI] [PubMed] [Google Scholar]
- Chen HY, Gilmore AW, Nelson SM, McDermott KB. Are There Multiple Kinds of Episodic Memory? An fMRI Investigation Comparing Autobiographical and Recognition Memory Tasks. J Neurosci. 2017;37(10):2764–2775. doi: 10.1523/JNEUROSCI.1534-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chételat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D … Group A. I. B. a. L. R. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67(3):317–324. doi: 10.1002/ana.21955. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. The Psychological Corporation; New York, NY: 2000. [Google Scholar]
- den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MM. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59(5):746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW. Subtle cognitive decline and biomarker staging in preclinical Alzheimer’s disease. Journal of Alzheimer’s Disease. 2015;47:231–242. doi: 10.3233/JAD-150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Haj M, Antoine P, Amouyel P, Lambert JC, Pasquier F, Kapogiannis D. Apolipoprotein E (APOE) e4 and episodic memory decline in Alzheimer’s disease: A review. Ageing Research Reviews. 2016;27:15–22. doi: 10.1016/j.arr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Haj M, Antoine P, Nandrino JL, Kapogiannis D. Autobiographical memory decline in Alzheimer’s disease, a theoretical and clinical overview. Ageing Research Reviews. 2015;23:183–192. doi: 10.1016/j.arr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fastenau PS, Denburg NL, Hufford BJ. Adult norms for the Rey-Osterrieth Complex Figure Test and for supplemental recognition and matching trials from the extended complex figure test. The Clinical Neuropsychologist. 1999;13(1):30–47. doi: 10.1076/clin.13.1.30.1976. doi/abs/10.1076/clin.13.1.30.1976. [DOI] [PubMed] [Google Scholar]
- Gamboz N, De Vito S, Brandimonte MA, Pappalardo S, Galeone F, Iavarone A, Della Sala S. Episodic future thinking in amnestic mild cognitive impairment. Neuropsychologia. 2010;48:2091–2097. doi: 10.1016/j.neuropsychologia.2010.03.030. [DOI] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory--one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42(10):1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. Boston diagnostic aphasia examination. 3. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Grilli MD, Wank AA, Verfaellie M. The life stories of adults with amnesia: Insights into the contribution of the medial temporal lobes to the organization of autobiographical memory. Neuropsychologia. 2018;110:84–91. doi: 10.1016/j.neuropsychologia.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli MD, Verfaellie M. Supporting the self-concept with memory: Insight from amnesia. Social Cognitive and Affective Neuroscience. 2015:1684–1692. doi: 10.1093/scan/nsv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli MD, Verfaellie M. Personal semantic memory: insights from neuropsychological research on amnesia. Neuropsychologia. 2014;61:56–64. doi: 10.1016/j.neuropsychologia.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Han DS, Nguyen CP, Stricker NH, Nation DA. Detectable Neuropsychological Differences in Early Preclinical Alzheimer's Disease: A Meta-Analysis. Neuropsychol Rev. 2017 doi: 10.1007/s11065-017-9345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci U S A. 2007;104(5):1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- Hodgetts CJ, Postans M, Warne N, Varnava A, Lawrence AD, Graham KS. Distinct contributions of the fornix and inferior longitudinal fasciculus to episodic and semantic autobiographical memory. Cortex. 2017;94:1–14. doi: 10.1016/j.cortex.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M, Landin-Romero R, Mothakunnel A, Ramanan S, Hseih S, Hodges JR, Piguet O. Evolution of autobiographical memory impairments in Alzheimer’s disease and frontotemporal dementia: A longitudinal neuroimaging study. Neuropsychologia. 2018;1110:14–25. doi: 10.1016/j.neuropsychologia.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Irish M, Halena S, Kamminga J, Tu S, Hornberger M, Hodges JR. Scene construction impairments in Alzheimer's disease - A unique role for the posterior cingulate cortex. Cortex. 2015;73:10–23. doi: 10.1016/j.cortex.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Irish M, Addis DR, Hodges JR, Piguet O. Considering the role of semantic memory in episodic future thinking: Evidence from semantic dementia. Brain. 2012;135:2178–2191. doi: 10.1093/brain/aws119. [DOI] [PubMed] [Google Scholar]
- Irish M, Hornberger M, Lah S, Miller L, Pengas G, Nestor PJ, Hodges JR, Piguet O. Profiles of recent autobiographical memory retrieval in semantic dementia, behavioural-variant frontotemporal dementia, and Alzheimer’s disease. Neuropsychologia. 2011;49:2694–2702. doi: 10.1016/j.neuropsychologia.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Irish M, Lawlor BA, O'Mara SM, Coen RF. Exploring the recollective experience during autobiographical memory retrieval in amnestic mild cognitive impairment. J Int Neuropsychol Soc. 2010;16(3):546–555. doi: 10.1017/S1355617710000172. [DOI] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, … Trojanowski JQ. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, … Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Weigand SD, Therneau TM, Knopman DS, Lowe V, … Petersen RC. Age-specific and sex-specific prevalence of cerebral β-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. Lancet Neurol. 2017;16(6):435–444. doi: 10.1016/S1474-4422(17)30077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. Autobiographical memory and the self in time: Brain lesion effects, functional neuroanatomy, and lifespan development. Brain & Cognition. 2004;55:54–68. doi: 10.1016/S0278-2626(03)00280-X. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging. 2002;17(4):677–689. [PubMed] [Google Scholar]
- Maguire EA, Mullally SL. The hippocampus: a manifesto for change. J Exp Psychol Gen. 2013;142(4):1180–1189. doi: 10.1037/a0033650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli P, Sperduti M, Piolino P. Neural substrates of the self-memory system: new insights from a meta-analysis. Hum Brain Mapp. 2013;34(7):1515–1529. doi: 10.1002/hbm.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer's disease. Nat Rev Dis Primers. 2015;1:15056. doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- McCormick C, St-Laurent M, Ty A, Valiante TA, McAndrews MP. Functional and effective hippocampalneocortical connectivity during construction and elaboration of autobiographical memory retrieval. Cereb Cortex. 2015;25(5):1297–1305. doi: 10.1093/cercor/bht324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon MC, Nica EI, Sengdy P, Kovacevic N, Moscovitch M, Freedman M, Miller BL, Black SE, Levine B. Autobiographical memory and patterns of brain atrophy in frontotemporal lobar degeneration. Journal of Cognitive Neuroscience. 2008;20:1839–1853. doi: 10.1162/jocn.2008.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TD, Chong TTJ, Aimola Davies AM, Ng TWC, Johnson MR, Irani SR, Vincent A, Husain M, Jacob S, Maddison P, Kennard C, Gowland PA, Rosenthal CR. Focal CA3 hippocampal subfield atrophy following LGII VGKC-complex antibody limbic encephalitis. Brain. 2017;140:1212–1219. doi: 10.1093/brain/awx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge ZA, Wing EA, Stokes J, Cabeza R. Search and recovery of autobiographical and laboratory memories: Shared and distinct neural components. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KJ, Troyer AK, Levine B, Moscovitch M. Episodic, but not semantic, autobiographical memory is reduced in amnestic mild cognitive impairment. Neuropsychologia. 2008;46(13):3116–3123. doi: 10.1016/j.neuropsychologia.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo DJ, Hayes SM, Peterson KM, Keane MM, Verfaellie M. Medial temporal lobe contributions to episodic future thinking: Scene construction or future projection? Cerebral Cortex. doi: 10.1093/cercor/bhw381. doi.org/10.1093/cercor/bhw381 (in press) [DOI] [PMC free article] [PubMed]
- Palombo DJ, Bacopulos A, Amaral RSC, Olsen RK, Todd RM, Anderson AK, Levine B. Episodic autobiographical memory is associated with variation in the size of hippocampal subregions. Hippocampus. 2018;28:69–75. doi: 10.1002/hipo.22818. [DOI] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Méndez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer's disease. Brain. 2010;133(9):2702–2713. doi: 10.1093/brain/awq148. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurements. 1977;1:385–401. [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, … Hardy J. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005;102(23):8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Haldstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson: Neuropsychology Press; 1993. [Google Scholar]
- Rentz DM, Amariglio RE, Becker JA, Frey M, Olson LE, Frishe K, … Sperling RA. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 2011;49(9):2776–2783. doi: 10.1016/j.neuropsychologia.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie. 1941;28:286–340. [Google Scholar]
- Roberts RP, Schacter DL, Addis DR. Scene Construction and Relational Processing: Separable Constructs? Cereb Cortex. doi: 10.1093/cercor/bhx081. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol. 2013;23(2):255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L, Walther K, Bendlin BB, Lue LF, Walker DG, Glisky EL. Age-related differences in white matter integrity and cognitive function are related to APOE status. Neuroimage. 2011;54(2):1565–1577. doi: 10.1016/j.neuroimage.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. "Preclinical" AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55(3):370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- Sekeres MJ, Bonasia K, St-Laurent M, Pishdadian S, Winocur G, Grady C, Moscovitch M. Recovering and preventing loss of detailed memory: differential rates of forgetting for detail types in episodic memory. Learn Mem. 2016;23(2):72–82. doi: 10.1101/lm.039057.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon S, Farb N, Palombo DJ, Levine B. Intrinsic medial temporal lobe connectivity relates to individual differences in episodic autobiographical remembering. Cortex. 2016;74:206–216. doi: 10.1016/j.cortex.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Sheldon S, Vandermorris S, Al-Haj M, Cohen S, Winocur G, Moscovitch M. Ill-defined problem solving in amnestic mild cognitive impairment: Linking episodic memory to effective solution generation. Neuropsychologia. 2015;68:168–175. doi: 10.1016/j.neuropsychologia.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME. Resting state functional connectivity in preclinical Alzheimer's disease. Biol Psychiatry. 2013;74(5):340–347. doi: 10.1016/j.biopsych.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, … Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Lockrow AW, DuPre E, Setton R, Spreng KAP, Turner GR. Semanticized autobiographical memory and the default-executive coupling hypothesis of aging. Neuropsychologia. 2018;1110:37–43. doi: 10.1016/j.neuropsychologia.2017.06.009. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Levine B. Ageing and autobiographical memory for emotional and neutral events. Memory. 2007;15:129–144. doi: 10.1080/09658210601119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. Differential engagement of brain regions within a 'core' network during scene construction. Neuropsychologia. 2010;48(5):1501–1509. doi: 10.1016/j.neuropsychologia.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg AJ, Filippini N, Mackay CE. The effects of APOE-ε4 on the BOLD response. Neurobiol Aging. 2012;33(2):323–334. doi: 10.1016/j.neurobiolaging.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Tramoni E, Felician O, Koric L, Balzamo M, Joubert S, Ceccaldi M. Alteration of autobiographical memory in amnestic mild cognitive impairment. Cortex. 2012;48:1310–1319. doi: 10.1016/j.cortex.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- van der Flier WM, Schoonenboom SN, Pijnenburg YA, Fox NC, Sheltens P. The effect of APOE genotype on clinical phenotype in Alzheimer’s disease. Neurology. 2006;67:526–527. doi: 10.1212/01.wnl.0000228222.17111.2a. [DOI] [PubMed] [Google Scholar]
- van der Vlies AE, Pijneburg YAL, Koene T, Klein M, Kok A, Sheltens P, van der Flier WM. Cognitive impairment in Alzheimer’s disease is modified by APOE genotype. Dementia and Geriatric Cognitive Disorders. 2007;24:98–103. doi: 10.1159/000104467. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Bousquet K, Keane MM. Medial temporal and neocortical contributions to remote memory for semantic narratives: evidence from amnesia. Neuropsychologia. 2014;61:105–112. doi: 10.1016/j.neuropsychologia.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-IV Manual. Psychological Corporation; New York, NY: 2008. [Google Scholar]