Abstract
Pediatric chronic pain is associated with numerous negative outcomes including increased physical disability, increased rates of depression and anxiety, and decreased quality of life. Pain catastrophizing – broadly conceptualized as including rumination, magnification, and helplessness cognitions surrounding one’s pain – has been linked with poor functional outcomes in children with chronic pain. Pain catastrophizing in pediatric chronic pain is often considered a key factor on which to focus treatment efforts. However, absent a systematic review that integrates the relevant literature, this call for routine assessment and targeted treatment may be premature. The present study aimed to: 1) meta-analytically quantify the relationship between catastrophizing and pain and functional/psychosocial outcomes (functional disability/physical functioning, anxiety, depression, and quality of life) in children with chronic pain, and 2) examine potential moderators of these relationships. Using a random effects model, a total of 111 effect sizes from 38 studies were analyzed. Effect sizes ranged from medium to large, with anxiety, depression, and quality of life demonstrating a strong association with catastrophizing. Pain intensity and physical disability had a moderate association with catastrophizing. These relationships were robust, minimizing potential publication bias. None of the examined moderators were significant. The strong relationships found between catastrophizing and anxiety, depression, and quality of life suggest that successfully intervening on catastrophizing could have far reaching implications in improving pain outcomes in pediatric chronic pain.
Keywords: meta-analysis, catastrophizing, child, adolescent, chronic pain, pediatric, functioning
Introduction
Chronic pain is associated with negative outcomes in children and adolescents, including worse physical and emotional functioning [5,9,33,50,53,54,66,75,81,83,100]. Many factors have been explored to determine why chronic pain leads to such poor outcomes. Pain catastrophizing is one such factor [19,104]. As detailed by Turner and Aaron (2001), catastrophizing is historically rooted in the broader psychological literature and is most commonly linked with anxiety disorders [12,102] and depression [32]. Nearly two decades after what many consider to be the first use of the term by Albert Ellis [24], scholars began characterizing and examining the impact of catastrophizing about pain specifically [13,14,89,101]. Several of the early self-report measures of pain-related coping, such as the Cognitive Error Questionnaire (CEQ) [63] and the Coping Strategies Questionnaire (CSQ) [89], included items assessing catastrophic thinking about pain. However, the content of these items differ across measures. Many of the items from the CEQ assess pessimistic pain-related thoughts about the future (e.g., “If I don’t get some time to relax during the day, I’m going to be bedridden and unable to work.”), whereas items from the CSQ focus solely on helplessness cognitions (e.g., “I feel like I can’t go on.”). Over ten years later, in an effort to integrate the various theories and measurements of pain catastrophizing, Sullivan and colleagues (1995) created the Pain Catastrophizing Scale [104], which includes three separate but related domains: rumination, magnification, and helplessness. Although the work of Sullivan and colleagues mark a turning point in our understanding of pain catastrophizing (at least as indicated by the exponential growth in the literature and the predominance of the PCS as the gold standard measure), Turner and Aaron (2001) argue that none of the currently available measures, including the PCS, fully capture the construct of catastrophizing as defined in the broader field of psychology. Specifically, they contend that none tap into thoughts about the worse possible outcomes of pain (e.g., paralysis, complete disability), although they do acknowledge that the CEQ comes closest. Given these historical developments, and the fact that the three-factor model is privileged in most of the contemporary work in this area, we adopted Sullivan and colleagues’ [104] conceptualization of pain catastrophizing for the current systematic review.
The aforementioned developments focused exclusively on adults with pain. Nearly a decade later, the pediatric literature began examining the impact of catastrophizing in pediatric pain experience. Self-report measures assessing pain catastrophizing in children and adolescents were created de novo (e.g., Pain Response Inventory [121] & Pain Coping Questionnaire [88]) or adapted from existing adult versions (e.g., CSQ – Child version [35], & PCS – Child version [19]). Research using these measures has found that catastrophizing in children with chronic pain is linked to greater pain and disability (cross-sectionally and longitudinally) [8,19], and to increased pain behaviors [74] and analgesic use [7]. In addition, previous research found that high catastrophizing children had significantly worse psychological outcomes than low catastrophizing children [21], with high catastrophizers scoring 4 times higher on a measure of depressive symptoms than their counterparts [74].
On account of these advancements in the theory and measurement of pain catastrophizing, along with the seemingly consistent pattern of findings suggesting that catastrophizing contributes to negative pain outcomes for children, several scholars have called for routine assessment of pain catastrophizing in pediatric chronic pain and consider it a key factor on which to focus treatment efforts [2,55,127]. However, this call for routine assessment and targeted treatment may be premature. To date, no study has systematically integrated the previous results, quantified the magnitude of the relationship between catastrophizing and poor pain outcomes in children, or explored possible moderating factors. Such information is critical for healthcare providers and administrators to best allocate finite clinical and financial resources to the patients that are most in need.
Given the rapid developmental changes – physical and psychosocial – that take place during childhood and adolescence, several moderators should be examined in the context of catastrophizing and its relation to poor outcomes in pediatric chronic pain. Age is one such moderator. Research suggests that increased age is associated with increased levels of catastrophizing [7]; thus, the strength of the relationships between catastrophizing and pain outcomes may vary with age. Gender may also moderate the relationship between catastrophizing and pain outcomes. Socialization in relation to pain differs for girls and boys. In both healthy and chronic pain samples, pain catastrophizing is associated with higher levels of pain expression [116,117]. This is potentially important in the context of gender differences given that girls display more pain behaviors than boys, which may result in girls receiving more comfort from others when in pain [28]. This differential reinforcement may, in turn, contribute to gender differences in catastrophizing and pain outcomes.
In addition to developmental factors that may affect the relationship between catastrophizing and pain outcomes, there are several other factors to consider. Pain severity may influence the relationship between catastrophizing and pain outcomes, given that increasing levels of pain are related to higher levels of catastrophizing [94,108]. Pain duration may also influence this relationship. A longer pain duration may exacerbate catastrophic and helpless perceptions (i.e., “It’s never going to get any better”), or it may attenuate them (i.e., “I need to accept this pain”). Another clinical factor to consider is type of chronic pain. Research suggests that levels of catastrophizing differ by pain diagnosis. For example, several studies have found that sickle cell patients have higher levels of pain catastrophizing than those with rheumatoid arthritis or musculoskeletal pain [16,47]. In addition to developmental and clinical factors, the specific measure used to assess catastrophizing may affect its relationship to pain. Measures of catastrophizing vary across clinical and research settings. These measures have different conceptualizations (unidimensional versus multidimensional) of catastrophizing and consequently, different psychometric properties. These differences between measures may influence the association between catastrophizing and pain outcomes.
In summary, catastrophizing is commonly touted as a key clinical construct to measure and focus treatment efforts on in the pediatric pain context. However, no systematic review has been conducted to integrate the relevant literature and guide these clinical efforts. Filling this knowledge gap would enhance treatment and guide allocation of resources to the patients who would benefit from treatment most. Thus, the aims of this study were to: 1) meta-analytically quantify the relationship between catastrophizing and pain and functional/psychosocial outcomes (physical functioning, anxiety, depression, and quality of life) in children with chronic pain, and 2) examine age, gender, pain intensity, pain duration, pain diagnosis, and measure type as moderators of these relationships.
Methods
Literature Search
Empirical studies were identified using PsychInfo, Medline, PubMed, and Embase databases. Relevant articles published through March 23, 2017 were selected based on searches defined by all possible keyword combinations of terms for 1) catastroph*, 2) child*, youth, adoles*, and 3) pain, chronic pain, nociception. Electronic mail alerts were created using these terms to identify articles published after the initial search. Reference sections of identified empirical studies were reviewed for additional relevant studies. In addition, forward searches were conducted using identified articles to find additional relevant articles. Study authors were contacted for any necessary information needed for analyses from studies that reported insufficient information (see Table 1).
Table 1.
Summary of Study Characteristics Across Independent Samples (k=38)
| Sample Characteristics | k/N | % |
|---|---|---|
| Median year (range) | 2013 (1998 – 2017) | |
| Mean sample size (SD) | 145 (159.4) | |
| Median sample size (Range) | 77 (6 – 725) | |
| Mean Age (SD) | 14.4 (1.50) | |
| Gender (% Female) | 72 | |
| Race (% White) | 80 | |
| Country of Study | ||
| United States | 24 | 63.2 |
| Belgium | 2 | 5.3 |
| Germany | 3 | 7.9 |
| Denmark | 4 | 10.5 |
| Netherlands | 1 | 2.6 |
| United Kingdom | 3 | 7.9 |
| Sweden | 1 | 2.6 |
| Catastrophizing Measure Used | ||
| PCS-C | 23 | 60.5 |
| PCS (adult) | 5 | 13.2 |
| PRI | 1 | 2.6 |
| CSQ-C | 1 | 2.6 |
| PCQ | 8 | 21.1 |
| PRCQ | 1 | 2.6 |
| Outcomes Measured | ||
| Pain Intensity | 35 | 92.1 |
| Physical Functioning | 30 | 78.9 |
| Anxiety | 15 | 39.5 |
| Depression | 19 | 50 |
| Quality of Life | 2 | 5.3 |
| Setting | ||
| Inpatient | 2 | 5.3 |
| Outpatient | 36 | 94.7 |
| Pain Diagnosis | ||
| Mixed Chronic Pain | 21 | 55.3 |
| Sickle Cell | 2 | 5.3 |
| Abdominal Pain | 4 | 10.5 |
| Headache | 2 | 5.3 |
| Fibromyalgia | 3 | 7.9 |
| Arthritis | 4 | 10.5 |
| Lupus | 1 | 2.6 |
| Chronic Low Back Pain | 1 | 2.6 |
Abbreviations: PCS = Pain Catastrophizing Scale (adult version), PCS-C = Pain Catastrophizing Scale for Children, PRCQ-C = Pain-Related Cognitions Questionnaire for Children, PCQ = Pain Coping Questionnaire, CSQ-C = Coping Strategies Questionnaire for Children, PRI = Pain Response Inventory
Inclusion and Exclusion Criteria
Studies were included if they 1) had measures of child-reported catastrophizing and one or more of the following: pain intensity, physical functioning, depression, anxiety, or quality of life (QOL), 2) were based on an independent child or adolescent sample (between the ages of 8 and 21) with chronic pain (i.e., persistent pain for 3 or more months), and 3) were available in English. Also, the studies needed to provide an effect size or contain information that allowed calculation of an effect size representing the relationship between catastrophizing and one or more of the pain outcomes of interest. Only effect sizes representing the relationship between baseline, or pre-intervention, levels of catastrophizing and functional/psychosocial outcomes were included.
Coding of Studies
Each article was read and coded independently by two study authors (M.M.M. and S.M.M.) using a standardized coding form.
Basic study information and study variables.
Sample-level information included publication year and type of publication. Sample characteristics included total sample size, race (percent White), gender (percent female), mean age, and whether or not the sample included participants over 18 years of age.
Moderator coding.
Gender (percent female), mean age of sample, mean pain intensity rating, and mean pain duration (in months) were coded as continuous moderators. Type of chronic pain and catastrophizing measure were coded as categorical moderators.
Quality Assessment
Studies were assessed for common sources of bias in observational studies [41,91]. Many items on the typical quality rubrics used for meta-analyses are not relevant for correlational designs [44,79]. Thus, for the current study, we used a modified version of the rubric from Salyers and colleagues’ [90] meta-analysis, such that study quality ratings ranged from 0 to 10. The initial rating system was tested and refined on several studies before applying it to the full sample of studies. Interrater reliability of the initial codes was strong (r=.81, p<.01), and disagreements were resolved through discussion.
Meta-Analytic Method
Mean Effect Size.
Pearson’s r was used as the effect size statistic for the relationship between catastrophizing and the pain outcomes of interest. Effect sizes were coded such that higher values reflect higher levels of catastrophizing and greater pain intensity, poorer physical functioning, higher levels of anxiety symptoms, higher levels of depressive symptoms, and worse QOL. All effect sizes were corrected using Fisher’s r-to-Z transformation, which mitigates the problematic standard error formulation inherent in using r in its raw form. Effect sizes at the study level were weighted by sample size in order to account for the standard error in effect size estimates [11]. When a study provided multiple values for an association, an average effect size was calculated to reduce bias [11].
A random effects model was used, when appropriate (k ≥ 6), due to effects of both within-study and between-study variability [65]; when k < 6, the fixed effects model is reported. Effect sizes, mean effect sizes, and moderation models were calculated using IBM SPSS Statistics 24 and macros provided by Wilson (2017) [126]. Effect sizes were transformed back to r for ease of interpretation using the inverse of the Fisher’s r-to-z transformation. Correlation coefficients of less than 0.10 were considered small, correlations of 0.25 were considered medium, and correlations greater than or equal to 0.40 were considered large [65].
Heterogeneity for each overall effect size was examined using the Q-statistic [11], with significant results (p < .10) suggesting moderation [45]. The I2 index was calculated to examine the extent of heterogeneity [45]. When I2 values were greater than or equal to 25%, moderator analyses were conducted [49], as this suggests that between-study variability in effect sizes exceeds levels that would be expected by chance [49].
A fail-safe N analysis was conducted to estimate how many studies with null findings would be necessary to reduce the effect sizes to non-significance [80]. Q-test effect size comparisons (with follow-up z tests) were conducted to determine the largest effect size for each outcome. The influence of publication bias was evaluated using Egger’s regression approach and funnel plots [23].
Moderation Analyses
Categorical moderators were tested using Q statistics and I2 indices (polarized effect sizes, decreased I2, and small confidence interval ranges indicating moderation), and continuous moderators were assessed for significant beta weights and decreased I2 using meta-regressions [49]. Because meta-regressions use list-wise deletion, each moderator was examined independently in order to maximize the number of studies included in the analysis. A minimum of two studies per comparison group for categorical moderators was considered necessary for conducting planned meta-analyses.
For the first aim, the mean effect sizes between catastrophizing and pain, physical functioning, anxiety, depression, and QOL were calculated. For the second aim, categorical and continuous moderators were explored when heterogeneity indexes indicated potential moderation.
Subgroup Analyses
In an effort to be inclusive, studies that included participants over the age of 18 or a small subset of participants reporting pain for < 3 months were included. Differences in mean effect size based on inclusion or exclusion of these samples were explored in subgroup analyses.
Results
Study Sample
One thousand two hundred and eighty-two records were identified through the initial database search. Sixty five studies met inclusion criteria. Of these 65 studies, 36 did not report sufficient effect size information. All corresponding authors were contacted via email to obtain required information. Twelve authors provided the necessary information and these data were included in the final sample, resulting in 38 unique samples of children with chronic pain (see Figure 1 for PRISMA flow diagram). Two studies were included that overlapped with other study samples but reported a unique catastrophizing – pain outcome relationship and so were retained for analysis. Descriptions of these 2 studies were omitted in the study summary (Table 1) and study quality (Table 2) tables to avoid duplication.
Figure 1.
PRISMA Flow Diagram
Table 2.
Ratings of Study Quality
| Study | Was the independent variable assessed with a validated measure? |
Was reliability information for the independent variable reported and above α=.70 for the current sample? |
Was the dependent variable clearly defined, using a measure that had been validated before? |
Was reliability information for the dependent variable reported and α=.70 for the current sample? |
Was the study part of a RCT? |
Were both variables continuous? |
Was the data collected as part of a research study |
Was the participation rate of eligible individuals at least 50% |
Single pain diagnosis pain sample? |
Power analysis? |
Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Benore et al. (2015) | Yes | No | Yes | No | No | Yes | No | No | No | No | 3 |
| Bhandari et al. (2016) | Yes | No | Yes | No | No | Yes | No | NR | No | No | 3 |
| Cousins et al. (2015) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | 8 |
| Crombez et al. (2003) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | 7 |
| Cunningham et al. (2014) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | 8 |
| Engel et al. (2013) | Yes | Yes | Yes | Yes | No | Yes | Yes | NR | No | No | 6 |
| Flink et al. (2016) | Yes | No | Yes | No | No | Yes | Yes | Yes | No | No | 5 |
| Guite et al (2011b) | Yes | Yes | Yes | Yes | No | Yes | Yes | NR | No | No | 6 |
| Guite et al (2011a) | Yes | Yes | Yes | Yes | No | Yes | No | NR | No | No | 5 |
| Heathcote et al. (epub) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | 7 |
| Hermann et al. (2007) | Yes | Yes | Yes | No | No | Yes | No | NR | No | No | 4 |
| Jones et al. (2016) | Yes | Yes | Yes | Yes | No | Yes | No | NR | Yes | No | 6 |
| Kashikar-Zuck et al. (2013) | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | No | 6 |
| Keogh & Eccleston (2006) | Yes | No | Yes | No | No | Yes | No | NR | No | No | 3 |
| Kroner-Herwig & Maas (2013) |
Yes | No | Yes | No | No | Yes | Yes | NR | Yes | No | 5 |
| Libby & Glenwick (2010) | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | 8 |
| Lomholt et al. (2013) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | 8 |
| Lomholt et al. (2015) | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | 7 |
| Lynch et al. (2007) | Yes | Yes | Yes | Yes | No | Yes | No | NR | No | No | 5 |
| Lynch et al. (2006) | Yes | Yes | Yes | No | No | Yes | No | NR | Yes | No | 5 |
| Lynch-Jordan et al. (2013) | Yes | Yes | Yes | Yes | No | Yes | No | NR | No | No | 5 |
| Mano et al. (2012) | Yes | Yes | Yes | Yes | No | Yes | No | NR | No | No | 5 |
| Mano et al. (2013) | Yes | No | Yes | No | Yes | Yes | Yes | No | No | No | 5 |
| Miller et al. (2016) | Yes | Yes | Yes | Yes | No | Yes | No | NR | No | No | 5 |
| Pielech et al. (2014) | Yes | Yes | Yes | Yes | No | Yes | No | NR | No | No | 5 |
| Sil et al. (2016a) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | 8 |
| Sil et al. (2016b) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 9 |
| Simons et al. (2015a) | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | No | 6 |
| Simons et al. (2015b) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | 7 |
| Thastum et al. (2005) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | 8 |
| Thastum et al. (1999) | Yes | Yes | Yes | No | No | Yes | Yes | NR | Yes | Yes | 7 |
| Tran et al. (2017) | Yes | No | Yes | No | No | Yes | Yes | N | Yes | No | 5 |
| Tran et al. (2015) | Yes | Yes | Yes | Yes | No | Yes | No | NR | No | No | 5 |
| van Tilburg et al. (2015) | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Verbunt et al. (2015) | Yes | No | Yes | No | No | Yes | Yes | N | No | No | 4 |
| Vervoort et al. (2009) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | 7 |
| Vervoort et al. (2008) | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No | 7 |
| Warschburger et al (2014) | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No | 7 |
| Chow et al. (2016) | Same sample as Simons, 2015 | ||||||||||
| Vervoort et al. (2006) | Same sample as Crombez 2003 | ||||||||||
NR=Not reported
A total of 111 effect sizes from 38 studies were included in the final sample. Each study provided an average of 2.5 effect sizes. Studies used the following self-report measures of pain catastrophizing: Pain Catastrophizing Scale – Child (PCS-C) and adult report (PCS), Pain Coping Questionnaire (PCQ), Coping Strategies Questionnaire – Child (CSQ-C), Pain Response Inventory (PRI), and Pain-related Cognitions Questionnaire (PRCQ). The most commonly used measures were PCS-C (k=23), PCQ (k=8), and PCS (k=5).
The overall sample size contained 6202 participants, with the mean sample size for included studies equal to 77 participants. Study samples were, on average, predominately female (M=72%) and White (M=80%). The mean age for the samples was 14.4 years (range: 11.2–18.3). Three studies provided multiple effect sizes for one of the catastrophizing-pain outcome relationships; these effect sizes were averaged within each study for subsequent analyses. Additional study summary characteristics are reported in Table 1.
Relationship between Catastrophizing and Pain Outcomes
Table 3 presents the original and corrected (Fisher’s r-to-Z transformed) effect sizes and sample level information for studies included in the final meta-analysis.
Table 3.
Description of Included Studies
| Study | Year | Sample Size |
Percent Female |
Percent White |
Mean Age |
Chronic Pain Diagnosis |
Catastrophizing Questionnaire |
Outcome Association |
Measure Used to Assess Outcome |
ES original |
ES corrected |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Benore et al. [8] | 2015 | 119 | 77% | 95% | 15.1 | Mixed Pain Sample |
PCS-C | Pain Intensity | NRS (0–10) | 0.18 | 0.18 |
| Physical Functioning |
BAPQ-PFss | 0.41 | 0.37 | ||||||||
| Physical Functioning |
PedsQL-PFss | 0.46 | 0.50 | ||||||||
| Anxiety | BAPQ - GAss | 0.57 | 0.65 | ||||||||
| Anxiety | BAPQ - PSAss | 0.77 | 1.02 | ||||||||
| Depression | BAPQ - Dss | 0.60 | 0.69 | ||||||||
| Bhandari et al. [4] |
2016 | 352 | 75% | 68% | 13.9 | Mixed Pain Sample |
PCS-C | Pain Intensity | NRS (0–10) | 0.26 | 0.26 |
| Physical Functioning |
PROMIS - mobility | 0.32 | 0.33 | ||||||||
| Anxiety | PROMIS - anxiety | 0.64 | 0.76 | ||||||||
| Depression | PROMIS - depression |
0.59 | 0.68 | ||||||||
| Chow et al. [15] | 2016 | 195 | 76% | 93% | 13.8 | Mixed Pain Sample |
PCS-C | Anxiety | RCMAS | 0.54 | 0.60 |
| Depression | CDI | 0.45 | 0.48 | ||||||||
| Cousins et al. [18] |
2015 | 58 | 79% | 60% | 14.6 | Mixed Pain Sample |
PCS-C | Pain Intensity | VAS | 0.44 | 0.47 |
| Physical Functioning |
FDI | 0.47 | 0.51 | ||||||||
| Quality of Life | PedsQL | −0.60 | −0.69 | ||||||||
| Crombez et al. [19] |
2003 | 43 | 54% | 100% | 11.83 | Mixed Pain Sample |
PCS-C | Pain Intensity | VAS | 0.49 | 0.54 |
| Physical Functioning |
FDI | 0.50 | 0.55 | ||||||||
| Cunningham et al. [20] |
2014 | 75 | 77% | 83% | 13.84 | Abdominal Pain | PCS-C | Pain Intensity | NRS (0–10) | 0.44 | 0.47 |
| Physical Functioning |
FDI | 0.53 | 0.59 | ||||||||
| Engel et al. [25] | 2013 | 80 | 43% | 77% | 14.35 | Mixed Pain Sample |
PCS | Pain Intensity | NRS (0–10) | −0.25 | −0.26 |
| Physical Functioning |
FDI | 0.19 | 0.19 | ||||||||
| Flink et al. [30] | 2016 | 6 | 83% | 100% | 18.33 | Mixed Pain Sample |
PCS-C | Physical Functioning |
FDI | 0.72 | 0.91 |
| Anxiety | HADS-A | 0.57 | 0.65 | ||||||||
| Depression | HADS-D | 0.01 | 0.01 | ||||||||
| Guite et al.(a) [39] |
2011 | 138 | 84% | 87% | 15.6 | Mixed Pain Sample |
PCS | Pain Intensity | VAS | 0.34 | 0.35 |
| Physical Functioning | FDI | 0.38 | 0.40 | ||||||||
| Guite et al.(b) [38] |
2011 | 259 | 78% | 93% | 15.1 | Mixed Pain Sample |
PCS-C | Pain Intensity | NRS (0–10) | 0.22 | 0.22 |
| Physical Functioning |
FDI | 0.20 | 0.20 | ||||||||
| Heathcote et al. [42] |
2017 | 66 | 83% | - | 13.97 | Mixed Pain Sample |
PCS-C | Pain Intensity | NRS (0–10) | 0.28 | 0.29 |
| Physical Functioning |
FDI | 0.31 | 0.32 | ||||||||
| Anxiety | RCADS-GADss | 0.46 | 0.49 | ||||||||
| Depression | RCADS-MDDss | 0.56 | 0.63 | ||||||||
| Hermann et al.* [43] |
2007 | 71 | - | - | - | Mixed Pain Sample |
PRCQ | Pain Intensity | CSI | 0.30 | 0.31 |
| 69 | - | - | - | Anxiety | CAT-II | 0.60 | 0.69 | ||||
| 106 | - | - | - | Depression | DTC-DMss | 0.30 | 0.31 | ||||
| Depression | DTC-ABss | 0.13 | 0.13 | ||||||||
| Depression | DTC-Ess | 0.21 | 0.21 | ||||||||
| Jones et al. [52] | 2016 | 60 | 88% | 50% | 16.1 | Lupus | PCS | Pain Intensity | VAS | 0.35 | 0.37 |
| Anxiety | SCARED | 0.52 | 0.58 | ||||||||
| Depression | CDI | 0.58 | 0.66 | ||||||||
| Quality of Life | PedsQL | −0.59 | −0.68 | ||||||||
| Kashikar-Zuck et al. [56] |
2013 | 100 | 93% | 90% | 15.02 | Juvenile Fibromyalgia |
PCQ | Physical Functioning |
FDI | 0.15 | 0.15 |
| Depression | CDI | 0.33 | 0.34 | ||||||||
| Keogh & Eccleston** [59] |
2006 | 46 (male) |
0% | - | - | Mixed Pain Sample |
PCQ | Pain Intensity | NRS (0–10) | 0.44 | 0.47 |
| Physical Functioning |
FDI | 0.32 | 0.33 | ||||||||
| Anxiety | SCAS | 0.53 | 0.59 | ||||||||
| Depression | CDI | 0.56 | 0.63 | ||||||||
| 115 (female) |
100% | - | - | Pain Intensity | NRS (0–10) | 0.21 | 0.22 | ||||
| Physical Functioning |
FDI | 0.33 | 0.34 | ||||||||
| Anxiety | SCAS | 0.50 | 0.55 | ||||||||
| Depression | CDI | 0.58 | 0.66 | ||||||||
| Kröner-Herwig & Maas [60] |
2013 | 60 | 55% | NR | 12.6 | Headache | PCS-C | Pain Intensity | NRS (0–10) | 0.25 | 0.26 |
| Libby & Glenwick [64] |
2010 | 57 | 93% | 2% | 15.5 | Juvenile Fibromyalgia |
CSQ-C | Pain Intensity | VAS | 0.33 | 0.34 |
| Depression | CDI | 0.46 | 0.50 | ||||||||
| Quality of Life | PedsQL | −0.67 | −0.81 | ||||||||
| Lomholt et al. (a) [69] |
2013 | 91 | 77% | NR | 12.7 | Arthritis | PCQ | Pain Intensity | FPS-R | 0.39 | 0.41 |
| Physical Functioning |
CHAQ | 0.22 | 0.22 | ||||||||
| Lomholt et al. (b) [68] |
2015 | 19 | 79% | NR | 11.72 | Arthritis | PCQ | Pain Intensity | FPS-R | 0.30 | 0.31 |
| Physical Functioning |
FDI | 0.45 | 0.48 | ||||||||
| Anxiety | BAI | 0.38 | 0.40 | ||||||||
| Depression | BDI | 0.72 | 0.91 | ||||||||
| Quality of Life | PedsQL | −0.53 | −0.59 | ||||||||
| Lynch et al.** [72] |
2007 | 70 | 0% | - | - | Mixed Pain Sample |
PCQ | Pain Intensity | VAS | 0.20 | 0.20 |
| 202 | 100% | - | - | Pain Intensity | VAS | 0.11 | 0.11 | ||||
| Lynch et al. [73] | 2006 | 65 | 80% | 91% | 14.9 | Chronic Back Pain | PCQ | Pain Intensity | VAS | 0.25 | 0.26 |
| Physical Functioning |
FDI | 0.57 | 0.65 | ||||||||
| Lynch-Jordan et al. [74] |
2013 | 240 | 77% | 89% | 14.76 | Mixed Pain Sample |
PCS-C | Pain Intensity | NRS (0–10) | 0.38 | 0.40 |
| Physical Functioning |
FDI | 0.51 | 0.56 | ||||||||
| Depression | CDI | 0.58 | 0.66 | ||||||||
| Quality of Life | PedsQL | −0.24 | −0.24 | ||||||||
| Mano et al. [76] | 2012 | 349 | 69% | 77% | 14.2 | Mixed Pain Sample |
PCS-C | Anxiety | SCARED | 0.56 | 0.63 |
| Quality of Life | PedsQL | −0.50 | −0.55 | ||||||||
| Mano et al. [77] | 2013 | 6 | 83% | 67% | 13.75 | Mixed Pain Sample |
PCS | Physical Functioning |
CALQ | −0.38 | −0.40 |
| Anxiety | STAI-C Trait Scale | 0.54 | 0.60 | ||||||||
| Anxiety | STAI-C State Scale | 0.68 | 0.83 | ||||||||
| Miller et al. [78] | 2016 | 139 | 72% | 92% | 15 | Mixed Pain Sample |
PCS-C | Pain Intensity | NRS (0–10) | 0.58 | 0.66 |
| Physical Functioning |
FDI | 0.34 | 0.35 | ||||||||
| Pielech et al. [84] | 2014 | 697 | 77% | 92% | 13.9 | Mixed Pain Sample |
PCS-C | Pain Intensity | NRS (0–10) | 0.26 | 0.27 |
| Physical Functioning |
FDI | 0.32 | 0.33 | ||||||||
| Anxiety | RCMAS | 0.45 | 0.48 | ||||||||
| Depression | CDI | 0.35 | 0.37 | ||||||||
| Sil et al. (a) [95] | 2016 | 40 | NR | NR | NR | Sickle Cell | PCS-C | Pain Intensity | NRS (0–10) | 0.05 | 0.05 |
| Physical Functioning |
FDI | 0.20 | 0.20 | ||||||||
| Depression | CDI | 0.04 | 0.04 | ||||||||
| Quality of Life | PedsQL | −0.43 | −0.46 | ||||||||
| Sil et al. (b) [96] | 2016 | 100 | 61% | 0% | 13.54 | Sickle Cell | PCS-C | Pain Intensity | NRS (0–10) | 0.26 | 0.27 |
| Physical Functioning |
FDI | 0.31 | 0.32 | ||||||||
| Simons et al. (a) [98] |
2015 | 206 | 73% | 90% | 13.6 | Headache | PCS-C | Pain Intensity | NRS (0–10) | 0.21 | 0.22 |
| Physical Functioning |
FDI | 0.46 | 0.49 | ||||||||
| Simons et al. (b) [99] |
2015 | 321 | 75% | 90% | 13.73 | Mixed Pain Sample |
PCS-C | Physical Functioning |
FDI | 0.40 | 0.42 |
| Thastum et al. [109] |
2005 | 56 | 80% | - | 11.4 | Arthritis | PCQ | Pain Intensity | VAS | 0.33 | 0.34 |
| Thastum et al. [110] |
1999 | 16 | - | - | - | Arthritis | PCQ | Pain Intensity | VAS | −0.02 | −0.02 |
| Tran et al. [112] | 2015 | 725 | 69% | 75% | - | Mixed Pain Sample |
PCS-C | Pain Intensity | NRS (0–10) | 0.38 | 0.40 |
| Physical Functioning |
CALQ | 0.48 | 0.52 | ||||||||
| Anxiety | SCARED | 0.53 | 0.59 | ||||||||
| Quality of Life | PedsQL | −0.50 | −0.55 | ||||||||
| Tran et al. [111] | 2017 | 13 | 100% | 92% | 15.94 | Juvenile Fibromyalgia |
PCS-C | Pain Intensity | VAS | 0.57 | 0.65 |
| Physical Functioning |
FDI | 0.50 | 0.55 | ||||||||
| Depression | CDI | 0.44 | 0.47 | ||||||||
| van Tilburg et al.t [113] |
2015 | 189 | 49% | 88% | 13.76 | IBD | PRI | Physical Functioning |
FDI | 0.55 | 0.62 |
| Depression | CDI | 0.54 | 0.61 | ||||||||
| 200 | 73% | 96% | 11.2 | Abdominal Pain | Physical Functioning |
FDI | 0.42 | 0.45 | |||
| Depression | CDI | 0.52 | 0.58 | ||||||||
| Verbunt et al. [115] |
2015 | 71 | 91% | - | 17 | Mixed Pain Sample |
PCS-C | Pain Intensity | VAS | 0.41 | 0.44 |
| Physical Functioning |
FDI | 0.52 | 0.58 | ||||||||
| Depression | CDI | 0.60 | 0.69 | ||||||||
| Vervoort et al. [119] |
2006 | 43 | 54% | 100% | 11.8 | Mixed Pain Sample |
PCS-C | Anxiety | STAI-C Trait Scale | 0.57 | 0.65 |
| Vervoort et al. [120] |
2009 | 38 | 76% | 100% | 15.74 | Mixed Pain Sample |
PCS-C | Pain Intensity | NRS (0–10) | 0.10 | 0.10 |
| Vervoort et al. [117] |
2008 | 61 | 57% | 96% | 13.33 | Mixed Pain Sample |
PCS | Pain Intensity | VAS | 0.32 | 0.33 |
| Warschburger et al. [122] |
2014 | 170 | 64% | NR | 11.7 | Abdominal Pain | PRCQ-R | Pain Intensity | FPS-R | 0.27 | 0.28 |
| Quality of Life | KINDL-R | −0.35 | −0.37 | ||||||||
Study reported different Ns for each measure;
Study reported separate correlations for boys and girls;
Study reported separate correlations by diagnosis subsample
Abbreviations: IBD = Inflammatory Bowel Disease, PCS = Pain Catastrophizing Scale (adult version), PCS-C = Pain Catastrophizing Scale for Children, PRCQ-C = Pain-Related Cognitions Questionnaire for Children, PCQ = Pain Coping Questionnaire, CSQ-C = Coping Strategies Questionnaire for Children, PRI = Pain Response Inventory, BAPQ - Dss = Bath Adolescent Pain Questionnaire - Depression subscale, BAPQ - GAss = Bath Adolescent Pain Questionnaire - General anxiety subscale, BAPQ - PSAss = Bath Adolescent Pain Questionnaire - Pain-specific anxiety subscale, BAPQ-PFss = Bath Adolescent Pain Questionnaire - Physical functioning subscale, CALQ = Child Activity Limitations Questionnaire, CAT-II = Children’s Anxiety Test - II, CDI = Children’s Depression Inventory, CHQ-CF87 - MHss = Child Health Questionnaire - Mental Health subscale, CSI = Children’s Somatization Inventory, DTC-ABss = Depression Test for Children - Agitated Behavior subscale, DTC-DMss = Depression Test for Children - Dysphoric Mood subscale, DTC-Ess = Depression Test for Children - Exhaustion/somatic complaints subscale, FDI = Functional Disability Inventory, HADS-A = Hospital Anxiety and Depression Scale - Anxiety subscale, HADS-D = Hospital Anxiety and Depression Scale - Depression subscale, NRS = Numeric Rating Scale, FPS-R = Faces of Pain Scale - Revised, CHAQ = Childhood Health Assessment Questionnaire, PedsQL = Pediatric Quality of Life, PedsQL - EFss = Pediatric Quality of Life - Emotional Functioning subscale, PedsQL-PFss = Pediatric Quality of Life - Physical Functioning subscale, BAI = Beck Anxiety Inventory, BDI = Beck Depression Inventory, PROMIS - anxiety = PROMIS Anxiety Question Bank, PROMIS - depression = PROMIS Depression Question Bank, PROMIS - mobility = PROMIS Mobility Question Bank, RCADS-GADss = Revised Child Anxiety and Depression Scale - Generalized Anxiety Disorder subscale, RCADS-MDDss = Revised Child Anxiety and Depression Scale - Major Depressive Disorder subscale, RCMAS = Revised Children’s Manifest Anxiety Scale, SCARED = Screen for Child Anxiety-Related Disorders, SCAS = Spence Children’s Anxiety Scale, STAI-C State Scale = State-Trait Anxiety Inventory for Children-State scale, STAI-C Trait Scale = State-Trait Anxiety Inventory for Children-Trait scale, VAS = Visual Analog Scale, PRCQ-R = Pain Related Coping Questionnaire - Revised
Pain intensity
The meta-analysis of the relationship between catastrophizing and pain intensity included 35 independent samples and resulted in a positive medium effect size, with r=0.29 (95% CI: 0.24 – 0.34, Table 4). The overall effect was significantly different from zero (z=11.37, p<0.01). Orwin’s fail safe N analysis indicated an additional 172 studies with null effects would be needed to reduce the overall mean effect to non-significance. Egger’s regression test of asymmetry resulted in a precision value = −0.17 (90% CI: −1.37 to 1.04, p=0.78), indicating no significant amount of asymmetry and suggesting no significant amount of publication bias [23]. The funnel plot indicates a roughly symmetric distribution of effect sizes, with most of the effects around the mean intercept, further suggesting that publication bias is unlikely (Figure 2). The Q-statistic of the overall effect was 85.12, with a substantial amount of heterogeneity (I2=60.06%) warranting additional moderation analyses.
Table 4.
Mean Effect Sizes
| Association | K | N | ES (r) | SE | 95% C.I. | Z | Q | I2 |
|---|---|---|---|---|---|---|---|---|
| Random-effects model | ||||||||
| Pain Intensity | 35 | 4661 | 0.29** | 0.03 | [0.24, 0.34] | 11.37 | 85.12** | 60.06 |
| Physical Disability | 30 | 4622 | 0.39** | 0.03 | [0.35, 0.43] | 15.26 | 72.33** | 59.91 |
| Anxiety | 15 | 2867 | 0.55** | 0.03 | [0.50, 0.59] | 18.96 | 28.04** | 50.07 |
| Depression | 19 | 2691 | 0.49** | 0.04 | [0.43, 0.55] | 12.58 | 66.96** | 73.12 |
| Quality of Life | 10 | 1724 | −0.48** | 0.06 | [−0.56, −0.39] | −9.32 | 30.31** | 70.31 |
p<.05
p<.01
Figure 2.
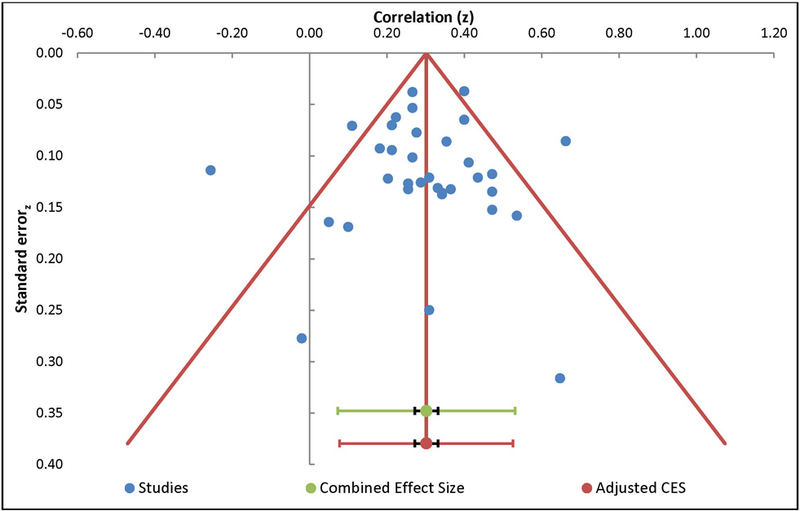
Funnel Plot – Pain Intensity
Physical functioning
The meta-analysis of the relationship between catastrophizing and physical functioning included 30 independent samples and resulted in a positive medium effect size, with r=0.39 (95% CI: 0.35 – 0.43, Table 4). The overall effect was significantly different from zero (z=15.26, p<0.01), and an additional 210 studies with null effects would be needed to reduce this to non-significance. Egger’s regression test of asymmetry resulted in a precision value = −0.17 (90% CI: −1.44 to 1.09, p=0.78), indicating no significant amount of asymmetry and minimal publication bias (Egger et al., 1997). The funnel plot was roughly symmetrical, further suggesting that publication bias is unlikely (Figure 3). The results of heterogeneity analyses (Q=72.33, I2=59.91%) supported additional moderation analyses.
Figure 3.
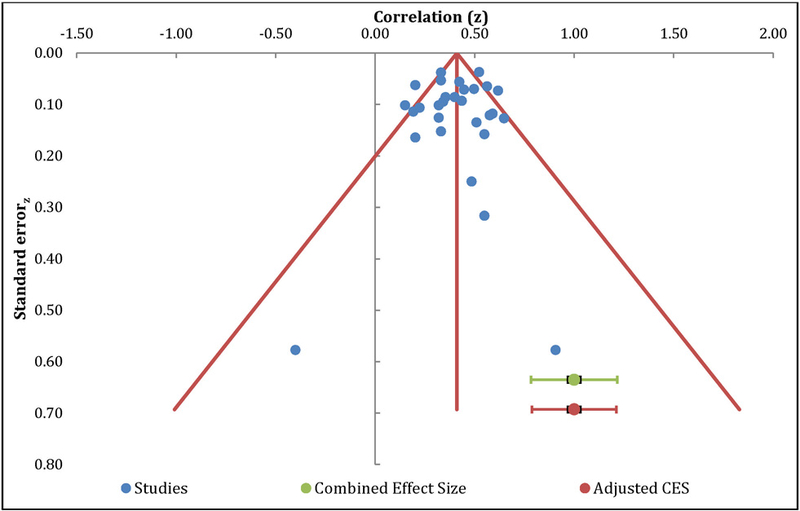
Funnel Plot – Physical Disability
Anxiety
The meta-analysis of the relationship between catastrophizing and anxiety included 15 independent samples and resulted in a positive large effect size, with r=0.55 (95% CI: 0.50 – 0.59, Table 4). The overall effect was significantly different from zero (z=18.96, p<0.01). Orwin’s fail safe N analysis indicated an additional 165 studies with null effects would be needed to reduce the overall mean effect to non-significance. Egger’s regression test of asymmetry resulted in a precision value = 0.37 (90% CI: −1.00 to 1.73, p=0.57), indicating no significant amount of asymmetry and minimal publication bias [23]. The funnel plot was roughly symmetrical, further suggesting that publication bias is unlikely (Figure 4). The Q-statistic of the overall effect was 28.04, with a moderate amount of heterogeneity (I2=45.91%).
Figure 4.
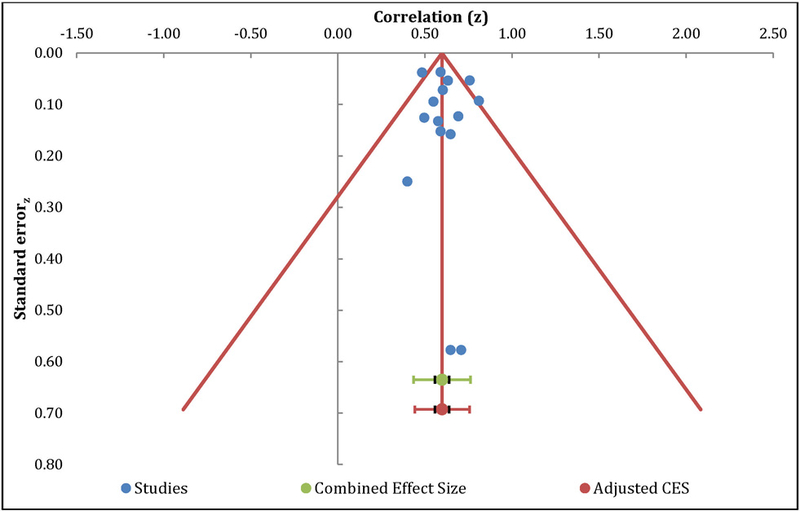
Funnel Plot – Anxiety
Depression
The meta-analysis of the relationship between catastrophizing and depression included 19 independent samples and resulted in a positive large effect size, with r=0.49 (95% CI: 0.43 – 0.55, Table 4). The overall effect was significantly different from zero (z=12.58, p<0.01) and an additional 180 studies with null effects would be needed to reduce this to non-significance. Egger’s regression test of asymmetry resulted in a precision value = 0.49 (90% CI: −1.48 to 2.46, p=0.61), indicating no significant amount of asymmetry, suggesting no significant amount of publication bias [23]. The funnel plot indicates a roughly symmetric distribution of effect sizes (Figure 5). The results of heterogeneity analyses (Q=66.96, I2=73.12%) supported additional moderation analyses.
Figure 5.
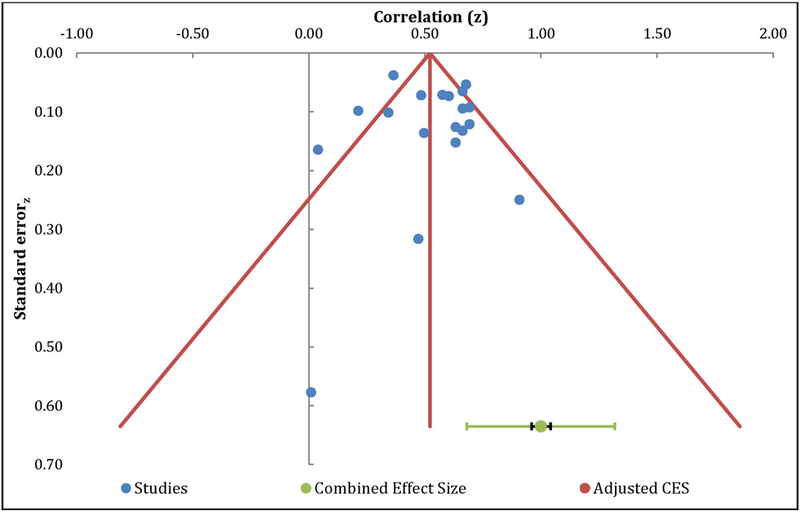
Funnel Plot – Depression
Quality of life
The meta-analysis of the relationship between catastrophizing and QOL included 10 independent samples and resulted in a positive large effect size, with r= −0.48 (95% CI: −0.56 – −0.39, Table 4). The overall effect was significantly different from zero (z=9.32, p<.01) with fail safe N analysis indicated 91 additional studies with null effects would be needed to reduce the overall mean effect to non-significance. Egger’s regression test of asymmetry resulted in a precision value = 0.37 (90% CI: −2.10 to 2.84, p=0.74), indicating no significant amount of asymmetry, suggesting no significant amount of publication bias [23]. The funnel plot indicates a roughly symmetric distribution of effect sizes, further suggesting that publication bias is unlikely (Figure 6). The Q-statistic of the overall effect was 30.31, with a substantial amount of heterogeneity (I2=70.31 %), indicating the need for additional moderation analyses.
Figure 6.
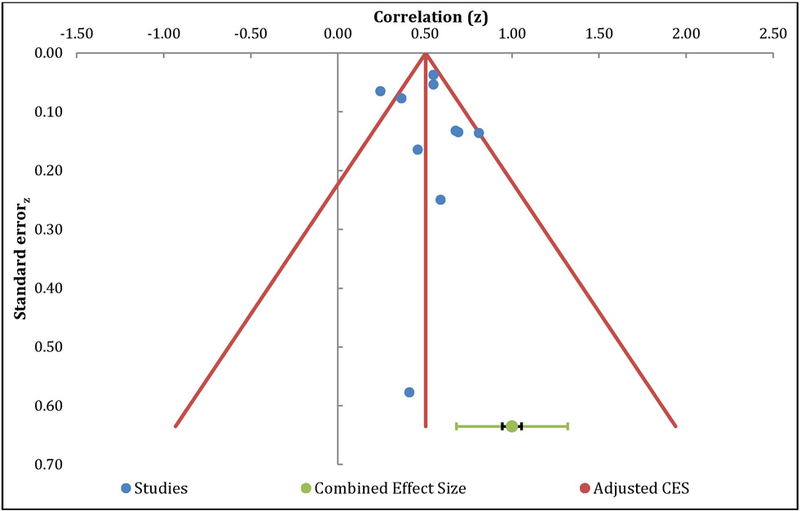
Funnel Plot – Quality of Life
Moderation
Moderator analyses for the relationship between catastrophizing, pain, and pain outcomes are shown in Tables 5 – 7. Among the continuous moderators and contrary to hypotheses, gender, age, pain intensity, and pain duration did not significantly moderate the relationship between catastrophizing and any of the pain outcomes. Similarly, among the categorical moderators, neither type of chronic pain nor catastrophizing measure significantly moderated (p>.05 for Q(b) values) any of the relationships between catastrophizing and pain outcomes. We could not conduct categorical moderation analyses of the relationship between catastrophizing and QOL due to having less than 2 studies for pain diagnosis or catastrophizing measure subgroups.
Table 5.
Continuous Moderator Analyses
| Association | K (N) | β (SE) | 95% CI | Z |
|---|---|---|---|---|
| Pain Intensity | ||||
| Gender | 32 (4534) | 0.04 (0.14) | [−0.23, 0.31] | 0.29 |
| Age | 27 (3376) | −0.002 (0.02) | [−0.05, 0.04] | −0.10 |
| Pain Intensity | 30 (4246) | −0.0002 (0.02) | [−0.05, 0.04] | −0.01 |
| Pain Duration | 20 (3082) | 0.001 (0.002) | [−0.002. 0.005] | 0.73 |
| Physical Disability | ||||
| Gender | 29 (4582) | −0.07 (0.16) | [−0.39, 0.25] | −0.42 |
| Age | 26 (3696) | −0.004 (0.02) | [−0.05, 0.04] | −0.16 |
| Pain Intensity | 25 (3900) | 0.03 (0.02) | [−0.01, 0.08] | 1.47 |
| Pain Duration | 17 (3228) | 0.001(0.003) | [−0.005, 0.006] | 0.28 |
| Anxiety | ||||
| Gender | 14 (2798) | −0.06 (0.19) | [−0.43, 0.32] | −0.29 |
| Age | 11 (1912) | 0.03 (0.04) | [−0.05, 0.12] | 0.74 |
| Pain Intensity | 11 (2591) | 0.02 (0.04) | [−0.05, 0.10] | 0.65 |
| Pain Duration | 11 (2434) | −0.002 (0.003) | [−0.008, 0.003] | −0.84 |
| Depression | ||||
| Gender | 17 (2545) | −0.08 (0.18) | [−0.43, 0.27] | −0.46 |
| Age | 15 (2384) | −0.001 (0.03) | [−0.05, 0.05] | −0.02 |
| Pain Intensity | 14 (1995) | −.01 (0.04) | [−0.10, 0.07] | −0.33 |
| Pain Duration | 11 (1728) | .01 (0.003) | [−0.0003, 0.01] | 1.87 |
| Quality of Life | ||||
| Gender | 9 (1684) | −1.07 (0.62) | [−2.29, 0.14] | −1.73 |
| Age | 8 (959) | −0.06 (0.15) | [−0.15, 0.04] | −1.20 |
| Pain Intensity | 9 (1718) | 0.04 (0.04) | [−0.04, 0.13] | 0.96 |
| Pain Duration | 6 (1451) | −0.002 (0.006) | [−0.01, 0.01] | −0.27 |
p<.05
p<.01
Table 7.
Categorical Moderator Analyses
| Catastrophizing Measure | ||||||||
| Association | ||||||||
| I2 | k (N) | r | 95% CI | z(SE) | Q(w) | Q(b) | ||
| CAT - PI | 33 (4434) | 0.29 | [0.24, 0.35] | 10.89** (0.03) |
32.79 | 3.93 | ||
| 0.00 | PCS-C (k=21) | 0.33 | [0.26, 0.38] | 9.96**(0.03) | 17.95 | |||
| 0.00 | PCQ (k=9) | 0.25 | [0.15, 0.35] | 4.48**(0.06) | 4.80 | |||
| 80.08 | PCS (k=3) | 0.16 | [−0.01, 0.33] | 1.80(0.09) | 10.04 | |||
| CAT - PF | 30 (4622) | 0.39 | [0.35, 0.43] | 17.00**(0.02) | 28.58 | 5.32 | ||
| 0.47 | PCS-C (k=20) | 0.40 | [0.35, 0.45] | 14.39**(0.03) | 19.09 | |||
| 7.41 | PRI (k=2) | 0.49 | [0.36, 0.60] | 6.81**(0.08) | 1.08 | |||
| 30.17 | PCQ (k=6) | 0.32 | [0.21, 0.42] | 5.41**(0.06) | 7.16 | |||
| 20.00 | PCS (k=2) | 0.30 | [0.21, 0.45] | 3.42**(0.09) | 1.25 | |||
| CAT - ANX | 14 (2807) | 0.55 | [0.50 , 0.59] | 18.53**(0.03) | 9.52 | 0.8 | ||
| 0.00 | PCS-C (k=11) | 0.56 | [0.51, 0.60] | 17.62**(0.04) | 9.16 | |||
| 0.00 | PCQ (k=2) | 0.49 | [0.42, 0.62] | 5.82**(0.09) | 0.36 | |||
| CAT - DEP | 17 (2574) | 0.49 | [0.42, 0.55] | 9.28**(0.05) | 17.62 | 0.71 | ||
| 29.28 | PCS-C (k=11) | 0.47 | [0.38, 0.55] | 9.28**(0.05) | 14.14 | |||
| 0.00 | PRI (k=2) | 0.53 | [0.35, 0.67] | 5.31**(0.11) | 0.02 | |||
| 13.29 | PCQ (k=4) | 0.52 | [0.37, 0.65] | 5.91**(0.10) | 3.46 | |||
p<.05
p<.01
CAT = catastrophizing, PI = pain intensity, PF = physical functioning, DEP = depression, ANX = anxiety
Study Quality
Study quality is reported in Table 2. The mean QR for studies was 5.97 (Median=6, Mode=5). In line with previous studies [17,27,48,90], quality rating (QR) was examined as a moderator of subgroup differences in effect sizes. Studies were grouped using sample mode (5) as a cut point [90], with studies 6 or above coded as “higher quality” and studies scoring at or below 5 considered “lower quality”. Effect sizes between these two groups were compared. Study quality was not a significant moderator (p>0.05 for Q(b) values) for any of the relationships between catastrophizing and pain outcomes. Detailed results are presented in Table 8.
Table 8.
Study Quality Moderator Analysis
| Association | ||||||||
| I2 | k (N) | r | 95% CI | z(SE) | Q(w) | Q(b) | ||
| CAT - PI | 34 (4618) | 0.29 | [0.24, 0.34] | 10.92** (0.03) | 34.12 | 0.91 | ||
| 0.00 | QR ≤ 5 (k=16) | 0.31 | [0.26, 0.37] | 8.64**(0.04) | 13.87 | |||
| 16.05 | QR ≥ 6 (k=18) | 0.26 | [0.19, 0.34] | 6.74**(0.04) | 20.25 | |||
| CAT - PF | 29 (4579) | 0.39 | [0.34, 0.43] | 15.34**(0.03) | 27.85 | 0.96 | ||
| 0.00 | QR ≤ 5 (k=14) | 0.41 | [0.36, 0.47] | 11.36**(0.04) | 10.32 | |||
| 20.14 | QR ≥ 6 (k=15) | 0.36 | [0.31, 0.42] | 10.35**(0.04) | 17.53 | |||
| CAT - ANX | 15(2867) | 0.55 | [0.55, 0.65] | 19.01**(0.03) | 9.95 | 0.66 | ||
| 0.00 | QR ≤ 5 (k=10) | 0.56 | [0.56, 0.60] | 17.09**(0.04) | 8.96 | |||
| 0.00 | QR ≥ 6 (k=5) | 0.52 | [0.44, 0.60] | 8.58**(0.07) | 0.99 | |||
| CAT - DEP | 19(2691) | 0.49 | [0.46, 0.55] | 13.47**(0.04) | 19.84 | 0.30 | ||
| 11.42 | QR ≤ 5 (k=10) | 0.51 | [0.45, 0.58] | 10.19**(0.06) | 10.16 | |||
| 0.00 | QR ≥ 6 (k=19) | 0.48 | [0.40, 0.56] | 8.82**(0.06) | 9.68 | |||
| CAT - QOL | 10 (1724) | −0.48 | [−0.62, −0.39] | −9.50*(0.05) | 7.84 | 1.37 | ||
| 5.96 | QR ≤ 5 (k=4) | −0.42 | [−0.61, −0 .29] | −5.82*(0.08) | 3.19 | |||
| 0.00 | QR ≥ 6 (k=6) | −0.52 | [−0.73, −0.41] | −7.60**(0.08) | 4.65 | |||
p<.05
p<.01
CAT = catastrophizing, PI = pain intensity, PF = physical functioning, DEP = depression, ANX = anxiety, QOL = quality of life
Sub-group Analyses
Two study characteristics were explored to assess their impact on the mean effect size: (1) whether or not samples included any participants older than 18 years of age, and (2) whether or not samples contained any participants reporting pain duration less than 3 months. Mean ESs were estimated including and excluding studies with these characteristics (i.e., participants older than 18 and participants with pain <3 months). As seen in Table 9, minimal differences in mean effect sizes were observed between the total study sample and the subgroups, indicating that these study characteristics did not substantially influence the results.
Table 9.
Comparison of Mean Effect Sizes
| Association | ||||||||
| Random-effects model | K | N | ES (r) | SE | 95% C.I. | Z | Q | I2 |
| Full Sample | ||||||||
| Pain Intensity | 35 | 4661 | 0.29** | 0.03 | [0.24, 0.34] | 11.37 | 85.12** | 60.06 |
| Physical Disability | 30 | 4622 | 0.39** | 0.03 | [0.35, 0.43] | 15.26 | 72.33** | 59.91 |
| Anxiety | 15 | 2867 | 0.55** | 0.03 | [0.50, 0.59] | 18.96 | 28.04** | 50.07 |
| Depression | 19 | 2691 | 0.49** | 0.04 | [0.43, 0.55] | 12.58 | 66.96** | 73.12 |
| Quality of Life | 10 | 1724 | −0.48** | 0.06 | [−0.56, −0.39] | −9.32 | 30.31** | 70.31 |
| 18 and under Sample | ||||||||
| Pain Intensity | 30 | 4369 | 0.30** | 0.02 | [0.27, 0.33] | 12.88 | 55.86** | 48.08 |
| Physical Disability | 26 | 4422 | 0.39** | 0.03 | [0.34, 0.43] | 14.65 | 65.28** | 61.70 |
| Anxiety | 12 | 2606 | 0.55** | 0.04 | [0.49, 0.60] | 16.07 | 27.99** | 60.70 |
| Depression | 15 | 2359 | 0.49** | 0.05 | [0.41, 0.56] | 10.62 | 62.77** | 77.70 |
| Quality of Life | 9 | 1664 | −0.47** | 0.06 | [−0.55, −0.37] | −8.56 | 28.53** | 71.96 |
| Exclusively Chronic Pain (3+ months) | ||||||||
| Pain Intensity | 27 | 2678 | 0.27** | 0.03 | [0.21, 0.34] | 8.17 | 67.46** | 61.46 |
| Physical Disability | 22 | 2374 | 0.36** | 0.04 | [0.30, 0.42] | 10.57 | 51.89** | 59.53 |
| Anxiety | 11 | 1207 | 0.58** | 0.04 | [0.53, 0.62] | 18.29 | 12.44** | 19.61 |
| Depression | 17 | 1799 | 0.51** | 0.04 | [0.45, 0.57] | 13.11 | 41.67** | 61.60 |
| Quality of Life | 9 | 999 | −0.48** | 0.07 | [−0.58, −0.36] | −7.36 | 27.74** | 71.16 |
p<.05
p<.01
Discussion
The current meta-analysis assessed the magnitude of the relationship between catastrophizing, pain, and functional outcomes in children with chronic pain. Effect sizes ranged from medium to large, with anxiety, depression, and QOL demonstrating strong associations with catastrophizing. Pain intensity and physical functioning had moderate associations with catastrophizing. These relationships were robust, minimizing the potential influence of publication bias and study quality. Gender, age, pain intensity rating, pain duration, pain diagnosis, and type of catastrophizing measure did not significantly moderate the relationship between catastrophizing and pain or functional/psychosocial outcomes.
Of the outcomes examined in the current meta-analysis, anxiety and depression had the strongest absolute relationships with catastrophizing. This strong relationship may be partially due to individuals having a general maladaptive thinking style that transcends pain-related stressors. Both in a broader psychological context and specifically within the chronic pain literature, catastrophizing has been characterized as a maladaptive thinking style in response to stress [6,24,104], frequently observed among individuals with anxiety [12,102] and depressive disorders [32], as well as those with chronic pain [87]. Thus, children who catastrophize in response to pain – and/or who appraise pain in catastrophic ways – may do similarly for non-pain stressors. This general cognitive-emotional style may explain the strong relationships between catastrophizing and anxiety and depression observed herein. Worth noting, a handful of studies point to conceptual overlap as a reason for the strong association between pain catastrophizing and negative mood (e.g., depression, anxiety) [1,46,105]. Although we acknowledge these blurry demarcations, cross-sectional [34,58,106] and longitudinal [57] evidence supports the conceptual distinctiveness of pain catastrophizing. These conceptual issues are paralleled by concerns about measurement overlap and common-method variance. Subscale or item level analysis between measures of catastrophizing, anxiety, and depression may clarify the relevance of these methodological issues. Unfortunately, because the majority of studies included in the current meta-analysis did not report subscale or item level scores, we could not undertake such analyses ourselves.
The strong relationship between catastrophizing and emotional outcomes suggests that intervention efforts aimed at decreasing catastrophic thinking may reduce anxiety and depressive symptoms among children with pain. Only a few studies have examined this issue. Kashikar-Zuck and colleagues [56] evaluated an 8-week CBT program for children with Juvenile Fibromyalgia and found that while catastrophizing decreased over the course of treatment, it did not mediate improvement in depressive symptoms. Similarly, Wicksell and colleagues [124] found that catastrophizing did not mediate changes in depression in response to an Acceptance and Commitment Therapy program (ranging from 7 to 20 therapy sessions) for pediatric chronic pain. Although these studies suggest that cognitive-behavioral and acceptance-based approaches can effectively reduce pain catastrophizing in children, much remains to be known about whether and how these changes translate into improvement in psychological functioning. Of note, these studies used less common measures of catastrophizing (PRI and PCQ), and neither assessed anxiety symptoms, thus constraining our understanding of these relationships.
Catastrophizing and physical functioning were moderately related in our analysis. The fear-avoidance model (FAM) of chronic pain provides one lens through which to interpret this relationship [3,62,85,97]. The FAM posits that poor physical functioning results from pain-related catastrophizing and threat appraisals. Thinking about pain in this way increases fear of future pain, leading to avoidance of activities and ultimately to disuse and disability [97]. This model has been applied to pediatric populations, lending support to the idea that intervening on pain catastrophizing could lead to improved physical functioning in children and adolescents with pain through decreases in their fear of pain and avoidance of activities. The biopsychomotor model offers another perspective on the connection between catastrophizing and physical functioning in children and adolescents. This model posits that pain behavior is an integral part of the pain system [103], serving communicative, protective, and social-relational purposes. Pain expression is a form of communication, transmitting information that has survival value while also soliciting attention and support for the pain sufferer [36,40,125]. Although catastrophizing has been linked to increased communicative pain behaviors, specifically facial expressions of pain [72,117,118], the protective and social-relational aspects of the biopsychomotor model seem particularly relevant to the current findings regarding the link between catastrophizing and physical functioning. Children who endorse high levels of catastrophizing about their pain may, consequently, engage in protective behaviors (e.g., guarding, bracing) that have been linked to increased functional disability [107]. These behaviors may also trigger the social response system of proximal others, leading to solicitous behaviors from parents, teachers, and peers. Such solicitousness may reinforce the child’s pain and avoidance behaviors thereby leading to continued physical disuse and worse functional disability.
QOL was strongly associated with catastrophizing. In child and adolescent chronic pain samples, QOL is often measured with the PedsQL [18,52,64,71,72,74,76,112], which assesses physical, emotional, social, and school domains [114]. Thus, the strong association between catastrophizing and QOL may be driven, in part, by the physical and emotional domains, which overlap with our other outcomes of interest (i.e., anxiety, depression, physical functioning) that demonstrated moderate-strong associations with catastrophizing. However, the PedsQL also assesses social and school functioning, domains of the pediatric pain experience that have received less attention in this literature. Children/adolescents with chronic pain frequently struggle with peer relations and academic achievement [31,82], and there is some research suggesting that catastrophizing contributes to poorer functioning in these domains [78]. Pain expressions and behaviors might tie these intra- and inter-personal factors together. As discussed above, although such behaviors may elicit emotional and functional support from others, they can also occasion more punitive responses, such as ignoring or expressions of anger or irritation [10], and are associated with interpersonal problems [61]. Although the current findings support the notion that pain catastrophizing and general QOL are strongly related in children with pain, future research should take a more nuanced approach, separating the construct into individual domains to elucidate their unique associations with pain catastrophizing.
Catastrophizing and pain intensity were moderately associated. This relationship was the most modest and varied (r= −.25 to .58) among those examined in our meta-analysis. Several physiological and neural explanations have been proposed to explain the link between catastrophizing and pain intensity; these include exaggerated muscle responses at the site of injury [86], altered hypothalamic-pituitary-adrenal axis activity [22,51], and lack of activation in brain regions responsible for top-down inhibitory control [92]. Although these studies suggest that pain catastrophizing is related to greater activity in brain regions involved in affective processing of pain, attention to pain, and pain behaviors, all were conducted in samples of adults with chronic pain. Whether and how such explanations apply to children remains an open question. Erpelding and colleagues [26] found that, among children with complex regional pain syndrome, pain catastrophizing was correlated with increased gray matter and activation in brain regions involved in motor function, sensorimotor integration, and anxiety-driven exacerbations of pain – these findings suggest that pain catastrophizing may predispose children and adolescents to develop chronic pain, experience greater pain intensity when they do have pain, and engage in particular behavioral and emotional responses to that pain. Additional studies are needed to better understand the extent to which catastrophizing is a cause and/or consequence of increased pain in children, as well as the neurophysiological underpinnings of these relationships.
The varying effect sizes – magnitude and direction – observed in the current meta-analysis suggest a complex relationship between catastrophizing and pain in children that is moderated by other factors. We examined several candidate moderators that were suggested by theory and prior studies, however, none significantly moderated the relationships examined herein. Though evidence suggests that the pain experience differs for boys and girls of varying ages [7,28], the samples included in our analyses were comprised mostly of adolescent girls. Additionally, because the majority (83%) of included samples reported moderate (NRS=4) to severe (NRS=7) average pain intensity, our moderation analyses of gender, age, and pain intensity may have lacked adequate power. Future, high-powered studies are needed to better understand whether and how these factors impact the relationship between catastrophizing and pain outcomes in children.
Pain duration and pain diagnosis were also not supported as moderators, which suggests that the catastrophizing-pain nexus is not altered by the length of time or the specific pain diagnosis a child/adolescent has been experiencing. However, it bears noting that the number of studies included in specific pain diagnoses subgroups was low (all Ns < 6), thus reducing confidence in the reliability of the null finding. Lastly, pain catastrophizing measure did not explain significant heterogeneity in the observed relationships. Nevertheless, both Q and I2 values were substantially reduced within categorical moderation subgroups for catastrophizing measure and pain diagnosis, indicating a reduction of variability in effect size heterogeneity between studies [49].
Findings from this meta-analysis have implications for treatment. Evidence supports the efficacy of several psychological interventions for reducing pain catastrophizing in pediatric samples – these include CBT for chronic pain [29,70], relaxation training [29], and Acceptance and Commitment Therapy [29,123]. These therapies often educate patients about pain catastrophizing, and some target self-reported catastrophic thoughts directly (i.e., through cognitive restructuring) or indirectly (i.e., through experiential exercises). Despite their wide use, much remains to be known about the effectiveness of such approaches – individually and/or collectively – as well their durability and mechanisms of action. Answers to these questions would provide insights for the further refinement of existing treatments and the formulation of new treatments to target catastrophic thinking and improve the functioning of children with pain. Results of the current meta-analysis suggest that such improvement may be especially achieved in the domains of anxiety and depression, as well as overall QOL.
Several limitations should be acknowledged. The majority of studies used clinical data, which introduces several forms of error, including selection bias and unsystematic administration of measures [93]. Additionally, clinical data sets are often used repeatedly for various publications, a detail that is not specifically and consistently disclosed, making it difficult to guarantee samples and corresponding effect sizes are unique. Furthermore, the variability in outcome measures used to assess a particular outcome (e.g. physical functioning) may influence effect sizes. Another limitation, common in meta-analyses, is the file drawer problem [80]. Though the results of fail-safe analyses instill confidence in the meta-analytic findings for all of our primary outcome variables, we cannot rule out the existence of unpublished studies that would have changed these findings had they been included. This meta-analysis only included a selection of outcomes that may be associated with catastrophizing. Unfortunately, other potentially important outcomes, such as school performance, are not commonly reported in the pediatric pain literature and thus were not included herein. Lastly, all measures were self-report and used similar item-response formats, thus, increasing the possibility that common method bias contributed to the observed associations.
Future studies should aim to elucidate possible moderators of the relationship between catastrophizing and pain outcomes, as the current results indicate considerable between-study variability in the nature of this relationship. Future research may also investigate the effectiveness of current interventions in reducing catastrophic thinking and the magnitude of these effects over time. These findings would serve to enhance the individualization of treatments for chronic pain in children. The role of parents is important to examine as well. Many studies have reported significant relationships between parental catastrophizing and child pain outcomes [37,67,74], but these relationships have yet to be meta-analytically quantified.
Table 6.
Categorical Moderator Analyses
| Chronic Pain Diagnosis | ||||||||
| Association | ||||||||
| I2 | k (N) | r | 95% CI | z(SE) | Q(w) | Q(b) | ||
| CAT - PI | 30 (4213) | 0.30 | [0.24, 0.35] | 9.90**(0.03) | 30.19 | 1.97 | ||
| 27.59 | Mixed Pain (k=20) | 0.28 | [0.22, 0.35] | 7.99**(0.04) | 26.24 | |||
| 58.33 | Arthritis (k=2) | 0.34 | [0.15, 0.50] | 3.48**(0.10) | 2.40 | |||
| 0.00 | Sickle Cell (k=2) | 0.18 | [−0.06, 0.41] | 1.45(0.13) | 0.68 | |||
| 0.00 | Abdominal Pain (k=2) | 0.35 | [0.14, 0.52] | 3.22**(0.11) | 0.75 | |||
| 0.00 | Musculoskeletal (k=2) | 0.37 | [0.16, 0.54] | 3.40**(0.11) | 0.12 | |||
| CAT - PF | 27 (4251) | 0.39 | [0.35, 0.42] | 17.18**(0.02) | 22.65 | 7.74 | ||
| 0.00 | Mixed Pain (k=20) | 0.37 | [0.33, 0.42] | 13.68**(0.03) | 18.59 | |||
| 0.00 | Arthritis (k=5) | 0.30 | [0.09, 0.48] | 2.81**(0.11) | 1.43 | |||
| 0.00 | Sickle Cell (k=2) | 0.27 | [0.08, 0.45] | 2.75**(0.10) | 0.29 | |||
| 28.06 | Abdominal Pain (k=2) | 0.50 | [0.40, 0.58] | 8.48**(0.06) | 1.39 | |||
| 0.00 | Musculoskeletal (k=2) | 0.44 | [0.29, 0.56] | 5.31**(0.09) | 0.95 | |||
| CAT - DEP | 16 (2520) | 0.50 | [0.44, 0.55] | 14.36**(0.04) | 14.14 | 2.52 | ||
| 28.68 | Mixed Pain (k=10) | 0.50 | [0.43, 0.56] | 11.93**(0.05) | 12.62 | |||
| 0.00 | Abdominal Pain (k=2) | 0.53 | [0.39, 0.65] | 6.42**(0.09) | 0.03 | |||
| 2.91 | Arthritis (k=2) | 0.62 | [0.31, 0.82] | 3.47**(0.21) | 1.03 | |||
| 0.00 | Fibromyalgia (k=2) | 0.39 | [0.18, 0.56] | 3.61**(0.11) | 0.46 | |||
p<.05
p<.01
CAT = catastrophizing, PI = pain intensity, PD = physical functioning, DEP = depression, ANX = anxiety
Acknowledgments:
This research was partially supported by the National Institutes of Health under award number T32 AR055885. There are no conflicts of interest to disclose.
Footnotes
There are no conflicts of interest that might be seen as influencing or prejudicing the research.
Disclosures:
This research was partially supported by the National Institutes of Health under award number T32 AR055885. We confirm that there have been no closely related manuscripts that have been submitted for simultaneous consideration to this or another journal.
References
- 1.Affleck G, Tennen H, Urrows S, Higgins P. Neuroticism and the pain-mood relation in rheumatoid arthritis: Insights from a prospective daily study. J Consult Clin Psychol 1992;60:119. [DOI] [PubMed] [Google Scholar]
- 2.American Pain Society. Pediatric chronic pain: A position statement for the American Pain Society. American Pain Society Bulletin 2001. (Jan/Feb):10–12. [Google Scholar]
- 3.Asmundson GJ, Noel M, Petter M, Parkerson HA. Pediatric fear-avoidance model of chronic pain: Foundation, application and future directions. Pain Res Manag 2012;17:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhandari RP, Feinstein AB, Huestis SE, Krane EJ, Dunn AL, Cohen LL, Kao MC, Darnall BD, Mackey SC. Pediatric-Collaborative Health Outcomes Information Registry (Peds-CHOIR): A learning health system to guide pediatric pain research and treatment. Pain 2016;157:2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker N, Thomsen AB, Olsen AK, Sjøgren P, Bech P, Eriksen,J. Pain epidemiology and health related quality of life in chronic non-malignant pain patients referred to a Danish multidisciplinary pain center. Pain 1998;73:393–400. [DOI] [PubMed] [Google Scholar]
- 6.Beck AT, Shaw BF, Emery G. Cognitive therapy of depression New York: Guilford Press, 1979. [Google Scholar]
- 7.Bedard GBV, Reid GJ, McGrath PJ, Chambers CT. Coping and self-medication in a community sample of junior high school students. Pain Res Manag 1997;2:151–156. [Google Scholar]
- 8.Benore E, D’Auria A, Banez GA, Worley S, Tang A. The influence of anxiety reduction on clinical response to pediatric chronic pain rehabilitation. Clin J Pain 2015;31:375–383. [DOI] [PubMed] [Google Scholar]
- 9.Brown GK. A causal analysis of chronic pain and depression. J Abnorm Psychol 1990;99: 127. [DOI] [PubMed] [Google Scholar]
- 10.Buenaver LF, Edwards RR, Haythornthwaite JA. Pain-related catastrophizing and perceived social responses: Inter-relationships in the context of chronic pain. Pain, 2007;127: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Card NA. Applied Meta-Analysis for Social Science Research New York, NY: Guilford Press, 2012 [Google Scholar]
- 12.Carleton RN, Sharpe D, Asmundson GJ. Anxiety sensitivity and intolerance of uncertainty: requisites of the fundamental fears?. Behav Res Ther 2007;45:2307–2316. [DOI] [PubMed] [Google Scholar]
- 13.Chaves JF, Brown JM. Self-generated strategies for the control of pain and stress In Annual Meeting of the American Psychological Association, Toronto, Canada, 1978. [Google Scholar]
- 14.Chaves JF, Brown JM. Spontaneous cognitive strategies for the control of clinical pain and stress. J Behav Med 1987;10:263–276. [DOI] [PubMed] [Google Scholar]
- 15.Chow ET, Otis JD, Simons LE. The longitudinal impact of parent distress and behavior on functional outcomes among youth with chronic pain. J Pain 2016;17:729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Citero VDA, Levenson JL, McClish DK, Bovbjerg VE, Cole PL, Dahman BA, Penberthy LT, Aisiku IP, Roseff SD, Smith WR. The role of catastrophizing in sickle cell disease–The PiSCES project. Pain 2007;133:39–46. [DOI] [PubMed] [Google Scholar]
- 17.Clair C, Cohen MJ, Eichler F, Selby KJ, Rigotti NA. The effect of cigarette smoking on diabetic peripheral neuropathy: A systematic review and meta analysis. J Gen Intern Med 2015; 30:1193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cousins LA, Cohen LL, Venable C. Risk and resilience in pediatric chronic pain: Exploring the protective role of optimism. J Pediatr Psychol 2015;40:934–942. [DOI] [PubMed] [Google Scholar]
- 19.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): A preliminary validation. Pain 2003;104:639–646. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham NR, Lynch-Jordan A, Barnett K, Peugh J, Sil S, Goldschneider K, Kashikar-Zuck S. Child pain catastrophizing mediates the relationship between parent responses to pain and disability in youth with functional abdominal pain. J Pediatr Gastroenterol Nutr 2014;59:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eccleston C, Crombez G, Scotford A, Clinch J, Connell H. Adolescent chronic pain: patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain 2004;108:221–229. [DOI] [PubMed] [Google Scholar]
- 22.Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain 2008;140:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis A A reason and emotion in psychotherapy New York: Lyle Stuart, 1962 [Google Scholar]
- 25.Engel JM, Wilson S, Tran ST, Jensen MP, Ciol MA. Pain catastrophizing in youths with physical disabilities and chronic pain. J Pediatr Psychol 2013;38:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erpelding N, Simons L, Lebel A, Serrano P, Pielech M, Prabhu S, Becerra L, Borsook D. Rapid treatment-induced brain changes in pediatric CRPS. Brain Struct Funct 2016;221:1095–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faragher EB, Cass M, Cooper CL. The relationship between job satisfaction and health: A meta-analysis. Occup Environ Med 2005;62:105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fearon I, McGrath PJ, Achat H. ‘Booboos’: the study of everyday pain among young children. Pain 1996;68:55–62. [DOI] [PubMed] [Google Scholar]
- 29.Fisher E, Heathcote L, Palermo TM, Williams ACDC, Lau J, Eccleston C. Systematic review and meta-analysis of psychological therapies for children with chronic pain. J Pediatr Psychol 2014;39:763–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flink IK, Sfyrkou C, Persson B. Customized CBT via internet for adolescents with pain and emotional distress: A pilot study. Internet Interv 2016;4:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forgeron PA, King S, Stinson JN, McGrath PJ, MacDonald AJ, Chambers CT. Social functioning and peer relationships in children and adolescents with chronic pain: A systematic review. Pain Res Manag 2010;15:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garnefski N, Kraaij V. Relationships between cognitive emotion regulation strategies and depressive symptoms: A comparative study of five specific samples. Pers Individ Dif 2006:40:1659–1669. [Google Scholar]
- 33.Gauntlett-Gilbert J, Eccleston C. Disability in adolescents with chronic pain: Patterns and predictors across different domains of functioning. Pain 2007;131:132–141. [DOI] [PubMed] [Google Scholar]
- 34.Geisser ME, Robinson ME, Keefe FJ, Weiner ML. Catastrophizing, depression and the sensory, affective and evaluative aspects of chronic pain. Pain 1994;59:79–83. [DOI] [PubMed] [Google Scholar]
- 35.Gil KM, Williams DA, Thompson RJ Jr Kinney TR Sickle cell disease in children and adolescents: The relation of child and parent pain coping strategies to adjustment. J Pediatr Psychol 1991;16: 643–663. [DOI] [PubMed] [Google Scholar]
- 36.Goubert L, Craig KD, Vervoort T, Morley S, Sullivan MJ, Williams ACDC, Cano A, Crombez G Facing others in pain: the effects of empathy. Pain 2005;118:285–288. [DOI] [PubMed] [Google Scholar]
- 37.Goubert L, Eccleston C, Vervoort T, Jordan A, Crombez G. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): a preliminary validation. Pain 2006;123:254–263. [DOI] [PubMed] [Google Scholar]
- 38.Guite JW, Logan DE, Simons LE, Blood EA, Kerns RD. Readiness to change in pediatric chronic pain: Initial validation of adolescent and parent versions of the Pain Stages of Change Questionnaire. Pain 2011(b);152:2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guite JW, McCue RL, Sherker JL, Sherry DD, Rose JB. Relationships among pain, protective parental responses, and disability for adolescents with chronic musculoskeletal pain: The mediating role of pain catastrophizing. Clin J Pain 2011(a);27:775–781. [DOI] [PubMed] [Google Scholar]
- 40.Hadjistavropoulos T, Craig KD. A theoretical framework for understanding self-report and observational measures of pain: a communications model. Behav Res Ther 2002;40:551–570. [DOI] [PubMed] [Google Scholar]
- 41.Hammer GP, du Prel JB, Blettner M. Avoiding bias in observational studies: part 8 in a series of articles on evaluation of scientific publications. Dtsch Arztebl Int 2009;106:664–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heathcote LC, Jacobs K, Eccleston C, Fox E, Lau JY. Biased interpretations of ambiguous bodily threat information in adolescents with chronic pain. Pain 2017;158:471–478. [DOI] [PubMed] [Google Scholar]
- 43.Hermann C, Hohmeister J, Zohsel K, Ebinger F, Flor H. The assessment of pain coping and pain-related cognitions in children and adolescents: Current methods and further development. J Pain 2007;8:802–813. [DOI] [PubMed] [Google Scholar]
- 44.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ;2011:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 46.Hirsh AT, George SZ, Riley JL, Robinson ME. An evaluation of the measurement of pain catastrophizing by the coping strategies questionnaire. Eur J Pain 2007;11:75–75. [DOI] [PubMed] [Google Scholar]
- 47.Hollins M, Stonerock GL, Kisaalita NR, Jones S, Orringer E, Gil KM. Detecting the emergence of chronic pain in sickle cell disease. J Pain Symptom Manage 2012;43:1082–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang RY, Huang CC, Hu FB, Chavarro JE. Vegetarian diets and weight reduction: a meta-analysis of randomized controlled trials. J Gen Intern Med 2016;31:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I² index? Psychol Methods 2006;11:193–206. [DOI] [PubMed] [Google Scholar]
- 50.Huguet A, Miró J. The severity of chronic pediatric pain: An epidemiological study. J Pain 2008;9:226–36. [DOI] [PubMed] [Google Scholar]
- 51.Johansson AC, Gunnarsson LG, Linton SJ, Bergkvist L, Stridsberg M, Nilsson O, Cornefjord M. Pain, disability and coping reflected in the diurnal cortisol variability in patients scheduled for lumbar disc surgery. Eur J Pain 2008;12:633–640. [DOI] [PubMed] [Google Scholar]
- 52.Jones JT, Cunningham N, Kashikar-Zuck S, Brunner HI. Pain, fatigue, and psychological impact on health-related quality of life in childhood-onset lupus. Arthritis Care Res 2016;68:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kashikar-Zuck S, Flowers SR, Claar RL, Guite JW, Logan DE, Lynch-Jordan AM, Palermo TM, Wilson AC. Clinical utility and validity of the Functional Disability Inventory among a multicenter sample of youth with chronic pain. Pain 2011;152:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clin J Pain 2001;17:341–349. [DOI] [PubMed] [Google Scholar]
- 55.Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, Passo MD, Schikler KN, Hashkes PJ, Spalding S, Lynch-Jordan AM, Banez G, Richards MM, Lovell DJ. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: A multisite, single-blind, randomized, controlled clinical trial. Arthritis Rheum 2012;64:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kashikar-Zuck S, Sil S, Lynch-Jordan AM, Ting TV, Peugh J, Schikler KN, Hashkes PJ, Arnold LM, Passo M, Richards MM, Powers SW, Lovell DJ. Changes in pain coping, catastrophizing, and coping efficacy after cognitive-behavioral therapy in children and adolescents with juvenile fibromyalgia. J Pain 2013;14:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain 1989;37:51–56. [DOI] [PubMed] [Google Scholar]
- 58.Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. ( The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain 2000;87:325–334. [DOI] [PubMed] [Google Scholar]
- 59.Keogh E, Eccleston C. Sex differences in adolescent chronic pain and pain-related coping. Pain 2006;123:275–284. [DOI] [PubMed] [Google Scholar]
- 60.Kröner-Herwig B, Maas J. The German Pain Catastrophizing Scale for Children (PCS-C)–psychometric analysis and evaluation of the construct. Psychosoc Med 2013;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lackner JM, Gurtman MB. Pain catastrophizing and interpersonal problems: a circumplex analysis of the communal coping model. Pain 2004;110:597–604. [DOI] [PubMed] [Google Scholar]
- 62.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med 2007;30:77–94. [DOI] [PubMed] [Google Scholar]
- 63.Lefebvre MF. Cognitive distortion and cognitive errors in depressed psychiatric and low back pain patients. . J Consult Clin Psychol 1981;49:517. [DOI] [PubMed] [Google Scholar]
- 64.Libby CJ, Glenwick DS. Protective and exacerbating factors in children and adolescents with fibromyalgia. Rehabil Psychol 2010;55:151. [DOI] [PubMed] [Google Scholar]
- 65.Lipsey MW, Wilson DB. Practical Meta-analysis (Vol. 49). Thousand Oaks, CA: Sage publications, 2001. [Google Scholar]
- 66.Logan DE, Scharff L. Relationships between family and parent characteristics and functional abilities in children with recurrent pain syndromes: An investigation of moderating effects on the pathway from pain to disability. J Pediatr Psychol 2005;30:698–707. [DOI] [PubMed] [Google Scholar]
- 67.Logan DE, Simons LE, Carpino EA. Too sick for school? Parent influences on school functioning among children with chronic pain. Pain 2012;153:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lomholt JJ, Thastum M, Christensen AE, Leegaard A, Herlin T. Cognitive behavioral group intervention for pain and well-being in children with juvenile idiopathic arthritis: a study of feasibility and preliminary efficacy. Pediatr Rheumatol Online J 2015;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lomholt JJ, Thastum M, Herlin T. Pain experience in children with juvenile idiopathic arthritis treated with anti-TNF agents compared to non-biologic standard treatment. Pediatr Rheumatol Online J 2013:11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lonergan A The effectiveness of cognitive behavioural therapy for pain in childhood and adolescence: a meta-analytic review. Ir J Psychol Med 2016;33:251–264. [DOI] [PubMed] [Google Scholar]
- 71.Lynch-Jordan AM, Kashikar-Zuck S, Goldschneider KR. Parent perceptions of adolescent pain expression: The adolescent pain behavior questionnaire. Pain 2010;151:834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lynch AM, Kashikar-Zuck S, Goldschneider KR, Jones BA. Sex and age differences in coping styles among children with chronic pain. J Pain Symptom Manage 2007;33:208–216. [DOI] [PubMed] [Google Scholar]
- 73.Lynch AM, Kashikar-Zuck S, Goldschneider KR, Jones BA. Psychosocial risks for disability in children with chronic back pain. J Pain 2006;7:244–251. [DOI] [PubMed] [Google Scholar]
- 74.Lynch-Jordan AM, Kashikar-Zuck S, Szabova A, Goldschneider KR. The interplay of parent and adolescent catastrophizing and its impact on adolescents’ pain, functioning, and pain behavior. Clin J Pain 2013;29:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: An examination in a nationally representative sample. Pain 2003;106:127–133. [DOI] [PubMed] [Google Scholar]
- 76.Mano KEJ, Evans JR, Tran ST, Khan KA, Weisman SJ, Hainsworth KR. The psychometric properties of the Screen for Child Anxiety Related Emotional Disorders in pediatric chronic pain. J Pediatr Psychol 2012;37:999–1011. [DOI] [PubMed] [Google Scholar]
- 77.Mano KEJ, Salamon KS, Hainsworth KR, Khan KJA, Ladwig RJ, Davies WH, Weisman SJ. A randomized, controlled pilot study of mindfulness-based stress reduction for pediatric chronic pain. Altern Ther Health Med 2013;19:8–14. [PubMed] [Google Scholar]
- 78.Miller MM, Scott EL, Trost Z, Hirsh AT. Perceived injustice is associated with pain and functional outcomes in children and adolescents with chronic pain: A preliminary examination. J Pain 2016;17:1217–1226. [DOI] [PubMed] [Google Scholar]
- 79.National Collaborating Centre for Methods and Tools Quality Assessment Tool for Quantitative Studies Hamilton: McMaster University, 2008. [Google Scholar]
- 80.Orwin RG. A fail-safe N for effect size in meta-analysis. J Educ Behav Stat 1983;157–159. [Google Scholar]
- 81.Paananen M, Taimela S, Auvinen J, Tammelin T, Zitting P, Karppinen J. Impact of self-reported musculoskeletal pain on health-related quality of life among young adults. Pain Med 2011;12:9–17. [DOI] [PubMed] [Google Scholar]
- 82.Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. J Dev Behav Pediatr 2000;21:58–69. [DOI] [PubMed] [Google Scholar]
- 83.Peterson CC, Palermo TM. Parental reinforcement of recurrent pain: The moderating impact of child depression and anxiety on functional disability. J Pediatr Psychol 2004;29:331–341. [DOI] [PubMed] [Google Scholar]
- 84.Pielech M, Ryan M, Logan D, Kaczynski K, White MT, Simons LE. Pain catastrophizing in children with chronic pain and their parents: Proposed clinical reference points and reexamination of the Pain Catastrophizing Scale measure. Pain 2014;155:2360–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pincus T, Smeets RJ, Simmonds MJ, Sullivan MJ. The fear avoidance model disentangled: improving the clinical utility of the fear avoidance model. Clin J Pain 2010;26:739–746. [DOI] [PubMed] [Google Scholar]
- 86.Quartana PJ, Burns JW, Lofland KR. Attentional strategy moderates effects of pain catastrophizing on symptom-specific physiological responses in chronic low back pain patients. J Behav Med 2007;30:221–231. [DOI] [PubMed] [Google Scholar]
- 87.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: A critical review. Expert Rev Neurother 2009;9:745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reid GJ, Gilbert CA, McGrath PJ. The pain coping questionnaire: preliminary validation. Pain 1988;76:83–96. [DOI] [PubMed] [Google Scholar]
- 89.Rosenstiel AK, Keefe FJ. (1983). The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain 1983;17:33–44. [DOI] [PubMed] [Google Scholar]
- 90.Salyers MP, Bonfils KA, Luther L, Firmin RL, White DA, Adams EL, Rollins AL. The relationship between professional burnout and quality and safety in healthcare: A meta-analysis. J Gen Intern Med 2017;32:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: A systematic review and annotated bibliography. Int J Epidemiol 2007;36:666–76. [DOI] [PubMed] [Google Scholar]
- 92.Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain 2006;120:297–306. [DOI] [PubMed] [Google Scholar]
- 93.Sessler DI, Imrey PB. Clinical research methodology 1: Study designs and methodologic sources of error. Anesth Analg 2015;121:1034–1042. [DOI] [PubMed] [Google Scholar]
- 94.Severeijns R, Vlaeyen JW, van den Hout MA, Weber WE. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin J Pain 2001;17:165–172. [DOI] [PubMed] [Google Scholar]
- 95.Sil S, Cohen LL, Dampier C. Psychosocial and functional outcomes in youth with chronic sickle cell pain. Clin J Pain 2016(a);32:527–533. [DOI] [PubMed] [Google Scholar]
- 96.Sil S, Dampier C, Cohen LL. Pediatric sickle cell disease and parent and child catastrophizing. J Pain 2016(b);17:963–971. [DOI] [PubMed] [Google Scholar]
- 97.Simons LE, Kaczynski KJ. The Fear Avoidance model of chronic pain: examination for pediatric application. J Pain 2012;13:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simons LE, Pielech M, Cappucci S, Lebel A. Fear of pain in pediatric headache. Cephalalgia 2015(a);35:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simons LE, Smith A, Kaczynski K, Basch M. Living in fear of your child’s pain: The parent fear of pain questionnaire. Pain 2015(b);156:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Skevington SM. Investigating the relationship between pain and discomfort and quality of life, using the WHOQOL. Pain 1998;76:395–406. [DOI] [PubMed] [Google Scholar]
- 101.Spanos NP, Radtke-Bodorik HL, Ferguson JD, Jones B. The effects of hypnotic susceptibility, suggestions for analgesia, and the utilization of cognitive strategies on the reduction of pain. J Abnorm Psychol 1979;88:282. [DOI] [PubMed] [Google Scholar]
- 102.Starcevic V, Berle D. Cognitive specificity of anxiety disorders: a review of selected key constructs. Depress and Anxiety 2006;23:51–61. [DOI] [PubMed] [Google Scholar]
- 103.Sullivan MJ. Toward a biopsychomotor conceptualization of pain: implications for research and intervention. Clin J Pain 2008;24:281–290. [DOI] [PubMed] [Google Scholar]
- 104.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524. [Google Scholar]
- 105.Sullivan MJ, D’Eon JL. Relation between catastrophizing and depression in chronic pain patients. J Abnorm Psychol 1990;99:260. [DOI] [PubMed] [Google Scholar]
- 106.Sullivan MJ, Stanish W, Waite H, Sullivan M, Tripp DA. Catastrophizing, pain, and disability in patients with soft-tissue injuries. Pain 1998;77:253–260. [DOI] [PubMed] [Google Scholar]
- 107.Sullivan MJ, Thibault P, Savard A, Catchlove R, Kozey J, Stanish WD. The influence of communication goals and physical demands on different dimensions of pain behavior. Pain 2006;125:270–277. [DOI] [PubMed] [Google Scholar]
- 108.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 2001;17:52–64. [DOI] [PubMed] [Google Scholar]
- 109.Thastum M, Herlin T, Zachariae R. Relationship of pain-coping strategies and pain-specific beliefs to pain experience in children with juvenile idiopathic arthritis. Arthritis Care Res 2005;53:178–184. [DOI] [PubMed] [Google Scholar]
- 110.Thastum M, Zachariae R, Schøler M, Herlin T. A Danish adaptation of the Pain Coping Questionnaire for children: Preliminary data concerning reliability and validity. Acta Paediatr 1999;88:132–138. [DOI] [PubMed] [Google Scholar]
- 111.Tran ST, Guite JW, Pantaleao A, Pfeiffer M, Myer GD, Sil S, Thomas SM, Ting TV, Williams SE, Edelheit B, Ounpuu S, Rodriguez-MacClintic J, Zempsky W, Kashikar-Zuck S. Preliminary outcomes of a cross–site cognitive–behavioral and neuromuscular integrative training intervention for juvenile fibromyalgia. Arthritis Care Res 2017;69:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tran ST, Mano KEJ, Hainsworth KR, Medrano GR, Khan KA, Weisman SJ, Davies WH. Distinct influences of anxiety and pain catastrophizing on functional outcomes in children and adolescents with chronic pain. J Pediatr Psychol 2015;40:744–755. [DOI] [PubMed] [Google Scholar]
- 113.Van Tilburg MA, Claar RL, Romano JM, Langer SL, Walker LS, Whitehead WE, Abdullah B, Christie DL, Levy RL. Role of coping with symptoms in depression and disability: Comparison between inflammatory bowel disease and abdominal pain. J Pediatr Gastroenterol Nutr 2015;61:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL™* 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003;3:329–341. [DOI] [PubMed] [Google Scholar]
- 115.Verbunt JA, Nijhuis A, Vikström M, Stevens A, Haga N, Jong JD, Goossens M. The psychometric characteristics of an assessment instrument for perceived harmfulness in adolescents with musculoskeletal pain (PHODA-youth). Eur J Pain 2015;19:695–705. [DOI] [PubMed] [Google Scholar]
- 116.Vervoort T, Caes L, Trost Z, Sullivan M, Vangronsveld K, Goubert L. Social modulation of facial pain display in high-catastrophizing children: An observational study in schoolchildren and their parents. Pain 2011;152:1591–1599. [DOI] [PubMed] [Google Scholar]
- 117.Vervoort T, Craig KD, Goubert L, Dehoorne J, Joos R, Matthys D, Buysse A, Crombez G Expressive dimensions of pain catastrophizing: A comparative analysis of school children and children with clinical pain. Pain 2008;134:59–68. [DOI] [PubMed] [Google Scholar]
- 118.Vervoort T, Gouber L, Crombez G. Parental responses to pain in high catastrophizing children: the moderating effect of child attachment. J Pain 2010;11:755–763. [DOI] [PubMed] [Google Scholar]
- 119.Vervoort T, Goubert L, Eccleston C, Bijttebier P, Crombez G. Catastrophic thinking about pain is independently associated with pain severity, disability, and somatic complaints in school children and children with chronic pain. J Pediatr Psychol, 2006;31:674–683. [DOI] [PubMed] [Google Scholar]
- 120.Vervoort T, Goubert L, Eccleston C, Vandenhende M, Claeys O, Clarke J, Crombez G Expressive dimensions of pain catastrophizing: An observational study in adolescents with chronic pain. Pain 2009;146:170–176. [DOI] [PubMed] [Google Scholar]
- 121.Walker LS, Smith CA, Garber J, Van Slyke DA. Development and validation of the pain response inventory for children. Psychol Assess 1997;9:392. [Google Scholar]
- 122.Warschburger P, Hänig J, Friedt M, Posovszky C, Schier M, Calvano C. Health-related quality of life in children with abdominal pain due to functional or organic gastrointestinal disorders. J Pediatr Psychol 2014;39:45–54. [DOI] [PubMed] [Google Scholar]
- 123.Wicksell RK, Melin L, Lekander M, Olsson GL Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain–a randomized controlled trial. Pain 2009;141:248–257. [DOI] [PubMed] [Google Scholar]
- 124.Wicksell RK, Olsson GL, Hayes SC. Mediators of change in acceptance and commitment therapy for pediatric chronic pain. Pain 2011;152:2792–2801. [DOI] [PubMed] [Google Scholar]
- 125.Williams ACDC. Facial expression of pain: An evolutionary account. Behav Brain Sci 2002;25:439–455. [DOI] [PubMed] [Google Scholar]
- 126.Wilson DB. Meta-analysis macros for SAS, SPSS, and Stata 2017. Retrieved, March 1st, 2017 from http://mason.gmu.edu/~dwilsonb/ma.html. [Google Scholar]
- 127.Zeltzer LK, Tsao JC, Bursch B, Myers CD. Introduction to the special issue on pain: From pain to pain-associated disability syndrome. J Pediatr Psychol 2006;31:661–666. [Google Scholar]


