Abstract
Purpose of review:
Poor sleep is a risk factor for cardiometabolic morbidity. The relationship of sleep and cardiometabolic health could be confounded, mediated, or modified by diet, yet the incorporation of diet in sleep-cardiometabolic health studies is inconsistent. This rapid systematic literature review evaluates the conceptualization of diet as a confounder, mediator, or effect modifier within sleep-cardiometabolic health investigations and the statistical approaches utilized.
Recent findings:
Of 4,692 studies identified, 60 were retained (28 adult, 32 pediatric). Most studies included diet patterns, quality, or energy intake as a confounder, while a few examined these dietary variables as a mediator or an effect modifier. There was some evidence, mostly in pediatric studies, that inclusion of diet altered sleep-cardiometabolic health associations.
Summary:
Diet plays a diverse role within sleep-cardiometabolic health associations. Investigators should carefully consider the conceptualization of diet variables in these relationships and utilize contemporary statistical approaches when applicable.
Keywords: nutrition, diet, total energy intake, dietary quality, sleep, sleep quality, sleep duration, cardiometabolic health, obesity, body mass index, confounding, mediation, effect modification
INTRODUCTION
Cardiometabolic syndrome consists of metabolic dysfunction characterized by insulin resistance and impaired glucose tolerance, atherogenic dyslipidemia, hypertension and intra-abdominal adiposity, and is linked to cardiovascular morbidity and mortality [1, 2]. Despite significant progress in diagnosis and treatment, the public health burden of cardiovascular diseases and diabetes is increasing globally [3, 4].
A myriad of studies have associated diet - dietary quality, dietary patterns, total energy intake, intake of specific foods, macronutrients and micronutrients - with the development and prevention of cardiometabolic morbidity [5–7]. Recently, poor sleep quality and short sleep duration have also been related to cardiometabolic health [8–10].
Despite their reported independent associations with cardiometabolic health, sleep and diet are in fact strongly interrelated. Moreover, their relationship is likely bidirectional; short sleep duration may lead to higher total energy intake and consumption of less-healthy foods [11, 12], while at the same time diet could alter sleep duration and quality [13, 14]. However, only the impact of sleep on diet has been consistently investigated. Robust experimental evidence has shown that poor sleep alters key appetite regulators, leptin and ghrelin, that in turn lead to dysregulated diet [15]. Poor sleep quality has been shown to impact leptin and ghrelin, key appetite regulators.
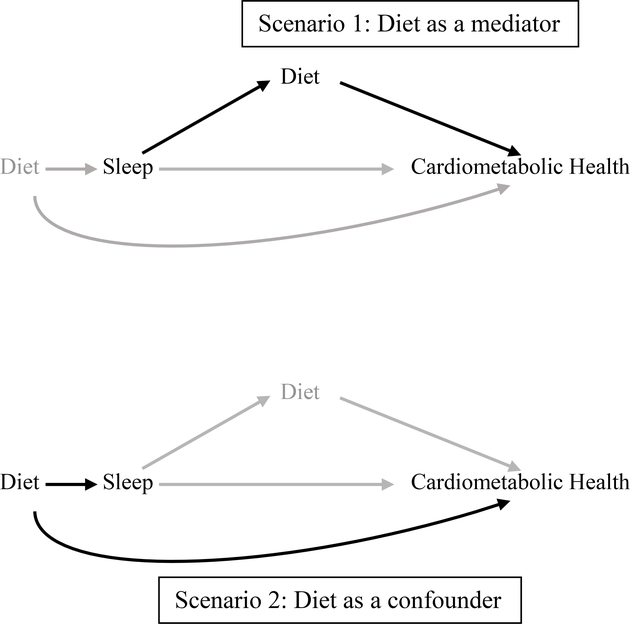
The observed bidirectional associations between sleep and diet presents a challenge for analysis and interpretation of current studies. Analyses should differ if diet is hypothesized to be caused by or alternatively to cause poor sleep. With respect to cardiometabolic health, this bidirectionality suggests two possible scenarios (Figure 1). For example, experimental studies have demonstrated that restricted sleep increases dietary intake on the following day [12]. Here, diet would act as a likely mediator on the pathway of sleep and cardiometabolic health. In contrast, consumption of a high glycemic index carbohydrate meal before bedtime has been shown to shorten sleep onset [16], a scenario in which diet would act as a potential confounder. In addition to its potential role as a confounder or as a mediator, diet could act as an effect modifier of the sleep-cardiometabolic health association. For example, an association between short sleep and obesity may be more evident among those with low quality diets [17]. Furthermore, diet is a multifaceted behavior that could be measured as total energy intake, diet composition and meal timing patterns, among others. Each of these dietary measures could have a differential role in the association of sleep and cardiometabolic health.
Figure 1.
Causal diagram illustrating the different roles of diet on the sleep-cardiometabolic health pathway
Despite increasing evidence that link diet and sleep, prior studies have inconsistently included diet in the analysis of sleep and cardiometabolic health. This oversight may threaten internal and external validity of these studies. Importantly, the distinction of diet as a confounder or a mediator requires different analytic strategies; whereas confounders should be accounted for in some way (e.g. through adjusting for them in regression models), it is advised that mediators should not be adjusted for in the analysis. Adjusting for mediators- one form of over-adjustment-can lead to bias when certain assumptions are not met, in particular when there are unmeasured confounders of the mediator-outcome pathway [18]. The lack of consensus in the literature on the inclusion or exclusion of diet in the context of sleep and cardiometabolic health, coupled with the increasing evidence that diet and sleep are intertwined, has motivated this systematic review. Specifically, we aimed to 1) examine the incorporation of diet as a confounder, mediator, or effect modifier of the sleep-cardiometabolic health associations; and 2) to guide future analyses that plan to incorporate diet in the context of sleep and cardiometabolic health.
METHODS
We applied systematic review methodology to conduct a rapid systematic review to examine the role of diet in sleep and cardiometabolic health. Specifically, we performed a systematic search, appraisal, and synthesis of the literature published within the last 5 years, hence a rapid process compared to a traditional systematic review [19]. In addition to significantly decreasing the length of the process, rapid review provides a quick assessment of a focused research question to inform investigators and public health professionals. We used the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to specify the systematic literature search protocol. An experienced health sciences informationist (CS) applied this protocol to systematically search within PubMed, EMBASE (biomedical and pharmaceutical literature), PsycINFO (psychology and behavioural sciences), CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature), and SCOPUS (Sciences, Engineering, Medicine, Social Sciences and some Arts) to identify peer-reviewed studies that examined sleep and diet in relation to cardiometabolic health within the last 5 years.
Search Strategy
The main search strategy, performed in PubMed and translated to other databases, was conducted by searching for selected keywords and medical subject headings in the titles/abstracts (tiab) and MeSH terms (mh) specific to sleep, diet and cardiometabolic health: sleep[mh] OR sleep[tiab] OR sleep wake disorders[mh] OR sleep wake disorders[tiab] OR sleep apnea syndromes[tiab] OR sleep quality OR sleep quantity[tiab]) AND (body mass index[mh] OR diet[mh] OR diet[tiab] OR dietary behavior[tiab] OR dietary pattern*[tiab] OR energy intake[mh] OR energy intake[tiab] OR feeding behavior[mh] OR feeding behavior[mh] OR nutrition[mh] OR nutrition[tiab] OR nutritional status[mh] OR nutritional status[tiab]) AND (adiposity[mh] OR adiposity[tiab] OR cardiometabolic health[tiab] OR cardiovascular health[tiab] OR cardiovascular disease[mh] OR cardiovascular diseases[tiab] OR diabetes [mh] OR diabetes [tiab] OR metabolic syndrome[mh] OR metabolic syndrome[tiab] OR obesity[mh] OR obesity[tiab]). All searches were completed by 20 March 2018. The full search strategy is available upon request. Citations were imported into EndNote (Thomson-Reuters, New York, NY).
Selection Criteria
This systematic review included peer-reviewed studies of both children and adults that were published in English. The following exclusion criteria were applied: conference abstracts and duplicate publications; studies within a population with underlying morbidity, e.g. diabetes, obstructive sleep apnea; studies that did not consider both nutrition and sleep in relation to cardiometabolic health; or if sleep or diet were not the primary exposure; studies that considered alcohol or caffeinated beverages as their only dietary variables; or studies that did not evaluate cardiometabolic outcomes or included participants younger than 2 years. Four of the authors (ECJ, GLD, MET, HG) independently assessed the eligibility of the studies by the specified criteria in a two-step process. In the initial screening step, publications were evenly divided among the four authors and were screened based on their title and abstract. In the second step, full-text articles of retained abstracts were reviewed by 2 or more authors. Any disagreements were resolved through arbitration. At the end of the screening process, there was a full inter-rater agreement among all reviewers.
RESULTS
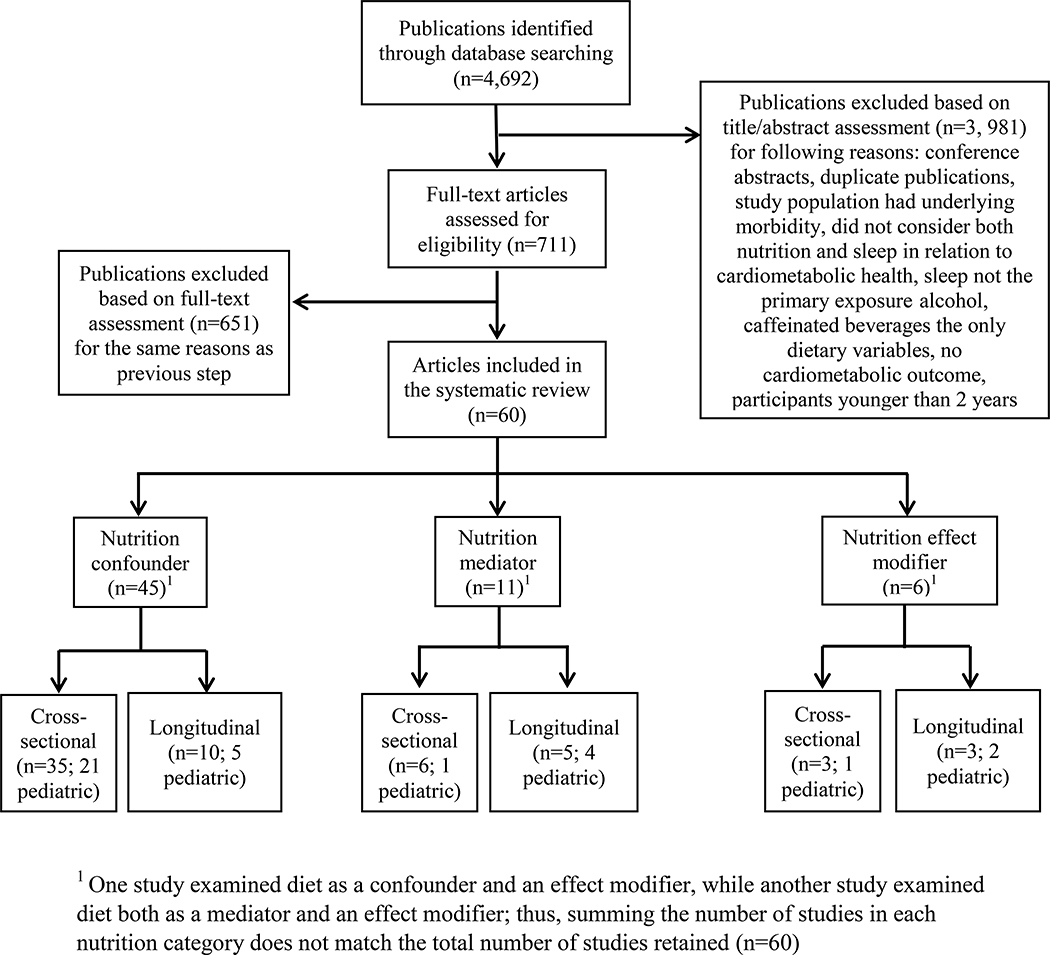
In total, 4,692 publications were identified. Following the first abstract review step, 711 articles were retained. A subsequent assessment of the 711 full-text by multiple authors yielded 60 articles (Figure 2). Of these, 44 used dietary variables strictly as a confounder or independent predictor, 10 studies considered diet strictly as a mediator, 4 studies considered diet only as an effect modifier; while one study examined diet both as a confounder and effect modifier and one study considered diet both as a mediator and effect modifier.
Figure 2.
Selection of studies
Diet as a confounder of the sleep and cardiometabolic health relationship
Ten longitudinal studies (Table 1) and thirty-five cross-sectional studies (Table 2) considered diet as a potential confounder in the relationship between sleep and cardiometabolic outcomes, or as an independent predictor of cardiometabolic outcomes. Of note, the majority of studies examined sleep duration, either continuously or defined as short sleep according to age-specific guidelines. Similarly, while the majority of cardiometabolic outcomes were a measure of adiposity or obesity. Of the prospective studies, half included children [20–24] while the remaining five studies included adults [25–28] or pregnant women [29].
Table 1.
Longitudinal studies with diet as a confounder or an independent risk factor
| Author, Year, Country | Study population | Study design | Sleep Assessment | Confounder Assessment | Outcome Assessmenta,b | Other Covariates | Statistical Analysis | Main findings |
|---|---|---|---|---|---|---|---|---|
| Children and adolescents | ||||||||
| Baird, 2016, UK [20] | 587 children, aged 3 years at baseline | Population-based longitudinal study of mothers and children followed from preconception | Sleep duration, including nighttime sleep and daytime naps, at age 3; parent-report | Diet quality at age 3, Prudent diet z-score; FFQ | BMI (height and weight); body composition (fat and fat-free mass index by DXA scan) at age 4 | Gestational age (GA), age at DXA, sex, pre-pregnancy maternal BMI, maternal education, prenatal smoking, parental SES, age last breastfed, activity level, TV watching time | Linear regression | Shorter sleep was associated with higher BMI, a greater fat mass index, and a greater fat-free mass index one year later; adjustment for confounders did not substantially alter estimates; diet was independently associated with adiposity |
| De Souzab, 2015, Portugal [21] | 6,894 adolescents, aged 10–18 years at baseline | Longitudinal study, annual measurements for 3 years | Sleep duration during the week; self-report | Fruit and vegetable intake (dichotomo us variable: every day vs. not every day); FFQ | BMI | Physical activity, physical fitness | Sex-stratified hierarchical linear models to model change in BMI | Sleep duration was not associated with BMI trajectories in fully adjusted models (crude association not reported) |
| Fairleyb, 2015, UK [24] | 987 participants, aged 3 years | Longitudinal multiethnic birth cohort | Sleep duration during day and night at 24 months; parent-report | Duration of any breastfeeding, age at weaning on to solids, infant’s total energy intake per day, infant’s total protein intake per day, caregivers feeding style; parent reported | BMI z-scores and child overweight; measured at the 36 month visit, based on the WHO 2006 growth standards | Ethnicity, infant sex, maternal age, maternal highest educational qualification, parity, birthweight, gestational age at delivery, mode of delivery | Linear regression models and Poisson regression models | No association between sleep duration and BMI-z scores (unadjusted analyses not shown). BMI z-scores were higher in children who breastfed between 1 day and 1 month (compared to those who never breastfed) and had an indulgent caregiver’s feeding style; similar patterns for overweight |
| Shang, 2014, Canada [23] | 613 children, aged 8–10 years | Longitudinal study of families | Sleep duration; 7-day actigraphy | 3 dietary patterns (traditional, healthy fast food); total energy intake; 24-hr recalls | BMI, WC, fat mass %, obesity defined as ≥ 95th percentile for age and sex | Age, sex, screen time, mother’s obesity, family income, daily steps; sweetened beverage intake | Multivariable logistic regressions | In fully adjusted multivariable models, fast food dietary pattern was associated with overweight and adiposity measures (BMI, WC, body fat mass %), sweetened beverage intake was associated with BMI and WC, but sleep duration was not significantly associated with adiposity (crude association not shown) |
| Lytle, 2013, USA [22] | 723 adolescents, mean age 14.7 years at baseline | Longitudinal study; one follow-up visit 2 years after baseline | Change in average daily sleep duration over follow-up; self-report | Total energy intake; average daily calories from three 24-h recalls via phone (two weekdays and a weekend) | BMI and % body fat; body size via bioelectrical impedance and hydrodensitometry | Sex, grade level, depression, screen time/sedentary behavior, physical activity, puberty, race, SES, parental education, study | Random coefficient models to account for within-person correlation, all conducted separately for males and females | Change in sleep duration was not associated with change in adiposity measures over two years, neither in analysis adjusting only for sociodemographic characteristics nor in fully adjusted analysis. |
| Adults | ||||||||
| Byrneb 2016, USA [25] | 10,248 employees of Vander bilt University, mean age 41 years | Longitudinal study, annual participation in health risk assessment | Sleep 7-8h per night (range from seldom or never to always); self-report | Dietary fat intake, unhealthy snacks, breads and grains, fruits and vegetables, regular breakfast; self-report | Cardiometabolic outcomes: hypercholester olemia, hypertension, obesity, diabetes mellitus, heart disease, stroke; self-report | Age, sex, race/ethnicity, physical activity, smoking, strength exercising, baseline BMI and comorbidities | Multivariable Cox regression, excluding individuals with outcomes at baseline | “Always” sleeping 7–8h per night compared to “never” was associated with lower risk of obesity, heart disease, hypercholester olemia, and stroke even after accounting for diet (largest effect estimate with high-fat diet) and other covariates |
| Hoevenaar-Blom, 2014, The Netherlands [27] | 17,887 adults age 20-79 years | Longitudinal study, 10–14 year follow up | Sleep duration (sufficient (≥7 h) versus insufficient); self-report | Mediterranean diet intake; FFQ | CVD events and mortality | Age, sex, physical activity, smoking, education, BMI, blood pressure, alcohol | Cox proportional hazards, Preventable fraction calculated | Sufficient sleep in addition to the healthy lifestyle score significantly reduced the hazard of CVD deaths or events (unadjusted estimates not substantially different from adjusted) |
| Li, 2014, Japan [28] | 12,883 adults aged 20–79 year at baseline | Longitudinal study with a 10-year follow-up | Sleep duration (<6h vs. ≥ 6 h); self-report | 3 dietary patterns: traditional, healthy, Western; FFQ | Diabetes mellitus, general CVD events and deaths | Occupation, age, current smoking, habitual drinking, regular physical activity, work intensity BMI, systolic BP, total cholesterol, and fasting blood glucose | Cox proportional hazard regression and logistic regression to predict 10-year risk of CVD events | Short sleep duration, traditional and Western diet were associated with CVD events after controlling for other lifestyle factors |
| Restall, 2014, multi-country: Australia, New Zealand, Ireland [29] | 1,950 nulliparous women, mean age 29 years, 14–16 weeks pregnant with a singleton | Longitudinal, multi-center cohort study, 2004–2011 | Nightti me sleep duration hours (<8h (referen ce), 8-9h, ≥10h); self report | Fish/seafood (including oily fish such as tuna or salmon and shellfish or shrimps) weekly intake (<3 vs. ≥3 servings); self-report | Excessive gestational weight gain (yes vs. no) according to Institute of Medicine 2009 guidelines | Exercise, infertility, behavioral responses to illness, smoking, immigration status, birth weight | Logistic regression; | High fish/seafood intake and sleeping for 10h+ per night were each associated with higher risk for excessive weight gain in pregnancy (unadjusted associations not shown) |
| Sayon-Orea, 2013, Spain [26] | 10,532 adults (n=9,470 for naps data), mean age 39 years at baseline | Longitudinal study, followed for median 6.5 years | Average night sleep duration (reference = 7–8 h) and average nap duration (reference=never/almost never nap); self report | Total energy intake, sugar-sweetened beverage intake, fast food intake, snacking between meals; FFQ | Incidence of obesity over follow-up period; self-reported height and weight | Age, sex, physical activity, smoking, sitting, regular snoring, insomnia, caffeine intake, alcohol intake, baseline BMI | Cox regression model | Sleeping <5 h per night was associated with higher risk of developing obesity and a nap of 30 min/day was associated with lower risk of developing obesity, accounting for potential confounders did not substantially alter estimates (except for adding baseline BMI). |
BMI=Body Mass index; CDC=Centers for Disease Control; CHO=carbohydrate; FFQ= food frequency questionnaire; IOM=Institute of Medicine; IOTF=International Obesity Taskforce; MetS=metabolic syndrome; SES=socioeconomic status; TV=television; UK=United Kingdom; USA=United States of America; WC=waist circumference; WHO=World Health Organization
Overweight defined as BMI≥25 and obesity defined as BMI≥30, WC≥40 in men, WC≥35 in women unless otherwise specified
Indicates a study where sleep was one of many risk factors (including nutrition) considered for cardiometabolic outcome
Table 2.
Cross-sectional studies with diet as a confounder or an independent risk factor
| Author, Year, Country | Study Population | Sleep Assessment | Confounder Assessment | Outcome Assessmenta,b | Other Covariates | Statistical Analysis | Main findings |
|---|---|---|---|---|---|---|---|
| Children and adolescents | |||||||
| Kelishadi, 2018, Iran [59] | 13,280 students, aged 6–18 years | Sleep duration as number of hours in a week; student and parent questionnaires | Intake of junk food, number of days eating breakfast in a week, breastfed | BMI, Waist-Height Ratio, WC | Provinces, Physical activity, family history of CVD, screen hours, smoking birth weight, psychologic al disorders, SES, age, sex | Bayesian multi-level linear regression | Higher sleep duration and breakfast intake was inversely associated with all three obesity measures. Junk food intake and breastfeeding were associated with higher BMI |
| Magriplis, 2018, Greece [61] | 4,434 children, aged 10–12 years | Sleep duration averaged for week nights and weekends; self-report | Non and potentially-obesogenic dietary patterns; self-report; 48-item FFQ | BMI, overweight based on IOTF | Age, sex, dietary pattern score, screen time, study hours | Logistic regression; Healthy and excessive weight | Higher sleep duration was associated with lower prevalence of excessive weight |
| Macwanac, 2016, India [58] | 1,050 adolescents, aged 11–19 years old | Sleep duration (categories: up to 7h or more than 7h); self-report, in analysis as predicted independent variable | Specific food items- pulse, dal rice, cheese/butter/paneer, wafer/fry items, pizza/burger/fast food; vegetarian or mixed diet, preference for outside food, breakfast skipping, meal skipping; self-report | Overweight and obesity based on BMI-for-age WHO reference: triceps skin fold thickness; waist and hip circumferences | Age group, sex, language of instruction, family history of obesity, daytime activities | Multiple logistic regression | Higher risk of overweight/obesity for inadequate sleep, intake of outside food, meal skipping habit, and infrequent intake of healthy food items |
| Roman-Vinãs, 2016, multi-country [37] | 6,128 children, aged 9-11 years | Sleep duration, dichotomous adherence to the recommended sleep duration (9–11 h/night), 7-day actigraphy | Unhealthy diet pattern (e.g. fast food, icecream, fried food);23- item FFQ | Obesity; categorical and continuous BMI-for- age z based on WHO reference | Age, sex, parental education | Generalized linear mixed model and linear mixed models Adjusted or stratified by sex |
Adherence to recommended sleep duration was associated with lower obesity prevalence and BMI z-score in the overall sample and in boys and girls |
| Wilkiec, 2016, UK [38] | 374 children, aged 9–11 years | Sleep duration, 1 week hip worn actigraphy | Dietary patterns-health and unhealthy (from principal components analysis); FFQ | Overweight/obese, based on BMI-for- age WHO reference | Age, sex, SES | Multilevel multiple logistic regression with schools as random effects; continuous sleep duration | Sleep duration was inversely associated with odds of overweight/obesity after accounting for potential confounders |
| Zhangc, 2016, China [39] | 3,766 children, aged 7–12 years | Average sleep duration (categories: <7h, 9–11h, and >11h); self-report | Average daily intake of fruits, vegetables, meat products, breakfast, sugar-sweetened beverages, high-energy snacks, fried food, western fast food, eating speed | Overweight and obesity based on Working Group of Obesity in China criteria | Age, sex, only child, parental education, parental occupation, monthly household income, sedentary time, physical activity | Multinomial logistic regression; | Compared to sleep duration <7h, children with 9–11 h of sleep were less likely to be overweight after accounting for potential confounders. The association with obesity was in the same direction but not statistically significant. |
| Arora, 2015, UK [30] | 511 children, aged 11–13 years | Sleep duration; 7-day actigraphy sub-sample n=236; sleep chronotype; self-report | Frequency of unhealthy foods/snacks (crisps, chocolate, biscuits, cake, sweets, etc.), daily intake of fruit and vegetables | BMI z-score (did not specify reference used) | Age, sex, school type, ethnicity, dietary behavior, parent obesity, depression, anxiety, daytime sleepiness, Confounder: intake of caffeinated beverages before bed | Linear regression | Weekday sleep duration and evening chronotype were each associated with higher BMI z-score. |
| Cao, 2015, China [31] | 8,760 children, aged 6–18 years | Sleep duration (categories: <7h, 7–9h, ≥9h); self-report, parent report for kids < 9 years | Daily food intake fruit, vegetables, meat, and sugar beverages; self-report, parent report for kids <9 years | Obesity, based on BMI z-scores by Chinese reference | Age, sex, dietary intake, physical activity, sedentary behavior | Logistic regression with robust standard errors | Boys: short sleep was associated with lower obesity prevalence vs. long sleep; Lower prevalence for boys aged 6–12, but higher prevalence for those aged 13–18 Girls: short sleep was associated with obesity vs. long sleep |
| D’Anielloc, 2015, Italy [33] | 54 children, aged 4–15 years | Sleep duration; sleep quality/fragm entation; parent-report | Weekly fructose intake from sugar sweetened beverages and fruit juice | BMI percentiles from Italian reference | BPA, WC, BP | ANCOVA, Spearman correlations, logistic regression | Short and fragmented sleep were associated with higher prevalence of overweight after taking into account fructose intake |
| Hunsberger, 2015, 12 countries (also found in effect modification) [62] | 5,944 children at baseline | Sleep duration hours (categories: short sleep <10h vs. reference ≥10h);parent-report | Breakfast, CHO from sugar and starch in morning, midday or evening, total energy intake; 24-hour diet recall by parent and school staff Monday through Thursday | BMI, BMI z-score,accord ing to IOTF criteria | Age, sex, highest parental education, country | Mutually adjusted multivariable linear regression | Short sleep was associated with higher BMI-z scores; association slightly attenuated in models adjusted for dietary variables and parental education |
| Katzmarzykc, 2015, multicountry [36] | 6,025 children, aged 9–11 | Nocturnal sleep duration; 7-day actigraphy | Unhealthy and healthy dietary patterns; FFQ | Obesity based on BMI-for- age WHO reference | Age, sex, SES, moderate to vigorous physical activity, TV viewing | Generalized linear mixed models to account for correlation within site and school | Sleep duration was inversely associated with odds of obesity, even after accounting for potential confounders |
| Labreec, 2015, The Netherlands [41] | 1,943 parent-child dyads, children at baseline were 8–9 years | Sleep duration based on time went to bed and awoke on an average school day; reported by primary caregiver | Four food categories: fruit, vegetables, sugar-sweetened beverages, and energy-dense snacks intake; primary caregiver reported children’s dietary intake with a FFQ | BMI and prevalence of overweight/obesity based on IOTF | Age, sex, parental educational level, and parental BMI | Linear regression and logistic regression | Sleep duration was inversely associated with BMI and a lower probability of overweight/obesity. The association did not change when dietary intake of snacks was added in the model |
| Peach, 2015, USA (cross-sectional analysis of longitudinal study) [64] | 541 6th grade students | Weekday sleep duration, weekend sleep duration from self-report; Daytime sleepiness; parent-report | Typical consumption habits of certain foods (soda, hotdogs/ham burgers, French fries, cookies/donuts); self-report | Hypertension, cutoffs determined by CDC sex, age, and height standards | Race/ethnicity, income, pubertal stage, depressive symptoms, physical activity, attention/be havioral problems, BMI | Structural equation models with BMI as mediator, full sample and stratified by sex | Weekend and weekday sleep duration as well as daytime sleepiness were associated with BMI in boys and had a direct effect on hypertension. In girls, daytime sleepiness was associated with BMI and had an indirect effect on hypertension. |
| Wijnhovenc, 2015, multicountry: Bulgaria, Lithuania, Portugal, Sweden, and Czech Republic [63] | 15,643 children, aged 6–9 years | Usual amount of sleep each day (categories: <9h/day (yes/no)), parent-report | Breakfast intake frequency, usual intake of fresh fruit, 100% fruit juice, vegetables, foods like potato chips, foods like candy bars or chocolate, foods like biscuits and cakes, food like pizza and French fries, hamburgers, sausages, or meat pies | Obesity, based on BMI-for- age WHO reference | Physical activity, screen time, sex, age, urbanicity, parental education, parental occupation | Multilevel logistic regression accounting for complex survey design | Sleep duration was not associated with obesity in any country (or pooled) after accounting for potential confounders |
| Carrillo-Larco, 2014, Peru [32] | 1,929 children, mean age 7.9 | Sleep duration as hours slept on a typical night (categories: <10h or 10–11h); self-report | Number of meals; self-report from previous day | BMI categorized into normal, overweight and obese by IOTF reference | Age, sex, urbanicity, birth weight, physical activity, parental education, maternal weight, wealth index | Generalized linear model with robust standard errors to account for clustering | Shorter sleep duration was associated with prevalence of obesity but not overweight; however the association was attenuated after accounting for all covariates |
| Chaputc, 2014, Canada [60] | 507 children, aged 9–11 years | Sleep duration; 7-day actigraphy | Fast food intake; self-reported frequency | % body fat (bioelectrical impedance), waist-to-height ratio | Age, sex, ethnicity, maturity offset, annual household income, parental education, sedentary time and physical activity | Multivariable linear regression models | Sleep duration was inversely associated with adiposity in unadjusted analyses, but not after accounting for potential confounders |
| Arora, 2013, UK [40] | 624 adolescents, aged 11–18 years | Weekday sleep duration, sleep onset latency, number of night-time awakening, sleep quality; self-report | Snacking before bedtime | Weight (kg) and height (cm), BMI z-score | Age, sex and ethnicity, quantity of weekday technology, snacking, activity, school, depression, potential OSA, bedroom sharing, morningness-eveningness and academic performance | Linear regression | An inverse linear relationship was observed between weekday sleep duration, weekday sleep onset latency and BMI z-score. No association was found between the number of night-time awakenings and BMI z-score |
| Devc, 2013, USA [35] | 329 children, aged 2 years, from USA | Nighttime sleep duration (≥9h vs. 8≤); parent-report | Mean intake/day of: milk, sugar beverages, juice, fresh fruits, French fries, vegetables, fast foods, candy sweets, salty snacks; sugar added to baby formula; perceived dietary quality | Overweight/obesity based on CDC reference | 20 other potential risk factors for childhood obesity (e.g. sex, ethnicity, TV habits, parental characteristi cs, SES, parental feeding practices) | Logistic regression | Sleep duration was one of three factors associated with obesity after mutual adjustment of all covariates. Dietary variables were not associated with obesity. |
| Pileggi, 2013, Italy [43] | 509 fifth graders, aged 10 years | Nighttime sleep duration, difference between weekday and weekend in hours of sleep; parent report; in analysis as continuous and dichotomous | Adequate intake calculated for key food groups including cereals, fruit and vegetables, milk, meat/fish and snacks based on Italian Institute of Nutrition age appropriate guidelines;self-report; FFQ | BMI percentile based on Italian Pediatrics Society of Diabetology and Endocrinology | Age, sex, physical activity, maternal BMI, parent’s education, hours watching TV, hours playing video games, breastfed | Linear regression | Higher BMI was found for short sleepers compared to long sleepers |
| Santiago, 2013, Spain [42] | 2,814 children, aged 6–12 years | Sleep duration (categories: <9h vs. >=9h), self-report to interviewer administered questionnaire | Eating patterns: number of meals, breakfast intake; from FFQ with daily/weekly intake assessed for recommended and not healthy food items | BMI classified as overweight/obese according to IOTF | Age, sex, physical activity, screen time | Logistic regression; stratified by sex | Boys and girls were less likely to be overweight if they slept >=9hrs per night in univariate models. In multi-variable models, sleep was no longer a significant predictor of higher BMI whereas dietary intake was associated with higher BMI in boys and, to a lesser degree, in girls. |
| Skidmore, 2013, New Zealand [34] | 685 adolescents, aged 15–18 years | Average sleep duration overall, on school nights and weekend nights; online questionnaire | Fruit and vegetable intake; self-report | BMI z-scores based on BMI-for- age WHO reference, Waist-to-height ratio, fat mass index, fat-free mass index (from bioelectrical impedance) | Sex, grade, ethnicity, number of screens in bedroom | GEE with robust standard errors, accounting for clustering within schools; full sample and stratified by sex | No differences in overall sample but sex-based differences were observed. Higher sleep duration in boys associated with lower BMI, WC and both fat mass index and fat-free mass index; no crude sex-stratified analyses shown |
| Young adults | |||||||
| Kjartansdottir, 2018, Iceland [57] | 199 students, aged 18 years | Sleep duration based on weighted average of self-reported duration during weekend and weekdays (categories: < 7h or ≥7 h), sleep debt defined as weekend sleep ≥2 hrs more than weekday; self-report | Meal frequency; healthy eating score calculated based on eight food groups; unhealthy eating score calculated based on five food groups; 47-item FFQ | BMI and WC | VO2 max | Linear regression, stratified by sex | Short sleep duration was associated with Higher BMI and WC in girls but not boys. |
| Pengpid, 2015, Thailand [44] | 860 college students, aged 17–25 | Sleep duration (reference category 7–8 hours), sleep disturbances; self-report | Weekly intake of fruits, vegetables, red meat, breakfast, snacks. | BMI categorized according to Asian guidelines for underweight, normal, overweight (>23) and obese (>25) | Perceived body size, belief in dietary benefits, physical activity, tobacco, alcohol, depression, PTSD, social support, religiousness, SES | Multinomial logistic regression; stratified by sex | Normal sleep duration was associated with being overweight in women whereas women with short sleep duration were more likely to be underweight |
| Adults | |||||||
| Jayawardana, 2017, Sri Lanka [45] | 2,469 males, aged 16–72 years | Sleep duration(cate gories: low (<6h), medium (7-8h), high (>8h)); self-reportduring an interviewer-administered interview | Average weekly intake of meat, fish, fruits, dairy, fried snacks and sweets, in last 6 months; FFQ | Obesity-WC, BMI (Asian cut-off values: underweight <18.5, normal 18.5–22.9, overweight 23–27.5 or obese >27.5) | Physical activity, smoking, alcohol, age, ethnicity, education, income | Multinomial logistic regression | Sleep and diet were not associated with higher BMI after adjusting for other covariates. Older age, ethnicity and income were associated with higher BMI. |
| Roosc, 2017, Sweden [51] | 18,880 adults, aged 45–75 years | General sleep quality, self-reported questionnaire | Irregular eating habits (not having breakfast, lunch and dinner every day); self-reported questionnaire | MetS | Age, sex, and BMI or fat mass, or fat-free mass, or WC, or waist/hip ratio. | Logistic regression | Sleep quality and irregular eating habits were not significantly related to MetS. |
| Yan, 2017, China [46] | 7,094 adults, aged 35–60 years | Sleep duration (categories: <6h, 6–7h, 7–8h (reference), 8–9h, ≥9h); self-report | Daily caloric intake, fat intake;FFQ (simplified) | BMI, WC; body fat (Bioelectrical Impedance Analysis) Obesity classified by BMI≥28, WC≥85 cm in men and ≥80 in women, body fat ≥25% in men and ≥35% in women |
Age, education occupation, marital status smoking, alcohol sedentary behavior hypertensio n, diabetes, dietary intake, physical activity | Logistic regression, quantile regression, stratified by sex | In men, long sleep >8h was associated with lower abdominal obesity vs. 7–8h of sleep; Negative associations between sleep duration >8h and median WC, but positive associations observed between 6–7h of sleep and WC >75th percentiles In women, sleep duration ≥9h vs. 7–8h was associated with higher probability of obesity and % body fat, but short sleep was not; Sleep ≥9h vs. 7–8h was associated with 10–75 BMI percentiles |
| Min, 2016, South Korea [56] | 8,505 women, aged 25–70 years | Average sleep duration per day (categories: short: ≤5h, short: 6h, reference: 7h, long: 8h, very long: ≥9h., self-report | Energy intake (kcal) 24-h recall method; self-report | MetS | BMI, smoking status, monthly income, alcohol intake, average physical activity per week, intense physical activity per week, education status. | Logistic regression | Both shorter (≤5h) and longer sleep duration (≥9h) were associated with higher probability of MetS and particular components of MetS compared to 7h. Longer sleep duration was also associated with lower abdominal adiposity in a linear manner. |
| Brocato, 2015, Saudi Arabia [47] | 2,686 adults, age not specified, from Saudi Arabia | Sleep duration (categories: <7h, 7h, 8h, >8h); self-report to interviewer administered questionnaire | Weekly intake of common food items, categorized as healthy (green leafy vegetables, fresh fruits, fish and green tea) and unhealthy (red meat, processed meat, pizza, croissants, ice cream, fried foods, soft drinks, etc.) | MetS componentsb | Age, education, physical activity, smoking, | Logistic regression | Longer sleep duration was associated with higher weight, BP and glycemic level. Unhealthy dietary intake was not a significant confounder in the association. |
| Grandner, 2015, USA [49] | 5,607 participants, aged 16+ years | Sleep duration (categories: very short (≤4h), short (5–6h), average (7-8h), long (≥9h)) self-report; in analysis evaluated continuously and categorically | Total daily caloric intake and food variety; 24-h recalls | BMI; height and weight measured by trained research assistants | Sex, race/ethnicity, marital status, education level, income to poverty ratio, exercise, smoking, alcohol, and depression | Linear regression | A fairly-linear relationship was seen among the youngest respondents, with the highest BMI associated with the shortest sleepers and the lowest BMI associated with the longest sleepers. This relationship became U- shaped in middle-age, and less of a relationship was seen among the oldest respondents. |
| Buman, 2014, USA [50] | 2,185 adults (923 fasting subsample), aged 20+ years | Sleep duration; self-report | Total energy, saturated fat; 24-h dietary recalls | WC and systolic and diastolic BPs, HDL-cholesterol and C-reactive protein, LDL- cholesterol, fasting TGL, plasma glucose, and insulin, Homeostasis model assessment of insulin sensitivity (HOMA-S) and homeostasis model assessment of β-cell function (HOMA-β) | Age, sex, race/ethnicity, marital status, education, work status, ratio of family income to poverty level, smoking, caffeine intake, alcohol intake, depressive symptoms, a general health rating, previous diagnosis of cancer or malignancy, CVD, or diabetes,and current use of diabetic, antihypertensive, lipidemic, or other CVD medication | Single-variable and partition models, isotemporal substitution models, interaction analyses | Sleep duration had inverse linear associations with WC and HOMA-β. Excessive or too little sleep was associated with higher diastolic BP, C-reactive protein, and LDL cholesterol. (Reallocating 30 minutes/day from sedentary behavior to sleep had beneficial associations with insulin, HOMA-S, and HOMA-β, as well as with LDL cholesterol in long sleepers only.) |
| Japas, 2014, USA and Canada [48] | 9,864 males, aged 40–60 years | Sleep duration; self-report on self-administered questionnaire | Vegetarian status (vegan, lacto-ovo, semi- and non- vegetarian); FFQ | Change in BMI; BMI at 20, 30, 40 years of age and currently, based on retrospective self-report | Physical activity, income, education | Logistic regression | BMI gain above the median was associated with lower sleep duration, non-vegetarian, less physical activity, and higher TV watching. |
| Parvaneh, 2014, Iran [53] | 226 adults, aged 20–55 years | Bed time, wake time and sleep duration during weekends and weekdays; calculated average duration weighted for weekends and weekdays; self-report | 24-hr food recall for 3 days (2 weekdays and 1 weekend), calculated servings and nutrients as mean values over 3 days; in analysis: mean intake values of energy, CHOs, protein and fat. | BMI (dichotomo us normal or overweight/obese according to WHO criteria), height, weight, hip circumference, WC, and body fat %; all measured by trained research assistants | Age, sex, physical activity | Correlations of continuous variables; stepwise logistic regression with overweight/obesity as the outcome. | Sleep duration was associated with higher caloric, CHO and fat intake. Going to bed later was associated with a higher probability of being overweight or obese. |
| Yu, 2014, China [52] | 11,496 adults, aged 35+ years | Average sleep duration (categories: ≤7h (reference), 7–8h, 8–9h, >9h); self-report obtained by trained research assistants | Weekly frequency of dietary intake of beans, bean products and tea; dietary score calculated based on food recall of average intake over the past year of vegetables and meat, fish and poultry. | MetS | Income, ethnicity, education, marital status, physical activity, smoking, alcohol, chronic diseases, sex, age | Logistic regression, total population and stratified by sex | In women, sleeping 8-9h was a risk factor for MetS compared to <7 h. Overall, those sleeping >9 h were 18% more likely to have MetS than those sleeping less than 7 h. Diet score was not associated with MetS whereas rarely consuming beans was associated with higher MetS in men and overall. |
| Older adults | |||||||
| Georgousop oulou, 2018, Greece [54] | 3,130 adults, aged 65–100 years | Sleep duration calculated from sleep and wake times on a typical day; self-report | Dietary habits; FFQ used and Mediterranea n Diet score calculated | MetS and its components | Age, gender, BMI, daily walking, smoking | Linear regression models with each MetS component as a separate dependent variable | Longer sleep duration was associated with greater WC, higher LDL and lower diastolic BP after adjustment for covariates. |
| Georgousopoulouc, 2017, Greece [55] | 1,369 men, aged 75±8 years and 1,380 women, aged 74±7 years | Siesta habit, considered as sleeping during the day for more than five days per week; self-report | Mean daily energy intake and mean percentage of total energy derived from dietary CHOs; from FFQ; level of adherence to the Mediterranean diet, from MedDietScore questionnaire | MetS | Age, sex, BMI, physical activity (daily walking time), smoking habits | Logistic regression models | Siesta habit was an independent positive predictor of the presence of MetS |
ANCOVA=Analysis of Covariance; BMI=Body Mass index; CDC=Centers for Disease Control; CHO=carbohydrate; CVD=Cardiovascular Disease; FFQ= food frequency questionnaire; HDL= high-density lipoprotein; IOM=Institute of Medicine; IOTF=International Obesity Taskforce; LDL= low-density lipoprotein; MetS=metabolic syndrome; MVPA=moderate-to-vigorous intensity physical activity; PSQI=Pittsburgh Sleep Quality Index; SES=socioeconomic status; TGL=triglycerides; TV=television; UK=United Kingdom; USA=United States of America; WC=waist circumference; WHO=World Health Organization
Overweight defined as BMI≥25 and obesity defined as BMI≥30, WC≥40 in men, WC≥35 in women unless otherwise specified
MetS defined with NCEP ATP III guidelines unless otherwise specified
Indicates a study where sleep was one of many risk factors (including nutrition) considered for cardiometabolic outcome
The pediatric longitudinal studies included children aged 2–18 years [20–24] with mostly subjective measures of baseline sleep duration as the primary exposure. Only one study used actigraphy to analyze sleep duration. Of note, one study assessed the change in average sleep duration over time as the exposure [22]. In these studies, the primary cardiometabolic outcome was adiposity assessed at follow-up. Adiposity was measured as BMI for age z-scores, percent body fat (measured using bioelectrical impedance or dual-energy x-ray absorptiometry), waist-to-height ratio and/or waist circumference. A range of dietary variables were considered as potential confounders, including diet quality, total energy intake, dietary patterns, and intake of specific foods (e.g. fruits and vegetables, soda intake, hamburgers/hotdogs). These measures were assessed at baseline with food frequency questionnaires[20, 21, 24] multiple 24-hour recalls [22, 23], or questions on specific foods or dietary habits [24].
A British study among 3-year old children found an association between shorter sleep duration and higher adiposity one year later [20], an association that remained unaltered by controlling for diet quality. However, other studies found no association between sleep and adiposity after adjusting for baseline dietary variables and other socio-demographic and behavioral confounders.
Studies in adult populations mostly focused on self-reported sleep duration [25–29]. In addition to sleep duration, one study included daytime napping [26]. The range of cardiometabolic outcomes assessed in adults included metabolic syndrome, gestational weight gain (in pregnant women), obesity, waist circumference, insulin resistance, and CVD events and mortality. The dietary confounders - measured concurrently with sleep - included total energy intake, dietary pattern intake, specific foods consumption or macronutrients (e.g. fish intake, unhealthy snacks, breads and grains, fruits and vegetables), and dietary habits (breakfast intake, snacking between meals). These studies found that short sleep duration was related to higher incidence of obesity and metabolic syndrome, even after accounting for dietary variables. Indeed, daily napping was associated with a lower probability of incident obesity after accounting for dietary confounders [26].
Among the 35 cross-sectional studies (21 in children and 14 in adults) [30–64], (Table 2) diet was incorporated as a confounder in the sleep-cardiometabolic health associations. As in the prospective studies, similar dietary confounders were included - total energy intake, dietary patterns, and intake of specific unhealthy or healthy foods. In contrast to findings in longitudinal pediatric studies, most cross-sectional reports suggested associations between sleep and adiposity or hypertension, [64] independent of dietary confounders. Regardless of study design, findings from adult populations were similar, as sleep duration was inversely associated with obesity and cardiometabolic risk after accounting for dietary confounders. Non-linear relations between sleep duration and cardiometabolic risk were examined in some studies that included a longer sleep duration category (e.g. >8 hours sleep duration) in addition to short sleep and sufficient sleep. A U-shape association was reported in 4 studies [49, 50, 52, 56], while one study found that only longer sleep (>8 hours) was associated with worse cardiometabolic outcomes compared to those with a 7 hour sleep duration [47].
Diet as a mediator of the sleep and cardiometabolic health relationship
Eleven studies evaluated diet as a potential mediator of the sleep-cardiometabolic health relationship, five of which were in pediatric populations [65–69] (Table 3). Within pediatric studies, four were longitudinal studies and one had a cross-sectional design. Sleep duration and sleep timing were examined as determinants of childhood obesity. With the exception of one study[67], sleep assessment of children and adolescents was subjective, either self-reported or parent-reported. Potential mediators included total energy intake, macronutrient intake, fast food consumption, diet quality, and food responsiveness (the degree to which a child expresses a desire for food, in particular for highly palatable food).[68] Evidence of partial mediation was observed in most studies. One study reported that energy intake or macronutrient intake fully mediated the longitudinal association between shorter sleep duration and a subsequent BMI Z-score change in children aged 2–6 y old. Only two studies[67, 68] applied formal mediation analysis to examine direct and indirect pathways - through diet - between sleep and childhood adiposity, while the other four informally evaluated the change in effect estimates upon addition of the mediator/s into the statistical models.
Table 3.
Studies with diet as a mediator
| Author Year | Study population | Study design | Exposure Assessment | Mediator Assessment | Outcome Assessment1,2 | Other Covariates | Statistical Analysis | Main findings |
|---|---|---|---|---|---|---|---|---|
| Children and adolescents | ||||||||
| Rangan, 2018, Denmark [65] | 368 children, aged 2–6 at baseline | 1.3 year randomized controlled trial, intervening on diet, physical activity, sleep quality, and stress | Sleep duration and variability based on 6 nights; 7-day parent report; in analysis as continuous or quartiles | Total energy, macronutrients, added sugars; based on 4-day food record from Wednesday to Saturday; parent report | Change in BMI z-score by Danish reference BMI | Baseline age, baseline BMI-z score, sex, physical activity, overweight intervention, parental baseline BMI & education | Multivariable linear regression | Shorter sleep duration, but not variability, was associated with higher BMI-z score change. When total energy or macronut rient intake used as mediators, association was fully attenuated. |
| Cespedes, 2016, USA (also found in effect modification) [17] | 1,046 children, aged 6 months at baseline | Longitudinal study, reports by parents started when children’s age 6 months and annually up to 7 years | Chronic insufficient sleep, quantified by a sleep score tallying the adequacy of parent- reported sleep duration, including naps and nighttime sleep, from infancy to mid-childhood according to National Sleep Foundation recommendations; in analysis continuous or categorical | Youth Healthy Eating Index (YHEI) score, and eating behaviors: fried foods outside the home, eating breakfast, and family dinner); parent report in mid-childhood | BMI z-score at mid-childhood according to CDC | Race/ethnicity, age, sex, maternal education and income, dietary factors at mid-childhood of an 18-item FFQ excluding YHEI factors | Multivari able linear regression models | Adequate sleep was inversely associated with BMI z-score; diet quality attenuated slightly this association in analysis with categorical sleep score, whereas it did not in analysis with continuous sleep score |
| Asamow, 2015, USA [66] | 3,342 adolescents, mean age 16 years at baseline | Nationally representative longitudinal study; | Workday bedtime; self-report | Fast food intake frequency; self-report | Change in BMI | Age, sex, race/ethnicity, welfare status, pubertal status, wave II BMI, television viewing (mediator), exercise (mediator), workday total sleep time (mediator) | Hierarchical linear models accounting for survey design weights and repeated measures sequentially added potential mediators | Later bedtimes were associated with greater change in BMI over followup period; estimates attenuated upon addition of fast food intake but not of sleep duration |
| He, 2015, USA [67] | 305 adolescents, mean age 16.7 years | Cross-sectional secondary analysis of a longitudinal study | Habitual sleep duration and variability; 7 nights of actigraphy and sleep diary | Daily total energy intake, total fat, protein, and CHO; Youth/Adolescent Questionnaire FFQ with 152 food items over 1 year | Abdominal obesity measures from DXA scans | Age, sex, race, BMI percentile according to CDC 2000 growth charts | Linear regression models accounting for both sleep variables and all covariates; separate mediation analysis for dietary variables (“Mediation” package in R) | In fully adjusted models, higher sleep variability was associated with higher abdominal fat, while sleep duration was no longer statistically significantly related to adipose markers. There was evidence of partial mediation by total energy intake, which may be attributed to CHO intake. |
| McDonald, 2015, England and Wales [68] | 1,008 children, each child from a twin pair, aged 5 years | Longitudinal twins study | Sleep duration (categories: shorter (<11), adequate (11–12), longer (>12)); parent-report | Appetite as food responsiveness (FR), measured using the 35-item Child Eating Behavior Questionnaire; parent-report | BMI, age- and sex- adjusted standard deviation scores according to UK 1990 reference data (n=494); height and weight parent-report | Age, sex, birthweight and maternal education | ANCOVA models; Hayes bootstrap ping PROCESS macro used to assess mediation | Shorter sleep was associated with higher FR and with higher BMI after adjusting for covariates; FR was a mediator in the association between short sleep and higher BMI |
| Adults | ||||||||
| Timmermans, 2017, multi-country: Belgium, France, Hungary, the Netherlands and the United Kingdom [75] | 5,900 adults, mean age 52 years | Cross-sectional | Sleep duration during an average night (categories: short: < 7 h, normal: 7 < 9 h, long: ≥ 9 h); self-report; in analysis as continuous or categorical | Dietary habits: short common FFQ about frequency of intake of: fruits, vegetables, fish, sugar sweetened beverages, sweets and fast food. number of times per week eating breakfast and cooking a meal at home using bought ingredients, rather than eating ready or takeaway meals. Variables dichotomized using the median. | BMI (dichoto mized according to WHO criteria into obesity and no obesity); self-reported weight and height | Covariates: age, gender, education, work type, alcohol intake, smoking, self-rated health, comorbidity, screen time, work hours; Effect modifiers: Age, gender, education and country; Other mediators: physical activity (leisure time and transport) and sedentary behaviors | Multilevel logistic regression and Mackinn on’s product-of-coefficients method | Longer sleeping time was associated with lower likelihood of being obese but no evidence for a mediating role of dietary habits |
| Kanagasabai, 2015, USA [71] | 2,079 adults, aged 20+ years | Cross-sectional study | Sleep duration per night on weekdays or workdays (categories ≤ 4, 5–6, 7–8, ≥9 h); self-report | Carotenoids, vitamins A, C, D, and E; laboratory measures from serum | MetSb; number of MetS components, and individual MetS components | Other mediators: CRP, GGT, bilirubin, and uric acid Confound ers: age, sex, ethnicity, income, education, alcohol intake, smoking history, and recreational physical activity (PA) adherence | Logistic regression for dichotomous outcomes and linear regression for continuous outcomes | Among women only, carotenoids and vitamins C and D were modest mediators of the sleep duration-MetS relationships; of the individual components of MetS, there was evidence of mediation for WC and BP |
| Wirth, 2015, USA [74] | 430 adults, aged 21 – 35 years | Cross-sectional analyses from an on-going longitudinal study | Nighttime sleep duration,Sleep/wake times,sleep efficiency, sleep latency, and wake after sleep onset (WASO), naps were removed; actigraphy | Dietary factors (i.e., calorie intake, percent calories from fat, and caffeine). | Body fat % measured by DXA; BMI | Total daily physical activity (also as potential mediator), smoking status, handednes s, sex, age, height, and weight | General linear models (final models developed with backward elimination) | Greater BMI and body fat % were associated with low sleep efficiency and high WASO. Elevated BMI or body fat percent also were observed for later wake times, shorter sleep duration, and longer sleep latency. Dietary factors did not mediate sleep-BMI or body fat association. |
| Chaputc, 2014, Canada [73] | 293 adults aged 18–65 years | Longitudinal study, followed for a mean of 6.0 ± 0.9 years | Sleep duration at baseline and year 6; selfreport; in analysis as continuous and categorical | Daily energy intake was assessed with a 3-day food record, including 2 week days and 1 weekend day | Visceral adipose tissue assessed using computed tomography | Age, sex, change in BMI, smoking habits, highest education level, total annual family income, and menopausal status, MVPA | Multivari able linear regression (accounting for within-individual repeated measures); sequential modeling including dietary variables as mediators | Change in sleep duration from <6 h/day to 7–8 h/day was inversely associated with VAT gain; estimates were attenuated upon adjustment for energy intake and physical activity. |
| Mesas, 2014, Spain [72] | 10,342 adults, aged 18+ years | Cross-sectional study | Poor sleep quality, difficulty falling asleep, difficulty maintaining sleep and sleeping pill ingestion; self-report | Energy intake and adherence to a Mediterranean dietary pattern (Mediterranean Diet Adherence Screener); from FFQ based on previous year | MetS | Age, sex, education and occupation-based social class, tobacco, alcohol and coffee intake, diagnosed morbidity, sleep duration, energy spent in physical activity and time watching TV, drug treatments | Logistic regression | Difficulty falling asleep was associated with higher frequency of MetS; the association was unchanged after accounting for sleep duration and for dietary variables. |
| Yeh, 2014, Australia [70] | 330 adults, mean age 27 years | Community-based and snowball recruitment for online survey | Sleep duration, poor sleep quality (defined as >5 score on PSQI); self-report | Binge-eating (>26 score on Binge Eating Scale), night eating (>24 score on Night-Eating Questionnaire); self-report | BMI (>25); self-report | Depression, age, sex, education, relationship status, employment status | Hierarchical multiple regression; mediation analysis using Sobel’s test | Binge eating and night eating were found to be partial mediators of the short sleep and BMI association. |
ANCOVA=Analysis of Covariance; BMI=Body Mass index; CDC=Centers for Disease Control; CHO=carbohydrate; CVD=Cardiovascular Disease; FFQ= food frequency questionnaire; HDL= high-density lipoprotein; IOM=Institute of Medicine; IOTF=International Obesity Taskforce; LDL= low-density lipoprotein; MetS=metabolic syndrome; MVPA=moderate-to-vigorous intensity physical activity; PSQI=Pittsburgh Sleep Quality Index; SES=socioeconomic status; TGL=triglycerides; TV=television; UK=United Kingdom; USA=United States of America; WC=waist circumference; WHO=World Health Organization
Overweight defined as BMI≥25 and obesity defined as BMI≥30, WC≥40 in men, WC≥35 in women unless otherwise specified
MetS defined with NCEP ATP III guidelines unless otherwise specified [83]
Indicates a study where sleep was one of many risk factors (including nutrition) considered for cardiometabolic outcome
Six adult studies[70–75], of which five were cross-sectional, examined sleep duration and quality with respect to adiposity or cardiometabolic health. Sleep duration and quality was self-reported in all but one study [74]. The range of cardiometabolic outcomes included BMI, metabolic syndrome, body fat and visceral adiposity. In these analyses, food groups (e.g. fruits, vegetables, fish, sugar-sweetened beverages), macronutrients, total energy intake, and micronutrients such as carotenoids were evaluated as potential mediators. Three of these studies reported no evidence of mediation by dietary factors, while three observed partial mediation. One study found that vitamins C and D partially mediated the sleep-obesity association, but only in women [71]. An Australian report suggested binge eating and night eating as partial mediators of short sleep and BMI association, [70]. Similarly, a Canadian study noted an attenuated association between decrease sleep duration over time and subsequent visceral adiposity gain upon adjustment for energy intake and physical activity [73]. None of these studies applied formal mediation analysis techniques.
Diet as an effect modifier of the sleep and cardiometabolic health relationship
Six studies evaluated diet as a potential effect modifier of the sleep-cardiometabolic health relationship in adults and children [62, 69, 76–79] (Table 4).
Table 4.
Studies with diet as an effect modifier
| Author Year | Study population | Study design | Exposure Assessment | Effect Modifier Assessment | Outcome Assessment | Other Covariates | Statistical Analysis | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Children or Adolescents | ||||||||
| Cespedes, 2016, USA (also found in mediation) [17] | 1,046 children, aged 6 months at baseline | Longitudinal study, reports by parents started when children aged 6-months and annually up to 7-years | Chronic insufficient sleep, from infancy to mid-childhood according to National Sleep Foundation recommendations; parent-report; in analysis dichotomous (≥11h vs. <11h, the median score) | Youth Healthy Eating Index (YHEI) and eating behaviors: fried foods outside the home, eating breakfast, and family dinner; parent report in mid-childhood; in analysis dichotomous (YHEI: >60 vs. <60, the median score) | BMI z-score at mid-childhood according to CDC reference | Race/ethnicity, age, sex, maternal education and income | Multivariable-adjusted models | Children in the least favorable category of sleep and diet had the highest estimated mid-childhood BMI z-scores |
| Hjorth, 2015, Denmark [76] | 834 children, 3rd −4th graders | Crossover trial, cluster-randomized, controlled, | Sleep duration in 7 consecutive days and 8 nights; accelerometer worn 7 consecutive days and 8 nights and sleep logs; sleep duration in tertiles | For 1 month children received school lunches based on Healthy New Nordic Diet (NND), for 1 month usual packed lunch from home | BMI z-score by WHO reference, total fat mass, abdominal fat mass, fat free mass, ratio of android from total fat and fat free mass, fat mass index | Age, sex, visit, order of dietary period (NND vs. packed lunch), height | Linear mixed models accounting for school, class, year group within school, sibling, and child | Among habitual short sleepers, intake of school lunches was associated with a gain in fat mass compared to usual packed lunches (control); there was no association with adiposity among long sleepers |
| Hunsberger, 2015, 12 countries (also found in confounder for cross-sectional analysis) [62] | 5,944 children at baseline 4,301 at followup, aged 2–9 years at baseline | Longitudinal study | Sleep duration hours (categories: short sleep <10h vs. reference ≥10h);parent-report | Breakfast, CHO from sugar and starch in morning, midday or evening, total energy intake; 24-hour diet recall by parent and school staff Monday through Thursday | BMI, BMI z-score, according to IOTF criteria | Age, sex, highest parental education,country | Mutually adjusted multivariable linear regression | Among short sleepers, those with high CHO from starch intake at midday had higher than additive BMI z-scores. There was a positive interaction between high midday sugar and short sleep with BMI z-scores at follow-up, but attenuated after adjusting for baseline BMI z-scores. |
| Adults | ||||||||
| Doo, 2017, South Korea [78] | 3,941 men, aged 40–69 years | Cross-sectional study | Sleep duration (categories: short sleep duration (≤6 h a day) and proper sleep duration (≥7 h a day)); self-report | Antioxidant Vitamins-vitamins A, C, E, retinol, carotene; FFQ | Obesity | Age, education, income, marital status, insomnia, smoking, drinking | Multinomial logistic regression; | Among short sleepers, certain antioxidants (e.g. carotene) may have higher than additive association with obesity among men (statistical tests did not evaluate interactions) |
| Doo, 2016, South Korea [77] | 14,680 adults, aged 19+ years | Cross sectional survey | Sleep duration per day (categories: <6h, 6–7h, 7–8h, 8+h), self-report | Total energy intake and the percentage of energy they obtained from protein, fat, and CHO; Dietary intake by a food frequency questionn aire with 63 food items | Body weight, height, WC, BMI, Systolic BPs, diastolic BPs, Venous blood samples collected after overnight fast. Fasting glucose (FG), total cholesterol, TGL, and HDL | Age, physical activity, current smoking, and alcohol drinking status | Multivariable logistic regression model | Among adults whose CHO intake was above the median and whose fat intake was above the median, those with sleep duration of <6 h per day had higher odds of obesity, while there was no association at other categories of sleep (interaction not evaluated statistically). |
| Kim, 2015, South Korea [79] | 12,999 adults, aged 20+ years | Cross-sectional study | Sleep duration per day (categories: <6h, 6–7h, 7-8h, 8+h), self-report | Eating every meal for the last 2 days (categories: (not skipping, skipping a meal in 1 day, skipping a meal both days); self-report | MetS | Age, sex, household income, education level, smoking status, alcohol sleep drinking, duration and physical eating activity, and total daily energy intake | Multiple logistic regression; participants divided into 12 groups, according to sleep duration and eating | Adults <50 y who reported skipping breakfast in the previous 2 days and sleeping less than 6 hours or >8 hours had higher prevalence of MetS compared to those eating breakfast in last 2 days and sleeping between 7–8 hours (no interaction terms tested). |
BMI=Body Mass index; CDC=Centers for Disease Control; CHO=carbohydrate; CVD=Cardiovascular Disease; DXA=dual-energy x-ray absorptiometry; FFQ= food frequency questionnaire; HDL= high-density lipoprotein; IOTF=International Obesity Taskforce; LDL= low-density lipoprotein; MetS=metabolic syndrome; TGL=triglycerides; TV=television; UK=United Kingdom; USA=United States of America; WC=waist circumference; WHO=World Health Organization
Overweight defined as BMI≥25 and obesity defined as BMI≥30, WC≥40 in men, WC≥35 in women unless otherwise specified
MetS defined with NCEP ATP III guidelines unless otherwise specified [83]
Indicates a study where sleep was one of many risk factors (including nutrition) considered for cardiometabolic outcome
The pediatric studies[69, 62, 76] evaluated interactions between short sleep duration and diet in relation to BMI z-scores. Two longitudinal studies[69, 62] suggested that short sleep duration in combination with diet, either poor dietary quality or high starch intake at midday, were associated with higher than additive BMI z-scores. A Danish crossover study[76] examined whether short sleep in combination with school lunch change (adoption of a New Nordic Diet school lunch vs. usual packed lunch) would change adiposity. This study suggested that only children with short sleep had gains in adiposity after school lunch changes, while children with long sleep had no difference in adiposity following the dietary changes.
In adult populations, there were three studies that examined diet as an effect modifier of the association between sleep duration and cardiometabolic health in Korean adults [77–79]. Several authors were involved in all three analyses, and two of the studies used overlapping data from the Korean National Health and Nutrition Survey. Analysis included self-reported sleep duration as the exposure, while adiposity measures and cardiometabolic syndrome were outcomes. Macronutrient intakes, meal skipping patterns, and antioxidant intake were considered as effect modifiers. One of the studies conducted among men only showed that lower intake of antioxidants was associated with higher odds of obesity among short sleepers [78], while another one of the studies including both men and women showed that a higher intake of carbohydrates and a lower intake of fat was associated with lower odds of obesity among short sleepers. In the third study, researchers found that adults between the ages of 20 and 50 years who reported skipping breakfast in the previous two days and having either short sleep (defined as sleeping <6 hours) or long sleep (defined as >8 hours) had higher odds of metabolic syndrome compared to those in the same categories of sleep duration but who reported to have eaten breakfast in the last two days [79]. Nonetheless, in these studies the statistical methodology of the interaction analyses was unclear.
CONCLUSIONS
This unique systematic review performed a rigorous evaluation of the conceptualization and analytic approaches implemented to incorporate diet within sleep and cardiometabolic health investigations. This assessment showed that the majority (two-thirds) of studies considered dietary variables as potential confounders, while only a few assessed dietary variables as mediators or effect modifiers of the sleep and cardiometabolic health relationship. Considerable evidence- particularly in pediatric populations- suggested that diet mediates the sleep-cardiometabolic health pathway, raising the possibility that the dietary variables previously used as confounders were in fact mediators. Therefore, investigators should carefully consider the conceptualization of diet variables in sleep-cardiometabolic health relationships and utilize contemporary statistical approaches when applicable.
As confounder, the most common dietary variables considered were total energy intake, dietary quality scores or dietary patterns. Of note, these dietary variables are known to be affected by sleep [80], thus potentially mediate the pathway from sleep to cardiometabolic health. Adjusting for a mediator on the causal pathway could cause an attenuation of effect estimates, but not necessarily[18]. In the worst-case scenario, this overadjustment may lead to invalid study estimates. The results of this potential overadjustment were inconsistent in the studies identified; in some studies adjustment for diet and other potential cofounders attenuated effect estimates. Interestingly, the largest impact of dietary variables on sleep and cardiometabolic health associations were observed in prospective studies within pediatric populations. These results may represent age-related heterogeneity of sleep and diet associations.
Despite the suggested impact of diet on sleep and cardiometabolic health associations, the independent effect of dietary variables could not be separated from that of other confounders as most studies implemented simultaneous confounding adjustment strategies. In contrast, sequential adjustment of potential confounders would allow to disentangle the impact of each additional variable (or a set of variables) on the effect estimates of interest. Further, the concurrent measurement of sleep and dietary variables presents another major limitation as it hinders temporal assessment of the examined associations.
Eleven studies evaluated dietary variables as potential mediators in the pathway between sleep and cardiometabolic health, with mostly supportive evidence. Similar to confounding dietary variables, the most common mediating dietary variables included total energy intake and unhealthy dietary patterns. Further, the impact of mediating dietary variables on effect estimates was again stronger in pediatric populations compared with adult studies. Potential explanations for this observation are unknown, yet worth mentioning. In adults, additional mediators in the pathway of sleep and cardiometabolic health, such as physical activity or altered metabolism [81], could exert a stronger indirect effect relative to dietary variables. Nonetheless, pediatric and adult study design heterogeneity may further explain the differential strength of dietary variables as mediators. Indeed, evidence from the pediatric populations was mostly obtained prospectively; while adult studies were mostly cross-sectional and thus subjected to reverse causation bias. In investigations of diet as a mediator, perhaps the main concern was the use of informal mediation analysis by most of these studies, which threatens internal validity of the reported results.
Finally, only 6 studies evaluated dietary variables as effect modifiers of sleep and cardiometabolic health associations. In children, associations between dietary variables and adiposity were more apparent among children with short sleep (based on age-specific recommendations). While adult studies echoed the pediatric findings, they lacked formal statistical tests of interaction, objective assessment of sleep, and temporal examination of sleep and cardiometabolic health. Although statistical interactions are not necessarily reflective of true biological interactions, underlying sleep-induced metabolic perturbations could alter the diet and cardiometabolic health associations. In support, experimental evidence showed that disrupted sleep schedules or a “cafeteria diet” alone in rats did not influence weight gain over a 12-week period; yet, the combination of disrupted sleep and a “cafeteria diet” caused weight gain and metabolic disruption in these rats [82].
Beyond the specific aforementioned analytical limitations, overarching methodological pitfalls could affect study validity regardless of the role of diet in these associations. First, measurement challenges pose a significant threat to internal validity, as sleep and diet were mostly self-reported, thus likely plagued by both random and systematic errors. Indeed, poor measurement of diet could explain the mild or null impact of dietary variables as confounders or mediators in some reports. Second, sleep and diet are multi-faceted behaviors with possible differential effects on cardiometabolic health. Yet, most studies focused on sleep duration but not sleep quality, timing, or weekday/weekend variability. Similarly, dietary variables mostly included measures of overall diet- e.g. total energy intake, diet patterns, diet quality- rather than meal timing or composition. Third, the majority of studies reported diet as either a confounder, mediator, or effect modifier in relation to sleep and cardiometabolic health. Certain aspects of diet may act as confounders (e.g. caffeinated beverages) while others (e.g. fast food consumption) could mediate the pathway from sleep to cardiometabolic health. Fourth, over the course of a longitudinal study diet is likely to change, however in none of the studies was diet evaluated as a time-varying confounder.
This systematic review fulfills a significant literature gap as it critically evaluates the incorporation of diet as a confounder, effect modifier, and mediator in sleep and cardiometabolic health associations. These findings will guide ongoing and future nutritional and epidemiological investigations. In particular, they may drive future investigations aimed to elucidate the complex associations between sleep, diet, and cardiometabolic health. As an additional strength, this rapid systematic review synthesizes the most recent studies, which ensures representation of the current knowledge and analytic techniques. Finally, careful attention was paid to causal inference implications. Namely, prospective studies were prioritized over cross-sectional, evaluation of the appropriateness of the statistical techniques and their applications was highlighted, and other important confounding factors such as age, sex, and physical activity were considered. Nonetheless, some limitations remain: as part of the dietary factor evaluations, alcohol and caffeine intake were excluded, as were publications written in languages other than English.
As researchers continue to uncover the sleep-related determinants of cardiometabolic health, the essential role of diet should not be overlooked. In light of the increasing evidence that diet could mediate the causal pathway between sleep and cardiometabolic health, we caution against habitual adjustment of dietary variables and instead recommend a careful consideration of the diverse role that dietary variables play in these associations. In addition, to evaluate the independent role of diet on sleep-cardiometabolic health associations, crude study estimates should be shown first, followed by sequential adjustment for potential confounders and then dietary variables. Finally, the use of advanced statistical techniques, particularly formal mediation methods and the consideration of time-varying confounding, could help to shed light on the directionality of sleep and diet associations and their impact on cardiometabolic health. Ultimately, a more careful consideration of diet in the sleep-cardiometabolic health pathway is instrumental to the elucidation of pathways linking modifiable behaviors with cardiometabolic health.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Erica C. Jansen, Galit Levi Dunietz, Maria-Efstratia Tsimpanouli, Heidi M. Guyer, Carol Shannon, Shelley D. Hershner, Louise M. O’Brien, and Ana Baylin declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
REFERENCES
- 1.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech 2009; 2:231–7. doi: 10.1242/dmm.001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mccullough AJ. Epidemiology of the metabolic syndrome in the USA. J. Dig. Dis 12:333–340 [DOI] [PubMed] [Google Scholar]
- 3.Ranasinghe P, Mathangasinghe Y, Jayawardena R, et al. Prevalence and trends of metabolic syndrome among adults in the Asia-pacific region: A systematic review. BMC Public Health 2017; 17:. doi: 10.1186/s12889-017-4041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin D, Kongpakpaisarn K, Bohra C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007–2014. Int J Cardiol 2018; 259:216–219. doi: 10.1016/j.ijcard.2018.01.139 [DOI] [PubMed] [Google Scholar]
- 5.Rimm EB, Appel LJ, Chiuve SE, et al. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2018; CIR.0000000000000574. doi: 10.1161/CIR.0000000000000574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen J, Wilmot KA, Ghasemzadeh N, et al. Mediterranean Dietary Patterns and Cardiovascular Health. Annu. Rev. Nutr 35:425–449 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO) & Food and Agriculture Organization of the United nations (FAO). Diet, nutrition and the prevention of chronic diseases
- 8.Shukla C, Basheer R. Metabolic signals in sleep regulation: Recent insights. Nat. Sci. Sleep 8:9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xi B, He D, Zhang M, et al. Short sleep duration predicts risk of metabolic syndrome: A systematic review and meta-analysis. Sleep Med Rev 2014; 18:293–297. doi: 10.1016/j.smrv.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 10.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 11:163–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart CN, Carskadon MA, Considine R V, et al. Changes in children’s sleep duration on food intake, weight, and leptin. Pediatrics 2013; 132:e1473–80. doi: 10.1542/peds.2013-1274 [DOI] [PubMed] [Google Scholar]
- 12.Mullins EN, Miller AL, Cherian SS, et al. Acute sleep restriction increases dietary intake in preschool-age children. J Sleep Res 2017; 26:48–54. doi: 10.1111/jsr.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howatson G, Bell PG, Tallent J, et al. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur J Nutr 2012; 51:909–916. doi: 10.1007/s00394-011-0263-7 [DOI] [PubMed] [Google Scholar]
- 14.Peuhkuri K, Sihvola N, Korpela R. Dietary factors and fluctuating levels of melatonin. Food Nutr Res 2012; 56:1–9. doi: 10.3402/fnr.v56i0.17252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141:846–850. doi: 10.7326/00034819-141-11-200412070-00008 [DOI] [PubMed] [Google Scholar]
- 16.Afaghi A, O’Connor H, Chow CM. High-glycemic-index carbohydrate meals shorten sleep onset. Am J Clin Nutr 2007; 85:426–430. doi: 10.1093/ajcn/85.2.426 [DOI] [PubMed] [Google Scholar]
- 17. •.Cespedes EM, Hu FB, Redline S, et al. Chronic insufficient sleep and diet quality: Contributors to childhood obesity. Obes (Silver Spring) 2016; 24:184–190. doi: 10.1002/oby.21196. This was a longitudinal study of US children with parent-reported sleep measures from age 6 months through 7 years. Investigators found that chronic insufficient sleep was associated with higher BMI z scores during mid-childhood, and they evaluated diet quality as a potential mediator and potential effect modifier, with partial evidence for both. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schisterman EF, Cole SR, Platf RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009; 20:488–495. doi: 10.1097/EDE.0b013e3181a819a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant MJ, Booth A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Info. Libr. J 26:91–108 [DOI] [PubMed] [Google Scholar]
- 20.Baird J, Hill CM, Harvey NC, et al. Duration of sleep at 3 years of age is associated with fat and fat-free mass at 4 years of age: the Southampton Women’s Survey. J Sleep Res 2016; 25:412–418. doi: 10.1111/jsr.12389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ••.de Souza MC, Eisenmann JC, e Santos D V, et al. Modeling the dynamics of BMI changes during adolescence. The Oporto Growth, Health and Performance Study. Int J Obes 2015; 39:1063–1069. doi: 10.1038/ijo.2015.60. Adolescents age 10–18 from the longitudinal Portuguese study Oporto Growth, Health and Performance Study were assessed over three years to determine changes in BMI during puberty. Weeknight sleep duration was not associated with BMI trajectories in fully adjusted models that included daily fruit and vegetable intake as dietary measures. [DOI] [PubMed] [Google Scholar]
- 22.Lytle LA, Murray DM, Laska MN, et al. Examining the longitudinal relationship between change in sleep and obesity risk in adolescents. Heal Educ Behav 2013; 40:362–370. doi: 10.1177/1090198112451446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shang L, O’Loughlin J, Tremblay A, Gray-Donald K. The association between food patterns and adiposity among Canadian children at risk of overweight. Appl Physiol Nutr Metab 2014; 39:195–201. doi: 10.1139/apnm-2012-0392 [DOI] [PubMed] [Google Scholar]
- 24.Fairley L, Santorelli G, Lawlor DA, et al. The relationship between early life modifiable risk factors for childhood obesity, ethnicity and body mass index at age 3 years: Findings from the Born in Bradford birth cohort study. BMC Obes 2015; 2:. doi: 10.1186/s40608-015-0037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. •.Byrne DW, Rolando LA, Aliyu MH, et al. Modifiable Healthy Lifestyle Behaviors: 10-Year Health Outcomes From a Health Promotion Program. Am J Prev Med 2016; 51:1027–1037. doi: 10.1016/j.amepre.2016.09.012. Frequency of sleeping 7–8 hours per night and dietary intake was assessed over time via self-report among university employees. Always sleeping 7–8 hours per night was associated with lower risk of cardiometabolic outcomes than never sleeping 7–8 hours per night after accounting for poor diet and additional covariates. [DOI] [PubMed] [Google Scholar]
- 26.Sayon-Orea C, Bes-Rastrollo M, Carlos S, et al. Association between sleeping hours and siesta and the risk of obesity: the SUN Mediterranean Cohort. Obes Facts 2013; 6:337–347. doi: 10.1159/000354746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, Verschuren WM. Sufficient sleep duration contributes to lower cardiovascular disease risk in addition to four traditional lifestyle factors: the MORGEN study. Eur J Prev Cardiol 2014; 21:1367–1375. doi: 10.1177/2047487313493057 [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Sato Y, Yamaguchi N. Lifestyle factors as predictors of general cardiovascular disease: use for early self-screening. Asia Pac J Public Heal 2014; 26:414–424. doi: 10.1177/1010539511423067 [DOI] [PubMed] [Google Scholar]
- 29.Restall A, Taylor RS, Thompson JM, et al. Risk factors for excessive gestational weight gain in a healthy, nulliparous cohort. J Obes 2014; 2014:148391. doi: 10.1155/2014/148391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int J Obes 2015; 39:39–44. doi: 10.1038/ijo.2014.157 [DOI] [PubMed] [Google Scholar]
- 31.Cao M, Zhu Y, He B, et al. Association between sleep duration and obesity is age- and gender-dependent in Chinese urban children aged 6–18 years: a cross-sectional study. BMC Public Health 2015; 15:1029. doi: 10.1186/s12889-015-2359-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrillo-Larco RM, Bernabe-Ortiz A, Miranda JJ. Short sleep duration and childhood obesity: cross-sectional analysis in Peru and patterns in four developing countries. PLoS One 2014; 9:e112433. doi: 10.1371/journal.pone.0112433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Aniello R, Troisi J, D’Amico O, et al. Emerging pathomechanisms involved in obesity. J Pediatr Gastroenterol Nutr 2015; 60:113–119. doi: 10.1097/mpg.0000000000000559 [DOI] [PubMed] [Google Scholar]
- 34.Skidmore PM, Howe AS, Polak MA, et al. Sleep duration and adiposity in older adolescents from Otago, New Zealand: relationships differ between boys and girls and are independent of food choice. Nutr J 2013; 12:128. doi: 10.1186/1475-2891-12-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dev DA, McBride BA, Fiese BH, et al. Risk factors for overweight/obesity in preschool children: an ecological approach. Child Obes 2013; 9:399–408. doi: 10.1089/chi.2012.0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katzmarzyk PT, Barreira T V, Broyles ST, et al. Relationship between lifestyle behaviors and obesity in children ages 9–11: Results from a 12-country study. Obes (Silver Spring) 2015; 23:1696–1702. doi: 10.1002/oby.21152 [DOI] [PubMed] [Google Scholar]
- 37.Roman-Vinas B, Chaput JP, Katzmarzyk PT, et al. Proportion of children meeting recommendations for 24-hour movement guidelines and associations with adiposity in a 12-country study. Int J Behav Nutr Phys Act 2016; 13:123. doi: 10.1186/s12966-016-0449-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkie HJ, Standage M, Gillison FB, et al. Multiple lifestyle behaviours and overweight and obesity among children aged 9–11 years: results from the UK site of the International Study of Childhood Obesity, Lifestyle and the Environment. BMJ Open 2016; 6:e010677. doi: 10.1136/bmjopen-2015-010677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang T, Cai L, Ma L, et al. The prevalence of obesity and influence of early life and behavioral factors on obesity in Chinese children in Guangzhou. BMC Public Health 2016; 16:954. doi: 10.1186/s12889-016-3599-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arora T, Hosseini-Araghi M, Bishop J, et al. The complexity of obesity in U.K. adolescents: relationships with quantity and type of technology, sleep duration and quality, academic performance and aspiration. Pediatr Obes 2013; 8:358–366. doi: 10.1111/j.2047-6310.2012.00119.x [DOI] [PubMed] [Google Scholar]
- 41.Labree W, van de Mheen D, Rutten F, et al. Differences in Overweight and Obesity among Children from Migrant and Native Origin: The Role of Physical Activity, Dietary Intake, and Sleep Duration. PLoS One 2015; 10:e0123672. doi: 10.1371/journal.pone.0123672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santiago S, Zazpe I, Marti A, et al. Gender differences in lifestyle determinants of overweight prevalence in a sample of Southern European children. Obes Res Clin Pr 2013; 7:e391–400. doi: 10.1016/j.orcp.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 43.Pileggi C, Lotito F, Bianco A, et al. Relationship between Chronic Short Sleep Duration and Childhood Body Mass Index: A School-Based Cross-Sectional Study. PLoS One 2013; 8:e66680. doi: 10.1371/journal.pone.0066680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pengpid S, Peltzer K. Prevalence of overweight and underweight and its associated factors among male and female university students in Thailand. Homo 2015; 66:176–186. doi: 10.1016/j.jchb.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 45.Jayawardana NW, Jayalath WA, Madhujith WM, et al. Lifestyle factors associated with obesity in a cohort of males in the central province of Sri Lanka: a cross-sectional descriptive study. BMC Public Health 2017; 17:27. doi: 10.1186/s12889-016-3963-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan LX, Chen XR, Chen B, et al. Gender-specific Association of Sleep Duration with Body Mass Index, Waist Circumference, and Body Fat in Chinese Adults. Biomed Env Sci 2017; 30:157–169. doi: 10.3967/bes2017.023 [DOI] [PubMed] [Google Scholar]
- 47.Brocato J, Wu F, Chen Y, et al. Association between sleeping hours and cardiometabolic risk factors for metabolic syndrome in a Saudi Arabian population. BMJ Open 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Japas C, Knutsen S, Dehom S, et al. Body mass index gain between ages 20 and 40 years and lifestyle characteristics of men at ages 40–60 years: the Adventist Health Study-2. Obes Res Clin Pr 2014; 8:e549–57. doi: 10.1016/j.orcp.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grandner MA, Schopfer EA, Sands-Lincoln M, et al. Relationship between sleep duration and body mass index depends on age. Obes (Silver Spring) 2015; 23:2491–2498. doi: 10.1002/oby.21247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buman MP, Winkler EA, Kurka JM, et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. Am J Epidemiol 2014; 179:323–334. doi: 10.1093/aje/kwt292 [DOI] [PubMed] [Google Scholar]
- 51.Roos V, Elmstahl S, Ingelsson E, et al. Alterations in Multiple Lifestyle Factors in Subjects with the Metabolic Syndrome Independently of Obesity. Metab Syndr Relat Disord 2017; 15:118–123. doi: 10.1089/met.2016.0120 [DOI] [PubMed] [Google Scholar]
- 52.Yu S, Guo X, Yang H, et al. An update on the prevalence of metabolic syndrome and its associated factors in rural northeast China. BMC Public Health 2014; 14:877. doi: 10.1186/1471-2458-14-877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parvaneh K, Poh BK, Hajifaraji M, Ismail MN. Sleep deprivation is related to obesity and low intake of energy and carbohydrates among working Iranian adults: a cross sectional study. Asia Pac J Clin Nutr 2014; 23:84–90. doi: 10.6133/apjcn.2014.23.1.02 [DOI] [PubMed] [Google Scholar]
- 54.Georgousopoulou EN, D’Cunha NM, Mellor DD, et al. The Association between Sleeping Time and Metabolic Syndrome Features, among Older Adults Living in Mediterranean Region: The MEDIS Study. Metab Syndr Relat Disord 2018; 16:. doi: 10.1089/met.2017.0113 [Google Scholar]
- 55.Georgousopoulou EN, Naumovski N, Mellor DD, et al. Association between siesta (daytime sleep), dietary patterns and the presence of metabolic syndrome in elderly living in Mediterranean area (MEDIS study): The moderating effect of gender. J Nutr Heal Aging 2017; 21:1118–1124. doi: 10.1007/s12603-016-0865-0 [DOI] [PubMed] [Google Scholar]
- 56.Min H, Um YJ, Jang BS, et al. Association between Sleep Duration and Measurable Cardiometabolic Risk Factors in Healthy Korean Women: The Fourth and Fifth Korean National Health and Nutrition Examination Surveys (KNHANES IV and V). Int J Endocrinol 2016; 2016:. doi: 10.1155/2016/3784210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kjartansdottir I, Arngrimsson SA, Bjarnason R, Olafsdottir AS. Cross-sectional study of randomly selected 18-year-old students showed that body mass index was only associated with sleep duration in girls. Acta Paediatr Int J Paediatr 2018; 107:1070–1076. doi: 10.1111/apa.14238 [DOI] [PubMed] [Google Scholar]
- 58.MacWana JI, Mehta KG, Baxi RK. Predictors of overweight and obesity among school going adolescents of Vadodara city in Western India. Int J Adolesc Med Health 2017; 29:. doi: 10.1515/ijamh-2015-0078 [DOI] [PubMed] [Google Scholar]
- 59.Kelishadi R, Heidari Z, Kazemi I, et al. A hierarchical Bayesian tri-variate analysis on factors associated with anthropometric measures in a large sample of children and adolescents: The CASPIAN-IV study. J Pediatr Endocrinol Metab 2018; 31:443–449. doi: 10.1515/jpem-2017-0213 [DOI] [PubMed] [Google Scholar]
- 60.Chaput JP, Leduc G, Boyer C, et al. Objectively measured physical activity, sedentary time and sleep duration: Independent and combined associations with adiposity in canadian childre. Nutr Diabetes 2014; 4:. doi: 10.1038/nutd.2014.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magriplis E, Farajian P, Panagiotakos DB, et al. The relationship between behavioral factors, weight status and a dietary pattern in primary school aged children: The GRECO study. Clin Nutr doi: 10.1016/j.clnu.2018.01.015 [DOI] [PubMed] [Google Scholar]
- 62.Hunsberger M, Mehlig K, Bornhorst C, et al. Dietary Carbohydrate and Nocturnal Sleep Duration in Relation to Children’s BMI: Findings from the IDEFICS Study in Eight European Countries. Nutrients 2015; 7:10223–10236. doi: 10.3390/nu7125529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wijnhoven TM, van Raaij JM, Yngve A, et al. WHO European Childhood Obesity Surveillance Initiative: health-risk behaviours on nutrition and physical activity in 6–9-year-old schoolchildren. Public Heal Nutr 2015; 18:3108–3124. doi: 10.1017/s1368980015001937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. •.Peach H, Gaultney JF, Reeve CL. Sleep characteristics, body mass index, and risk for hypertension in young adolescents. J Youth Adolesc 2015; 44:271–284. doi: 10.1007/s10964-014-0149-0. Among US sixth-graders, parent-reported sleep duration and daytime sleepiness was associated with BMI among boys whereas only daytime sleepiness was associated with BMI among girls. Daytime sleepiness had a direct effect on hypertension in boys and an indirect effect (through BMI) on hypertension in girls. Associations were independent of self-reported consumption of unhealthy foods such as soda, hotdogs, French fries and cookies. [DOI] [PubMed] [Google Scholar]
- 65.Rangan A, Zheng M, Olsen NJ, et al. Shorter sleep duration is associated with higher energy intake and an increase in BMI z-score in young children predisposed to overweight. Int J Obes (Lond). doi: 10.1038/ijo.2017.216 [DOI] [PubMed] [Google Scholar]
- 66.Asarnow LD, McGlinchey E, Harvey AG. Evidence for a Possible Link between Bedtime and Change in Body Mass Index. Sleep 2015; 38:1523–1527. doi: 10.5665/sleep.5038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. ••.He F, Bixler EO, Liao J, et al. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med 2015; 16:1489–94. doi: 10.1016/j.sleep.2015.07.028. This study of US adolescents found that actigraphyassessed sleep duration variability was associated with higher abdominal fat measured by dual X-ray absorptiometry (DXA). Further, they found evidence of partial mediation by total energy intake, measured by a food frequency questionnaire. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McDonald L, Wardle J, Llewellyn CH, Fisher A. Nighttime sleep duration and hedonic eating in childhood. Int J Obes 2015; 39:1463–1466. doi: 10.1038/ijo.2015.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cespedes EM, Rifas-Shiman SL, Redline S, et al. Longitudinal associations of sleep curtailment with metabolic risk in mid-childhood. Obes (Silver Spring) 2014; 22:2586–2592. doi: 10.1002/oby.20894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yeh SS, Brown RF. Disordered eating partly mediates the relationship between poor sleep quality and high body mass index. Eat Behav 2014; 15:291–297. doi: 10.1016/j.eatbeh.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 71. •.Kanagasabai T, Ardern CI. Contribution of Inflammation, Oxidative Stress, and Antioxidants to the Relationship between Sleep Duration and Cardiometabolic Health. Sleep 2015; 38:1905–1912. doi: 10.5665/sleep.5238. The association between vitamins and carotenoids and metabolic syndrome was assessed in a nationally-representative study of US adults. Carotenoids, vitamins C and D were modest mediators of the relationship between sleep duration and the metabolic syndrome among women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mesas AE, Guallar-Castillon P, Lopez-Garcia E, et al. Sleep quality and the metabolic syndrome: the role of sleep duration and lifestyle. Diabetes Metab Res Rev 2014; 30:222–231. doi: 10.1002/dmrr.2480 [DOI] [PubMed] [Google Scholar]
- 73.Chaput JP, Bouchard C, Tremblay A. Change in sleep duration and visceral fat accumulation over 6 years in adults. Obes (Silver Spring) 2014; 22:E9–12. doi: 10.1002/oby.20701 [DOI] [PubMed] [Google Scholar]
- 74.Wirth MD, Hébert JR, Hand GA, et al. Association between actigraphic sleep metrics and body composition. Ann Epidemiol 2015; 25:773–778. doi: 10.1016/j.annepidem.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. •.Timmermans M, Mackenbach JD, Charreire H, et al. Exploring the mediating role of energy balance-related behaviours in the association between sleep duration and obesity in European adults. The SPOTLIGHT project. Prev Med (Baltim) 2017; 100:25–32. doi: 10.1016/j.ypmed.2017.03.021. Mediation of the association between longer sleeping (≥9 hours) and obesity by dietary intake behaviors was assessed in urban-dwelling European adults. Longer sleep duration was associated with a lower likelihood of obesity than short sleep duration (<7 hours), but there was no evidence of mediation by diet. [DOI] [PubMed] [Google Scholar]
- 76. ••.Hjorth MF, Sjodin A, Dalskov SM, et al. Sleep duration modifies effects of free ad libitum school meals on adiposity and blood pressure. Appl Physiol Nutr Metab 2016; 41:33–40. doi: 10.1139/apnm-2015-0319. Children from Denmark were enrolled in a crossover trial, in which they were fed a New Nordic Diet (NND) lunch for 1 month and had usual packed lunches for one month. Among children with habitual short sleep (assessed with actigraphy), the NND resulted in fat mass gain compared usual lunch consumption. [DOI] [PubMed] [Google Scholar]
- 77.Doo H, Chun H, Doo M. Associations of daily sleep duration and dietary macronutrient consumption with obesity and dyslipidemia in Koreans: A cross-sectional study. Med 2016; 95:e5360. doi: 10.1097/md.0000000000005360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doo M, Kim Y. The Consumption of Dietary Antioxidant Vitamins Modifies the Risk of Obesity among Korean Men with Short Sleep Duration. Nutrients 2017; 9:E780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim NH, Shin DH, Kim HT, et al. Associations between metabolic syndrome and inadequate sleep duration and skipping breakfast. Korean J Fam Med 2015; 36:273–277. doi: 10.4082/kjfm.2015.36.6.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chaput J-P. Sleep patterns, diet quality and energy balance. Physiol Behav 2014; 134:86–91. doi: 10.1016/j.physbeh.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 81.Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. [DOI] [PubMed] [Google Scholar]
- 82.Espitia-Bautista E, Velasco-Ramos M, Osnaya-Ramírez I, et al. Social jet-lag potentiates obesity and metabolic syndrome when combined with cafeteria diet in rats. Metabolism. doi: 10.1016/j.metabol.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 83.National Cholesterol Education Program Expert Panel, National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421. doi: 10.1161/01.CIR.0000048067.86569 [PubMed] [Google Scholar]