Abstract
The excitotoxicity of glutamate plays an important role in the progression of various neurological disorders via participating in inflammation and neuronal damage. In this study, we identified the role of excessive glutamate stimulation in the modulation of angiotensin-converting enzyme type 2 (ACE2), a critical component in the compensatory axis of the renin–angiotensin system (RAS). In primary cultured cortical neurons, high concentration of glutamate (100 µM) significantly reduced the enzymatic activity of ACE2. The elevated activity of ADAM17, a member of the ‘A Disintegrin And Metalloprotease’ (ADAM) family, was found to contribute to this glutamate-induced ACE2 down-regulation. The decrease of ACE2 activity could be prevented by pre-treatment with antagonists targeting ionotropic glutamate receptors. In addition, the glutamate-induced decrease in ACE2 activity was significantly attenuated when the neurons were co-treated with MitoTEMPOL or blockers that target oxidative stress-mediated signaling pathway. In summary, our study reveals a strong relationship between excessive glutamate stimulation and ADAM17-mediated impairment in ACE2 activity, suggesting a possible cross-talk between glutamate-induced excitotoxicity and dysregulated RAS.
Keywords: Glutamate, Excitotoxicity, ACE2, ADAM17
Introduction
Within the renin–angiotensin system (RAS), angiotensin-converting enzyme type 2 (ACE2) has been established as a major component of the compensatory axis for its role in the metabolism of angiotensin II (Ang II). ACE2 transforms Ang II into Ang-(1–7), thus turning the vasoconstrictor into a vasodilator peptide, promoting nitric oxide (NO) release, reducing sympathetic outflow, increasing baroreflex sensitivity, and ultimately reducing high blood pressure (BP). In addition to cardiovascular diseases, the brain RAS also participates in the pathological process of various neurological diseases, including stroke, traumatic brain injury, and neurodegenerative diseases, such as Alzheimer’s disease, as evidenced by both the protective and therapeutic effects of RAS blockers (Villapol et al. 2015; Kusaka et al. 2004; Ongali et al. 2014). The ACE2/Ang-(1–7) pathway also exhibits neuroprotective effects by reducing oxidative stress and suppressing inflammation. For example, overexpression of ACE2 exhibited a protective role in brain ischemic damage by decreasing the detrimental effects of Ang II-induced reactive oxygen species (ROS) production (Zheng et al. 2014). And the anti-inflammatory action of ACE2/Ang-(1–7), including the inhibition of microglial activation and inflammatory cytokine expression (Liu et al. 2016), was found to contribute to the attenuation in chronic heart failure-induced cognitive impairment (Hay et al. 2017). Despite those protective actions, down-regulated activity of ACE2-Ang-(1–7)-Mas axis in the brain is observed in patients or experimental models of Alzheimer’s disease and ischemic stroke (Jiang et al. 2013a; Kehoe et al. 2016), suggesting that impaired ACE2/Ang-(1–7) compensatory activity might play a role in the neurodegeneration and its related process.
During brain injury and inflammation, the neurons can be damaged by excessive stimulation of glutamate, which is released from the activated glial cells surrounding those neurons. The pathologically high concentration of glutamate can cause excitotoxicity through massive Ca2+ influx, which will damage the function of mitochondria, increase oxidative stress, and go on to damage cell structures and DNA through various downstream signals, including Ca2+/calmodulin-dependent protein kinase II, protein kinase C (PKC), nitric oxide synthase, calpain, and mitogen-activated protein kinase (MAPK) (Bal-Price and Brown 2001; Minger et al. 1998; Chaparro-Huerta et al. 2005). It has been shown that Ang II can inhibit the function of glutamate transporter (GLT1) on astrocytes, which is the major source of glutamate release, increasing the extracellular glutamate levels and extra-synaptic glutamate excitatory tone (Stern et al. 2016). In this case, if the compensatory activity of ACE2 is also impaired by glutamate-induced excitotoxicity, in turn, the inflammatory process and glial activation could be exacerbated via the over-activated RAS, further increasing the level of local glutamate.
In addition to internalization (Deshotels et al. 2014), cell membrane-bound ACE2 can be impaired through ectodomain shedding. ADAM17, a member of the ‘A Disintegrin And Metalloprotease’ (ADAM) family, is known to cleave a variety of membrane-anchored proteins, including ACE2 (Xu et al. 2016; Lambert et al. 2005). We previously demonstrated the opposite relationship between ADAM17 and the compensatory activity of ACE2 in the mouse brain, especially in neurons (Xu et al. 2017; Xia et al. 2013). Upon brain RAS over-activation, ADAM17 is up-regulated through increased ROS and activated MAPK pathway, leading to an increased ectodomain shedding of ACE2.
Glutamate contributes to neuronal injury not only through the ionotropic glutamate receptors (iGluR)-mediated excitotoxic pathway, but also via oxidative stress, including the ROS/MAPK pathway (Bossy-Wetzel et al. 2004; Molz et al. 2008; Stanciu et al. 2000). Several pro-inflammatory cytokines can be up-regulated during glutamate-induced excitotoxicity. Among them, tumor necrosis factor α (TNFα) production in hippocampus is significantly increased by glutamate in neonatal rats, but inhibited by a p38 MAPK blocker, which has no effect on the glutamate-induced increase in TNFα gene expression (Chaparro-Huerta et al. 2005), indicating that the shedding activity of ADAM17 (also known as TNFα converting enzyme) could be increased during glutamate stimulation. In addition, stress-induced increase in ADAM17 activity in the cortex of adult male rats can be blocked by treatment with MK-801, an antagonist of the N-methyl-d-aspartate (NMDA) subtype of glutamate receptor (Madrigal et al. 2002). Altogether, these studies suggest that the shedding activity of ADAM17 can be elevated during excessive glutamate stimulation. This increase in ADAM17 activity might compromise the compensatory effects of ACE2/Ang-(1–7) mediated signaling, in favor of inflammatory progression and glial activation, which can cause further neuronal damage through this vicious cycle.
In this study, we investigated the relationship between excessive glutamate stimulation and ADAM17-mediated impairment of ACE2 activity. In primary cultured cortical neurons, high dose of glutamate treatment significantly decreased the enzymatic activity of ACE2, while blockade of iGluR with specific antagonists restored the ACE2 activity completely. Via specific ADAM17 inhibitor, it was demonstrated later that glutamate-induced ADAM17 activation contributed to this reduction in ACE2 activity. Although blocking iGluR had limited effect on ADAM17 activation, signals like mitochondrial ROS, p38 MAPK, and inducible nitric oxide synthase (iNOS), which are known to be involved in glutamate-induced neuronal damage, seemed to contribute to the ADAM17-mediated ACE2 shedding during excessive glutamate stimulation.
Materials and Methods
All procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Louisiana State University Health Sciences Center Animal Care and Use Committee (#3418). Dams (C57BL/6j) were housed under standard 12-h light/dark cycle with ad libitum access to food and water.
Cortical Primary Neurons Culture
Cortical primary neurons were isolated from neonatal or 1-day-old wild type or pups lacking AT1a receptors (AT1R) specifically on neurons (AT1N) (Xu et al. 2017). Brains were dissected immediately and the cortex was collected in ice-cold Hank’s balanced salt solution (HBSS) (Gibco 14175-079). Following brief dissociation, the cortices were washed and dispersed in HBSS containing 1% trypsin (Sigma-Aldrich T1426) and 1.5 kU/mL DNaseI (Sigma-Aldrich D5025), and then digested for 10 min at 37 °C. The digested samples were then dissociated by gentle pipetting in HBSS containing 1.5 KU/mL DNase I. After brief centrifugation, the cell pellets were re-suspended in complete Neurobasal culture medium, supplemented with 2% B27 and 0.5 mM GlutaMax (Gibco), and plated at a density of 106 cells/ml onto poly-L-lysine-coated plates. Cytosine arabinofuranoside (Ara-C, 2 µM, Sigma-Aldrich C1768) was added to the cultures 48 h after plating to arrest the growth of non-neuronal cells. Cultured cortical neurons were validated via immunofluorescence labeling with MAP2 (microtubule-associated protein 2), as shown in Fig. 1a. Cortical primary neurons were cultured for at least 10 days and then treated with specific agonist or blockers. All blockers, except for MitoTEMPOL and 1400W, were given 1 h before l-glutamic acid treatment (100 µM): NBQX (AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, receptor antagonist, 100 µM), D-AP5 (NMDA receptor, 50 µM), and DL-AP4 (non-selective ionotropic receptors antagonist, 50 µM), SB203580 (p38 MAPK blocker, 10 µM). MitoTEMPOL (mitochondria-targeted antioxidant, 10 µM) and 1400W (iNOS inhibitor, 5 µM) were given 30 min before l-glutamic acid treatment.
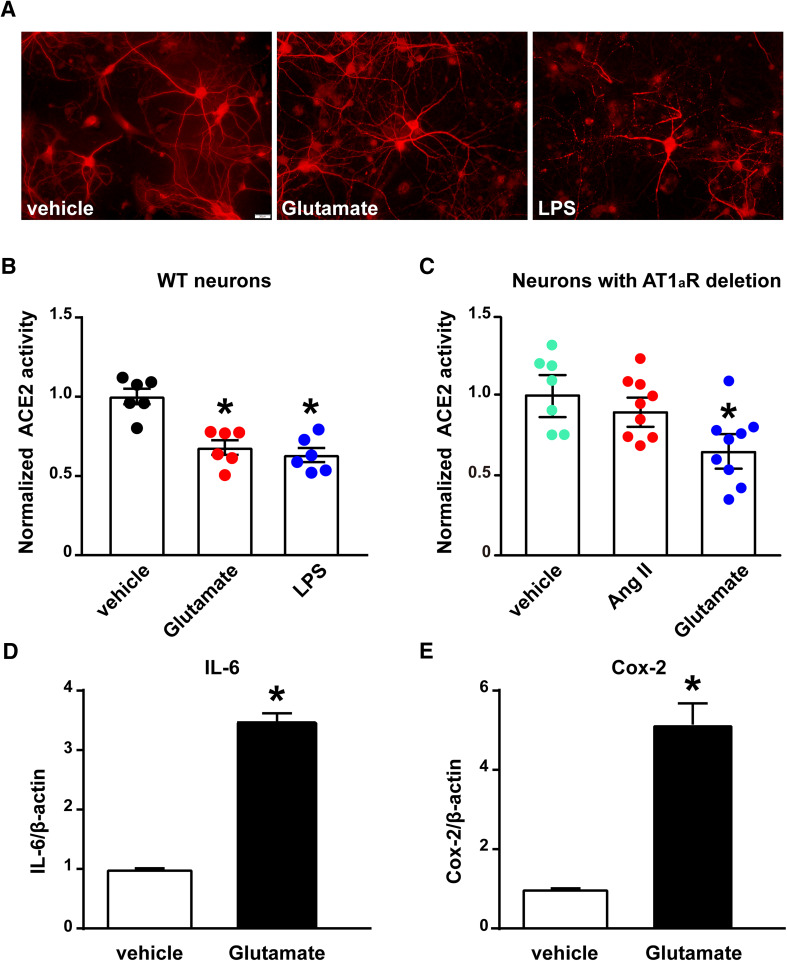
Fig. 1.
The compensatory activity of ACE2 is impaired by glutamate and inflammatory stimuli in cultured cortical neurons. a Immunofluorescence labeling of primary cultured cortical neurons for visualization of MAP2 (red). The scale bar represents 20 µm. b ACE2 activity assay was performed in primary cultured cortical neurons isolated from wild-type neonates. The cultured neurons were treated with l-glutamic acid (glutamate, 100 µM) or lipopolysaccharide (LPS, 100 ng/ml) for 18 h (n = 3 independent cultures/group). c ACE2 activity assay was performed in cultured cortical neurons isolated from neonates with neuronal AT1aR deficiency. The cultured neurons were treated with glutamate (100 µM) or Angiotensin II (Ang II, 300 nM) for 18 h (n = 4 independent cultures/group). Statistical significance: One-way ANOVA: *P < 0.05 versus vehicle. d, e Glutamate significantly upregulates the gene expression of pro-inflammatory cytokines in cultured cortical neurons: qPCR was performed to assess the mRNA levels of IL-6 (d), and Cox-2 (e) in neurons treated with glutamate (100 µM) or vehicle. Statistical significance: Student t test: *P < 0.05 versus vehicle
ACE2 and ADAM17 Activity Assay
Primary cultured neurons collected from drug- or vehicle-treated groups were processed for ACE2 and ADAM17 activities, as reported previously (Pedersen et al. 2015, 2011). Data are normalized according to the activity of vehicle-treated group in each experiment.
RNA Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
Primary cultured neurons were extracted with TRIzol, phase separated with 1-bromo-3-chloropropane (BCP, MRC BP151) and spun at 13,000×g for 15 min at 4 °C. For primary neurons, the supernatant was added to an equal volume of ethanol and then processed using an RNA Mini-Prep kit from ZYMO Research (R2070). Real-time RT-PCR amplification reactions were performed with Power SYBR Green RNA-to-CT one-step kit (Thermo Fisher 4389986). Data were normalized to β-actin expression by the ΔΔCT comparative method and expressed as fold change compared to sham.
Results
To test the hypothesis that stimuli (e.g., glutamate and inflammation), other than Ang II, can reduce the compensatory activity of ACE2 in the central nervous system, cultured primary cortical neurons were treated with l-glutamic acid (glutamate, 100 µM) and lipopolysaccharide (LPS, 100 ng/ml), respectively. Glutamate significantly reduced the enzymatic activity of ACE2 (Fig. 1b) in cultured cortical neurons, after 18 h of treatment. Similarly, an inflammatory stimulus, such as LPS, also compromised the activity of ACE2. Since the treatment with glutamate can also promote neuronal inflammation, evidenced by up-regulated expression of interleukin 6 (IL-6) and cyclooxygenase-2 (COX-2, Fig. 1d, e) (Sriramula et al. 2015), in the following experiments we investigated the contribution of glutamate to reduced ACE2 activity in neurons.
We previously found that neuronal AT1R plays a pivotal role in DOCA-salt-induced reduction of ACE2 compensatory activity in the mouse hypothalamus (Xu et al. 2017). To further investigate the involvement of neuronal AT1R, we treated cortical neurons, isolated from mice lacking AT1R selectively from neurons, with glutamate. As shown in Fig. 1c, the enzymatic activity of ACE2 was still significantly decreased in the glutamate-treated neurons, compared to vehicle-treated controls, while the effect of Ang II was completely abolished. This result indicates that glutamate can decrease ACE2 activity through a neuronal AT1R-independent mechanism.
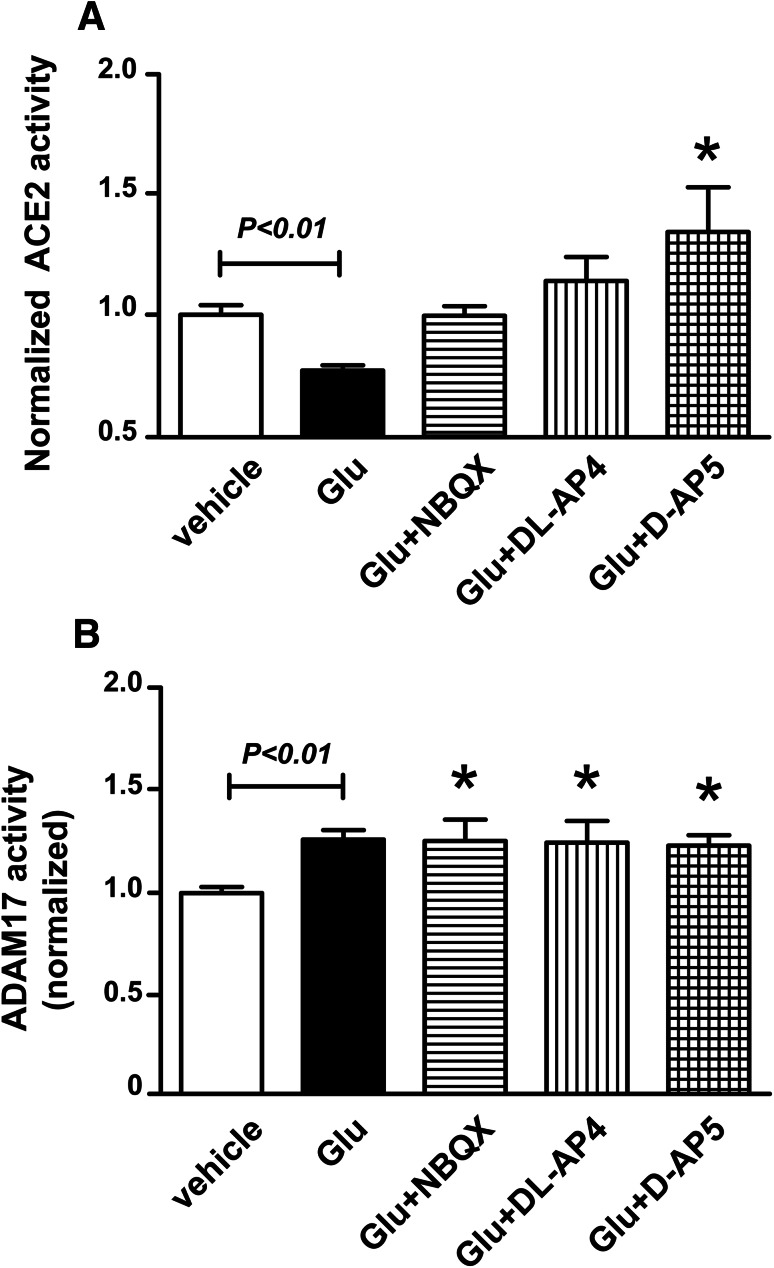
To determine whether ionotropic glutamatergic receptors are involved in the glutamate-induced impairment of ACE2, the cultured primary neurons were pre-treated with NBQX (an AMPA receptor antagonist), D-AP5 (a NMDA receptor blocker), and DL-AP4 (a non-selective ionotropic receptors antagonist), before neurons exposure to glutamate (100 µM) for 18 h. As previously, glutamate reduced ACE2 activity in cortical neurons and this effect was completely prevented by all three ionotropic antagonists (Fig. 2a). Interestingly, NMDA blockade resulted in significant increase in ACE2 activity above baseline levels, suggesting that this receptor is involved in some intrinsic inhibition of ACE2, while the others appear to be involved in the excitotoxic inhibition of the carboxypeptidase.
Fig. 2.
The glutamate-induced reduction of ACE2 activity, but not the ADAM17 activation, is mediated via the ionotropic glutamate receptors. ACE2 (a) and ADAM17 (b) activity assay were performed in cultured cortical neurons isolated from wild-type neonates. After 1 h of pre-treatment with NBQX (an AMPA receptor antagonist, 100 µM), d-2-amino-5-phosphonopentanoate (d-AP5, a NMDA receptor antagonist, 50 µM), or d,l-2-amino-4-phosphonobutyric acid (dl-AP4, a non-selective ionotropic glutamate receptors antagonist, 50 µM), the cultured neurons were then treated with glutamate (100 µM) for 18 h (n = 6 independent cultures/group). Statistical significance: One-way ANOVA: *P < 0.05 versus vehicle
We previously demonstrated the opposite relationship between ACE2 and ADAM17, a protease responsible for ACE2 ectodomain shedding, in mouse hypothalamus during deoxycorticosterone acetate (DOCA)-salt hypertension (Xia et al. 2013). Here, we hypothesized that glutamate-induced inhibition of ACE2 activity could also be the consequence of ADAM17-mediated shedding. Interestingly, although we did observe a significant increase in ADAM17 activity in glutamate-treated neurons, none of the three ionotropic antagonists, NBQX, D-AP5, or DL-AP4, could block this effect (Fig. 2b).
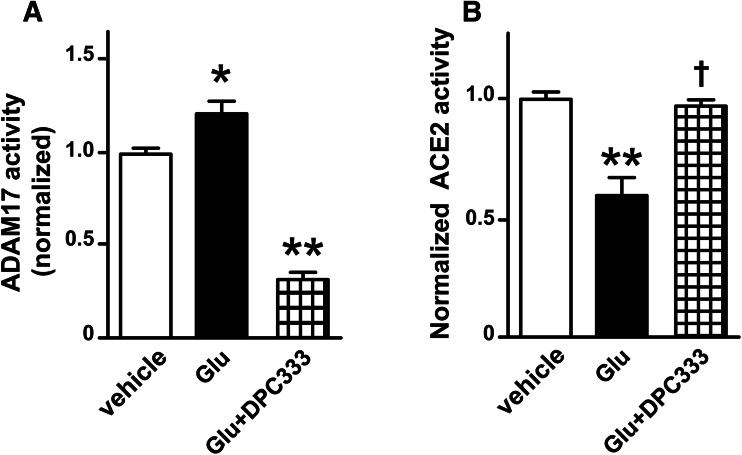
To confirm the involvement of ADAM17 in the glutamate-induced reduction of ACE2 activity, neurons were pre-treated with DPC333, a specific ADAM17 inhibitor (Fig. 3a) (Le Gall et al. 2010). Fig. 3b shows that blockade of ADAM17 fully restored the glutamate-impaired ACE2 activity, implicating the role of this sheddase in the decrease of ACE2 activity in the glutamate-treated neurons.
Fig. 3.
ADAM17-mediated shedding is responsible for the glutamate-induced impairment of ACE2 activity in cortical neurons. ADAM17 (a) and ACE2 (b) activity assay were performed, respectively, in cultured cortical neurons isolated from wild-type neonates. The cultured neurons were treated with glutamate (100 µM) or glutamate with 1 h of pre-treatment with DPC333 (a specific ADAM17 inhibitor, 10 µM) for 18 h (n = 3–6 independent cultures/group). Statistical significance: One-way ANOVA: *P < 0.01 and **P < 0.001 versus vehicle; †P < 0.001 versus glutamate
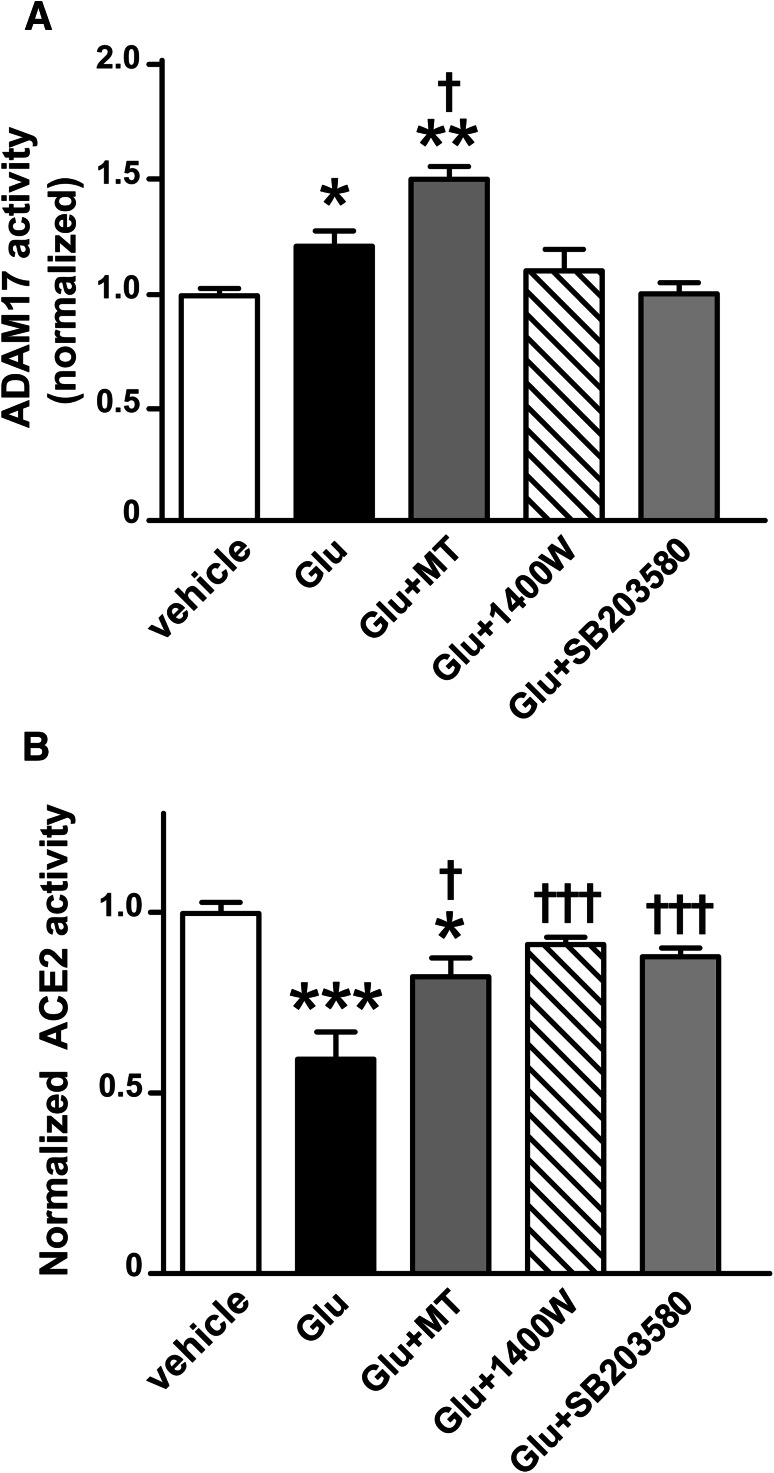
Mitochondrial function is impaired by excitotoxicity upon excessive glutamate stimulation (Pereira and Oliveira 2000). Meanwhile, oxidative stress and the p38 MAPK pathway activation are also found to be critical to ADAM17 activation (Patel et al. 2014; Xu and Derynck 2010; Xu et al. 2017). Accordingly, we further investigated the participation of mitochondrial ROS, iNOS, and p38 MAPK in the following experiments. While both iNOS and p38 MAPK inhibitors blocked the glutamate-induced increase in ADAM17 activity, MitoTEMPOL (MT), a mitochondrial ROS scavenger, potentiated the sheddase activity (Fig. 4a). This is most likely due to the conversion of ROS into H2O2 after restoration of manganese-dependent superoxide dismutase (MnSOD) activity, as H2O2 was previously shown to increase ADAM17 activity in monocytes (Scott et al. 2011). Although MitoTEMPOL failed to prevent ACE2 impairment, it partially restored the ACE2 activity in cortical neurons after glutamate stimulation (Fig. 4b). Unlike MitoTEMPOL, the p38 MAPK blocker and iNOS inhibitor completely prevented the increase in ADAM17 activity (Fig. 4a) and the inhibition of ACE2 activity (Fig. 4b) suggesting that these signaling components are part of the same pathway.
Fig. 4.
Downstream signals of mitochondrial ROS, MAPK, and iNOS are involved in glutamate-induced ADAM17 activation and the reduction of ACE2 activity in cortical neurons. ADAM17 (a) and ACE2 (b) activity assay were performed, respectively, in cultured cortical neurons isolated from wild-type neonates. The cultured neurons were treated with glutamate (100 µM), glutamate plus co-treatment with MitoTEMPOL (MT, a mitochondrial ROS scavenger, 10 µM), or glutamate plus 0.5–1 h of pre-treatment with 1400W (an iNOS inhibitor, 5 µM), SB203580 (a p38MAPK blocker, 10 µM), for 18 h (n = 3–6 independent cultures/group). Statistical significance: One-way ANOVA: *P < 0.05, **P < 0.01 and ***P < 0.001 versus vehicle; †P < 0.05 and †††P < 0.001 versus glutamate
Discussion
The inhibition of ACE2 enzymatic activity has been observed in the central nervous system and peripheral organs, such as heart and kidney, during cardiovascular diseases and diabetes (Cherney et al. 2014; Epelman et al. 2008; Úri et al. 2014). In the brain, through its conversion of Ang II into the vasodilator peptide Ang-(1–7), ACE2 promotes the reduction of oxidative stress and attenuates neuronal apoptosis, in addition to its protective role in the maintenance of blood–brain barrier function (Wu et al. 2015; Jiang et al. 2013b). Therefore, reduced ACE2 activity favors the progression of pathological processes. Our group previously identified the deleterious effects of an over-activated RAS in blunting ACE2 activity (Xia et al. 2013). By activating AT1R, Ang II induces both internalization and ectodomain shedding of ACE2, inhibiting both ACE2 expression and enzymatic activity (Deshotels et al. 2014; Xia et al. 2013). In this study, we demonstrate that an excitotoxic concentration of glutamate can markedly reduce ACE2 activity in cultured primary cortical neurons. Excessive stimulation by glutamate can induce excitotoxicity and damage the nerve cells. The excitotoxicity of glutamate is known to be involved in stroke, traumatic brain injury, and neurodegenerative diseases, such as Alzheimer’s disease, amyotrophic lateral sclerosis, and Parkinson’s disease (Doble 1999). Therefore, loss of ACE2 compensatory activity might also contribute to the development of those neurological disorders or to the progression of their pathological mechanisms, such as inflammation and apoptosis.
We previously discovered that ACE2 is undergoing ectodomain shedding, mediated by ADAM17 and possibly resulting in compromised ACE2 efficiency in transforming Ang II to Ang-(1–7). Interestingly, unlike the previous studies in hypertensive models, the role of glutamate in ACE2 inhibition is independent of neuronal AT1R, suggesting that glutamate signaling might have a direct effect on ACE2. To further identify the mechanism of the glutamate-induced inhibition of ACE2 activity, we used DPC333, a specific ADAM17 inhibitor, and showed that blockade of the sheddase activity completely abolished the reduction of ACE2 activity in glutamate-treated neurons, indicating that excessive glutamate stimulation decreases ACE2 activity though an ADAM17-mediated mechanism. Moreover, our data showing that none of the ionotropic inhibitors was able to reduce the glutamate-mediated increase in ADAM17 activity, pointing to a contribution of the metabotropic receptors. This is in agreement with previous observations by Cho et al. that stimulation of mGluR1/5, the Group I metabotropic glutamate receptors (mGluR), can activate ADAM17, further promoting the shedding of neuronal pentraxin receptor and endosome-dependent removal of AMPA receptors from the synapses (Cho et al. 2008). Stimulation of Group I mGluR leads to activation of a wide variety of signaling pathways. mGluR couple to Gαq/11 proteins, activating phospholipase C and resulting in both diacylglycerol and inositol-1,4,5-triphosphate formation followed by the activation of PKC. It is well known that activation on PKC-mediated signaling can increase ADAM17 enzymatic activity, evidenced by the prominent effect of phorbol myristate acetate (PMA), a potent PKC activator, on ADAM17 activation (Zhang et al. 2000; Kveiborg et al. 2011). Moreover, a study by Horiuchi et al. pointed that the activation of ADAM17 did not rely on the existence of calcium (Ca2+), unlike other member of its family (Horiuchi et al. 2007). This might explain why pre-incubating the neurons with ionotropic antagonists has no effect in diminishing glutamate-induced ADAM17 activity. Interestingly, unlike their role in ADAM17 modulation, those iGluR antagonists successfully prevented the inhibition of ACE2 activity in glutamate-treated neurons. This result seemingly goes against the previous findings on the opposite relationship between ADAM17 and ACE2. A previous study from the group that originally reported ADAM17-mediated ACE2 shedding indicated that the interaction between ACE2 and calmodulin (CaM), a ubiquitous Ca2+ sensing protein, could inhibit the shedding of its ectodomain (Lambert et al. 2008). Furthermore, our group found that this ACE2-CaM interaction was significantly down-regulated in the brain when local Ang II stimulation was increased by DOCA-salt treatment (Xia et al. 2013). Ang II is known to have direct effects on multiple Ca2+ channels and can rapidly increase the level of intracellular Ca2+ (Zimmerman et al. 2005). In the event of a transient rise in [Ca2+]i, the Ca2+ ion will interact with each Ca2+-binding loop on CaM (Chin and Means 2000) and the structural rearrangements in CaM might result in decreased binding with ACE2. In this case, it is conceivable that glutamate activates ADAM17, while ACE2 activity remains intact due to the inadequate intracellular Ca2+ level, considering that the major Ca2+ influx is blocked by the treatment of iGluR antagonists.
The neurotoxicity of glutamate is triggered primarily by a massive Ca2+ influx arising from over-stimulation of the iGluR (Szydlowska and Tymianski 2010). The elevation of intracellular [Ca2+] can primarily cause mitochondrial dysfunction and increase ROS accumulation (Pereira and Oliveira 2000; Almeida et al. 1998), which plays a positive role on ADAM17 via increasing its translocation and activation (Patel et al. 2014; Dreymueller et al. 2012). However, we did not see any significant inhibition but a small elevation in ADAM17 activity when the neurons were co-treated with MitoTEMPOL; this is possibly because MitoTEMPOL restores MnSOD activity, which causes H2O2 accumulation, and H2O2 was previously shown to increase ADAM17 activity in monocytes (Scott et al. 2011). Upon mitochondrial ROS blockade, ACE2 activity was partially restored in the neurons subjected to high dose of glutamate. Again, these results confirm that activation of iGluR through excessive glutamate stimulation does not significantly contribute to ADAM17 activation, but it is critical to the glutamate-induced process of ACE2 shedding.
The excitotoxicity induced by excessive glutamate stimulation contributes to the development of inflammatory neurodegeneration through oxidative stress and/or iNOS-mediated mechanisms (Chang et al. 2008; Ward et al. 2009). It has been shown that chronic NMDA receptor activation increases inflammatory markers, including iNOS expression, in rat frontal cortex (Chang et al. 2008). In addition, the production of iNOS is also found to be downstream of PKC-mediated signaling in various cell systems (McKenna et al. 1995; Ginnan et al. 2006; Salonen et al. 2006). Increased iNOS can promote the release of glutamate from neighboring astrocytes and furtherly worsen the neuro-inflammation (Brown and Bal-Price 2003). Interestingly, iNOS leads to the translocation and activation of ADAM17 through its NO/cGMP/protein kinase G (PKG) signaling in hepatocytes (Chanthaphavong et al. 2012). Here, we show that iNOS-mediated signaling is also involved in the glutamate-induced ACE2 impairment through an increase in ADAM17 shedding activity, supported by an attenuated reduction in ACE2 activity after excessive glutamate stimulation.
Although ADAM17-mediated ACE2 shedding could contribute to the neurotoxicity of glutamate because of the impaired compensatory role of ACE2/Ang-(1–7), blocking ADAM17 activation may not improve neuronal survival. Indeed, it was shown in rat cortical cultures that inhibiting ADAM17 worsens neuronal apoptosis after glutamate exposure (Hurtado et al. 2002). ADAM17 promotes the shedding of multiple substrates, some involved in cell survival while others are pro-inflammatory and pro-apoptotic (Dreymueller et al. 2012). For example, by shedding TNFα from activated glial cells, ADAM17 is pro-inflammatory, but by cleaving its receptor from the plasma membrane, the sheddase disconnects this receptor from its downstream signaling and thus regulate the inflammatory process and help to reduce the neuronal damage induced by TNFα (Marchetti et al. 2004; Tellier et al. 2006)
Although the current study identifies a new stimulus and potential receptors leading to ADAM17-mediated inhibition of ACE2 activity in the brain, further investigation is needed to elucidate the mechanisms leading to ADAM17 substrate selectivity and uncover effective new therapeutic targets for treatment of related neurological diseases.
Acknowledgements
The authors would like to thank Brystol-Myers Squibb for providing the DPC333 compound.
Author Contributions
JX, SS, and EL designed the experiments; experiments and data collection were performed by JX and SS; data analysis was performed by JX and SS; data interpretation was conducted by JX, SS, and EL; and all authors contributed to and approved the final manuscript.
Funding
This work was supported by research grants from the National Institutes of Health (HL093178 to E. Lazartigues and COBRE P30GM106392).
Compliance with Ethical Standards
Conflict of interest
The authors report no conflict of interest.
References
- Almeida A, Heales SJ, Bolanos JP, Medina JM (1998) Glutamate neurotoxicity is associated with nitric oxide-mediated mitochondrial dysfunction and glutathione depletion. Brain Res 790(1–2):209–216 [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Brown GC (2001) Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci 21(17):6480–6491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Talantova MV, Lee WD, Scholzke MN, Harrop A, Mathews E, Gotz T, Han J, Ellisman MH, Perkins GA, Lipton SA (2004) Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron 41(3):351–365 [DOI] [PubMed] [Google Scholar]
- Brown GC, Bal-Price A (2003) Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol 27(3):325–355. 10.1385/MN:27:3:325 [DOI] [PubMed] [Google Scholar]
- Chang YC, Kim HW, Rapoport SI, Rao JS (2008) Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: cross-talk between excitotoxicity and neuroinflammation. Neurochem Res 33(11):2318–2323. 10.1007/s11064-008-9731-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanthaphavong RS, Loughran PA, Lee TY, Scott MJ, Billiar TR (2012) A role for cGMP in inducible nitric-oxide synthase (iNOS)-induced tumor necrosis factor (TNF) alpha-converting enzyme (TACE/ADAM17) activation, translocation, and TNF receptor 1 (TNFR1) shedding in hepatocytes. J Biol Chem 287(43):35887–35898. 10.1074/jbc.M112.365171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro-Huerta V, Rivera-Cervantes MC, Flores-Soto ME, Gomez-Pinedo U, Beas-Zarate C (2005) Proinflammatory cytokines and apoptosis following glutamate-induced excitotoxicity mediated by p38 MAPK in the hippocampus of neonatal rats. J Neuroimmunol 165(1–2):53–62. 10.1016/j.jneuroim.2005.04.025 [DOI] [PubMed] [Google Scholar]
- Cherney DZI, Xiao F, Zimpelmann J, Har RLH, Lai V, Scholey JW, Reich HN, Burns KD (2014) Urinary ACE2 in healthy adults and patients with uncomplicated type 1 diabetes. Can J Physiol Pharmacol 92(8):703–706. 10.1139/cjpp-2014-0065 [DOI] [PubMed] [Google Scholar]
- Chin D, Means AR (2000) Calmodulin: a prototypical calcium sensor. Trends Cell Biol 10(8):322–328 [DOI] [PubMed] [Google Scholar]
- Cho RW, Park JM, Wolff SB, Xu D, Hopf C, Kim JA, Reddy RC, Petralia RS, Perin MS, Linden DJ, Worley PF (2008) mGluR1/5-dependent long-term depression requires the regulated ectodomain cleavage of neuronal pentraxin NPR by TACE. Neuron 57(6):858–871. 10.1016/j.neuron.2008.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM (2014) Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension 64(6):1368–1375. 10.1161/HYPERTENSIONAHA.114.03743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble A (1999) The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther 81(3):163–221 [DOI] [PubMed] [Google Scholar]
- Dreymueller D, Pruessmeyer J, Groth E, Ludwig A (2012) The role of ADAM-mediated shedding in vascular biology. Eur J Cell Biol 91(6–7):472–485. 10.1016/j.ejcb.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Epelman S, Tang WHW, Chen SY, Van Lente F, Francis GS, Sen S (2008) Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin–angiotensin–aldosterone system. J Am Coll Cardiol 52(9):750–754. 10.1016/j.jacc.2008.02.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginnan R, Guikema BJ, Singer HA, Jourd’heuil D (2006) PKC-delta mediates activation of ERK1/2 and induction of iNOS by IL-1beta in vascular smooth muscle cells. Am J Physiol Cell Physiol 290(6):C1583–C1591. 10.1152/ajpcell.00390.2005 [DOI] [PubMed] [Google Scholar]
- Hay M, Vanderah TW, Samareh-Jahani F, Constantopoulos E, Uprety AR, Barnes CA, Konhilas J (2017) Cognitive impairment in heart failure: a protective role for angiotensin-(1–7). Behav Neurosci 131(1):99–114. 10.1037/bne0000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K, Le Gall S, Schulte M, Yamaguchi T, Reiss K, Murphy G, Toyama Y, Hartmann D, Saftig P, Blobel CP (2007) Substrate selectivity of epidermal growth factor-receptor ligand sheddases and their regulation by phorbol esters and calcium influx. Mol Biol Cell 18(1):176–188. 10.1091/mbc.E06-01-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado O, Lizasoain I, Fernandez-Tome P, Alvarez-Barrientos A, Leza JC, Lorenzo P, Moro MA (2002) TACE/ADAM17-TNF-alpha pathway in rat cortical cultures after exposure to oxygen-glucose deprivation or glutamate. J Cereb Blood Flow Metab 22(5):576–585. 10.1097/00004647-200205000-00009 [DOI] [PubMed] [Google Scholar]
- Jiang T, Gao L, Lu J, Zhang YD (2013a) ACE2-Ang-(1–7)-Mas axis in brain: a potential target for prevention and treatment of ischemic stroke. Curr Neuropharmacol 11(2):209–217. 10.2174/1570159X11311020007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Gao L, Shi J, Lu J, Wang Y, Zhang Y (2013b) Angiotensin-(1–7) modulates renin–angiotensin system associated with reducing oxidative stress and attenuating neuronal apoptosis in the brain of hypertensive rats. Pharmacol Res 67(1):84–93. 10.1016/j.phrs.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Kehoe PG, Wong S, Al Mulhim N, Palmer LE, Miners JS (2016) Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-beta and tau pathology. Alzheimers Res Ther 8(1):50. 10.1186/s13195-016-0217-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka I, Kusaka G, Zhou C, Ishikawa M, Nanda A, Granger DN, Zhang JH, Tang J (2004) Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol 286(6):H2442–H2451. 10.1152/ajpheart.01169.2003 [DOI] [PubMed] [Google Scholar]
- Kveiborg M, Instrell R, Rowlands C, Howell M, Parker PJ (2011) PKCalpha and PKCdelta regulate ADAM17-mediated ectodomain shedding of heparin binding-EGF through separate pathways. PLoS ONE 6(2):e17168. 10.1371/journal.pone.0017168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ (2005) Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 280(34):30113–30119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DW, Clarke NE, Hooper NM, Turner AJ (2008) Calmodulin interacts with angiotensin-converting enzyme-2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett 582(2):385–390. 10.1016/j.febslet.2007.11.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall SM, Maretzky T, Issuree PD, Niu XD, Reiss K, Saftig P, Khokha R, Lundell D, Blobel CP (2010) ADAM17 is regulated by a rapid and reversible mechanism that controls access to its catalytic site. J Cell Sci 123(Pt 22):3913–3922. 10.1242/jcs.069997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Shi P, Sumners C (2016) Direct anti-inflammatory effects of angiotensin-(1–7) on microglia. J Neurochem 136(1):163–171. 10.1111/jnc.13386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal JL, Hurtado O, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, Leza JC (2002) The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology 26(2):155–163. 10.1016/S0893-133X(01)00292-5 [DOI] [PubMed] [Google Scholar]
- Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL (2004) Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-d-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem 279(31):32869–32881. 10.1074/jbc.M311766200 [DOI] [PubMed] [Google Scholar]
- McKenna TM, Li S, Tao S (1995) PKC mediates LPS- and phorbol-induced cardiac cell nitric oxide synthase activity and hypocontractility. Am J Physiol 269(6 Pt 2):H1891–H1898. 10.1152/ajpheart.1995.269.6.H1891 [DOI] [PubMed] [Google Scholar]
- Minger SL, Geddes JW, Holtz ML, Craddock SD, Whiteheart SW, Siman RG, Pettigrew LC (1998) Glutamate receptor antagonists inhibit calpain-mediated cytoskeletal proteolysis in focal cerebral ischemia. Brain Res 810(1–2):181–199 [DOI] [PubMed] [Google Scholar]
- Molz S, Decker H, Dal-Cim T, Cremonez C, Cordova FM, Leal RB, Tasca CI (2008) Glutamate-induced toxicity in hippocampal slices involves apoptotic features and p38 MAPK signaling. Neurochem Res 33(1):27–36. 10.1007/s11064-007-9402-1 [DOI] [PubMed] [Google Scholar]
- Ongali B, Nicolakakis N, Tong XK, Aboulkassim T, Papadopoulos P, Rosa-Neto P, Lecrux C, Imboden H, Hamel E (2014) Angiotensin II type 1 receptor blocker losartan prevents and rescues cerebrovascular, neuropathological and cognitive deficits in an Alzheimer’s disease model. Neurobiol Dis 68:126–136. 10.1016/j.nbd.2014.04.018 [DOI] [PubMed] [Google Scholar]
- Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, Putko B, Kassiri Z, Turner AJ, Oudit GY (2014) Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol 66:167–176. 10.1016/j.yjmcc.2013.11.017 [DOI] [PubMed] [Google Scholar]
- Pedersen KB, Sriramula S, Chhabra K, Xia H, Lazartigues E (2011) Species-specific inhibitor sensitivity of angiotensin-converting enzyme 2 (ACE2) and its implication for ACE2 activity assays. Am J Physiol Regul Integr Comp Physiol 301(5):1293–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen KB, Chodavarapu H, Porretta C, Robinson LK, Lazartigues E (2015) Dynamics of ADAM17-mediated shedding of ACE2 Applied to pancreatic islets of Male db/db mice. Endocrinology 156(12):4411–4425. 10.1210/en.2015-1556 doi [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira CF, Oliveira CR (2000) Oxidative glutamate toxicity involves mitochondrial dysfunction and perturbation of intracellular Ca2+ homeostasis. Neurosci Res 37(3):227–236 [DOI] [PubMed] [Google Scholar]
- Salonen T, Sareila O, Jalonen U, Kankaanranta H, Tuominen R, Moilanen E (2006) Inhibition of classical PKC isoenzymes downregulates STAT1 activation and iNOS expression in LPS-treated murine J774 macrophages. Br J Pharmacol 147(7):790–799. 10.1038/sj.bjp.0706672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AJ, O’Dea KP, O’Callaghan D, Williams L, Dokpesi JO, Tatton L, Handy JM, Hogg PJ, Takata M (2011) Reactive oxygen species and p38 mitogen-activated protein kinase mediate tumor necrosis factor alpha-converting enzyme (TACE/ADAM-17) activation in primary human monocytes. J Biol Chem 286(41):35466–35476. 10.1074/jbc.M111.277434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriramula S, Xia H, Xu P, Lazartigues E (2015) Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension 65(3):577–586. 10.1161/hypertensionaha.114.04691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW, DeFranco DB (2000) Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem 275(16):12200–12206 [DOI] [PubMed] [Google Scholar]
- Stern JE, Son S, Biancardi VC, Zheng H, Sharma N, Patel KP (2016) Astrocytes contribute to angiotensin II stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension 68(6):1483–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M (2010) Calcium, ischemia and excitotoxicity. Cell Calcium 47(2):122–129. 10.1016/j.ceca.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Tellier E, Canault M, Rebsomen L, Bonardo B, Juhan-Vague I, Nalbone G, Peiretti F (2006) The shedding activity of ADAM17 is sequestered in lipid rafts. Exp Cell Res 312(20):3969–3980. 10.1016/j.yexcr.2006.08.027 [DOI] [PubMed] [Google Scholar]
- Úri K, Fagyas M, Mányiné Siket I, Kertész A, Csanádi Z, Sándorfi G, Clemens M, Fedor R, Papp Z, Édes I, Tóth A, Lizanecz E (2014) New perspectives in the renin–angiotensin–aldosterone system (RAAS) IV: circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure. PLoS ONE 9(4):e87845. 10.1371/journal.pone.0087845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S, Balarezo MG, Affram K, Saavedra JM, Symes AJ (2015) Neurorestoration after traumatic brain injury through angiotensin II receptor blockage. Brain 138(Pt 11):3299–3315. 10.1093/brain/awv172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, de Witte P, Ballini C, Corte LD, Dexter D (2009) Neuro-inflammation induced in the hippocampus of ‘binge drinking’ rats may be mediated by elevated extracellular glutamate content. J Neurochem 111(5):1119–1128. 10.1111/j.1471-4159.2009.06389.x [DOI] [PubMed] [Google Scholar]
- Wu J, Zhao D, Wu S, Wang D (2015) Ang-(1–7) exerts protective role in blood–brain barrier damage by the balance of TIMP-1/MMP-9. Eur J Pharmacol 748:30–36. 10.1016/j.ejphar.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Xia H, Sriramula S, Chhabra KH, Lazartigues E (2013) Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ Res 113(9):1087–1096. 10.1161/CIRCRESAHA.113.301811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Derynck R (2010) Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell 37(4):551–566. 10.1016/j.molcel.2010.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mukerjee S, Silva-Alves C, Carvalho-Galvão A, Cruz J, Balarini C, Braga V, Lazartigues E, França-Silva MdS (2016) A disintegrin and metalloprotease 17 in the cardiovascular and central nervous systems. Front Physiol. 10.3389/fphys.2016.00469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Sriramula S, Xia H, Moreno-Walton L, Culicchia F, Domenig O, Poglitsch M, Lazartigues E (2017) Clinical relevance and role of neuronal AT1 receptors in ADAM17-mediated ACE2 shedding in neurogenic hypertension. Circ Res 121(1):43–55. 10.1161/CIRCRESAHA.116.310509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jiang J, Black RA, Baumann G, Frank SJ (2000) Tumor necrosis factor-alpha converting enzyme (TACE) is a growth hormone binding protein (GHBP) sheddase: the metalloprotease TACE/ADAM-17 is critical for (PMA-induced) GH receptor proteolysis and GHBP generation. Endocrinology 141(12):4342–4348. 10.1210/endo.141.12.7858 [DOI] [PubMed] [Google Scholar]
- Zheng J, Li G, Chen S, Bihl J, Buck J, Zhu Y, Xia H, Lazartigues E, Chen Y, Olson JE (2014) Activation of the ACE2/Ang-(1–7)/Mas pathway reduces oxygen-glucose deprivation-induced tissue swelling, ROS production, and cell death in mouse brain with angiotensin II overproduction. Neuroscience 273:39–51. 10.1016/j.neuroscience.2014.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MC, Sharma RV, Davisson RL (2005) Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension 45(4):717–723. 10.1161/01.HYP.0000153463.22621.5e [DOI] [PubMed] [Google Scholar]