Abstract
We evaluated the heat shock system 70 (HSP70) in patients with chronic glomerulonephritis (CGN). Seventy-six patients with CGN patients were included in our study. Ten patients with mild proteinuria (median 0.48 [0.16–0.78] g/24 h) and ten healthy subjects served as positive and negative controls, respectively. Urinary levels of HSP70, interleukin-10, and serum levels of anti-HSP70 were measured by ELISA. The immunohistochemical peroxidase method was used to study the expression of HSP70 and Foxp3+ in kidney biopsies. TregFoxP3+ cells in the interstitium were determined morphometrically. Median urinary HSP70 levels in patients with nephrotic syndrome (NS) [6.57 (4.49–8.33) pg/mg] and subnephrotic range proteinuria [5.7 (4.12–6.9) pg/mg] were higher (p < 0.05) than in positive [3.7 (2.5–4.82) pg/mg] and negative [3.78 (2.89–4.84) pg/mg] controls. HSP70 expression index in tubular cells positively correlated with urinary HSP70 (Rs = 0.948, р < 0.05) and proteinuria (Rs = 0.362, p < 0.05). The number of TregFoxp3+ cells in the kidney interstitium and interleukin-10 excretion were lower in patients with NS. Anti-HSP70 antibody serum levels in patients with NS [21.1 (17.47–29.72) pg/ml] and subnephrotic range proteinuria [24.9 (18.86–30.92) pg/ml] were significantly higher than in positive [17.8 (12.95–23.03) pg/ml] and negative [18.9 (13.5–23.9) pg/ml] controls. In patients with CGN, increasing proteinuria was associated with higher HSP70 renal tissue and urinary levels. However, activation of HSP70 in patients with nephrotic syndrome did not lead to an increase in tissue levels of TregFoxp3+ cells or to the release of IL-10.
Electronic supplementary material
The online version of this article (10.1007/s12192-018-0928-8) contains supplementary material, which is available to authorized users.
Keywords: Heat shock protein 70, Anti-HSP-70-antibodies, Chronic glomerulonephritis, TregFoxp3+ cells, Interleukin-10
Introduction
Inflammation and renal tissue damage in patients with chronic glomerulonephritis (CGN) activate the glomerular self-defense mechanisms that counter-balance offending factors and can be responsible for the attenuation and resolution of disease (Kitamura and Fine 1999). Current therapy for CGN relies mainly on the pharmacological inhibition of immune cell-driven injury underlying the inflammatory response, while blockade of the renin angiotensin system remains the cornerstone of nephroprotective treatment. Alternatively, elucidation of the endogenous defense mechanisms can pave the way for new pharmacological interventions that may improve the efficacy of treatment for glomerular disease. However, there is a significant gap in our knowledge of kidney self-defense, particularly of the role of heat shock proteins (HSPs).
HSPs, or stress proteins, are a family of highly conserved molecules that constitute an important part of the architecture of the kidney self-defense system. HSPs have been classified into five groups according to their molecular weight, i.e., small HSP, HSP60, HSP70, HSP90, and HSP110 (Beck et al. 2000). The constitutively expressed intracellular HSPs control maturation and turnover of intracellular proteins and contribute to the maintenance of cellular integrity, while the inducible HSPs act as intracellular chaperones, protecting protein structure and folding under stress condition, and also participate in the numerous reparative processes including the refolding or removal of denatured proteins (Kampinga and Craig 2010).
Many HSPs are expressed in normal kidneys, i.e., HSP73, a constitutive 73 kDa protein belonging to the HSP70 family, was ubiquitously present in rats podocytes, Bowman’s capsule, tubular epithelial cells papillary epithelium, and interstitium (Komatsuda et al. 1992). The expression of the inducible HSP72 that belongs to the same HSP family was also detected both in normal kidney and after exposure to ischemia or toxic injury (Harrison et al. 2008; Guo et al. 2014; O’Neill et al. 2013). The experiments in vivo suggested that the downregulation of HSP70 might contribute to the progression of chronic kidney disease, given the anti-inflammatory and immunoregulatory effects of these proteins in addition to their chaperone function (Pockley 2003; Wang et al. 2011). The immunomodulatory activity of the extracellular HSPs is partly mediated by the induction of the regulatory T cells (Treg) that are characterized by the expression of the transcription factor Forkhead box P3 (Foxp3). Tregs produce different anti-inflammatory cytokines, including interleukin (IL)-10, and are responsible for the maintenance of peripheral tolerance and suppression of exacerbated immune responses (Kim et al. 2014).
In the present study, we studied the urinary levels of HSP70 and IL-10 and the expression of HSP70 and TregFoxp3+ in renal tissue in patients with chronic glomerulonephritis. Moreover, we measured serum levels of anti-HSP70 antibodies that were implicated as biomarkers of activity or severity in patients with different autoimmune diseases (Dhillon et al. 1991; Georgopoulos and McFarland 1993; Birnbaum et al. 1998; Wu and Tanguay 2006).
Materials and methods
Study subjects
Adult patients with CGN (n = 76) were recruited from the Clinic of Nephrology of the Sechenov University (Moscow). Exclusion criteria were age < 18 years, pregnancy, active urinary infection, heart failure, diabetes mellitus, diabetes insipidus, severe arterial hypertension, obesity, liver disease, diffuse connective tissue diseases, and chronic kidney disease stage 5. Venous blood samples were taken at the time of recruitment to assay serum creatinine, total protein, and albumin levels. Morning urine samples were assayed for protein and red blood cells. Daily urine was collected for daily proteinuria assessment. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula.
For positive and negative controls, we studied ten patients with mild proteinuria (median 0.48 [0.157–0.78] g/24 h) probably related to latent glomerulonephritis and ten healthy subjects, respectively.
The study was approved by the Ethics Committee of the Sechenov University. All subjects provided informed written consent before participation in the study that conformed with the Declaration of Helsinki.
Determination of HSP70 and IL-10 in urine and anti-HSP70 antibodies in serum
Ten-milliliter samples of morning urine were collected in dry plastic test tubes and centrifuged at room temperature for 15 min with rotational frequency of 3000 rpm. The urine supernatant was immediately frozen and stored in a refrigerator at the temperature of − 20 °C. We used the commercial enzyme-linked immunosorbent assay (ELISA) kits to determine the levels of HSPs 70 (ADI-EKS-715, Enzo Life Science, USA) and IL-10 (BMS 21512/BMS215/2TEN, eBiosciеnce, Austria).
For detection of anti-HSP70 antibodies, serum was obtained by centrifugation of the whole blood at room temperature for 20 min at 3000 rpm. Antibody levels were measured using commercial ELISA kits (ADI-EKS-750, Enzo Life Science, USA).
Histological study
We used the immunohistochemical peroxidase method to study the expression of HSP70 and TregFoxp3+ in kidney bioptates. Tissue sections were dewaxed with xylene and alcohols in different concentrations. In order to unmask antigens, the sections were boiled in citrate buffer. After deparaffinization and endogenous peroxidase inhibition, the sections were incubated with primary antibodies for 30 min at room temperature. We used rabbit polyclonal HSP70 antibody for HSP70 (1:800 dilution; E-12330, Spring Bioscience, USA) and rabbit polyclonal Foxp3 antibody for TregFoxp3+ (1:200 dilution; E-18490, Spring Bioscience, USA). Staining was performed using EnVision visualization kit (Dako; Denmark): specimens were incubated for 30 min with a polymer conjugated to anti-mouse and anti-rabbit IgG secondary antibodies and peroxidase. For the detection of peroxidase activity, the DAB+ Substrate Chromogen System (Dako, Denmark) was used. After the system produced a brown stain, the specimens were washed and immersed in hematoxylin solution for staining nuclei (for 1 min).
HSP70 and FoxP3 expression in kidney tissue was evaluated using a semiquantitative score: (−) 0 for absence, + for mild, ++ for moderate, and +++ for strong expression. The number of Treg cells in the interstitium of the renal cortex (1.5 mm2) was determined morphometrically by calculating of FoxP3+ nuclei.
Statistical analysis
The data were summarized with descriptive statistics. Normality tests were performed with the Shapiro–Wilk test. Results are presented as the number and percentage for categorical variables, as the mean ± SD for continuous variables with a normal distribution, and the median and interquartile range (IQR) for continuous variables with a non-normal distribution. Differences in proportions and continuous data were tested using the Pearson chi-square test and Mann–Whitney U test, respectively. Correlations between laboratory variables were evaluated by the Spearmen correlation coefficient. A two-sided p value < 0.05 was considered to indicate statistical significance. Statistical analysis was performed using StatSoft STATISTICA version 10.0 (StatSoft Inc., Tulsa, OK, USA).
Results
Subject characteristics
Characteristics of the study participants are shown in Table 1. Patients with CGN were distributed into two groups depending on the presence of nephrotic syndrome. In all patients, diagnosis of CGN was confirmed histologically.
Table 1.
Characteristic of the study subjects
| CGN with NS (n = 42) | CGN without NS (n = 34) | Positive controls (n = 10) | Negative controls (n = 10) | |
|---|---|---|---|---|
| Age, years | 40.4 [24–53] | 36.4 [26–49] | 34 [23–45] | 31 [28–37] |
| Gender (males), n (%) | 24 (57) | 19 (56) | 5 (50) | 5 (50) |
| Kidney histology, n (%) | 40 (95.2) | 22 (64.7) | – | – |
| MCD | 5 (12.5) | 0 | – | – |
| FSGS | 5 (12.5) | 2 (9.1) | – | – |
| MN | 10 (25) | 3 (13.6) | – | – |
| MPGN | 6 (15) | 5 (22.7) | – | – |
| IgA nephropathy | 18 (45) | 12 (54.5) | – | – |
| Arterial hypertension, n (%) | 37 | 30 | 2 | 0 |
| Proteinuria, g/24 h | 6.12 [4.37–10.0] | 1.9 [1.4–3.2] | 0.48 [0.16–0.78] | 0 |
| Serum albumin, g/L | 25.6 [21.3–30.7] | 39.8 [37.5–42.4] | 43.8 [41.2–46.1] | – |
| Creatinine, μmol/L | 1.1 [0.85–1.65] | 1.06 [0.8–1.5] | 1.03 [0.84–1.5] | – |
| eGFR, ml/min/1.73 m2 | 87.29 [50–116] | 81.0 [54–112] | 98 [68–125] | – |
| eGFR < 60 ml/min/1.73 m2, n (%) | 14 (33.3) | 7 (20.6) | 2 (20) | – |
| Immunosupressive therapy, n (%) | 23 (54.8) | 15 (44.1) | 0 | 0 |
| Oral corticosteroids | 6 (26) | 4 (26.7) | 0 | 0 |
| Pulse MP/CYC | 12 (52.2) | 7 (46.6) | 0 | 0 |
| Cyclosporine A | 5 (21.7) | 4 (26.7) | 0 | 0 |
MP methylprednisolone, CYC cyclophosphamide, MCD minimal change disease, FSGS focal segmental glomerular sclerosis, MN membranous nephropathy, MPGN membranoproliferative glomerulonephritis, eGFR estimated glomerular filtration rate
HSP70 in urine and HSP70 and Treg Foxp3+ cells kidney tissue
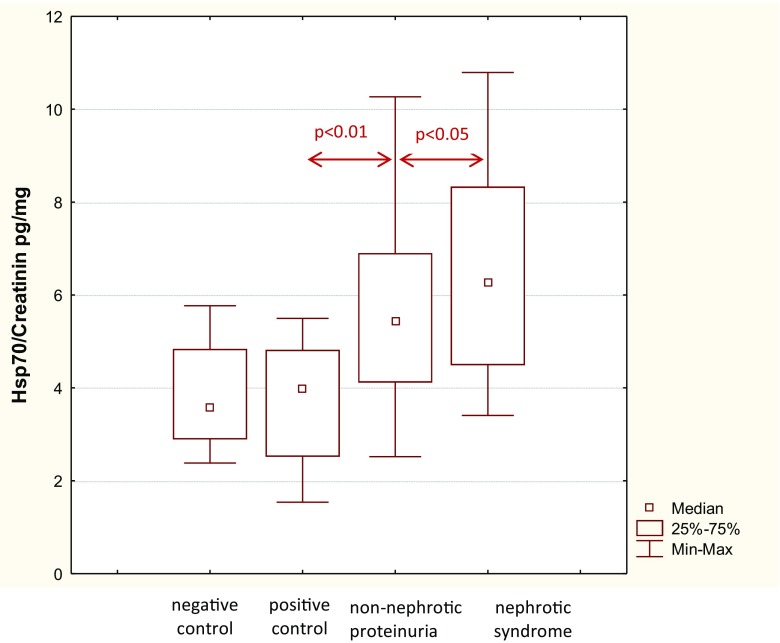
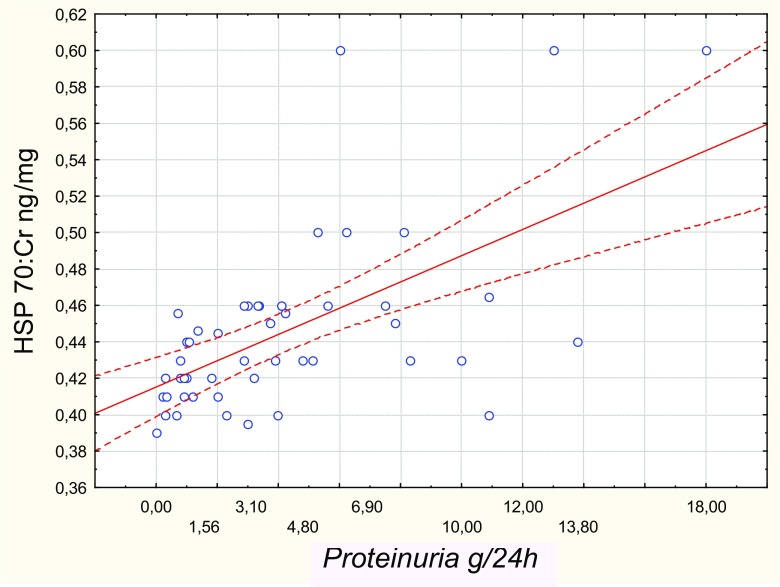
Median urinary HSP70 levels in patients with nephrotic syndrome [6.57 (4.49–8.33) pg/mg] and the non-nephrotic range proteinuria [5.7 (4.12–6.9) pg/mg) were higher (p < 0.05) than in positive [3.7 (2.5–4.82) pg/mg] and negative [3.78 (2.89–4.84) pg/mg] controls, while the difference between the first two groups did not reach statistical significance (Fig. 1). Moreover, there was a direct correlation between urinary HSP70 levels and proteinuria (Rs = 0.59, p < 0.01, Fig. 2). Urinary HSP70 concentration did not depend on the histological type of glomerulonephritis and was similar in patients with minimal change disease [5.99 (4.16–7.83) pg/mg], focal segmental glomerular sclerosis [6.05 (4.16–8.27) pg/mg], membranous nephropathy [6.5 (5.56–7.26) pg/mg], membranoproliferative glomerulonephritis [4.46 (4.03–4.49) pg/mg] or IgA-nephropathy [5.6 (3.57–8.29) pg/mg]. Impairment of kidney function was not associated with changes in urinary HSP70, since its median levels were comparable in patients with eGFR < 60 ml/min/1.73 m2 and ≥ 60 ml/min/1.73 m2 [5.7 (4.9–7.91) vs. 5.55 (4.34–7.64) рg/mg, respectively, p = 0.56]. Urinary HSP70 did not correlate with serum creatinine (Rs = 0.061, p = 0.67) or eGFR (Rs = − 0.26, p = 0.07), blood pressure (Rs = 0.25, p = 0.118), did not depend on the duration of kidney disease (Rs = 0.16, p = 0.32), and was similar in treated and untreated patients [5.56 (4.6–8.1) vs 5.2 (3.87–6.22) pg/mg, p = 0.102].
Fig. 1.
Hsp70 excretion in patients with chronic glomerulonephritis
Fig. 2.
Correlation between HSP70 in the urine and proteinuria (g/24 h)
HSP70 expression in renal tissue was found in 62 patients and was similarly distributed between different kidney structures in patients with nephrotic syndrome and non-nephrotic range proteinuria (Fig. 1 Suppl.). In patients with nephrotic syndrome, tubular epithelial cells of convoluted tubules and Henle loop expressed HSP70 more intensely compared with patients with non-nephrotic proteinuria (Table 2). HSP70 expression score positively correlated with the urinary HSP70 (Rs = 0.948, р < 0.05) and proteinuria (Rs = 0.362, p < 0.05).
Table 2.
HSP70 and Foxp3 expression in kidney tissue in patients with glomerulonephritis
| Non-nephrotic proteinuria (n = 22) | Nephrotic syndrome (n = 40) | p value | |
|---|---|---|---|
| HSP70 expression, n of biopsies (%) | |||
| Podocytes | 10 (45.5) | 21 (52.5) | 0.1 |
| Parietal cells | 9 (40.9) | 21 (52.5) | 0.23 |
| Convoluted tubuli | 11 (50.0) | 22 (55.0) | 0.03* |
| Henle loop | 4 (18.2) | 17 (42.5) | 0.04* |
| Cellular interstitial infiltrates | 12 (54.5) | 23 (57.5) | 0.04* |
| Vascular endothelium | 6 (27.2) | 10 (25.0) | 0.33 |
| HSP70 expression, score | |||
| Podocytes | 1.00 ± 0.54 | 1.21 ± 0.55 | 0.60 |
| Parietal cells | 1.10 ± 0.68 | 1.22 ± 0.42 | 0.57 |
| Convoluted tubuli | 1.42 ± 0.60 | 1.90 ± 0.22 | 0.04* |
| Henle loop | 1.09 ± 0.50 | 1.75 ± 0.50 | 0.04* |
| Cellular interstitial infiltrates | 0.93 ± 0.56 | 0.87 ± 0.25 | 0.56 |
| Vascular endothelium | 0.25 ± 0.12 | 0.75 ± 0.42 | 0.32 |
| FoxP3 in interstitial infiltrates, score | 0.30 ± 0.10 | 0.04 ± 0.03 | 0.004* |
| The number Treg Foxp3 in interstitial infiltrates (nuclei/1.5mm2) | 8.5 [3.0–16.0] | 2.0 [0.4–4.0] | 0.012* |
*p ≤ 0.05
The number of Treg Foxp3+ cells in the kidney interstitium in patients with nephrotic syndrome was significantly lower than in patients with non-nephrotic range proteinuria (p = 0.004) and negatively correlated with proteinuria (Rs = − 0.407, р = 0.006) (Fig.2 Suppl.).
Serum anti-Hsp70 antibodies and IL-10
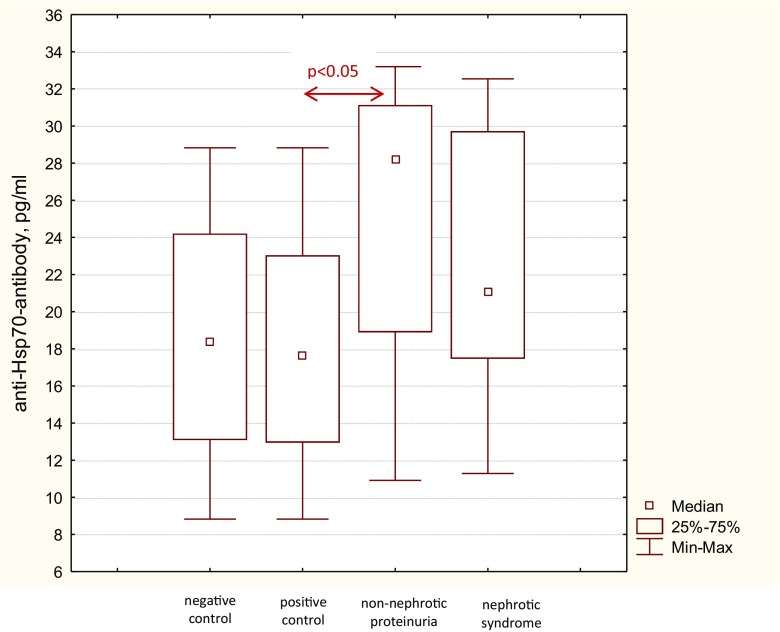
Anti-HSP70 antibody serum levels in patients with nephrotic syndrome [21.1 (17.47–29.72) pg/ml] and non-nephrotic range proteinuria [24.9 (18.86–30.92) pg/ml] were significantly higher than in positive [17.8 (12.95–23.03) pg/ml] and negative [18.9 (13.5–23.9) pg/ml] controls, while the difference between the first two groups did not reach statistical significance (p = 0.27) (Fig. 3). In patients with nephrotic syndrome, eGFR < 60 ml/min/1.73 m2 was associated with a lower anti-HSP70 antibody serum level [20.3 (15.3–24.3) pg/ml] compared with that in patients with eGFR ≥ 60 ml/min/1.73 m2 [22.7 (17.6–31.36) pg/ml, p < 0.05], while in patients with subnephrotic range proteinuria anti-HSP70 antibody serum level did not depend on eGFR [27.34 (14.71–31.27) pg/ml vs. 28.1 (19.27–30.72) pg/ml].
Fig. 3.
Serum anti-Hsp70-antibody levels in patients with chronic glomerulonephritis
Anti-HSP70 antibody titers did not correlate with proteinuria (Rs = 0.10, p = 0.39), blood pressure (Rs = − 0.11, p = 0.36), and duration of disease (Rs = − 0.13, p = 0.411). In treated patients, anti-HSP70 antibody levels were numerically lower than in untreated patients [20.1 (14.25–24.5) vs 22 (17.24–29.87) pg/ml]. However, the difference was insignificant (p = 0.081).
IL-10 was found in urine in 16/34 patients (47.1%) with non-nephrotic range proteinuria, 5/42 patients (11.9%) with nephrotic syndrome, 3/10 positive controls (30.0%), and 1/10 negative controls (10.0%). The difference between the first two groups and between the first group and negative controls was significant (p = 0.012 and p = 0.034, respectively). Median urinary IL-10 levels were similar in patients with non-nephrotic range proteinuria [22.9 (6.5–40.0) pg/mg] and nephrotic syndrome [18.9 (8.05–28.2) pg/ml]. IL-10 was not present in urine in patients with impaired kidney function.
Discussion
The results of our study demonstrate the distinct changes of the HSP70 system in patients with chronic glomerulonephritis. Median urinary HSP70 levels were higher in patients with nephrotic syndrome and non-nephrotic range proteinuria compared with the control groups and directly correlated with proteinuria, while impairment of kidney function was not associated with a further increase in the urinary HSP70 levels. Notably, the levels of HSP70 in urine were similar in patients with the different histological types of nephropathy. As expected, the standard immunosuppressive treatment had no impact on the urinary HSP70 levels. Expression of HSP70 in renal tissue was found in all patients with active chronic glomerulonephritis, though we did not study it in the control groups for ethical reasons. A direct correlation between the urinary and tissue HSP70 levels may indicate the renal origin of HSP70 detected in urine.
In patients with glomerulonephritis, HSP70 was expressed in the various kidney structures, particularly in the tubular epithelial cells. HSP70 expression in the tubulointerstitium was more intense in patients with nephrotic syndrome compared with that in patients with a lower proteinuria. Furthermore, the HSP70 expression index directly correlated with proteinuria. Given the nephrotoxic effect of proteinuria, accumulation of HSP70 mainly in the tubulointerstitial tissue can be regarded as a response of tubular cells to damage and activation of remodeling processes. HSP 70 expression is induced (mainly in tubules) in patients with different renal diseases such as acute vascular rejection, acute tubular necrosis, CMV infection, and minimal change disease (Dodd et al. 1993). In the study of Venkataseshan and Marque (1996), normal renal tissue and tissues from patients with idiopathic nephrotic syndrome or noninflammatory diseases showed a uniform pattern of fine-granular HSP70 staining of low intensity in the glomerular epithelial cells, in the cells of the distal convoluted tubules and cortical and medullary collecting tubules. However, in all cases of active or acute interstitial inflammation, there was a significant but variable increase of stainable HSP70 in the tubular cells.
The immunoregulatory effect of HSPs in inflammatory diseases was first shown in experimental animals with autoimmune arthritis (van Eden et al. 1988). Intracellular HSP70 can be expressed on the surface of inflammatory cells or released into extracellular space (Multhoff and Hightower 1996; Asadullah et al. 2010). HSP70 epitopes are recognized by T lymphocytes which form a pool of Treg cells. These cells express Foxp3 (Fontenot et al. 2003; Hori et al. 2003) and produce IL-10 that provides a powerful anti-inflammatory effect, inhibiting the production of IL-1, IL-6, tumor necrosis factor-α (de Waal Malefyt et al. 1991), oxygen reactive radicals, and prostaglandins (Dokka et al. 2001), and reducing the inflow of leukocytes into the area of inflammation. In our study, Foxp3 expression and the number of TregFoxp3+ cells in patients with nephrotic syndrome were significantly lower than in patients with non-nephrotic range proteinuria despite a direct correlation between the urinary HSP70 level and proteinuria. Moreover, IL-10 was not detected in urine in the majority of the former patients. These data may indicate an impaired anti-inflammatory function of HSP70 in patients with a more severe nephropathy.
HSPs can stimulate the immune system, leading to the production of autoantibodies. Antibodies against HSPs, are found under normal physiological conditions, after exposure to environmental stresses, and in many diseases. Increased anti-HSP70 levels were shown in patients with systemic lupus erythematosus, immune thrombocytopenic purpura, asthma, primary biliary cirrhosis, and Meiniere’s disease (Wu and Tanguay 2006). The clinical significance of the immune response to HSPs remains largely unknown. We have found no previous data on the autoantibodies against HSP in patients with kidney diseases. In our study, anti-HSP70 antibody serum levels in patients with nephrotic syndrome and the non-nephrotic range proteinuria were higher than in positive and negative controls, while the difference between the first two groups was not significant. These data suggest that anti-HSP70 antibodies may indicate activity of glomerulonephritis. However, this hypothesis should be confirmed by further studies.
Our study has limitations. The sample of patients was relatively small, and we did not study HSP70 expression in patients with normal histology of kidney tissue for ethical reasons. However, the urinary HSP70 probably reflects its expression in the kidney tissues given a strong correlation between HSP70 expression score and the urinary HSP70 level.
Conclusion
Increasing proteinuria in patients with CGN was associated with higher urinary HSP70 levels and its expression in kidney biopsies. However, activation of HSP70 in patients with nephrotic syndrome did not lead to an increase in tissue levels of TregFoxp3+ cells or release of IL-10. These data may indicate a disorder of anti-inflammatory function of HSP70 or a switch of its anti-inflammatory effect to pro-inflammatory action in patients with a more severe nephropathy.
Electronic supplementary material
(DOCX 2832 kb)
Acknowledgements
The authors thank Professor Vladimir Varshavsky for morphological analysis. The authors also thank all patients and site staff who participated in this study.
Abbreviations
- HSPs
Heat shock proteins
- CGN
Chronic glomerulonephritis
- Tregs
Regulatory T cells
- Foxp3
Transcription factor Forkhead box P3
- IL-10
IL-1, IL-6 - interleukin -10, -1, and -6
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- eGFR
Estimated glomerular filtration rate
- ELISA
Enzyme-linked immunosorbent assay
- MCD
Minimal change disease
- FSGS
Focal segmental glomerular sclerosis
- MN
Membranous nephropathy
- MPGN
Membranoproliferative glomerulonephritis
- CMV
Cytomegalovirus
Compliance with ethical standards
The study was approved by the Ethics Committee of the Sechenov University. All subjects provided informed written consent before participation in the study that conformed with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy – review of a new approach. Pharmacol Rev. 2010;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- Beck F-X, Neuhofer W, Muller E. Molecular chaperones in the kidney: distribution, putative roles and regulation. Am J Physiol Ren Physiol. 2000;279:203–215. doi: 10.1152/ajprenal.2000.279.2.F203. [DOI] [PubMed] [Google Scholar]
- Birnbaum G, Kotilinek L, Miller SD, Raine CS, Gao YL, Lehmann PV, et al. Heat shock proteins and experimental autoimmune encephalomyelitis. II: environmental infection and extra-neuraxial inflammation after the course of chronic relapsing encephalomyelitis. J Neuroimmunol. 1998;90:149–161. doi: 10.1016/S0165-5728(98)00141-6. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon V, Latchman D, Isenberg D. Review: heat shock proteins and systemic lupus erythematosus. Lupus. 1991;1:3–8. doi: 10.1177/096120339100100102. [DOI] [PubMed] [Google Scholar]
- Dodd SM, Martin JE, Swash M, Mather K. Expression of heat shock protein epitopes in renal disease. Clin Nephrol. 1993;39(5):239–244. [PubMed] [Google Scholar]
- Dokka S, Shi X, Leonard S, Wang L, Castranova V, Rojanasakul Y. Interleukin-10-mediated inhibition of free radical generation in macrophages. Am J Phys Lung Cell Mol Phys. 2001;280(6):1196–1202. doi: 10.1152/ajplung.2001.280.6.L1196. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C, McFarland H. Heat shock protein in multiple sclerosis and other autoimmune diseases. Immunol Today. 1993;14:373–375. doi: 10.1016/0167-5699(93)90135-8. [DOI] [PubMed] [Google Scholar]
- Guo Q, Du X, Zhao Y, Zhang D, Yue L, Wang Z. Ischemic postconditioning prevents renal ischemia reperfusion injury through the induction of heat shock proteins in rats. Mol Med Rep. 2014;10:2875–2881. doi: 10.3892/mmr.2014.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EM, Sharpe E, Bellamy CO, McNally SJ, Devey L, Garden OJ, et al. Heat shock protein 90-binding agents protect renal cells from oxidative stress and reduce kidney ischemia reperfusion injury. Am J Physiol Ren Physiol. 2008;295:397–405. doi: 10.1152/ajprenal.00361.2007. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-G, Cho EG, Lee JW, Ko YS, Lee HY, Jo S-K, et al. The heat-shock protein-70-induced renoprotective effect is partially mediated by CD4+ CD25+ Foxp3 + regulatory T cells in ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2014;85(1):62–71. doi: 10.1038/ki.2013.277. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Fine LG. The concept of glomerular self-defense. Kidney Int. 1999;55:1639–1671. doi: 10.1046/j.1523-1755.1999.00425.x. [DOI] [PubMed] [Google Scholar]
- Komatsuda A, Wakui H, Imai H, Nakamoto Y, Miura AB, Itoh H. Renal localization of the constitutive 73-kDa heat shock protein in normal and PAN rats. Kidney Int. 1992;41:1204–1212. doi: 10.1038/ki.1992.182. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Hightower LE. Cell surface expression of heat shock proteins and the immune response. Cell Stress Chaperones. 1996;1:167–176. doi: 10.1379/1466-1268(1996)001<0167:CSEOHS>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill S, Ingman TG, Wigmore SJ, Harrison EM, Bellamy CO. Differential expression of heat shock proteins in healthy and diseased human renal allografts. Ann Transplant. 2013;18:550–557. doi: 10.12659/AOT.889599. [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- van Eden W, Tholet JER, van der Zee R, Noordzij A, van Embden JDA, Hensen EJ, et al. Cloning of the mycobacterial epitope recognized by T lymphocyte in adjuvant arthritis. Nature. 1988;331:171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- Venkataseshan V, Marque S. Heat shock protein 72/73 in normal and diseased kidneys. Nephron. 1996;73(3):442–449. doi: 10.1159/000189108. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gall JM, Bonegio RG, Havasi A, Hunt CR, Sherman MY, et al. Induction of heat-shock protein 70 inhibits ischemic renal injury. Kidney Int. 2011;79:861–870. doi: 10.1038/ki.2010.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Tanguay RM. Antibodies against heat shock proteins in environmental stresses and diseases: friend or foe? Cell Stress Chaperones. 2006;11:1–12. doi: 10.1379/CSC-155R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 2832 kb)