Abstract
Diapause is an important strategy for certain insect species to survive unfavorable environmental conditions, including low temperatures experienced when they overwinter in cold climate. Many studies have indicated that the increased expression of heat shock proteins during diapause improves the thermal tolerance of insects. However, the relationship between small heat shock proteins (sHSPs) and diapause is not clear or well-researched. In this study, we investigated the transcript levels of 14 sHSP genes in the spruce budworm, Choristoneura fumiferana, a major pest of spruce and fir in Canada, during pre-diapause, diapause, and post-diapause under normal rearing conditions and in response to a heat shock treatment. We found that sHSP expression profiles could be classified into five patterns under normal laboratory conditions: pattern I was upregulated only during pre-diapause, pattern II was upregulated only during diapause, pattern III was constantly expressed throughout diapause, pattern IV was upregulated in both pre-diapause and diapause, and pattern V was upregulated only during post-diapause. After heat shock, five different expression patterns were observed: pattern I responded weakly or not at all throughout diapause, pattern II responded weakly during the diapause stage but strongly at the onset of diapause and in the post-diapause period, pattern III was upregulated only during post-diapause, pattern IV was strongest during diapause, and pattern V was strongest only in early diapause. These complex expression profiles lead us to suggest that most of the sHSP genes are involved in the diapause process and that they may have multiple and important roles in different phases of this process.
Keywords: Diapause, Spruce budworm, sHSP, Chaperone, Overwinter, Gene expression
Introduction
Diapause is an important strategy employed by many species of insects to enable them to survive unfavorable environmental conditions such as cold winters, hot summers, or dry seasons (Denlinger 2002; MacRae 2010; King and MacRae 2015). Insect diapause is a physiological process characterized by behavioral modification, metabolic suppression, and increased stress tolerance. It is controlled externally by environmental factors such as temperature and day length and internally by a complex combination of processes involving endocrine, neuroendocrine, metabolic, molecular, cellular, and enzymatic changes (Denlinger 2002; MacRae 2010 and King and MacRae 2015). Many studies have demonstrated that diapausing insects can enhance stress tolerance or resistance through production of polyols and other low-molecular-weight compounds, such as glycerol, sorbitol, mannitol, trehalose, proline, and antifreeze proteins, to depress their body’s supercooling point and to non-colligatively stabilize proteins. Cold-induced damage to certain species of insects’ metabolism can also be mitigated through modification of the cytoskeleton by enhancing the elasticity of cellular membranes (Denlinger 2002; MacRae 2010; King and MacRae 2015). In contrast, scant information exists on the regulation of diapause at the molecular level.
Recent studies have provided evidence that heat shock proteins (HSPs) may be major contributors to stress tolerance, allowing certain species of insects to withstand severe weather conditions (Rinehart et al. 2007; Basha et al. 2012; Zhao and Jones 2012; King and MacRae 2015). Heat shock proteins are a group of molecular chaperones that are ubiquitous in both prokaryotes and eukaryotes. They assist in ensuring the correct folding of nascent proteins and help to refold proteins misfolded due to cumulative cell stressors, thus preventing their aggregation (Waters and Rioflorido 2007; Waters 2013).
Insect HSPs can be classified into four major families based on their molecular weights and structural characteristics (King and MacRae 2015): HSP90, HSP70, HSP60, and small heat shock proteins (sHSPs). Many HSPs are upregulated by diverse stresses, including heat, cold, starvation, dehydration, infection, ultraviolet light, and exposure to wide range of chemicals (Rinehart et al. 2007; Basha et al. 2012; Zhao and Jones 2012, King and MacRae 2015). It has been demonstrated that members of the HSP70 and HSP60 families are upregulated during diapause in insects from different orders. For example, HSP70s are upregulated in the gypsy moth (Lymantria dispar) embryo (Rinehart et al. 2007), the European corn borer (Ostrinia nubilalis) larva (Rinehart et al. 2007), and the flesh fly (Sarcophaga crassipalpis) pupa (Hayward et al. 2005). HSP60s are upregulated in the Sarcophaga crassipalpis pupa (Rinehart et al. 2007). Increased HSP70 or HSP60 levels may also contribute to the enhancement of cold tolerance and the interruption of development in various insect orders (Rinehart et al. 2007). In contrast to the genes of the HSP70 and HSP60 families, those of the HSP90 family are downregulated during diapause in most species examined, e.g., in S. crassipalpis pupa (Hayward et al. 2005; Rinehart et al. 2007) and in bamboo borer (Omphisa fuscidentalis) larva (Tungitwitayakul et al. 2008). Decreased HSP90 levels during diapause suggest possible critical roles in shutting down hormonal signals and in arrest of the cell cycle (Tammariello and Denlinger 1998; Rinehart and Denlinger 2000; MacRae 2010).
Members of the sHSP family have a molecular weight range of 12–43 kDa, are characterized by the presence of a conserved α-crystallin domain, and exhibit ATP-independent chaperone-like activity (Basha et al. 2012; King and MacRae 2015). These proteins have been implicated in many physiological processes, including cellular stress resistance (Landry et al. 1989), cytoskeleton stabilization (Wieske et al. 2001; Quinlan 2002), apoptosis inhibition (Arrigo 1998), membrane fluidity (Tsvetkova et al. 2002), cellular longevity (Wood et al. 2010), and resistance to various diseases (MacKay et al. 2003; Evgrafov et al. 2004). The roles of sHSP in diapause, however, have been less studied and are thus poorly understood.
Insect sHSPs are expressed in a wide array of patterns under stress and non-stress conditions. A number of sHSPs have been isolated and studied from the insect orders Diptera (Drosophila melanogaster, Haass et al. 1990; Morrow et al. 2006), Lepidoptera (Bombyx mori, Li et al. 2009; Chilo suppressalis, Lu et al. 2014), Coleoptera (Tribolium castaneum, Mahroof et al. 2005), and Hymenoptera (Apis cerana, Liu et al. 2012). The expression of an individual sHSP is regulated in a tissue- and developmental stage-specific manner in the absence of stress, suggesting that insect sHSPs play important roles in various developmental processes as well as metabolic activities and reproduction (Dubrovsky et al. 1996; Huet et al. 1996; Joanisse et al. 1998; Sonoda et al. 2006; Kokolakis et al. 2008; Takahashi et al. 2010).
Currently, the role of sHSPs in insect diapause is poorly understood although evidence suggests they are important during the diapause developmental process. For example, in the freeze-tolerant gall fly, Euros solidaginis, the expression of sHSP increases before and during diapause in late fall and winter (Zhang et al. 2011). In silkworm (B. mori), the expression of HSP20.4, HSP20.8, HSP40, HSP70, and HSP90 remains constant in diapausing eggs, whereas HSP20.8A is upregulated (Saravanakumar et al. 2008; Hwang et al. 2005). In the corn stalk borer (Sesamia nonagrioides), SnoHSP19.5 is expressed consistently, whereas SnoHSP20.8 is initially downregulated and then upregulated as diapause comes to completion (Gkouvitsas et al. 2008). In diapausing larvae of the blow fly (Calliphora vicina), HSP23 and HSP24 are upregulated (Fremdt et al. 2014).
Despite the likelihood that sHSPs act during diapause, there is no clear and concrete picture of their expression profiles in any single species during this developmental phase. Furthermore, phylogenetic and biological analyses suggest that most insect sHSPs have evolved independently in different insect orders (Sakano et al. 2006, Huang et al. 2008; Li et al. 2009; Zhang et al. 2015). It may be that species-specific sHSPs strongly contribute to the adaptability of insects in diverse environments.
Investigating the roles of sHSPs during the diapause of a single species will improve our understanding of how an insect adapts to environmental conditions and how the changing climate affects its geographic distribution and population dynamics. As such information becomes available for more insects, we may gain an appreciation of how specific sHSPs act in each species.
The spruce budworm (SBW) (Choristoneura fumiferana; Lepidoptera: Tortricidae) is the most widely distributed and destructive defoliator of spruce-fir forests in North America (Stehr 1967, Régnière et al. 2012). In the past few hundred years, periodic outbreaks have occurred across millions of hectares of spruce-fir forest (Blais 1983, Boulanger and Arseneault 2004). The SBW has an obligatory winter diapause of approximately 8 months as a second-instar larva within a hibernaculum spun on sheltered structures of host trees. Overwintering survival greatly affects SBW population size, dynamics, and geographical distribution (Royama 1984; Han and Bauce 1993).
Previously, we identified 15 sHSP genes in the SBW transcriptomes, including CfHSP20.3, which has no expression during development and may be a pseudogene (Quan et al. 2018). Phylogenetic analysis indicated that at least five of these sHSPs are SBW specific. Under non-stress conditions, transcript levels of various sHSPs differ according to life stage and tissue. Upon heat stress, the expression of nine sHSP genes increases (Quan et al. 2018). In the present study, we analyzed 14 sHSP genes during different diapause stages in the laboratory and their response to heat shock during diapause. Here, we present our findings and discuss the potential roles of these sHSPs before, during, and after diapause.
Materials and methods
Experimental insects
Second instar larvae during or after diapause were provided by Insect Production Services, Great Lakes Forestry Centre, Sault Ste. Marie, ON, Canada, and were reared on an artificial diet modified from McMorran (1965). The insects were maintained at 22–23 °C, 70% relative humidity, and under a 12-h light:12-h dark photoperiod regime.
Sample collection and heat shock treatment
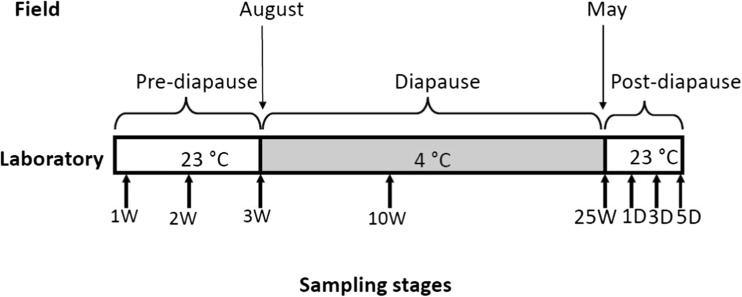
Approximately 50 first instar larvae were collected within 12 h after hatching. The larvae were reared in a 15-mL cup with diet at 23 °C for 3 weeks then transferred to 4 °C for 25 weeks. They were thereafter transferred to a cup with fresh diet and reared at 23 °C for a final week. Larvae at different diapause stages were collected at different time intervals as indicated in Fig. 1. For heat shock treatment, approximately 25 larvae at various diapause stages were transferred to an incubator at 37 °C for 1 h and then homogenized in TRIzol reagent (Thermo Fisher Scientific, USA). The homogenates were stored at − 20 °C until RNA extraction.
Fig. 1.
Schematic of the experimental design used in this study. Sampling times occurred at 1, 2, and 3W (first instar larvae held at 23 °C for 1, 2, or 3 weeks after hatch); 10 and 25 W (second instar larvae held at 4 °C for 10 and 25 weeks); and 1, 3, and 5D (second instar larvae returned to 23 °C for 1, 3, and 5 days after 25 weeks at 4 °C). Note that spruce budworm diapause extends from August to May in the field
RNA isolation and RT-qPCR
Total RNA isolation, removal of genomic DNA contamination, and first-strand complementary DNA (cDNA) generation were conducted as previously reported (Quan et al., 2018). The real-time quantitative polymerase chain reaction (RT-qPCR) was conducted in 20 μL volumes comprising 10 μL 2xSensiFAST SYBR No-ROX Mix (Bioline USA Inc.), 2 μL cDNA template, and 8 μL gene-specific primers (Quan et al. 2018). A CFX Connect Real-time PCR Detection (Bio-Rad) system was used as follows: 40 cycles at 95 °C for 10 s and 65 °C for 20 s. The relative quantity of each transcript was assessed using the 2–∆∆CT method (Livak and Schmittgen 2001). Three (Tef-1α, actin and HSC72.5) genes were evaluated as potential internal reference genes for calculation of relative transcript levels of the genes under heat shock and diapause development. The relative mRNA levels of these genes were similar in the control and heat-treated samples, but the actin and HSC72.5 were unstable during diapause (data not shown). Therefore, the Tef-1α gene was used to normalize transcript abundance in each sample. The expression levels of each transcript were measured in at least four independent biological samples and in two technical replicates for each biological sample.
Statistical analysis
All data are presented as mean ± SD (standard deviation). Differences between treatment groups and the control group were analyzed by one-way ANOVA, followed by student’s t tests.
Results
Expression profiles of 14 sHSP transcripts under laboratory conditions in SBW in pre-diapause, diapause, and post-diapause
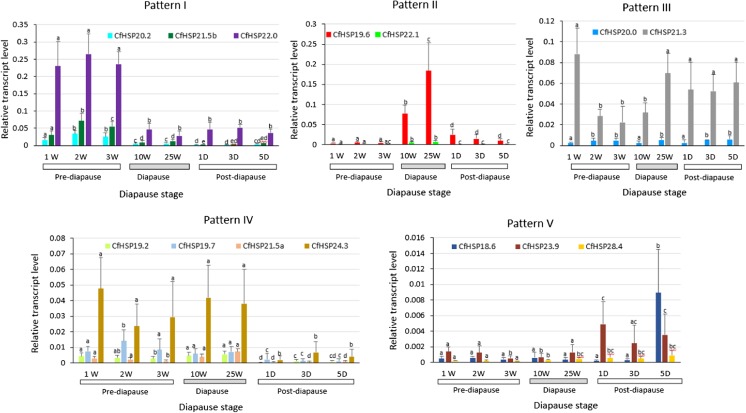
The transcript levels of 14 C. fumiferana sHSPs genes (CfHSPs) were measured using RT-qPCR. Samples from eight time points from pre- to post-diapause were collected (Fig. 1). The sHSP transcript expression profiles under laboratory conditions were classified into five patterns (Fig. 2): pattern I was upregulated only during pre-diapause, pattern II was upregulated only during diapause, pattern III was constantly expressed throughout diapause, pattern IV was upregulated in both pre-diapause and diapause, and pattern V was upregulated only during post-diapause. Pattern I included three CfHSP: CfHSP20.2, CfHSP21.5b, and CfHSP22.0. These transcripts were highly expressed during pre-diapause, but once the larvae were confirmed to be in diapause and were transferred to 4 °C, their expression levels dropped to low levels and such a drop in the expression levels was also observed even when subjects were transferred to room temperature. The expression of pattern I genes during pre-diapause was approximately 5 times higher than that during any later stage tested. Pattern II occurred with two genes: CfHSP19.6 and CfHSP22.1. Transcript levels were dramatically upregulated during the development of diapauses, but they exhibited low expression levels at early and post-diapause. CfHSP19.6 transcript levels were 60-fold higher in larvae that had been in diapause for 25 weeks compared to those in pre-diapause. Pattern III occurred with two genes: CfHSP20.0 and CfHSP21.3. Their transcripts were constitutively expressed during diapause with little variation, particularly CfHSP20.0. In contrast, transcripts of CfHSP21.3 were higher 1 week after the larvae hatched than those in larvae further along in the pre-diapause stage or 10 weeks into diapause. At 25 weeks of diapause, transcript abundance increased again and remained relatively constant throughout post-diapause. Pattern IV occurred with four genes: CfHSP19.2, CfHSP19.7, CfHSP21.5a, and CfHSP24.3. The transcripts of these CfHSPs were highly expressed during pre-diapause and diapause but were then downregulated in post-diapause when development resumed. Pattern V occurred with three genes: CfHSP18.6, CfHSP23.9, and CfHSP28.4. The common feature of this pattern was that transcripts were upregulated only in post-diapause, that is, after larvae were transferred back to room temperature. However, the expression pattern of CfHSPs in pattern V was somewhat hypervariable: CfHSP18.6 was extremely highly expressed on day 5 post-diapause, CfHSP23.9 was upregulated on day 1 post-diapause, and CfHSP28.4 started to increase after 10 weeks at 4 °C, maintaining a relatively high expression until development resumes. In summary, the transcript levels of most CfHSPs varied across diapause stages.
Fig. 2.
Expression profiles of small CfHSP transcripts during pre-diapause, diapause, and post-diapause. Total RNAs were isolated from pre-diapause (1, 2, and 3 W), diapause (10 and 25W), and post-diapause (1, 3, and 5D) larvae. The small CfHSP transcript levels in the larvae were quantified with RT-qPCR (see “Materials and methods”). Values are means ± SD of four biological replicates, with two technical replicates each and are expressed as apparent expression levels relative to the control gene, translation elongation factor-1α (Tef-1α). For each gene, different letters above the bars indicate significant differences from the other developmental times (P < 0.05)
Effect of heat stress on expression profiles of 14 CfHSPs during pre-diapause, diapauses, and post-diapause in SBW
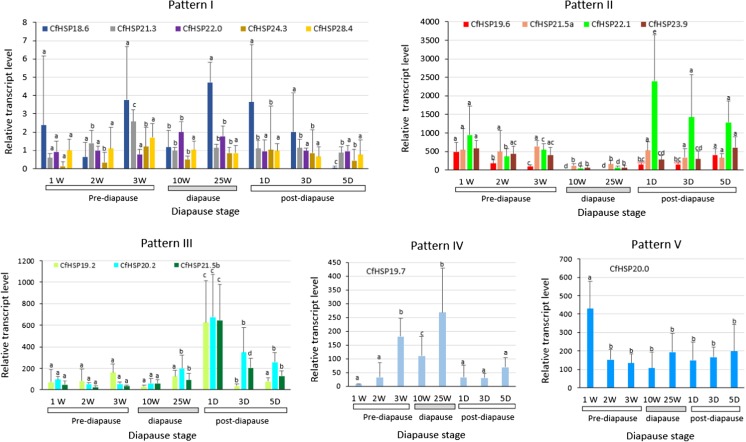
We compared the relative expression levels of 14 CfHSP transcripts after heat shock treatments to those of controls using RT-qPCR during pre-diapause, diapause, and post-diapause stages of second instar larvae of SBW. The results revealed that the expression profiles could be classified into five patterns after heat shock (Fig. 3): pattern I responded weakly or not at all throughout diapause, pattern II responded weakly during diapause but strongly responded at the onset of diapause and in the post-diapause period, pattern III was upregulated on day 1 when larvae were transferred to room temperature but declined on days 3 and 5 after transfer to room temperature, pattern IV was strongest during diapause, and pattern V was strongest only in early diapause. Five genes were classified as showing pattern I: CfHSP18.6, CfHSP21.3, CfHSP22.0, CfHSP24.3, and CfHSP28.4. Pattern II was shown by four genes: CfHSP19.6, CfHSP21.5a, CfHSP22.1, and CfHSP23.9. Pattern III was shown by three genes: CfHSP19.2, CfHSP20.2, and CfHSP21.5b. Pattern IV was shown by one gene: CfHSP19.7. Interestingly, one gene, CfHSP 20.0 (Pattern V), exhibited a relatively constant response to heat shock during all the stages tested, although the response in the first week after hatching was two to four times stronger than at other sampling times.
Fig. 3.
Relative transcript levels of CfHSPs in SBW responding to heat stress during pre-diapause, diapause, and post-diapause. For heat shock treatment, larvae at pre-diapause (1, 2, and 3W), diapause (10 and 25W), and post-diapause (1, 3, and 5D) were heat stressed at 37 °C for 1 h or left at room temperature for 1 h as a control. The total RNAs were isolated from the heat-treated or control larvae, and the transcript levels of CfHSPs in the samples were quantified using RT-qPCR. Values are means ± SD of four biological replicates and are presented as relative expression levels between the stress and control after normalization to the expression of translation elongation factor-1α (Tef-1α). For each gene, different letters above the bars indicate significant differences compared with the other developmental times (P < 0.05)
In summary, CfHSP genes exhibited complex expression patterns after heat shock. Five genes showed no or very weak responses to heat shock, whereas nine genes showed responses that were variable in strength and dependent on the gene and stage of diapause. Four of the nine heat-inducible genes showed relatively weaker responses during the main stage of diapause, and three displayed stronger responses in post-diapause. One gene displayed a similar response to heat shock in most diapause stages and one gene had a stronger response during the main stage of diapause.
Discussion
The complex expression profiles of 14 small CfHSP genes in second-instar C. fumiferana larvae during diapause as well as pre- and post-diapause lead us to suggest that most of the sHSP genes are involved in this developmental process. Diapause is not just an interruption in development; it is an alternate developmental pathway, characterized by metabolic suppression, cell cycle arrestment, and increased resistance to environmental stress. All of these steps can be associated with gene expression patterns not observed in non-diapause-destined individuals (Flannagan et al. 1998; Denlinger 2002; Rinehart et al. 2010; King and MacRae 2015; Poupardin et al. 2015).
Adequate preparation for diapause is extremely important because failure to do so will reduce the likelihood of survival (Denlinger 2002). The early diapause genes represent a subcategory of upregulated genes that are expressed in pre- and early diapause, but that cease to be expressed at high levels in late diapause. They may be involved in the processes that bring development to a halt or remove developmental blocks that enable the termination of diapause (Denlinger 2002). The upregulation of HSPs including sHSPs in diapause has often been explained in terms of increased cold tolerance, suppression of development, cytoskeleton stabilization, or protein sequestering (Rinehart et al. 2010; King and MacRae 2015). We found three members showing pattern I (CfHSP20.2, CfHSP21.5b and CfHSP22.0) and four members showing pattern IV (CfHSP19.2, CfHSP19.7, CfHSP21.5a, and CfHSP24.3) that were highly expressed in the early stage of diapause. We propose that these sHSPs may be involved in the events cited above; however, further investigations are required to verify this.
It has been demonstrated in C. fumiferana as well as in other insect species such as L. dispar, Galleria mellonella, and Leptinotarsa decemlineata that proteins associated with diapause begin to accumulate just before the onset of diapause and are utilized when development resumes at the termination of diapause (de Kort 1996; Lee et al. 1998; Palli et al. 1998; Godlewski et al. 2001). The expression of mRNA during diapause has been previously studied in C. fumiferana. Feng et al. (2001) found that C. fumiferana glutathione S-transferase (CfGST) is variably expressed throughout developmental stages; they also determined that CfGST is highly expressed before and throughout diapause and that levels do not begin to decline until well after diapause has ended. These findings led Feng et al. (2001) to suggest that CfGST may have roles in detoxification. It is unclear whether or not the upregulated genes that code for these proteins have some relationship with the upregulated CfHSPs studied (pattern IV; Fig. 2). What is known is that early diapause is a complex process. This was recently demonstrated in the drosophilid fly Chymomyza costata, where early transcriptional events linked to the induction of diapause are involved in cell cycle regulation, metabolism of lipids, amino acids, organic acids, detoxification, redox balance, protection against oxidative stress, cuticle formation, and synthesis of larval storage proteins (Poupardin et al. 2015).
Once in diapause, the insect’s development is interrupted and new metabolic machinery that will sustain the larva during metabolic suppression is switched on. Development resumes only after environmental cues activate changes within the insect’s body. Genes associated with the action of ecdysteroids may be critical for regulating such diapause events. In C. fumiferana, both the ecdysteriod receptor (EcR) and the ultraspiracle (USP) are expressed throughout diapause. However, the ecdysone-inducible transcription factors Choristoneura hormone receptor 5 (CHR75A and CHR75B) and Choristoneura hormone receptor 3 (CHR3) are absent during diapause and reappear when diapause is terminated (Palli et al. 2001).
In the current study, we found six CfHSP genes upregulated in diapause, including four members showing pattern IV (CfHSP19.2, CfHSP19.7, CfHSP21.5a, CfHSP24.3) and two members showing pattern II (CfHSP19.6 and CfHSP22.1). In S. crassipalpis, at least six sHSPs are upregulated in overwintering pupae (Yocum et al. 1998; Rinehart and Denlinger 2000). These sHSPs do not determine the onset of diapause nor do they affect its duration. They do, however, have a profound effect on the pupa’s ability to survive at low temperature (Rinehart and Denlinger 2000). This cryoprotection may result from the maintenance of the integrity of key metabolic enzymes and/or structural proteins and/or the direct contribution to the regulation of diapause by ensuring the cell cycle is interrupted (Yocum et al. 1998; Rinehart and Denlinger 2000, Rinehart et al. 2006, 2007). The upregulated CfHSPs may also contribute to cold tolerance in C. fumiferana. However, the four genes showing pattern IV display different expression patterns from those of their counterparts in S. crassipalpis, i.e., they have relatively high expression during the pre- and main diapause periods. It is possible that this group has one or more other roles, such as triggering the onset of diapause and possibly regulating the anti-apoptosis process, a process crucial for maintaining cellular integrity during diapause. Certain sHSPs can control apoptosis during diapause by interacting with β-arrestin, pro-apoptotic members of the Bcl-2 family such as Bax and Bcl-Xs, cytochrome c, and caspases (Villeneuve et al. 2006; MacRae 2010). Whether this is the case in C. fumiferana can be determined through further experiments.
During the late or post-diapause stages, genes involved in controlling the mechanisms that suppress development are switched off and genes involved in development are switched on (Denlinger 2002). These late-diapause genes may be involved in the construction of new tissues and in meeting new metabolic requirements. For example, the activity of several digestive enzymes is promptly increased in the mid-gut of L. dispar upon the termination of diapause (Lee et al. 1998). In B. mori, the level of sorbitol dehydrogenase (SDH), the enzyme that catalyzes the conversion of sorbitol into glycogen for energy, increases at the termination of diapause (Niimi et al. 1993). In the sweet potato hornworm, Agrius convolvuli, a cytochrome c oxidase subunit is upregulated in the brain 24 h after the pupae are transferred for diapause termination (Uno et al. 2004). The increase in abundance of this electron transfer protein is associated with the boost in oxygen demand that accompanies the termination of diapause and resumption of development.
This study found that CfHSP18.6, CfHSP23.9, and CfHSP28.4 had their highest expression after the larvae were returned to room temperature (pattern V; Fig. 2). Under laboratory conditions, the previously diapausing larvae started to consume the diet provided after 24 h at room temperature and by 3–5 days post-diapause; 80–100% were actively feeding. This suggests that the upregulated expression of the three sHSPs may be involved in the completion of diapause and the resumption of development. Curiously, the upregulation of CfHSP18.6 was not observed until day 5 post-diapause. The reason for this late increase is unknown and needs to be deciphered via further experimentation.
Rezende et al. (2011) noted that the responses of sHSPs to heat shock can be complex. They depend on the speed of onset and the intensity of the stress. In this study, second instar larvae from pre- to post-diapause were heat treated using the conditions previously described by Quan et al. (2018). Interestingly, nine CfHSPs that were induced by heat in non-diapausing life stages had marked increases in transcript levels (~ 250 to 2400 times the control values), whereas the five non-heat-inducible CfHSPs responded weakly or not at all (~ 0 to 5 times the controls; Figs. 1 and 2). For example, CfHSP22.1 strongly responded to heat shock in SBW eggs, larvae, pupae, and adults and also did so during diapause in second instar larvae. CfHSP20.0 responded to heat shock during diapause in a manner similar to that observed during non-diapause (Fig. 2; Table 1).
Table 1.
Summary of the abundance of CfHSPs transcripts at several points throughout the life cycle of spruce budworm, including diapause and non-diapause developmental stages under non-stress and heat shock conditions. Data for non-diapause are taken from Quan et al. (2018). Developmental stages: Eg day 4 eggs, La day 4 sixth instar, Pu day 4 pupae, Ad day 0 adults, E early diapause, M mid diapause, L late diapause. Day 4 sixth instar tissues: He head, Ep epidermis, Fb fat body, Mg mid-gut, Mt Malpighian tubule
| Non-stress | Heat shock response | Non-stress | Heat shock response | |||
|---|---|---|---|---|---|---|
| Gene name | Stage | Tissue | Stage | Tissue | Diapause stage | Diapause stage |
| CfHsp18.6 | Eg = La = Pu = Ad | He = Ep = Fb = Mg = Mt | No | No | L > E = M | No |
| CfHsp19.2 | La = Pu = Ad>Eg | He = Ep = Fb = Mg = Mt | La = Pu = Ad>Eg | Mt ≥ Fb = He>Ep = Mg | E = M > L | L > E = M |
| CfHsp19.6 | Pu > La = Ad>Eg | Ep = Mg = Mt. > Fb = He | Eg > La = Pu = Ad | He>Ep = Fb = Mt. > Mg | M > L > E | L ≥ E > M |
| CfHsp19.7 | Ad>Eg = La > Pu | He = Ep = Fb = Mg = Mt | Ad>Eg = La = Pu | Fb = Mt. ≥ Ep = He = Mg | E = M > L | M > L = E |
| CfHsp20.0 | Ad>Eg = La > Pu | He = Ep = Mg = Mt. > Fb | Eg = La = Pu = Ad | Fb = He = Mg = Mt. > Ep | E = M = L | E = M = L |
| CfHsp20.2 | Ad>La = Pu > Eg | Mt > He = Ep = Mg > Fb | La = Pu > Ad>Eg | He = Ep = Fb = Mg > Mt | E > M = L | L > E = M |
| CfHsp21.3 | Ad>La > Eg = Pu | He = Ep > Fb = Mt. > Mg | No | No | E = M = L | No |
| CfHsp21.5a | Ad>Eg = La = Pu | He = Ep = Fb = Mg = Mt | La = Pu= > Ad>Eg | Fb = Mg = Mt. ≥ He = Ep | E = M > L | L ≥ E > M |
| CfHsp21.5b | Ad>La = Pu > Eg | He = Ep = Mt. > Fb > Mg | Ad>Eg = La = Pu | He = Ep = Fb = Mg > Mt | E > M = L | L > E = M |
| CfHsp22.0 | Ad>Eg = La > Pu | He = Ep > Mt. > Mg > Fb | No | No | E > M = L | No |
| CfHsp22.1 | Eg = La = Pu = Ad | He = Ep = Fb = Mg = Mt | Eg = La = Pu = Ad | He = Ep = Fb = Mg = Mt | M > E = L | L ≥ E > M |
| CfHsp23.9 | Eg = La = Pu = Ad | He = Ep = Fb = Mg = Mt | Eg = Pu = Ad>La | He≥Mt. = Ep = Mg > Mg | L > E = M | L ≥ E > M |
| CfHsp24.3 | Ad>Pu > Eg = La | He = Ep > Mt. > Fb = Mg | No | No | E = M > L | No |
| CfHsp28.4 | Ad>La = Pu > Eg | He = Ep = Fb = Mg = Mt | No | No | L > E = M | No |
These results may imply that the heat response regulating system is similar in diapausing and non-diapausing insects. Genes showing pattern II had high transcript levels before and after diapause, but relatively low levels during diapause; genes showing pattern III had the highest response after diapause, and CfHSP19.7 (Pattern IV) was highest during diapause. The heat-inducible CfHSP genes were sometimes strongly and sometimes weakly induced at different stages of diapause. It may be that different genes are responsible for managing stress at different times and that multiple genes are required to provide ongoing stress protection throughout the diapause process.
Phylogenetic and biological analyses suggest that most insect sHSPs have evolved independently in different insect orders (Huang et al. 2008; Li et al. 2009; Zhang et al. 2015), implying that species-specific sHSPs may greatly contribute to the adaptability of these insects in diverse environments. Previously, we compared the CfHSP genes with the 67 sHSPs isolated from four other lepidopteran species. We found that five CfHSPs are probably SBW-specific (CfHSP19.2, CfHSP 20.0, CfHSP21.5a, CfHSP22.1, and CfHSP23.9) (Quan et al. 2018). Remarkably, although these five SBW-specific CfHSPs displayed similar or different expression patterns, all are heat inducible in diapause (Figs. 2 and 3). It would be interesting to determine whether other species-specific lepidopteran sHSPs show similar expression patterns during diapause, and whether these genes significantly enhance stress tolerance, compared to other sHSPs. Studies of these genes may greatly contribute to our understanding why different pest insects are able to adapt to different environments, under what conditions their numbers may be repressed, and under what conditions they may thrive and possibly expand to outbreak status.
To understand how the expression of sHSPs might influence diapause, investigations are required at the level of the proteome and metabolome. Zhao and Jones (2012) reported that an increase of sHSPs at the transcript level is not always correlated with higher protein levels under heat stress. Therefore, further research is necessary to clarify which, if not all, of the sHSP RNAs are actually translated and at what diapause stages. The downregulation of sHSP using RNAi might dissect the roles played by the individual sHSPs by looking for lethality or for non-lethal morphological, temporal, or metabolic changes. Small HSPs work as molecular chaperones to bind substrate proteins, especially improperly folded proteins, and also work with other proteins, such as other ATP-dependent HSP, to form networks during most biological events (Jaya et al. 2009; Mymrikov et al. 2011; King and MacRae 2015). Therefore, identification of their substrates during each stage of diapause is important to understand the roles of individual sHSPs in diapause and their contribution to insect survival during various times of increased stress.
In this study, we established that many sHSP transcript levels changed dramatically during diapause initiation, maintenance, and termination. These pronounced changes may be useful as biomarkers to monitor diapause development and eventually to predict the distribution and outbreaks of C. fumiferana in the field.
We analyzed sHSP expression profiles under ideal laboratory conditions, but the field environment has very different conditions with fluctuations in temperature. Such fluctuations may have a stimulating or synchronizing effect on post-diapause development (Règnière 1987, 1990). Furthermore, thermal stress is usually accompanied by one more additional stress factors such as drought or high levels of solar irradiation. Therefore, it is important to investigate the relationship between CfHSP expression and diapause development under field conditions.
Acknowledgments
We greatly thank Insect Production Services at the Great Lake Forestry Centre for providing insect materials. We thank two anonymous reviewers for their constructive comments.
Funding information
This work was supported by grants from the Genomics Research and Development Initiative (GRDI) and the Canadian Forest Service, Natural Resources Canada.
References
- Arrigo AP. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol Chem. 1998;379:19–26. [PubMed] [Google Scholar]
- Basha E, O’Neill H, Vierling E. Small heat shock proteins and α-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci. 2012;37:106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais JR. Trends in the frequency, extent, and severity of spruce budworm outbreaks in eastern Canada. Can J For Res. 1983;13:539–547. doi: 10.1139/x83-079. [DOI] [Google Scholar]
- Boulanger Y, Arseneault D. Spruce budworm outbreaks in eastern Quebec over the last 450 years. Can J For Res. 2004;34:1035–1043. doi: 10.1139/x03-269. [DOI] [Google Scholar]
- Denlinger DL. Regulation of diapause. Annu Rev Entoml. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- de Kort CAD. Cosmic influences on the expression of a specific gene in the Colorado potato beetle: the diapause protein 1 gene. Arch Insect Biochem Physiol. 1996;32:567–573. doi: 10.1002/(SICI)1520-6327(1996)32:3/4<567::AID-ARCH28>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Dubrovsky EB, Dretzen G, Berger EM. The broad-complex gene is a tissue-specific modulator of the ecdysone response of the Drosophila hsp23 gene. Mol Cell Biol. 1996;16:6542–6552. doi: 10.1128/MCB.16.11.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, Schagina O, Verpoorten N, Van Impe K, Fedotov V, Dadali E. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004;36:602–606. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- Feng QL, Davey KG, Pang ASD, Ladd TR, Retnakaran A. Developmental expression and stress induction of glutathione S-transferase in the spruce budworm, Choristoneura fumiferana: developmental expression and the induction by various stresses. J Insect Physiol. 2001;47:1–10. doi: 10.1016/S0022-1910(00)00093-7. [DOI] [PubMed] [Google Scholar]
- Flannagan RD, Tammariello SP, Joplin KH, Cikra-Ireland RA, Yocum GD, Denlinger DL. Diapause-specific gene expression in pupae of the flesh fly Sarcophaga crassipalpis. Proc Natl Acad Sci U S A. 1998;95:5616–5620. doi: 10.1073/pnas.95.10.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremdt H, Amendt J, Zehner R. Diapause-specific gene expression in Calliphora vicina (Diptera: Calliphoridae)—a useful diagnostic tool for forensic entomology. Int J Legal Med. 2014;128:1001–1011. doi: 10.1007/s00414-013-0920-x. [DOI] [PubMed] [Google Scholar]
- Gkouvitsas T, Kontogiannatos D, Kourti A. Differential expression of two small Hsps during diapause in the corn stalk borer Sesamia nonagrioides (Lef.) J Insect Physiol. 2008;54:1503–1510. doi: 10.1016/j.jinsphys.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Godlewski J, Kludkiewicz B, Grzelak K, Cymborowski B. Expression of larval hemolymph proteins (Lhp) genes and protein synthesis in the fat body of greater wax moth (Galleria mellonella) larvae during diapause. J Insect Physiol. 2001;47:759–766. doi: 10.1016/S0022-1910(01)00050-6. [DOI] [PubMed] [Google Scholar]
- Haass CH, Klein UL, Kloetzel PM. Developmental expression of Drosophila melanogaster small heat-shock proteins. J Cell Sci. 1990;96:413–418. doi: 10.1242/jcs.96.3.413. [DOI] [PubMed] [Google Scholar]
- Han EN, Bauce E. Physiological changes and cold hardiness of spruce budworm larvae, Choristoneura fumiferana (Clem.), during pre-diapause and diapause development under laboratory conditions. Can Ent. 1993;125:1043–1053. doi: 10.4039/Ent1251043-6. [DOI] [Google Scholar]
- Hayward SA, Pavlides SC, Tammariello SP, Rinehart JP, Denlinger DL. Temporal expression patterns of diapause-associated genes in flesh fly pupae from the onset of diapause through post-diapause quiescence. J Insect Physiol. 2005;51:631–640. doi: 10.1016/j.jinsphys.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Huang LH, Wang HS, Kang L. Different evolutionary lineages of large and small heat shock proteins in eukaryotes. Cell Res. 2008;18:1074–1076. doi: 10.1038/cr.2008.283. [DOI] [PubMed] [Google Scholar]
- Huet F, Lage JD, Ruiz C, Richards G. The role of ecdysone in the induction and maintenance of hsp27 transcripts during larval and prepupal development of Drosophila. Dev Genes Evol. 1996;206:326–332. doi: 10.1007/s004270050059. [DOI] [PubMed] [Google Scholar]
- Hwang JS, Go HJ, Goo TW, Yun EY, Choi KH. The analysis of differentially expressed novel transcripts in diapausing and diapause-activated eggs of Bombyx mori. Arch Insect Biochem Physiol. 2005;59:197–201. doi: 10.1002/arch.20057. [DOI] [PubMed] [Google Scholar]
- Jaya N, Garcia V, Vierling E. Substrate binding site flexibility of the small heat shock protein molecular chaperones. Proc Natl Acad Sci U S A. 2009;106:15604–15609. doi: 10.1073/pnas.0902177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanisse DR, Michaud S, InagumaY TRM. Small heat shock proteins of Drosophila: developmental expression and functions. J Biosci. 1998;23:369–376. doi: 10.1007/BF02936130. [DOI] [Google Scholar]
- King AM, MacRae TH. Insect heat shock proteins during stress and diapause. Annu Rev Entomol. 2015;60:59–75. doi: 10.1146/annurev-ento-011613-162107. [DOI] [PubMed] [Google Scholar]
- Kokolakis G, Tatari M, Zacharopoulou A, Mintzas AC. The hsp27 gene of the Mediterranean fruit fly, Ceratitis capitata: structural characterization, regulation and developmental expression. Insect Mol Biol. 2008;17:699–710. doi: 10.1111/j.1365-2583.2008.00840.x. [DOI] [PubMed] [Google Scholar]
- Landry J, Chrétien P, Lambert H, Hickey E, Weber LA. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989;109:7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Valaitis AP, Denlinger DL. Activity of gut alkaline phosphatase, proteases and esterase in relation to diapause of pharate first instar larvae of the gypsy moth, Lymantria dispar. Arch Insect Biochem Physiol. 1998;37:197–205. doi: 10.1002/(SICI)1520-6327(1998)37:3<197::AID-ARCH2>3.0.CO;2-Q. [DOI] [Google Scholar]
- Li ZW, Li X, Yu QY, Xiang ZH, Kishino H, Zhang Z. The small heat shock protein (sHSP) genes in the silkworm, Bombyx mori, and comparative analysis with other insect sHSP genes. BMC Evol Biol. 2009;9:1–14. doi: 10.1186/1471-2148-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xi D, Kang M, Guo X, Xu B. Molecular cloning and characterization of Hsp27.6: the first reported small heat shock protein from Apis cerana cerana. Cell Stress Chaperones. 2012;17:539–551. doi: 10.1007/s12192-012-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu MX, Hua J, Cui YD, Du YZ. Five small heat shock protein genes from Chilo suppressalis: characteristics of gene, genomic organization, structural analysis, and transcription profiles. Cell Stress Chaperones. 2014;19:91–104. doi: 10.1007/s12192-013-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alpha A-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–793. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- MacRae TH. Gene expression, metabolic regulation and stress tolerance during diapause. Cell Mol Life Sci. 2010;67:2405–2424. doi: 10.1007/s00018-010-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahroof R, Zhu KY, Subramanyam B. Changes in expression of heat shock proteins in Tribolium castaneum (Coleoptera: Tenebrionidae) in relation to developmental stage, exposure time, and temperature. Ann Entomol Soc Am. 2005;98:100–107. doi: 10.1603/0013-8746(2005)098[0100:CIEOHS]2.0.CO;2. [DOI] [Google Scholar]
- McMorran A. A synthetic diet for the spruce budworm, Choristoneura fumiferana (Clem.)(Lepidoptera: Tortricidae) Can Ent. 1965;97:58–62. doi: 10.4039/Ent9758-1. [DOI] [Google Scholar]
- Morrow G, Heikkila JJ, Tanguay RM. Differences in the chaperone-like activities of the four main small heat shock proteins of Drosophila melanogaster. Cell Stress Chaperones. 2006;11:51–60. doi: 10.1379/CSC-166.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol Rev. 2011;91:1123–1159. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- Niimi T, Yamashita O, Yaginuma T. A cold-inducible Bombyx gene encoding a protein similar to mammalian sorbitol dehydrogenase. FEBS J. 1993;213:1125–1131. doi: 10.1111/j.1432-1033.1993.tb17862.x. [DOI] [PubMed] [Google Scholar]
- Palli SR, Kothapalli R, Feng Q, Ladd T, Perera SC, Zheng S-C, Gojtan K, Pang ASD, Primavera M, Tomkins W, Retnakaran A. Molecular analysis of overwintering diapause. In: Denlinger DL, Giebultowicz JM, Saunders DS, editors. Insect Timing: Circadian Rhythmicity to Seasonality (133–144) Amsterdam: Elsevier; 2001. [Google Scholar]
- Palli SR, Ladd TR, Ricci AR, Primavera M, Mungrue IN. Synthesis of the same two proteins prior to larval diapause and pupation in the spruce budworm, Choristoneura fumiferana. J Insect Physiol. 1998;44:509–524. doi: 10.1016/S0022-1910(97)00123-6. [DOI] [PubMed] [Google Scholar]
- Poupardin R, Schöttner K, Korbelová J, Provazník J, Doležel D, Pavlinic D, Beneš V, Koštál V. Early transcriptional events linked to induction of diapause revealed by RNAseq in larvae of drosophilid fly, Chymomyza costata. BMC Genomics. 2015;16:720. doi: 10.1186/s12864-015-1907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan G, Duan J, Ladd L, Krell PJ. Identification and expression analysis of multiple small heat shock protein genes in spruce budworm, Choristoneura fumiferana (L.) Cell Stress Chaperones. 2018;23:141–154. doi: 10.1007/s12192-017-0832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R. Cytoskeletal competence requires protein chaperones. Pro Mol Subcell Biol. 2002;28:219–234. doi: 10.1007/978-3-642-56348-5_12. [DOI] [PubMed] [Google Scholar]
- Rezende EL, Tejedo M, Santos M. Estimating the adaptive potential of critical thermal limits: methodological problems and evolutionary implications. Fun Ecol. 2011;25:111–121. doi: 10.1111/j.1365-2435.2010.01778.x. [DOI] [Google Scholar]
- Régnière J. Temperature-dependent development of eggs and larvae of Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae) and simulation of its seasonal history. Can Entomol. 1987;119:717–728. doi: 10.4039/Ent119717-7. [DOI] [Google Scholar]
- Régnière J. Diapause termination and changes in thermal responses during postdiapause development in larvae of the spruce budworm, Choristoneura fumiferana. J Insect Physiol. 1990;36:727–735. doi: 10.1016/0022-1910(90)90046-I. [DOI] [Google Scholar]
- Régnière J, St-Amant R, Duval P. Predicting insect distributions under climate change from physiological responses: spruce budworm as an example. Biol Invasions. 2012;14:1571–1586. doi: 10.1007/s10530-010-9918-1. [DOI] [Google Scholar]
- Rinehart JP, Denlinger DL. Heat shock protein 90 is downregulated during pupal diapause in the flesh fly, Sarcophaga crassipalpis, but remains responsive to thermal stress. Insect Mol Biol. 2000;9:641–645. doi: 10.1046/j.1365-2583.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- Rinehart JP, Hayward SA, Elnitsky MA, Sandro LH, Lee RE, Denlinger DL. Continuous up-regulation of heat shock proteins in larvae, but not adults, of a polar insect. Proc Natl Acad Sci U S A. 2006;103:14223–14227. doi: 10.1073/pnas.0606840103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart JP, Li A, Yocum GD, Robich RM, Hayward SA, Denlinger DL. Up-regulation of heat shock proteins is essential for cold survival during insect diapause. Proc Natl Acad Sci U S A. 2007;104:11130–11137. doi: 10.1073/pnas.0703538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart JP, Robich RM, Denlinger DL. Isolation of diapause-regulated genes from the flesh fly, Sarcophaga crassipalpis by suppressive subtractive hybridization. J Insect Physiol. 2010;56:603–609. doi: 10.1016/j.jinsphys.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Royama TO. Population dynamics of the spruce budworm Choristoneura fumiferana. Ecol Monogr. 1984;54:429–462. doi: 10.2307/1942595. [DOI] [Google Scholar]
- Saravanakumar R, Ponnuvel KM, Qadri SMH. Expression of metabolic enzyme genes and heat shock protein genes during embryonic development in diapause and non-diapause egg of multivoltine silkworm Bombyx mori. Biologia. 2008;63:737–744. doi: 10.2478/s11756-008-0124-x. [DOI] [Google Scholar]
- Sakano D, Li B, Xia Q, Yamamoto K, Fujii H, Aso Y. Genes encoding small heat shock proteins of the silkworm, Bombyx mori. Biosci Biotechnol Biochem. 2006;70:2443–2450. doi: 10.1271/bbb.60176. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Ashfaq M, Tsumuki H. Cloning and nucleotide sequencing of three heat shock protein genes (hsp90, hsc70, and hsp19.5) from the diamondback moth, Plutella xylostella (L.) and their expression in relation to developmental stage and temperature. Arch Insect Biochem Physiol. 2006;62:80–90. doi: 10.1002/arch.20124. [DOI] [PubMed] [Google Scholar]
- Stehr GW. On coniferophagous species of Choristoneura (Lepidoptera: Tortricidae) in North America: II. Geographic distribution in accordance with forest regions. Can Entomol. 1967;99:456–463. doi: 10.4039/Ent99456-5. [DOI] [Google Scholar]
- Takahashi KH, Rako L, Takano-Shimizu T, Hoffmann AA, Lee SF. Effects of small Hsp genes on developmental stability and microenvironmental canalization. BMC Evol Biol. 2010;10:1–11. doi: 10.1186/1471-2148-10-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammariello SP, Denlinger DL. G0/G1 cell cycle arrest in the brain of Sarcophaga crassipalpis during pupal diapause and the expression pattern of the cell cycle regulator, proliferating cell nuclear antigen. Insect Biochem Mol Biol. 1998;28:83–89. doi: 10.1016/S0965-1748(97)00082-9. [DOI] [PubMed] [Google Scholar]
- Tsvetkova NM, Horváth I, Török Z, Wolkers WF, Balogi Z, Shigapova N, Crowe LM, Tablin F, Vierling E, Crowe JH, Vígh L. Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci U S A. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungjitwitayakul J, Tatun N, Singtripop T, Sakurai S. Characteristic expression of three heat shock-responsive genes during larval diapause in the bamboo borer Omphisa fuscidentalis. Zool Sci. 2008;25:321–333. doi: 10.2108/zsj.25.321. [DOI] [PubMed] [Google Scholar]
- Uno T, Nakasuji A, Shimoda M, Aizono Y. Expression of cytochrome c oxidase subunit 1 gene in the brain at an early stage in the termination of pupal diapause in the sweet potato hornworm, Agrius convolvuli. J Insect Physiol. 2004;50:35–42. doi: 10.1016/j.jinsphys.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Villeneuve TS, Ma X, Sun Y, Oulton MM, Oliver AE, MacRae TH. Inhibition of apoptosis by p26: implications for small heat shock protein function during Artemia development. Cell Stress Chaperones. 2006;11:71–80. doi: 10.1379/CSC-154R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters ER, Rioflorido I. Evolutionary analysis of the small heat shock proteins in five complete algal genomes. J Mol Evol. 2007;65:162–174. doi: 10.1007/s00239-006-0223-7. [DOI] [PubMed] [Google Scholar]
- Waters ER. The evolution, function, structure, and expression of the plant sHSPs. J Exp Bot. 2013;64:391–403. doi: 10.1093/jxb/ers355. [DOI] [PubMed] [Google Scholar]
- Wieske M, Benndorf R, Behlke J, Dölling R, Grelle G, Bielka H, Lutsch G. Defined sequence segments of the small heat shock proteins HSP25 and αB-crystallin inhibit actin polymerization. FEBS J. 2001;268:2083–2090. doi: 10.1046/j.1432-1327.2001.02082.x. [DOI] [PubMed] [Google Scholar]
- Wood KL, Voss OH, Huang Q, Parihar A, Mehta N, Batra S, Doseff AI. The small heat shock protein 27 is a key regulator of CD8+ CD57+ lymphocyte survival. J Immunol. 2010;184:5582–5588. doi: 10.4049/jimmunol.0902953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum GD, Joplin KH, Denlinger DL. Upregulation of a 23 kDa small heat shock protein transcript during diapause in the flesh fly, Sarcophaga crassipalpis. Insect Biochem Mol Biol. 1998;28:677–682. doi: 10.1016/S0965-1748(98)00046-0. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zheng J, Peng Y, Liu X, Hoffmann AA, Ma CS. Stress responses of small heat shock protein genes in Lepidoptera point to limited conservation of function across phylogeny. PLoS One. 2015;10:e0132700. doi: 10.1371/journal.pone.0132700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Storey JM, Storey KB. Chaperone proteins and winter survival by a freeze tolerant insect. J Insect Physiol. 2011;57:1115–1122. doi: 10.1016/j.jinsphys.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Zhao L, Jones WA. Expression of heat shock protein genes in insect stress responses. Invertebrate Surviv J. 2012;90:93–101. [Google Scholar]