Abstract
The purpose of this study was to evaluate whether exposure to particles induces an imbalance in 70-kDa heat shock proteins (HSP70). Since intracellularly (iHSP70) it has anti-inflammatory roles whereas extracellularly (eHSP70) it has pro-inflammatory roles, we evaluate the effect of residual oil fly ash (ROFA) exposure on eHSP70-to-iHSP70 ratio (H index), a biomarker of inflammatory status that is related to oxidative stress in plasma and lymphoid tissue. Wistar rats that received ROFA suspension for three consecutive days (750 μg) showed an increase in plasma eHSP70 levels (mainly the 72-kDa inducible form). Also, ROFA promoted alterations on plasma oxidative stress (increased protein carbonyl groups and superoxide dismutase activity, and decrease sulfhydryl groups). There was an increase in H index of the plasma/thymus with no changes in circulating leukocyte level, iHSP70, or oxidative stress markers in lymphoid tissues. Our results support the hypothesis that eHSP70 content and H index represent inflammatory and oxidative biomarkers.
Keywords: Residual oil fly ash, eHSP70, iHSP70, Oxidative stress, Lymphoid tissue
Introduction
Fine particulate matter (PM2.5) is considered one of the most important environmental pollutants contributing to the global human disease burden (Mehta et al. 2013). PM2.5 originates primarily from the combustion of fossil compounds for energy generation and transport, and is associated with progressive changes in atmospheric composition (Ghio et al. 2012). The residue that remains after incomplete oxidation of carbon-containing compounds present in fuel is called residual oil fly ash (ROFA). ROFA is rich in metals and significantly contributes to urban PM2.5 (Ghio et al. 2002; Heck et al. 2015). ROFA exposure promotes oxidative stress (OS) in experimental models (Zanchi et al. 2008) and triggers a cell stress response, thereby contributing to changes in immune response (Delfosse et al. 2012, 2015).
The cellular stress response is essential for protecting cells and tissues against environmental stress, marked by increased expression of heat shock proteins (HSP), especially increased synthesis of 70-kDa heat shock proteins (HSP70) (Mukhopadhyay et al. 2003). This heat shock response (HSR) involves at least 13 genes in humans which are responsible for encoding proteins of the HSP70 family, such as HSPA1A (72-kDa inducible form, also known as HSP72) and HSPA8 (73-kDa constitutive form, also known as HSP73) (De Maio 2014; Heck et al. 2011). Intracellular HSP70 protein (iHSP70) have cytoprotective functions; however, during homeostasis-threatening situations, such as OS, many cells are able to release these proteins into the extracellular milieu (eHSP70), including lymphocytes (Heck et al. 2011, 2017; Hunter-Lavin et al. 2004; Scholer et al. 2016).
A pro-oxidant state evokes the HSR, and consequently, increases the levels of iHSP70 in order to maintain homeostasis by avoiding the formation of toxic polypeptide aggregates that may trigger apoptosis or inflammation (Ludwig et al. 2014; Newsholme and de Bittencourt 2014). Oxidative stress is also related to alterations in eHSP70 levels (Miragem et al. 2015), including under PM2.5 exposure (Gelain et al. 2011; Goettems-Fiorin et al. 2016). In this way, systemic inflammation and OS induced by PM2.5 in the bloodstream (Yamawaki and Iwai 2006) may be accompanied by increased plasma eHSP70 levels (Xia et al. 2017; Yang et al. 2007), suggesting that these proteins may represent biomarkers of environmental air pollution. Extracellular HSP70 also acts as a danger signal and activates pro-inflammatory pathways associated with conditions of OS (Heck et al. 2011, 2017). Thus, these proteins are pro-inflammatory biomarkers that reflect the activation of signaling pathways in response to environmental challenges (Bianchi 2007). Considering that eHSP70 has a pro-inflammatory role and iHSP70 does the opposite, the extracellular-to-intracellular HSP70 ratio (also known as the H index of HSP70 status) has previously been related to the immune inflammatory balance (Goettems-Fiorin et al. 2016; Heck et al. 2017; Scholer et al. 2016). As alterations in OS, eHSP70, and the H index can act as markers for the subclinical processes of tissue and systemic injury, we investigated whether ROFA exposure induces OS and an imbalance in stress-related proteins (H index).
Methods
Animals and ethics
Male Wistar rats (n = 24; 90 days old, weighing approximately 250 g) obtained from the Regional University of Northwestern State’s Rio Grande do Sul (UNIJUI) were housed in plastic cages (49 × 34 × 16 cm) and maintained at a controlled temperature (23 ± 1 °C) under a 12/12-h light/dark cycle (lights on at 7 a.m.). Throughout the experiments, the animals had free access to water and were fed with standard pelleted laboratory chow (NUVILAB CR-1; Nuvital Nutrients S.A., Curitiba, Brazil) ad libitum. All procedures were approved by the Committee of Animal Welfare (CEUA-UNIJUÍ, protocol no. 005/2014).
Experimental design
Animals were divided into a control group (n = 12) that received nasotropic instillation of 100 μL of saline or a ROFA group (n = 12) that received 100 μL of ROFA suspension (750 μg/100 μL; 2.5 mg/kg) once a day for three consecutive days. Rats were euthanized by decapitation 24 h after the last instillation, and blood samples were collected for eHSP70, OS, and leukogram analyses. The mesenteric lymph nodes, thymus, and spleen were collected for iHSP70 and OS analyses.
Characterization of particles
ROFA was obtained from the chimneys of a large steel plant in São Paulo, Brazil, using an electrostatic precipitator. Almost all particles had a diameter of less than 10 μm. The mean and standard deviation was 1.7 ± 2.56 μm for carbon particles and 1.2 ± 2.24 μm for transition metal particles. About 78% of carbon particles and 98% of transition metal particles had a diameter less than 2.5 μm. The elemental composition of particles in the ROFA suspension was determined by neutron activation analysis, and was found to have the following composition (ng/m3): Fe 1058.9 ± 2.37, Rb 719.7 ± 1.0, Zn 491.9 ± 3.1, As 154.4 ± 0.8, Cr 107.7 ± 1.4, V 35 ± 4, Ce 16.3 ± 0.3, La 10.3 ± 0.1, Co 9.9 ± 0.25, Mn 3.8 ± 24, Sb 2.2 ± 0.9, and Br 1.5 ± 19.
Sample processing
The rats were euthanized by decapitation and whole blood was collected into EDTA tubes (2 mg/mL); then, samples were centrifuged at 1800×g for 10 min to obtain the plasma. Lymphoid tissues were surgically excised. Plasma and lymphoid tissues were frozen, and samples of lymphoid tissues were homogenized in either 0.1% sodium dodecyl sulfate solution (Western blot), potassium phosphate solution (OS assays), or 0.02 mol/L EDTA solution (tissue sulfhydryl group assay), then centrifuged at 1800×g for 10 min at room temperature. The supernatant was frozen and stored in a freezer at − 20 °C. Protease inhibitors, including PMSF (100 μmol/L), leupeptin (4.2 μmol/L), aprotinin (0.31 μmol/L), TLCK (20 μmol/L), Na3VO4 (1 mmol/L), Na2MoO4 (1 mmol/L), and β-glicerophosphate, were added to the samples.
Determination of leukocyte content
Total leukocytes in the blood were quantified using a Neubauer chamber. The leukocyte differential count was performed using slides stained with the May-Grunwald-Giemsa method.
Protein quantification
eHSP70 and iHSP70 measurements and H index
In order to examine the eHSP70 content in the plasma and iHSP70 in the mesenteric lymph nodes, thymus, and spleen, the samples were processed and submitted to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli 1970). The amount of protein in the samples was assessed by the Bradford technique (Bradford 1976). An equivalent amount of protein from each tissue sample (~40 μg) and equivalent volume of plasma sample (10 μL) were mixed with Laemmli’s gel loading buffer and electrophoresed in a polyacrylamide mini-gel. Proteins were transferred onto nitrocellulose membranes and for the immunoblotting procedures with mouse anti-HSP70 monoclonal antibody. HSP70 detection was performed by the enhanced chemiluminescence method and protein bands were revealed in film. Actin detection in tissues was performed by Coomassie Blue staining of the gel. All bands were photodocumented and quantified using ImageJ software (http://imagej.nih.gov/). Full procedures details were described in Heck et al. (2017).
The extracellular to intracellular HSP70 ratio (H index) measured in each lymphoid tissue in relation to plasma values was calculated. Then, H index was calculated as the quotient of any RROFA = [eHSP70]/[iHSP70] by respective RCONTROL = [eHSP70]/[iHSP70], which will be therefore considered as the unity (RCONTROL = 1.0) in each plasma/tissue relation. Thus, we considered the RROFA/RCONTROL as the H index of each tissue (Goettems-Fiorin et al. 2016; Heck et al. 2017).
Protein carbonyls
Samples were incubated with 2,4-dinitrophenylhydrazine (DNPH) for 1 h, and proteins were precipitated with 3% sodium dodecyl sulfate denaturing solution; then, ethanol and hexane were added followed by vigorous shaking and centrifugation. Blanks were made for all samples, prepared according to the above description but containing hydrochloric acid solution (2 mol/L) instead of DNPH. Absorbance was measured at 370 nm and protein carbonyl groups were determined using the DNPH molar extinction coefficient (22,000 M−1 cm−1) (Levine et al. 1990).
Lipid peroxidation
Lipid peroxidation was assessed by the thiobarbituric acid reactive substance (TBARS) method (Buege and Aust 1978). The protein in homogenates was precipitated with 10% trichloroacetic acid and centrifuged; then, the supernatant was incubated with thiobarbituric acid (Sigma Chem. Co.) for 1 h at 100 °C. Afterwards, the absorbance was recorded by spectrophotometry at 535 nm using a malondialdehyde standard (Sigma).
Sulfhydryl groups
Free sulfhydryl groups in the plasma were measured by the 5′5′dithio-bis(2-nitrobenzoic acid) (DTNB) reaction from the consequent release of 5′thio-2-nitrobenzoic acid (TNB) (Himmelfarb et al. 2000). Aliquots of 50-μL plasma were added to 1 mL of Tris-EDTA solution (0.1 mol/L Tris and 10 mmol/L EDTA; pH 8.2), followed by the addition of 50 μL of DTNB (10 mmol/L in methanol). Blanks were made for all samples containing methanol instead of DTNB. Preparations were incubated for 15 min, and the absorbance was measured at 412 nm. The concentration was determined using the molar extinction coefficient of the TNB (14,100 M−1 cm−1). Aliquots of 100 μL of tissue homogenates were added to 300 μL of Tris solution (0.02 mol/L Tris; pH 8.2), 20 μL of DTNB (0.01 mol/L in methanol), and 1.58 mL of methanol. Blanks (without sample) and sample blanks (without DTNB) were prepared similarly. The final solutions were incubated for 15 min, then centrifuged at 1800×g for 15 min. Supernatant absorbance was measured at 412 nm. Total sulfhydryl groups were calculated using the DTNB molar extinction coefficient (13,100 M−1 cm−1) (Sedlak and Lindsay 1968).
Catalase and superoxide dismutase activities
In order to identify altered anti-oxidant enzyme activities in the plasma, we spectrophotometrically evaluated superoxide dismutase (SOD) and catalase (CAT) activities. SOD activity was measured by the inhibition of pyrogallol autooxidation, whereas CAT activity was evaluated by assessing the decomposition of hydrogen peroxide (Marklund and Marklund 1974).
Statistical analysis
The Kolmogorov-Smirnov normality test was applied before all analyses. Values are presented as the mean and standard deviation. The Mann-Whitney test was performed to compare the nonparametric variables (HSP70, SOD, and MDA from lymph nodes, and CAT from spleen), and the Student’s t test was performed to compare parametric variables (all other variables). The significance level was set at 5%.
Results
Animals exposed to ROFA showed higher total eHSP70 levels (P = 0.038; Fig. 1a). This increase was marked by higher levels of the inducible form, HSP72 (29% increase; P = 0.042; Fig. 1b), and not by changes in the constitutive form, HSP73 (P = 0.102; Fig. 1c), as observed in the representative immunoblot presented in Fig. 1d.
Fig. 1.
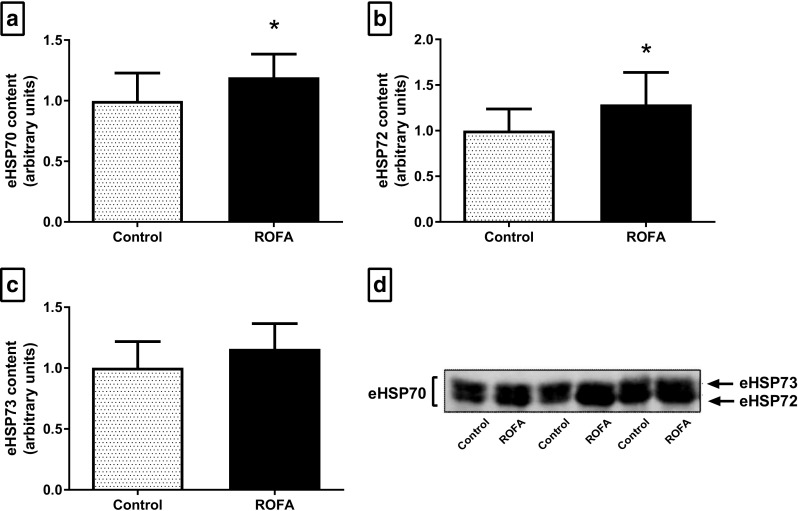
Extracellular HSP70 content in the plasma of animals submitted to subacute nasotropic instillation of residual oil fly ash (ROFA). a Total eHSP70 content. b Inducible 72-kDa isoform content. c Constitutive 73-kDa isoform content. d Representative immunoblot data. C control, R ROFA. c Values are presented as mean ± SD, n = 10–12 rats per group. Statistical analysis was performed by Student’s t test. *P < 0.05
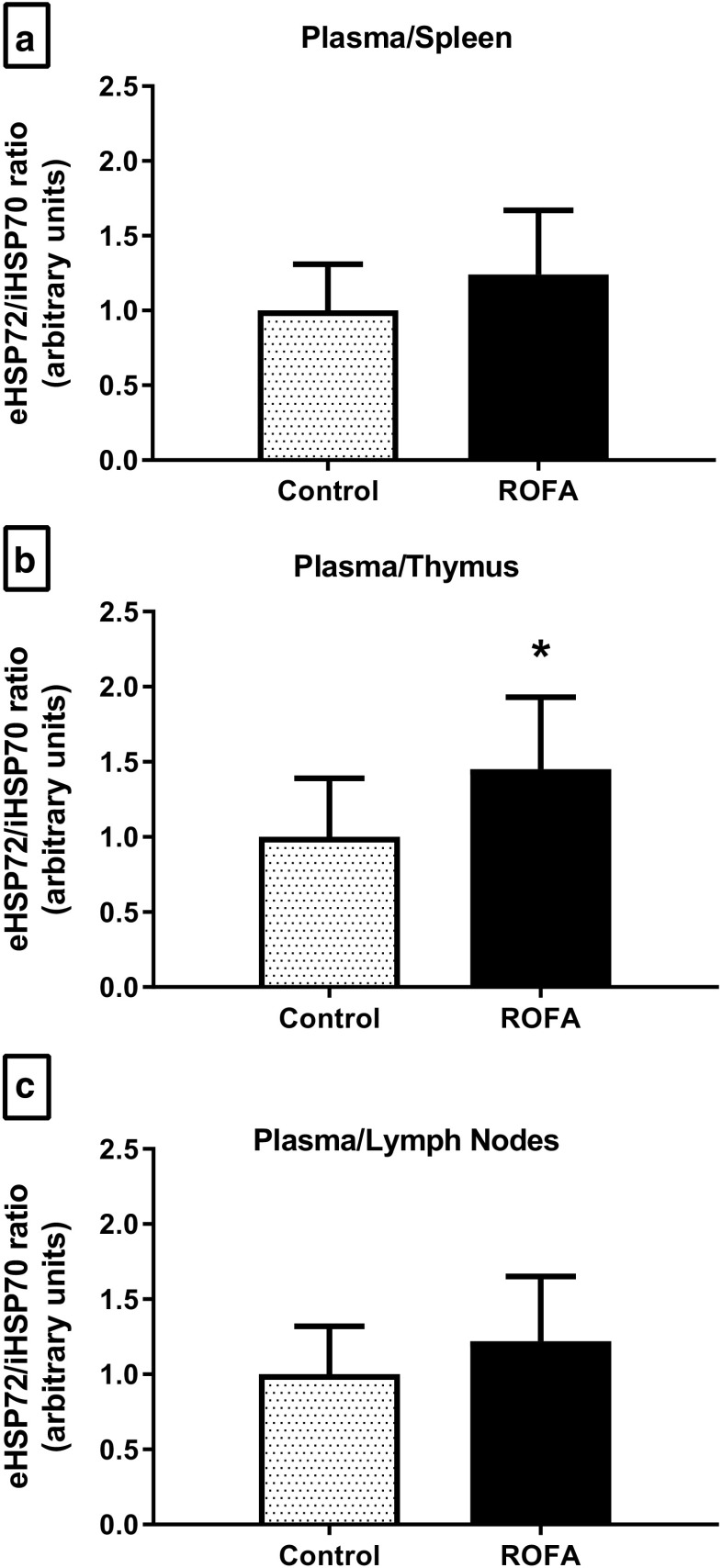
The lymphoid tissues studied (mesenteric lymph nodes, thymus, and spleen) showed no alterations in iHSP70 levels, nor in any of the OS-related variables tested (Fig. 2a–c). The extracellular-to-intracellular HSP70 ratio (H index) was measured by the plasma/lymphoid tissue relationship. Following ROFA exposure, the H index of animals showed an increase in the plasma/thymus ratio (P = 0.036; Fig. 3b), but there were no alterations in the plasma/spleen and plasma/lymph nodes ratios (Fig. 3a, c, respectively).
Fig. 2.
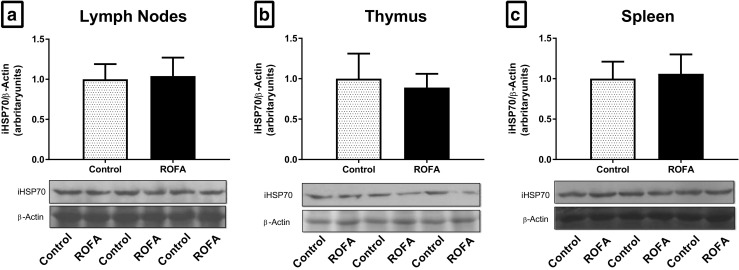
Intracellular HSP70 content in the lymphoid tissue of animals submitted to subacute nasotropic instillation of residual oil fly ash (ROFA). Intracellular HSP70 (iHSP70) content in a lymph nodes, b thymus, and c spleen with the respective representative immunoblots. C control, R ROFA. Values are presented as mean ± SD, n = 10–12 rats per group. Statistical analysis was performed by Student’s t test. *P < 0.05
Fig. 3.
Extracellular to intracellular HSP70 ratio (H index) in the plasma/lymphoid tissue of animals submitted to subacute nasotropic instillation of residual oil fly ash (ROFA). Extracellular to intracellular HSP70 ratio (plasma/tissue eHSP70/iHSP70 ratio, H index) in a lymph nodes, b thymus, and c spleen. C control, R ROFA. Values are presented as mean ± SD, n = 10–12 rats per group. Statistical analysis was performed by Student’s t test. *P < 0.05
Exposure to ROFA triggered redox imbalance in the plasma, as observed by an increased carbonyl group content (P = 0.044; Fig. 4a) and decreased total sulfhydryl group content (P = 0.007; Fig. 4b). ROFA exposure also promoted an imbalance in plasma anti-oxidant enzymes, as observed by increased SOD activity (P = 0.027; Fig. 4c) but no change in CAT activity (P = 0.347; Fig. 4d), and thus, an increased SOD/CAT ratio (P = 0.041; Fig. 4e). However, we found no alterations in the lipid peroxidation levels of plasma samples (P = 0.910; Fig. 4f), nor in the circulating leucocyte profile (Table 1).
Fig. 4.
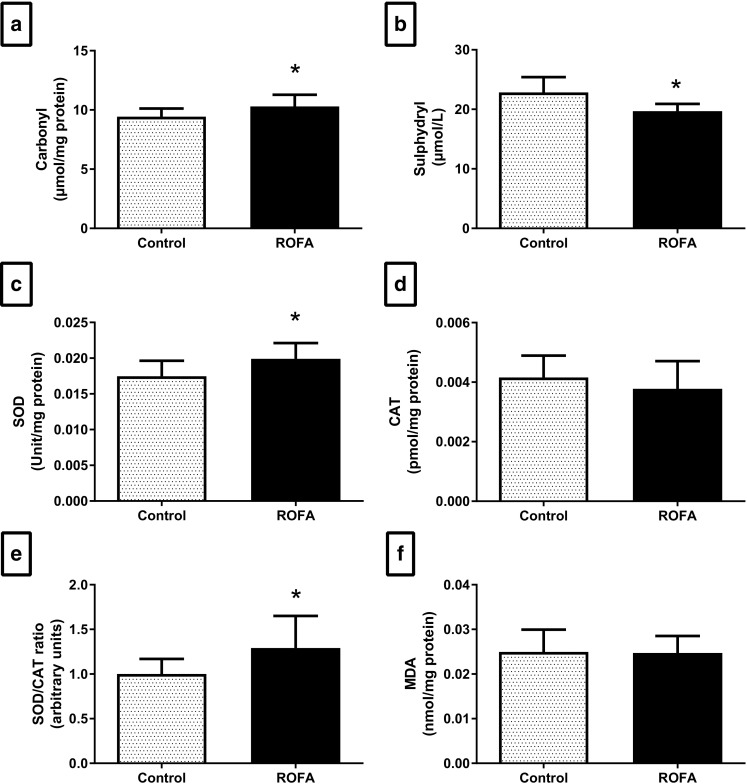
Plasma oxidative stress of animals submitted to subacute nasotropic instillation of residual oil fly ash (ROFA). a Protein carbonyl groups. b Superoxide dismutase (SOD) activity. c Catalase (CAT) activity. d Sulfhydryl groups. e SOD/CAT activity ratio. f Lipid peroxidation. Values are presented as mean ± SD, n = 8–10 rats per group. Statistical analysis was performed by Student’s t test. *P < 0.05
Table 1.
Leukocyte levels of animals submitted to subacute nasotropic instillation of residual oil fly ash (ROFA)
| Leukocytes | Control | ROFA | P value |
|---|---|---|---|
| Total leukocytes (cells/mm3) | 4879 ± 745 | 4388 ± 1512 | 0.323 |
| Lymphocytes (%) | 74 ± 8 | 74 ± 13 | 0.981 |
| Neutrophils (%) | 18 ± 6 | 19 ± 8 | 0.812 |
| Monocytes (%) | 10 ± 3 | 7 ± 5 | 0.122 |
| Band neutrophils (%) | 1 ± 0.4 | 2 ± 0.8 | 0.418 |
Values are presented as mean ± SD. Total leukocytes, lymphocytes, neutrophils, monocytes, and band neutrophils (n = 9–12 rats per group). Mann-Whitney test (to compare band neutrophils) and Student’s t test (to compare all other variables) were applied
Discussion
Our study is the first to show an increase in plasma eHSP70 levels and H index (plasma/tissue HSP70 ratio) in response to a few days of air pollutant exposure. Molecular weight analysis of HSP70 isoforms in the plasma samples also revealed an increase in eHSP72, but not eHSP73 content. Thus, our data reinforces the hypothesis of eHSP72 as the major stress response component in the extracellular milieu, with eHSP72 able to act as a danger signal between immune cells under adverse conditions (Heck et al. 2017; Ludwig et al. 2014; Scholer et al. 2016). The evidence accumulated over the past few years has indicated that the presence of eHSP70 in plasma or serum is a coordinated biological event (De Maio 2014), stimulating many discussions regarding the role of these proteins as signaling molecules and biomarkers. Although increased eHSP70 levels have been reported under adverse conditions, including environmental exposure (Goettems-Fiorin et al. 2016; Kido et al. 2011; Ye et al. 2014), our study reports the acute aspects of HSR and OS under an environmental pollution challenge.
Regarding environmental pollution extrapolation, in our study, we used three consecutive days of particle exposure in rats based on two aspects. First, we try to represent an urban risk condition. Since there is a high probability of the next day being polluted when the previous day was polluted (Mohamad et al. 2018), the polluted days may reach 200 days in a year (Wang et al. 2018). The formation of severe air pollution episodes is closely linked with the high emissions and secondary particle formation associated with unfavorable and stagnant meteorological conditions (e.g., when the daily wind speed is less than 3.2 m s−1, and there is no precipitation), representing an insufficient condition to disperse accumulated air pollutants (Wang et al. 2018). Thus, it is possible to observe that less than a half of pollution episodes have a 1-day duration only and that 2–3 days of recurrence of the polluted days represents one third of polluted days (Mohamad et al. 2018). Second, 3 days of ROFA exposure have been used as a toxicological protocol in different studies to investigate subacute inhalation toxicity, which means adverse effects occurring as a result of the repeated daily exposure of experimental animals to a chemical by inhalation for part of a life span (less than 10%) (as defined by the United States Environmental Protection Agency; https://www.epa.gov/). Also, this protocol has been used to investigate potential effects of particles regarding clinical and subclinical outcomes as mortality, impaired cardiovascular function, and increased oxidative stress damage (Killingsworth et al. 1997; Kodavanti et al. 1999).
Our data show a redox imbalance in the plasma that occurs concomitant with increasing levels of eHSP70. Intracellular HSP70 has previously been described to recognize redox changes and act to defend against cellular OS (Kalmar and Greensmith 2009; Miragem et al. 2015). In the same way, extracellular OS appears to be related to an increase in eHSP70 levels (Gelain et al. 2011), possibly to protect the components of the extracellular milieu from oxidative damage (Franco et al. 2016). The innate immune response, which recognizes, binds, and internalizes potential harmful particles by phagocytosis, is characterized by a dramatic increase in the production of reactive oxygen and nitrogen species. Thus, the innate immune response and HSP70 present two-way signaling in which the oxidative state produced by phagocytic cells induces HSR, which then induces iHSP70 synthesis to protect the immune system against OS and to facilitate phagocytosis. The HSR of the immune system generates a great release of eHSP70. In turn, eHSP70 has the capacity to activate phagocytic cells and modulate the immune response by interacting with several cell surface receptors, including those involved in phagocytosis. This two-way network promotes tissue repair by promoting the phagocytosis of cellular debris or damaged tissue, representing the positive aspects of increased eHSP70 levels and OS (Bianchi 2007; Calderwood et al. 2016; De Maio 2014; Franco et al. 2016).
The increase in circulating eHSP70 levels before challenges has a beneficial effect by improving animal survival (Tsai et al. 2015). Because eHSP70 has bivalent effects, either anti- or pro-inflammatory depending on the milieu and the receptors present on the surface of immune cells that encounter them (Calderwood et al. 2016), the release of eHSP70 is a key factor that triggers the immune response, and identifying the underlying mechanisms could contribute to developing strategies to prevent detrimental health effects. However, the pro-oxidant status may induce structural changes in eHSP70 that could lead to functional impairment of eHSP70 in the bloodstream (Grunwald et al. 2014). Thus, the combination of high levels of eHSP70 and a pro-oxidant status in plasma, as observed in our study, may represent a pathological biomarker (Gelain et al. 2011) and dysfunction between eHSP70 and the immune system.
It is well established that lymphocytes are able to release a greater amount of eHSP70 under stress conditions than macrophages (Heck et al. 2011, 2017; Hunter-Lavin et al. 2004). In our study, exposure to ROFA did not modify the iHSP70 content in the mesenteric lymph nodes, thymus, or spleen, all of which are tissues that could potentially be affected by systemic OS. Thus, we postulate that the high plasma eHSP72 content observed in animals exposed to ROFA was not derived from lymphoid tissues. Despite this, the levels of eHSP72 in the circulation, and importantly the increase in H index, indicate an unfavorable condition. The increased plasma/tissue HSP70 ratio may signal that a particular organ is at risk. Thus, the increased plasma/thymus HSP70 ratio (H index of 1.45) represents a subclinical pro-inflammatory state, as quantification of total and differential leukocytes (considered clinical parameters) were not altered.
Although cells may release iHSP70 after cell disruption, eHSP70 is mainly exported through a specialized non-canonical secretory mechanism via exosomes and lipid rafts (Asea 2007; Heck et al. 2017). Once secreted, eHSP70 works by binding to toll-like receptors on a variety of cells, thereby leading to the activation of pro-inflammatory pathways. Also, lung epithelial cells of the respiratory tract are able to increase both iHSP70 expression and eHSP70 release, whereas the last represents an important endogenous danger signal to activate the host innate immune response, a process mediated by induced cytokine gene expression in neutrophils dependent upon activation of both TLR-4 and NF-κB (Wheeler et al. 2009). In this way, eHSP70 may act as an effector molecule downstream of TLR4 signaling involved in the regulation pollution-induced lung inflammation (Bauer et al. 2011). Remarkably, the H index is directly correlated with the ratio of pro/anti-inflammatory cytokines, which, in turn, are positively associated with a pro-inflammatory state (Heck et al. 2017; Ye et al. 2014). Our results support the hypothesis that high eHSP70 content and extracellular-to-intracellular HSP70 ratio (H index) represent a warning signal and oxidative biomarker under environmental challenges. These measures can be used as subclinical biomarkers of the effects of exposure to environmental pollutants. As a perspective, we recommended future studies that investigate two major issues regarding stress response against air pollutant challenge: the intracellular location of iHSP70 in lymphoid issues and the source of eHSP70 that is found as a signaling molecule in the bloodstream.
Acknowledgments
The authors would like to thank R.D.B. Basso and E.G. de P. Basso (UNIJUI), P.I.H. Bittencourt (UFRGS), P.H.N. Saldiva (USP), and colleagues from the Laboratory of Atmospheric Pollution (UFCSPA) for their technical support. Also we would like to thank professor John Willians (Chester University) for scientific discussion.
Financial support
This work was supported by the Federal University of Health Sciences of Porto Alegre (UFCSPA) and by grants awarded to TGH from the Research Support Foundation of the State of Rio Grande do Sul (PqG-2013-FAPERGS process #002106–2551/13–5 and ARD/PPP/FAPERGS/CNPq-08/2014 process #16/2551–0000196-6), the The Brazilian National Council for Scientific and Technological Development (UNIVERSAL MCTI/CNPq process #407329/2016–1), and Coordination for the Improvement of Higher Education Personnel Development (CAPES-PGCI process #88887.141981/2017–00). FGB, PBGF, and ABS were recipients of scholarships from CAPES.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32:579–584. doi: 10.1007/s12038-007-0057-5. [DOI] [PubMed] [Google Scholar]
- Bauer AK, Rondini EA, Hummel KA, Degraff LM, Walker C, Jedlicka AE, Kleeberger SR. Identification of candidate genes downstream of TLR4 signaling after ozone exposure in mice: a role for heat-shock protein 70. Environ Health Perspect. 2011;119(8):1091–1097. doi: 10.1289/ehp.1003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Gong J, Murshid A. Extracellular HSPs: the complicated roles of extracellular HSPs in immunity. Front Immunol. 2016;7:159. doi: 10.3389/fimmu.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maio A. Extracellular Hsp70: export and function. Curr Protein Pept Sci. 2014;15:225–231. doi: 10.2174/1389203715666140331113057. [DOI] [PubMed] [Google Scholar]
- Delfosse VC, Gioffre AK, Tasat DR. Low levels of residual oil fly ash (ROFA) impair innate immune response against environmental mycobacteria infection in vitro. Toxicol in Vitro. 2012;26:1001–1006. doi: 10.1016/j.tiv.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Delfosse VC, Tasat DR, Gioffre AK. In vivo short-term exposure to residual oil fly ash impairs pulmonary innate immune response against environmental mycobacterium infection. Environ Toxicol. 2015;30:589–596. doi: 10.1002/tox.21936. [DOI] [PubMed] [Google Scholar]
- Franco L, Terrinca J, Rodriguez AB, Espino J, Pariente JA. Extracellular heat shock proteins protect U937 cells from H2O2-induced apoptotic cell death. Mol Cell Biochem. 2016;412:19–26. doi: 10.1007/s11010-015-2604-y. [DOI] [PubMed] [Google Scholar]
- Gelain DP, de Bittencourt Pasquali MA, M Comim C, Grunwald MS, Ritter C, Tomasi CD, Alves SC, Quevedo J, Dal-Pizzol F, Moreira JC. Serum heat shock protein 70 levels, oxidant status, and mortality in sepsis. Shock. 2011;35:466–470. doi: 10.1097/SHK.0b013e31820fe704. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Silbajoris R, Carson JL, Samet JM. Biologic effects of oil fly ash. Environ Health Perspect. 2002;110(Suppl 1):89–94. doi: 10.1289/ehp.02110s1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev. 2012;15:1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- Goettems-Fiorin PB, Grochanke BS, Baldissera FG, dos Santos AB, Homem de Bittencourt PI, Ludwig MS, Rhoden CR, Heck TG. Fine particulate matter potentiates type 2 diabetes development in high-fat diet-treated mice: stress response and extracellular to intracellular HSP70 ratio analysis. J Physiol Biochem. 2016;72:643–656. doi: 10.1007/s13105-016-0503-7. [DOI] [PubMed] [Google Scholar]
- Grunwald MS, Pires AS, Zanotto-Filho A, Gasparotto J, Gelain DP, Demartini DR, Schöler CM, de Bittencourt PIH, Moreira JCF. The oxidation of HSP70 is associated with functional impairment and lack of stimulatory capacity. Cell Stress Chaperones. 2014;19:913–925. doi: 10.1007/s12192-014-0516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck TG, Scholer CM, de Bittencourt PI. HSP70 expression: does it a novel fatigue signalling factor from immune system to the brain? Cell Biochem Funct. 2011;29:215–226. doi: 10.1002/cbf.1739. [DOI] [PubMed] [Google Scholar]
- Heck TG, Nunes RB, Petry MR, Maslinkiewicz A, Saldiva PHN, Lago PD, Rhoden CR. Residual oil fly ash (ROFA) inhalation promotes lung and heart oxidative stress without hemodynamic effects in exercising rats. J Exerc Physiol Online. 2015;17:11. [Google Scholar]
- Heck TG, Scomazzon SP, Nunes PR, Schöler CM, da Silva GS, Bittencourt A, Faccioni-Heuser MC, Krause M, Bazotte RB, Curi R, Homem de Bittencourt PI. Acute exercise boosts cell proliferation and the heat shock response in lymphocytes: correlation with cytokine production and extracellular-to-intracellular HSP70 ratio. Cell Stress Chaperones. 2017;22:271–291. doi: 10.1007/s12192-017-0771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000;58:2571–2578. doi: 10.1046/j.1523-1755.2000.00443.x. [DOI] [PubMed] [Google Scholar]
- Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Kalmar B, Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev. 2009;61:310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Kido T, Bai N, Yatera K, Suzuki H, Meredith A, Mukae H, Rosenfeld ME, van Eeden SF. Diesel exhaust inhalation induces heat shock protein 70 expression in vivo. Inhal Toxicol. 2011;23:593–601. doi: 10.3109/08958378.2011.595843. [DOI] [PubMed] [Google Scholar]
- Killingsworth CR, Alessandrini F, Murphy GGK, Catalano PJ, Paulauskis JD, Godleski JJ. Inflammation,chemokine expression, and death in monocrotaline-treated rats following fuel oil fly ash inhalation. Inhal Toxicol. 1997;9:541–565. doi: 10.1080/089583797198060. [DOI] [Google Scholar]
- Kodavanti UP, Jackson MC, Ledbetter AD, Richards JR, Gardner SY, Watkinson WP, Campen MJ, Costa DL. Lung injury from intratracheal and inhalation exposures to residual oil fly ash in a rat model of monocrotaline-induced pulmonary hypertension. J Toxicol Environ Health A. 1999;57:543–563. doi: 10.1080/009841099157502. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine RL, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-H. [DOI] [PubMed] [Google Scholar]
- Ludwig MS, Minguetti-Camara VC, Heck TG, Scomazzon SP, Nunes PR, Bazotte RB, Homem de Bittencourt PI., Jr Short-term but not long-term hypoglycaemia enhances plasma levels and hepatic expression of HSP72 in insulin-treated rats: an effect associated with increased IL-6 levels but not with IL-10 or TNF-alpha. Mol Cell Biochem. 2014;397:97–107. doi: 10.1007/s11010-014-2176-2. [DOI] [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem / FEBS. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Mehta S, Shin H, Burnett R, North T, Cohen AJ. Ambient particulate air pollution and acute lower respiratory infections: a systematic review and implications for estimating the global burden of disease. Air Qual Atmos Health. 2013;6:69–83. doi: 10.1007/s11869-011-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miragem AA, Ludwig MS, Heck TG, Baldissera FG, dos Santos AB, Frizzo MN, Homem de Bittencourt PI., Jr Estrogen deprivation does not affect vascular heat shock response in female rats: a comparison with oxidative stress markers. Mol Cell Biochem. 2015;407:239–249. doi: 10.1007/s11010-015-2472-5. [DOI] [PubMed] [Google Scholar]
- Mohamad NS, Deni SM, Ul-Saufie AZ. Application of the first order of markov chain model in describing the pm10 occurrences in shah alam and jerantut, Malaysia Pertanika. J Sci Technol. 2018;1:367–378. [Google Scholar]
- Mukhopadhyay I, Nazir A, Saxena DK, Chowdhuri DK. Heat shock response: hsp70 in environmental monitoring. J Biochem Mol Toxicol. 2003;17:249–254. doi: 10.1002/jbt.10086. [DOI] [PubMed] [Google Scholar]
- Newsholme P, de Bittencourt PI., Jr The fat cell senescence hypothesis: a mechanism responsible for abrogating the resolution of inflammation in chronic disease. Curr Opin Clin Nutr Metab Care. 2014;17:295–305. doi: 10.1097/MCO.0000000000000077. [DOI] [PubMed] [Google Scholar]
- Scholer CM, Marques CV, da Silva GS, Heck TG, de Oliveira Junior LP, Homem de Bittencourt PI., Jr Modulation of rat monocyte/macrophage innate functions by increasing intensities of swimming exercise is associated with heat shock protein status. Mol Cell Biochem. 2016;421:111–125. doi: 10.1007/s11010-016-2791-1. [DOI] [PubMed] [Google Scholar]
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Tsai TN, Lee TY, Liu MS, Chuang IC, Lu MC, Dong HP, Lue SI, Yang RC. Release of endogenous heat shock protein 72 on the survival of sepsis in rats. J Surg Res. 2015;198:165–174. doi: 10.1016/j.jss.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Wang X, Dickinson RE, Su L, Zhou C, Wang K. PM2.5 pollution in China and how it has been exacerbated by terrain and meteorological conditions. Bull Am Meteorol Soc. 2018;99:105–119. doi: 10.1175/bams-d-16-0301.1. [DOI] [Google Scholar]
- Wheeler DS, Chase MA, Senft AP, Poynter SE, Wong HR, Page K. Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via toll-like receptor (TLR)-4. Respir Res. 2009;10:31. doi: 10.1186/1465-9921-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia B, Chen K, Lv Y, Huang D, Liu J, Liang G, Zhang L’, Wang F, Su C, Zou Y, Yang X. Increased oxidative stress and plasma Hsp70 levels among gasoline filling station attendants. Toxicol Ind Health. 2017;33:171–181. doi: 10.1177/0748233715616554. [DOI] [PubMed] [Google Scholar]
- Yamawaki H, Iwai N. Mechanisms underlying nano-sized air-pollution-mediated progression of atherosclerosis: carbon black causes cytotoxic injury/inflammation and inhibits cell growth in vascular endothelial cells. Circ J. 2006;70:129–140. doi: 10.1253/circj.70.129. [DOI] [PubMed] [Google Scholar]
- Yang X, Zheng J, Bai Y, Tian F, Yuan J, Sun J, Liang H, Guo L, Tan H, Chen W, Tanguay RM, Wu T. Using lymphocyte and plasma Hsp70 as biomarkers for assessing coke oven exposure among steel workers. Environ Health Perspect. 2007;115:1573–1577. doi: 10.1289/ehp.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Zhu R, He X, Feng Y, Yang L, Zhu X, Deng Q, Wu T, Zhang X. Association of plasma IL-6 and Hsp70 with HRV at different levels of PAHs metabolites. PLoS One. 2014;9:e92964. doi: 10.1371/journal.pone.0092964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchi AC, Venturini CD, Saiki M, Nascimento Saldiva PH, Tannhauser Barros HM, Rhoden CR. Chronic nasal instillation of residual-oil fly ash (ROFA) induces brain lipid peroxidation and behavioral changes in rats. Inhal Toxicol. 2008;20:795–800. doi: 10.1080/08958370802009060. [DOI] [PubMed] [Google Scholar]