Abstract
Heat shock protein 60 (HSP60) is a highly conserved chaperone molecule that plays important roles in mediating some physiological and pathological functions. However, researchers have not yet determined whether HSP60 is expressed in the mammalian cochlea. This study constitutes the first investigation of the expression of HSP60 in the postnatal rat cochlea. We also examined the expression of HSP60 in rats with drug-induced hearing loss. Auditory thresholds were assessed by monitoring the auditory brainstem response (ABR) prior to and after drug injection. Expression levels of the HSP60 gene (Hsp60) and HSP60 protein in the rat cochlea were detected by quantitative real-time polymerase chain reaction and Western blotting, respectively. The distribution of HSP60 in the rat cochlea was further examined by immunofluorescence staining. We have demonstrated that HSP60 was expressed in the postnatal rat cochlea in an age-dependent and cell-specific manner. In addition, after drug exposure, the average hearing threshold of rats in the experimental group was significantly higher than that in the control group, with increased HSP60 expression level in response to kanamycin and furosemide treatments. HSP60 expression was observed in the supporting cells (SCs) within the organ of Corti in both the uninjured and the injured cochlea, but it was undetectable in the mechanosensory hair cells (HCs) and spiral ganglion neurons. Therefore, our research suggests that HSP60 may play an important role in auditory function.
Keywords: Heat shock protein 60, Hearing loss, Cochlea, Supporting cell, Hair cell
Introduction
Sensorineural hearing loss (SNHL) is a common sensory impairment in humans that not only affect the quality of life of the affected individuals but also impose a heavy social and economic burden on families and the community. The WHO estimates that over 360 million people worldwide have disabling hearing loss (Yu et al. 2017). Aging, noise trauma, infections, hereditary mutations, and ototoxic drugs are all causes of hearing loss. Therapeutic drugs with ototoxic side effects include aminoglycoside antibiotics, loop diuretics, nonsteroidal anti-inflammatory drugs, and some anticancer medications (Alharazneh et al. 2011; Liu et al. 2011; Sun et al. 2015; Jadali et al. 2017). In most situations, these drugs primarily induce the degeneration of or damage to HCs. Because cochlear HCs are considered terminally differentiated cells without the ability to regenerate and the loss of HCs is permanent in mammals, the survival of HCs is indispensable for the preservation of hearing.
Heat shock proteins (HSPs) are highly conserved and widely expressed proteins in both prokaryotes and eukaryotes. Heat shock preconditioning results in the upregulation of inducible HSP family members, which exert protective effects on a wide variety of cellular stresses (Richter et al. 2010; Rampelt et al. 2012). Heat shock protein 60 (HSP60), a member of the HSP family, has a molecular weight of 60 kDa. HSP60 is localized in the mitochondria and cytosol and translocates to the plasma membrane or is released from apoptotic and necrotic cells to perform extracellular functions (Lin et al. 2007; Cappello et al. 2009; Osterloh et al. 2009). It has been proposed that HSP60 is involved in the initiation of cancer and autoimmune, metabolic, neurodegenerative, cardiovascular diseases. Most HSPs are prosurvival proteins, although both prosurvival and prodeath roles of HSP60 have been reported. Several studies have demonstrated that upregulated HSP60 expression provided antiapoptotic roles in cancers, such as colorectal, cervical, and prostate cancers (Ghosh et al. 2008). However, HSP60 is likely an endogenous inducer of dopaminergic cell death in Parkinson’s disease (Noelker et al. 2014). HSP60-induced neonatal heart failure likely involves developmental defects and excessive apoptosis (Heiserman et al. 2015).
To date, the expression and function of HSP60 in the mammalian inner ear have not been explored. The present study was designed to determine the expression pattern of HSP60 and to investigate the primary function of HSP60 in the mammalian inner ear. First, we observed the expression pattern of HSP60 in the mammalian inner ear. Furthermore, we evaluated the role of HSP60 in the process of HC death during drug-induced hearing loss following co-administration of kanamycin and furosemide. HSP60 was expressed in the postnatal cochlea in an age-dependent and cell-specific manner, the expression levels of HSP60 were significantly upregulated 7 days after drug administration, and HSP60 expression was observed in the cytoplasm of the cochlear supporting cells (SCs) through immunofluorescence staining. In summary, we assumed that HSP60 may play a crucial role in the cochlea.
Materials and methods
Animal experiments and ethics statement
In the first part of the study, 40 Sprague-Dawley (SD) rats were analyzed at different ages, including postnatal day (P) 0, P3, P7, P14, and P28. Then, 20 SD rats (weighing 100–120 g, aged 4–5 weeks) with Preyer’s reflex were randomly allocated into two groups: the experimental group, which was administered drugs, and the control, which was administered a saline solution. Seven days after drug administration, the animals’ hearing levels were measured using auditory brainstem response (ABR). All animals were euthanized within 24 h after the ABR test. The cochleae were separated and collected for quantitative real-time polymerase chain reaction (qRT-PCR), Western blot, and immunofluorescence assays.
Procedures for drug administration
The protocols used in the present study were performed as described by Liu et al. (2011). Briefly, the animals were anesthetized with an intraperitoneal injection of pentobarbital sodium (40 mg/kg). During surgery, the rats were fixed in place and kept warm with a heating pad. In the experimental group, furosemide (100 mg/kg, Kingyork Group, China) was intravenously injected through the rat external carotid vein. Immediately afterward, kanamycin sulfate (500 mg/kg, Amresco) was intramuscularly injected into the inside of the thigh. Each animal in the control group was injected with the same amount of saline solution. All solutions mentioned above were freshly prepared before use.
ABR measurements
ABR was detected before and after drug administration to evaluate the level of hearing loss. ABR was detected in a sound-attenuated and electrically shielded room. The animals were anesthetized, fixed in place, and kept warm. An active electrode was inserted under the skin of the scalp, a reference electrode was inserted beneath the pinna of the measured ear, and a ground electrode was placed beneath the root of the tail; all needle electrodes were placed subcutaneously. The ABR in response to a click stimulus and the thresholds were recorded for each ear. The stimulus signal was generated using an Intelligent Hearing Systems device (Bio-logic Systems, USA) and was delivered by an earphone. The responses to 1024 click presentations were amplified and synchronously averaged. The stimulus was changed systematically in steps of 5-dB sound pressure levels (SPLs) until the response disappeared. The ABR threshold was defined using the third wave in the present study.
qRT-PCR
The protocols used in this study were performed as described by Ding et al. (2015). The rats were decapitated after receiving anesthesia. Temporal bones were placed in ice-cold normal saline, and the cochleae were quickly removed by dissection. The cochleae with the temporal bone were stored at − 80 °C until use. Total RNA was isolated using an RNA extraction kit (Qiagen), and genomic DNA was extracted using the QuantiTect Reverse Transcription Handbook (Qiagen), according to the manufacturer’s instructions. The quantitative PCR analysis was performed using a PCR kit (Qiagen) and RNase-free 96-well PCR plates. The primers for Hspd1 and GAPDH were purchased from GeneCopoeia (catalog nos.: RQP050362, RQP049537). The qRT-PCR assays were repeated three times using an Applied Biosystems 7300 real-time PCR system (Bio-Rad, CFX96™ Real-Time System). GAPDH was used as an endogenous reference (Wu et al. 2007; Yu et al. 2017).
Western blot analysis
The cochleae were harvested and lysed with RIPA buffer (Protein Biotechnology) containing protein inhibitor cocktail (Sigma) for 30 min at 4 °C. The samples were centrifuged at 14000 rpm for 30 min at 4 °C, and protein concentrations were calculated using the BCA Protein Assay Kit (Protein Biotechnology). A total of 30 μg of each protein sample was denatured, separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels, and transferred to polyvinylidene fluoride (PVDF) membranes (Roche Diagnostics). After blocking in 5% skim milk, the membranes were incubated for 2 h at room temperature. The membranes were then incubated with mouse anti-HSP60 monoclonal antibody (1:1000, Millipore) or rabbit anti-GAPDH polyclonal antibody (1:1000, Proteintech) overnight at 4 °C. The membranes were incubated with peroxidase-labeled secondary antibodies (1:2000, Santa Cruz Biotechnology) at room temperature for 2 h and were detected by enhanced chemiluminescence (Millipore). The band intensity was measured, and the values were normalized to GAPDH (Song et al. 2018).
Tissue preparation and immunofluorescence assays
As reported previously (Zhao et al. 2015), the rats were deeply anesthetized and perfused through the heart with normal saline. Next, the cochleae were fixed with 4% paraformaldehyde overnight. Samples were decalcified in 10% EDTA for 5 to 7 days. Subsequently, some cochleae were dissected to obtain the cochlear sensory epithelium, and the others were dehydrated in 30% sucrose solution for 12 h, sectioned into 10-μm-thick slices across the modiolus, and stored at − 80 °C until immunofluorescence staining.
For the immunofluorescence assays, the samples were treated with 1% Triton X-100 for 10 min and then incubated for 30 min in a blocking solution containing 5% bovine serum albumin (BSA, Sigma). The samples were then incubated with the following primary antibodies overnight at 4 °C: mouse anti-HSP60 monoclonal antibody (1:100, Millipore), rabbit anti-myosin7a monoclonal antibody (1:1000, Proteus Bioscience), and goat anti-SOX2 polyclonal antibody (1:100, Santa Cruz Biotechnology). Alexa Fluor 488-conjugated donkey anti-mouse (1:200, Life Technologies), Alexa Fluor 594-conjugated donkey anti-goat (1:200, Invitrogen), and Alexa Fluor 594-conjugated donkey anti-rabbit (1:200, Invitrogen) secondary antibodies were subsequently incubated with the sections overnight at 4 °C. Sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI) (1:1000, Sigma) for 10 min to stain the nuclei. Finally, the specimens were evaluated under a confocal microscope (Olympus FV1000). Negative control experiments were performed as described above, but the primary antibody was omitted.
Statistical analysis
All data are expressed as means ± standard deviations (SD). The statistical analyses were performed using one-way analysis of variance (ANOVA) or t tests, as appropriate, with Statistical Package for the Social Sciences software (SPSS, version 19.0). P values < 0.05 were considered statistically significant.
Results
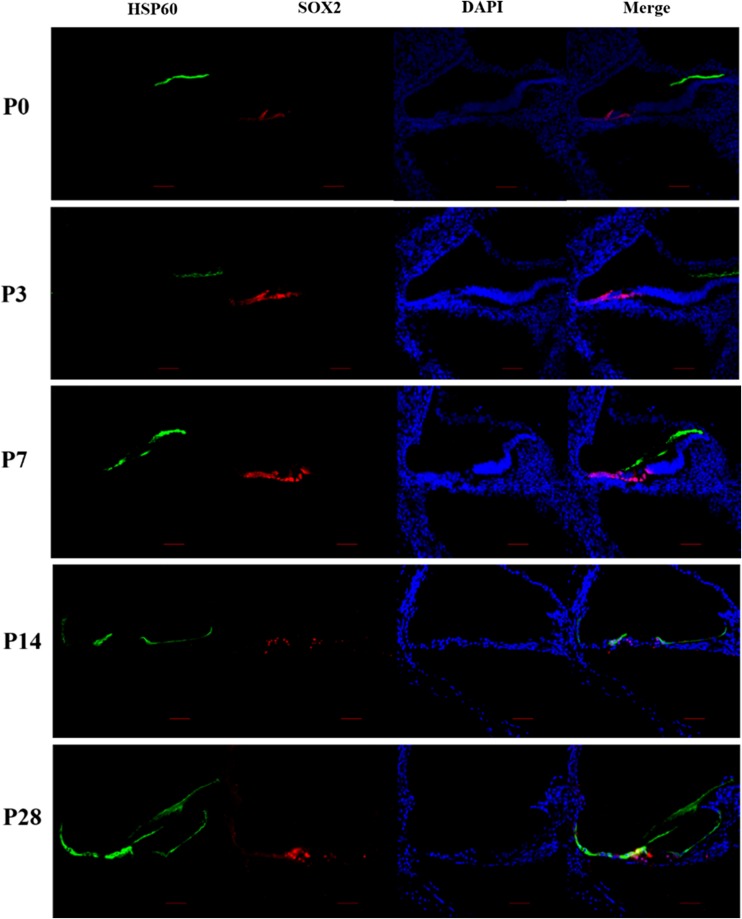
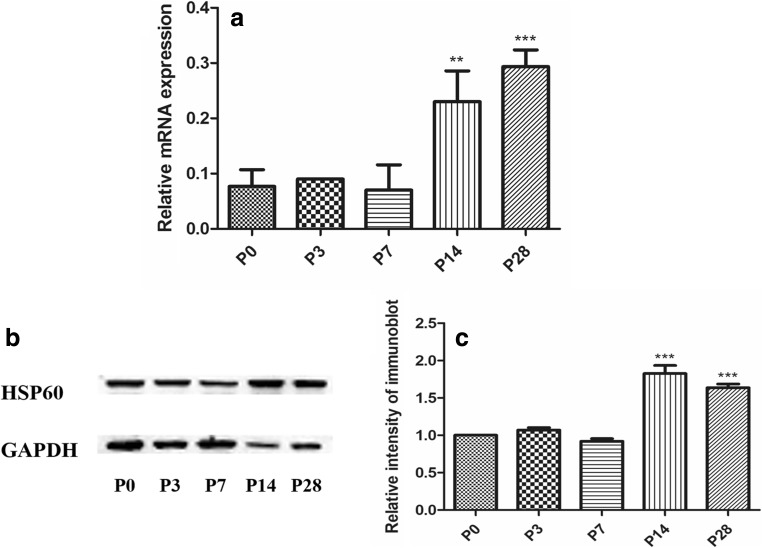
To characterize the expression pattern of HSP60 in the cochlea, we performed immunofluorescence on cochlear cryosections from rats at different postnatal ages. Sox2 staining was used to label the nuclei in SCs within the organ of Corti, serving as a marker of the SCs (Hume et al. 2007). The expression of HSP60 in the cochlea changed from P0 to P28 (Fig. 1). Immunostaining did not detect HSP60 expression in the P0, P3, and P7 cochlea. At P14, robust HSP60 expression was observed in SCs. Beginning at P28, HSP60 was expressed at high levels in SCs but was still not expressed in hair cell (HCs) and spiral ganglion neurons. HSP60 was expressed in SCs in the organ of Corti, including inner sulcus cells, inner border cells, Hensen’s cells, and Claudius cells. Then, qRT-PCR and Western blotting assays were performed to confirm the levels of HSP60 mRNA and protein, respectively, in the SD rat cochlea (Fig. 2). qRT-PCR revealed that the levels of Hsp60 mRNA were higher at P14 and P28 (Fig. 2a). Similarly, HSP60 protein expression increased at P14 and P28 as shown by Western blot (Fig. 2b, c). Based on these results, HSP60 is expressed in an age-dependent and cell-specific manner in the organ of Corti of postnatal rats.
Fig. 1.
HSP60 expression in the rat cochlea. Immunofluorescence staining in cryosections of the cochlea showed the expression pattern of HSP60 (green) in rats at different postnatal ages. Sox2 (red) was used as a SC marker. Scale bars = 20 μm
Fig. 2.
The expression level of HSP60 was upregulated during later postnatal development. qRT-PCR and WB showed the Hsp60 mRNA and the protein levels of HSP60 at different time points, respectively. GAPDH severed as a housekeeping gene **p < 0.01, ***p < 0.001, versus P0
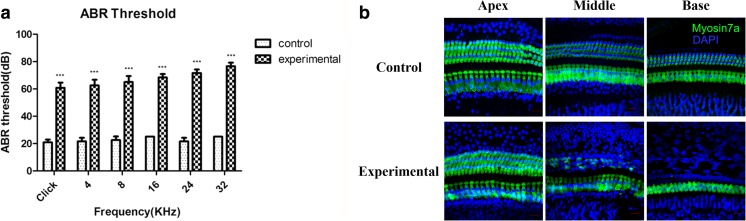
Furthermore, all rats in the experimental group were administered furosemide and kanamycin to induce hearing loss, and we examined whether HSP60 expression in the cochlea was affected by the drug treatment. Prior to the injection, all animals had hearing levels that were essentially equivalent to normal, as determined by ABR measurements. During drug treatment, the rats in the control group were administered sterile saline. ABR was detected 7 days after drug administration to evaluate functional hearing impairments (Versnel et al. 2007; Liu et al. 2011). In contrast to the control group, the ABR thresholds were increased significantly in the experimental group (P < 0.001), and the higher thresholds measured at the higher frequency (Fig. 3a). In the experimental group, HC loss was observed in a gradient from the apex to the base, with the basal turns displaying greater HC loss than the apical turns. This pattern of HC loss corresponded to that of ABR threshold increasement recorded at different sound frequencies (Fig. 3b). These results confirmed the successful establishment of a drug-induced hearing loss model.
Fig. 3.
Establishment and validation of the drug-induced hearing loss model. a ABR thresholds of the experimental group increased after drug injection. ***p < 0.001. b Immunofluorescence staining demonstrated HCs loss in the experimental group. Myosin7a (green) was used as a HC marker. Scale bar = 20 μm
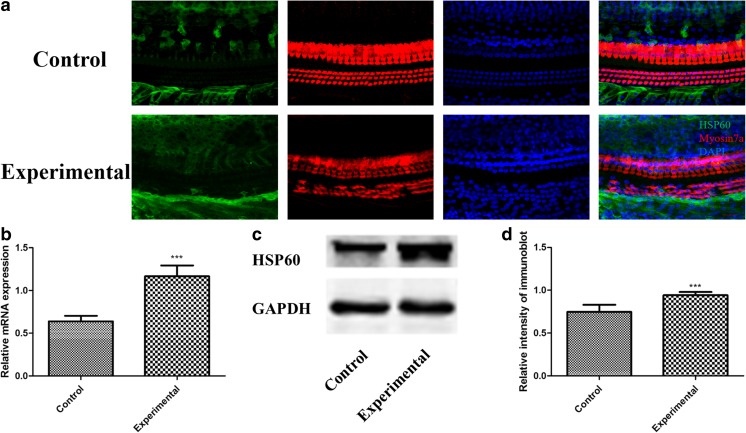
Because HSP60 had the same expression pattern in all three turns of the cochlea, we selected the middle turn as a representative area (Fig. 4a). Myosin7a was used as an HC marker (Guan et al. 2016). Immunostaining of both control and experimental cochleae showed HSP60 expression was observed in SCs but absent in HCs (Fig. 4a). HSP60 expression was increased in the SCs after drug treatment compared with the undamaged control. qRT-PCR was performed to determine the levels of Hsp60 mRNA in the cochlea and confirmed that Hsp60 mRNA levels increased in the cochlea after drug treatment (Fig. 4b). Similarly, Western blotting analyses confirmed that HSP60 protein levels increased in the cochlea following drug administration (Fig. 4c, d). Thus, drug-induced injury increased HSP60 expression in the cochlea, indicating that HSP60 might have an essential role in the cochlea.
Fig. 4.
Upregulation of HSP60 expression in the cochlea after drug injury. a Immunofluorescence staining showed that HSP60 (green) expression was increased in the experimental group. Myosin7a (red) was used as a HC marker. Scale bar = 20 μm. b, c, and d qRT-PCR and Western blot analysis confirmed that the expression levels of Hsp60 gene and HSP60 protein in the experimental group were significantly higher than those in the control group ***p < 0.001
Discussion
On the basis of present work, we demonstrated the expressions of HSP60 by immunofluorescence, RT-PCR and Western blot analysis, with the aim of identifying their localizations, expression levels, and the potential functions within the cochlea form postnatal rats. After 7 days from drug administration, the saline-administrated animals in the control group whose hearing levels were equivalent to normal had no HCs loss seen in the sensory epithelium, and their cochlear HSP60 immunoreactivity together with mRNA and protein expression levels were lower at a baseline level (compared with the experimental group). While the aminoglycoside-administrated animals in the experimental group had developed a substantial hearing impairment, including a gradient HCs loss from the apical to basal turn, and ABR threshold shifts recorded at different sound frequencies corresponding to the cochlear sensory HCs distribution, and HSP60 immunoreactivity together with mRNA and protein expression levels were significantly elevated compared with those in the control group. Therefore, an indirect correlation between HSP60 expression and the auditory test results could be seen from the “Results” section.
The heat shock protein (HSP) family includes both constitutive and inducible proteins; the constitutive forms are involved in a variety of critical cell functions, whereas the inducible forms are transcriptionally upregulated in response to stress. HSPs are known to have cytoprotective functions, often as molecular chaperones participating in folding, targeting, and degradation of proteins and as inhibitors of apoptotic pathways. HSP is involved in hepatocellular carcinoma development, and its overexpression may be a useful early diagnostic marker (Wu et al. 2007). HSP might play a critical and fundamental role in the capacity of the kidney to modulate both the apoptotic pathway and oxidative stress during development (Mazzei and Manucha 2017). Particularly, some previous studies have described the importance of HSP60 in the nervous system, cardiomyocytes, the colonic epithelium, and certain other tissues. HSP60 is expressed in the developing tooth germs, and increased levels of HSP60 might cause abnormalities in the morphological development of the tooth germ (Papp et al. 2016). Since HSP60 has not been investigated in the mammalian cochlea, our study shows the first demonstration of HSP60 expression in the mammalian cochlea. In the postnatal organ of Corti, HSP60 is specifically expressed in SCs and is upregulated during later postnatal development. Thus, we speculate that HSP60 is specifically expressed in SCs in the mammalian cochlea and might play a role in the maturation and differentiation of the postnatal cochlear sensory epithelium.
Aminoglycosides, such as kanamycin, are widely used in clinical practice to treat bacterial infections, but their accompanying ototoxic side effects greatly limit their clinical use (Zimmerman and Lahav 2013). Loss of HCs after exposure to ototoxic agents causes hearing loss. In the current study, we confirmed that co-administration of furosemide and kanamycin induced HC loss in a gradient from the base to the apex, a typical manifestation of drug-induced ototoxicity. HSP60 was expressed in SCs, but not in HCs, in both control and experimental cochleae. In cochlear SCs, HSP60 expression increased after drug exposure. HC damage results in an intercellular calcium wave that propagates in the SCs and moves away from the site of damage, suggesting that SCs generate a specific response to HC stress (Lahne and Gale 2010). HSP60 was shown to be released into the extracellular space by cardiomyocytes during heart failure, inducing apoptosis by binding to Toll-like receptor (TLR)-4 (Lin et al. 2007; Heiserman et al. 2015). In cultured utricles, SCs were shown to secrete HSPs, which could protect HCs from damage (May et al. 2013). Thus, HSP60 might play important roles in stress-induced apoptosis or in protecting cells against drug-induced damage, and SCs might be involved in mediating HCs damage by secreting HSP60. However, further studies are needed to confirm the exact role of HSP60 in aminoglycoside antibiotic-induced damage to cochlear HCs and to determine the possible mechanisms.
In conclusion, we provide the first report of the dynamic expression pattern of HSP60 in the rat cochlea. Moreover, HSP60 expression was increased in cochlear SCs after drug exposure. This initial study revealed the upregulation of HSP60 in cochlear SCs, which was correlated with the damage to sensory HCs in the cochlea of rats with drug-induced hearing loss. Many previous studies investigating the mechanisms underlying drug-induced hearing loss focused on the HCs of the inner ear, whereas few study has examined the SCs that contact these cells. In the last decade, numerous studies have sought to clarify the roles of HSP60 in the immune system, carcinogenesis, and apoptosis, with the aim of finding new therapies for a wide array of human diseases (Nakamura and Minegishi 2013). HSP60 might provide a new target for studying the role of SCs in the inner ear. Further in-depth studies are required to clearly define the function and mechanism of HSP60 in cochlear cells.
Funding information
This study was supported by the National Natural Science Foundation of China (grant numbers 81120108008, 81400459, 81271069 and 8110097).
Compliance with ethical standards
All procedures related to the use and care of animals in this study were approved by the Institutional Animal Care and Use Committee of the Fourth Military Medical School.
References
- Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, Ricci AJ. Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One. 2011;6:e22347. doi: 10.1371/journal.pone.0022347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello F, Conway de Macario E, Di Felice V, Zummo G, Macario AJ. Chlamydia trachomatis infection and anti-Hsp60 immunity: the two sides of the coin. PLoS Pathog. 2009;5:e1000552. doi: 10.1371/journal.ppat.1000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZJ, Chen X, Tang XX, Wang X, Song YL, Chen XD, Mi WJ, Wang J, Lin Y, Chen FQ, Qiu JH. Calpain inhibitor PD150606 attenuates glutamate induced spiral ganglion neuron apoptosis through apoptosis inducing factor pathway in vitro. PLoS One. 2015;10:e0123130. doi: 10.1371/journal.pone.0123130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JC, Dohi T, Kang BH, Altieri DC. Hsp60 regulation of tumor cell apoptosis. J Biol Chem. 2008;283:5188–5194. doi: 10.1074/jbc.M705904200. [DOI] [PubMed] [Google Scholar]
- Guan M, Fang Q, He Z, Li Y, Qian F, Qian X, Lu L, Zhang X, Liu D, Qi J, Zhang S, Tang M, Gao X, Chai R. Inhibition of ARC decreases the survival of HEI-OC-1 cells after neomycin damage in vitro. Oncotarget. 2016;7:66647–66659. doi: 10.18632/oncotarget.11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiserman JP, Chen L, Kim BS, Kim SC, Tran AL, Siebenborn N, Knowlton AA. TLR4 mutation and HSP60-induced cell death in adult mouse cardiac myocytes. Cell Stress Chaperones. 2015;20:527–535. doi: 10.1007/s12192-015-0577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume CR, Bratt DL, Oesterle EC. Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expr Patterns. 2007;7:798–807. doi: 10.1016/j.modgep.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadali A, Ying YM, Kwan KY. Activation of CHK1 in supporting cells indirectly promotes hair cell survival. Front Cell Neurosci. 2017;11:137. doi: 10.3389/fncel.2017.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahne M, Gale JE. Damage-induced cell-cell communication in different cochlear cell types via two distinct ATP-dependent ca waves. Purinergic Signal. 2010;6:189–200. doi: 10.1007/s11302-010-9193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Kim SC, Wang Y, Gupta S, Davis B, Simon SI, Torre-Amione G, Knowlton AA. HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H2238–H2247. doi: 10.1152/ajpheart.00740.2007. [DOI] [PubMed] [Google Scholar]
- Liu H, Ding DL, Jiang HY, Wu XW, Salvi R, Sun H. Ototoxic destruction by co-administration of kanamycin and ethacrynic acid in rats. J Zhejiang Univ Sci B. 2011;12:853–861. doi: 10.1631/jzus.B1100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May LA, Kramarenko II, Brandon CS, Voelkel-Johnson C, Roy S, Truong K, Francis SP, Monzack EL, Lee FS, Cunningham LL. Inner ear supporting cells protect hair cells by secreting HSP70. J Clin Invest. 2013;123:3577–3587. doi: 10.1172/JCI68480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzei L, Manucha W. Growing evidence suggests WT1 effects in the kidney development are modulated by Hsp70/NO interaction. J Nephrol. 2017;30:11–18. doi: 10.1007/s40620-016-0302-9. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Minegishi H. HSP60 as a drug target. Curr Pharm Des. 2013;19:441–451. doi: 10.2174/138161213804143626. [DOI] [PubMed] [Google Scholar]
- Noelker C, Morel L, Osterloh A, Alvarez-Fischer D, Lescot T, Breloer M, Gold M, Oertel WH, Henze C, Michel PP, Dodel RC, Lu L, Hirsch EC, Hunot S, Hartmann A. Heat shock protein 60: an endogenous inducer of dopaminergic cell death in Parkinson disease. J Neuroinflamm. 2014;11:86. doi: 10.1186/1742-2094-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh A, Geisinger F, Piédavent M, Fleischer B, Brattig N, Breloer M. Heat shock protein 60 (HSP60) stimulates neutrophil effector functions. J Leukoc Biol. 2009;86:423–434. doi: 10.1189/jlb.0109011. [DOI] [PubMed] [Google Scholar]
- Papp T, Polyak A, Papp K, Meszar Z, Zakany R, Meszar-Katona E, Tünde PT, Ham CH, Felszeghy S. Modification of tooth development by heat shock protein 60. Int J Oral Sci. 2016;8:24–31. doi: 10.1038/ijos.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampelt H, Kirstein-Miles J, Nillegoda NB, Chi K, Scholz SR, Morimoto RI, Bukau B. Metazoan Hsp70 machines use Hsp110 to power protein disaggregation. EMBO J. 2012;31:4221–4235. doi: 10.1038/emboj.2012.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Song YL, Tian KY, Mi WJ, Ding ZJ, Qiu Y, Chen FQ, Zha DJ, Qiu JH. Decreased expression of TERT correlated with postnatal cochlear development and proliferation reduction of cochlear progenitor cells. Mol Med Rep. 2018;17:6077–6083. doi: 10.3892/mmr.2018.8565. [DOI] [PubMed] [Google Scholar]
- Sun W, Liu J, Zhang C, Zhou N, Manohar S, Winchester W, Miranda JA, Salvi RJ. Potassium channel activator attenuates salicylate-induced cochlear hearing loss potentially ameliorating tinnitus. Front Neurol. 2015;6:77. doi: 10.3389/fneur.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versnel H, Agterberg MJ, de Groot JC, Smoorenburg GF, Klis SF. Time course of cochlear electrophysiology and morphology after combined administration of kanamycin and furosemide. Hear Res. 2007;231:1–12. doi: 10.1016/j.heares.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Wu XH, Yao DF, Su XQ, Tai BJ, Huang H, Qiu LW, Wu W, Shao YX. Dynamic expression of rat heat shock protein gp96 and its gene during development of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2007;6:616–621. [PubMed] [Google Scholar]
- Yu X, Liu W, Fan Z, Qian F, Zhang D, Han Y, Xu L, Sun G, Qi J, Zhang S, Tang M, Li J, Chai R, Wang H. c-Myb knockdown increases the neomycin-induced damage to hair-cell-like HEI-OC1 cells in vitro. Sci Rep. 2017;7:41094. doi: 10.1038/srep41094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HB, Zhu Y, Liang C, Chen J. Pannexin 1 deficiency can induce hearing loss. Biochem Biophys Res Commun. 2015;463:143–147. doi: 10.1016/j.bbrc.2015.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman E, Lahav A. Ototoxicity in preterm infants: effects of genetics, aminoglycosides, and loud environmental noise. J Perinatol. 2013;33:3–8. doi: 10.1038/jp.2012.105. [DOI] [PubMed] [Google Scholar]