Abstract
Heat shock proteins (HSPs) are a family of proteins produced by cells in response to exposure to stressful conditions. In addition to their role as chaperones, they also play an important role in the cardiovascular, immune, and other systems. Normal bone tissue is maintained by bone metabolism, particularly by the balance between osteoblasts and osteoclasts, which are physiologically regulated by multiple hormones and cytokines. In recent years, studies have reported the vital role of HSPs in bone metabolism. However, the conclusions remain largely controversial, and the exact mechanisms are still unclear, so a review and analyses of previous studies are of importance. This article reviews the current understanding of the roles and effects of HSPs on bone cells (osteoblasts, osteoclasts, and osteocytes), in relation to bone metabolism.
Keywords: Heat shock proteins, Bone metabolism, Osteoblasts, Osteoclasts, Osteocytes
Introduction
Bone metabolism
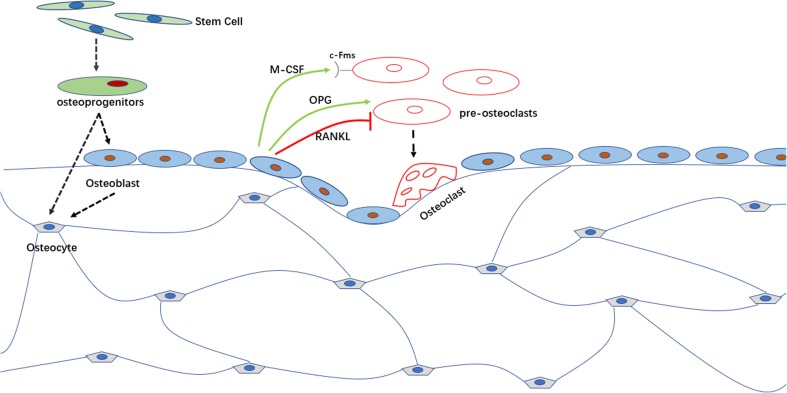
Bone tissue is a dynamic system that is controlled mainly by three types of cells: osteoblasts, which form bone, osteoclasts, which resorb bone (Raggatt and Partridge 2010), and osteocytes, which are derived from osteoprogenitors, are the most numerous cells in bone, representing 90–95% of the total amount (Capulli et al. 2014). The osteocyte is an important regulator of bone mass and a key endocrine regulator of phosphate metabolism (Dallas and Bonewald 2010). Osteocytes not only synthesize sclerostin, which inhibits bone formation by binding to LRP5/LRP6 co-receptors and blunting Wnt signaling (Nakashima 2015), but also express a great amount of RANKL (Nakashima et al. 2012) that drives bone resorption (see Fig. 1). In addition, osteal macrophages near the bone surface also play an important role in bone remodeling. They create a regenerative microenvironment in the fracture healing processes, construct a cellular canopy structure over bone remodeling sites, coordinate osteoclast-to-osteoblast coupling, and drive anabolic cytokines for bone formation (Nakashima et al. 2012). Maintenance of bone homeostasis requires tight collaboration between osteoclasts and osteoblasts, as well as between other cell populations and factors present at bone-remodeling sites, which involve complex signaling pathways (Sims and Martin 2014). Regardless of which type of cell is dysfunctional, the bone system balance will be perturbed, resulting in many metabolic bone diseases.
Fig. 1.
Bone tissue is a dynamic system that is driven mainly by two types of cells: osteoblasts, which promote bone formation, and osteoclasts, which promote bone resorption. Aging osteoblasts will ultimately undergo apoptosis or become osteocytes. Osteoblasts also influence osteoclast formation through a paracrine manner. Osteoblasts can secret macrophage colony-stimulating factor (M-CSF) that promotes osteoclast proliferation and differentiation by interacting with its receptor c-Fms expressed on the surface of pre-osteoclasts. Osteoblasts also regulate osteoclast differentiation by RANKL/RANK/OPG pathway. Receptor activator of nuclear kappaB ligand (RANKL) that is produced by osteoblasts can bind to its receptor RANK expressed by osteoclast precursors and activate the fusion and differentiation of pre-osteoclasts into mature osteoclast. Osteoblasts also produce another factor called osteoprotegerin (OPG) which inhibits osteoclastogenesis and the subsequent bone resorption by binding to RANKL and avoiding its interaction with RANK
Multiple factors control the state of osteoblasts and osteoclasts. 1,25-Dihydroxy vitamin D formation from (inactive) vitamin D is a critical point of control in bone metabolism. Several hormones, including parathyroid hormone, growth hormone, steroids, and calcitonin, as well as bone marrow-derived membrane and soluble cytokines and growth factors are involved in these controls. Thus, a disorder of the extracellular environment can also cause bone diseases, including osteoarthritis (OA) and rheumatoid arthritis.
The heat shock protein family
Heat shock proteins (HSPs), also referred to as molecular chaperones or cell stress proteins, are a family of proteins produced by cells in response to exposure to stressful conditions such as heat shock (Ritossa 1962), cold (Matz et al. 1995), UV (ultraviolet) light (Cao et al. 1999), during wound healing or tissue remodeling (Laplante et al. 1998), and many other environmental stress conditions. HSPs are found in virtually all living organisms, from bacteria to humans. The expression of the HSPs is regulated by transcription factors (Morimoto 1993; Sorger 1991). Based on their molecular weights, HSPs are classified into different families, such as HSP40, HSP60, HSP70, HSP90, HSP110, and small HSPs (sHSPs) (Schlesinger 1990). Kampinga et al. (Kampinga et al. 2009) provided a new nomenclature, classifying the HSP family into seven groups, including HSPA (HSP70), HSPB (small HSP), HSPC (HSP90), HSPD/E (HSP60/HSP10), HSPH (HSP110), DNAJ (HSP40), and CCT (TRiC).
HSPs mainly function as intracellular chaperones for other proteins. They facilitate the correct folding of newly synthesized peptide chains to ensure the proper protein conformation, and they also prevent the aggregation of abnormal proteins. HSPs can transport unfolded proteins across membranes within the cell by partially stabilizing the proteins (Walter and Buchner 2002; Julio and Carlos 2005). In cancer cells, where they participate in oncogenesis and resistance to chemotherapy, the expression of intracellular HSPs is abnormally high. In addition to these essential intracellular functions, several molecular chaperones play an important role in communication between cells. For example, in the cardiovascular system, HSP90 binds both endothelial nitric oxide synthase and soluble guanylate cyclase that in turn are involved in vascular relaxation (Antonova et al. 2007). HSP70 forms a complex with tumor-related antigens via its polypeptide-binding domain, to elicit greater antigen-specific immune responses (Nishikawa et al. 2008). Moreover, HSPs may be involved in binding protein fragments from dead malignant cells, to present them to antigen-presenting cells via MHC class I and class II molecules, leading to the activation of anti-tumor CD8+ and CD4+ T cells. In addition, HSP-based vaccines and small molecular inhibitors of HSPs have shown promise as anticancer agents (Didelot et al. 2007). Clinical studies have been carried out and are presently in progress, using HSP-based anticancer vaccines or immunizing cancer patients with autologous tumor-derived HSP-peptide complexes (HSPPCs) (Ciocca et al. 2012).
Overall, HSPs may play important roles in some bone diseases. The aim of this review is therefore to explore the relationship between the HSPs and bone metabolism, with insights into novel ways to treat bone diseases (see Fig. 2, Table 1).
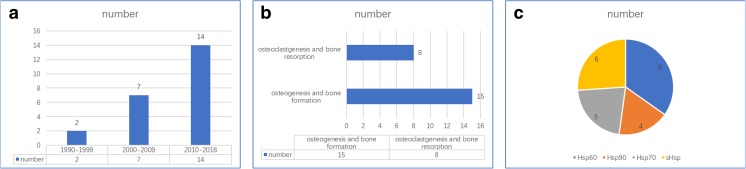
Fig. 2.
Publications on bone metabolism, formation, resorption, and HSPs. a The number of papers studying the role of HSPs in bone metabolism in different periods. b The number of studies involved in bone formation and bone resorption. c The number of various HSP families in the studies involved in bone metabolism
Table 1.
Studies in HSPs and bone metabolism
| Studies | Class | Factors | Model | Dose | Inhibitor | OE/KD/KO | Conclusion | Pathway |
|---|---|---|---|---|---|---|---|---|
| Yamamoto, N. (2016) | sHSPs family | HSP22 | Mc3T3-E1 | NA | NA | KD | TGF-β-induced migration of osteoblasts↑ | R-Smads signaling pathway |
| Flanagan M. (2017) | HSP22 | DPSCs | NA | NA | KD | Osteogenic differentiation potential of DPSCs↓ | – | |
| Kato, K. (2011) | HSP27 | Mc3T3-E1 | NA | NA | KD | TGF-β-stimulated VEGF induction↓ | – | |
| Kato, K. (2011) | Unphos-HSP27 | Mc3T3-E1 | NA | NA | NA | Osteocalcin synthesis↓ and mineralization↑ | p38 MAPK and cAMP pathway | |
| Kondo, A. (2013) | Unphos-HSP27 | Mc3T3-E1 | NA | NA | OE | VEGF release stimulated by FGF-2↓ | – | |
| Kainuma, S. (2017) | HSP27 | Mc3T3-E1 | NA | NA | NA | PDGF-BB-stimulated migration of osteoblasts↓ | – | |
| Kirby, A. C. (1995) | HSP60 family | Cpn60 | Mouse | 100 ng/mL | NA | NA | Bone resorption↑ | – |
| Reddi, K. (1998) | Cpn60 | mBMCs | 1–1000 ng/mL | NA | NA | Osteoclasts formation↑ (dose-dependent) | – | |
| Meghji, S. (2003) | HSP60 | Mouse | 1–1000 ng/mL | NA | NA | Bone resorption↑ | RANK/RANKL/OPG pathway | |
| Winrow, V. R. (2008) | Cpn60.1 | RAW264.7 | 0.1 ng/mL-10μg/mL | NA | NA | Osteoclast formation↓ | RANKL/RANK pathway | |
| Koh, J. M. (2009) | HSP60 | BMM | 10–100 ng/mL | NA | NA | Osteoclast formation and bone resorption↑ | Toll-like receptor (TLR)-2 pathway | |
| Kim, Y. S. (2009) | HSP60 | Osteoblasts | 100 ng/mL | NA | NA | Induced TLR-dependent apoptosis↑ | p38 MAPK and NF-κB pathway | |
| Wang, F. S. (2011) | HSP60 | Osteoblasts | NA | NA | OE | Inhibitory effects of glucocorticoid treatment on osteoblast survival bone↓ | ERK and Akt pathway | |
| Lu, M. C. (2016) | citHSP60 | Saos-2 | NA | NA | NA | ACPA-mediated Saos-2 cell apoptosis↓ | Toll-like receptor 4 pathway | |
| Chen, E. (2015) | HSP70 | hMSCs | 200 ng/mL | NA | NA | Alkaline phosphatase activity↑ and hMSC mineralization↑ | ERK pathway | |
| Zhang, W. (2016) | HSP70 family | HSPA1A | BMSCs | NA | NA | OE | Osteogenic differentiation of BMSCs↑ | Wnt/β-catenin pathway |
| Notsu, K. (2016) | HSP70-8 | Raw264.7 | NA | NA | KO | Osteoclastogenesis↑ | RANKL/ERK1/2, p38 pathway | |
| Sakai, G. (2017) | HSP70 | Mc3T3-E1 | 10–30μm/mL | NA | NA | TGF-β-stimulated VEGF release↑ (dose-dependent) | p38 MAP pathway | |
| Li, C. (2018) | HSP70 | hMSCs | NA | NA | KD | hMSC osteogenesis↓ and chondrogenic markers↓ | – | |
| Francis, L. K. (2006) | HSP90 | MMCs | NA | 17-AAG | NA | Osteoclast formation↑ | – | |
| Yano, A. (2008) | HSP90 family | HSP90 | PC-3M | NA | 17-AAG | NA | Growth of prostate carcinoma cells in bone↓ | Src kinase and Akt pathway |
| Okawa, Y. (2009) | HSP90 | Osteoclasts | NA | SNX-2112 | NA | Osteoclast formation↑ | ERK/c-fos and PU.1 pathway | |
| Miyasaka M. (2015) | HSP90 | Osteoblasts | NA | NA | NA | Cell viability and proliferation↑ | Smad1 and Smad5 pathway |
HSP heat shock protein; Unphos-HSP27 unphosphorylated HSP27; Cpn60 Escherichia coli chaperonin 60; citHSP60 citrullinated HSP60; HSP70-8 heat shock 70-kDa protein 8; DPSCs dental pulp stem cells; mBMCs mouse bone marrow cells; BMM bone marrow macrophage; hMSCs human mesenchymal stem cells; MMCs multiple myeloma cells; NA not available; OE overexpression; KD knockdown; KO knockout; TGF-β transforming growth factor-β; VEGF vascular endothelial growth factor; FGF-2 fibroblast growth factor-2; PDGF-BB platelet-derived growth factor-BB; TLR Toll-like receptor; ACPA anti-citrullinated protein antibodies; "↑" effect enhanced; "↓" effect weaked
HSPs and bone resorption
The HSP60 family and osteoclast/bone resorption
HSP60 is a mitochondrial chaperonin that cooperates with HSP10 in the transportation and refolding of proteins from the cytoplasm into the mitochondrial matrix. HSP60 aids in the folding and conformation maintenance of approximately 15–30% of all cellular proteins (Ranford et al. 2000) to enable cells to adapt to environment stress. HSP60 can also be an immune modulator and biomarker (Quintana and Cohen 2011), and can promote development of infections caused by viruses (Wyzewski et al. 2018). HSP60 has been implicated in cancer; however, there are different hypotheses to explain the effects of positive versus negative expressions of this protein (Cappello et al. 2006; Urushibara et al. 2007).
In 1995, the potent osteolytic activity of molecular chaperones extracted from Escherichia coli was reported in a murine calvarial bone resorption assay (Kirby et al. 1995). To characterize the effects of groEL (a lipopolysaccharide-free recombinant cpn60 of E. coli) on the formation of osteoclasts in culture, Reddi et al. (Reddi et al. 1998) used 12-day cultures of mouse bone marrow to assess osteoclast recruitment. GroEL (1–1000 ng/mL) remarkably stimulated the formation of TRAP multinucleated cells, as osteoclasts, that can secrete various proteinases and seem to participate in cartilage destruction in a dose-dependent manner (Reddi et al. 1998). Indomethacin, an inhibitor of cyclooxygenase, almost completely abolished the osteoclast formation induced by groEL. Lipopolysaccharide-low human recombinant chaperonin 60 (HSP60) is a potent stimulator of the bone resorption on murine calvarial bone, and that resorption activation of murine calvarial bone resorption by HSP60 is dose-dependent over a range of 0.001–1 μg/mL (Meghji et al. 2003). The bone-resorbing activity of HSP60 is significantly inhibited by both indomethacin and high concentrations of the natural IL-1 antagonist, IL-1ra. The osteoclast inhibitor, osteoprotegerin (OPG), almost totally inhibits bone resorption induced by HSP60.
The RANK/RANKL/OPG signaling pathway controls the differentiation and activation of osteoclasts. In this system, RANKL is expressed in several tissues and organs including the following: skeletal muscle, thymus, liver, colon, small intestine, adrenal gland, osteoblast, mammary gland epithelial cells, prostate, and pancreas (Wada et al. 2006), and RANKL binds RANK on cells of the myeloid lineage and functions as a key factor for osteoclast differentiation and activation. Osteoprotegerin is mainly produced by osteoblasts and is a decoy receptor for RANKL, which inhibits the activation of RANK-dependent pathways by competing for RANKL binding. Furthermore, bone resorption regulated by HSP60 may involve the RANKL/RANK system. HSP60 causes osteoclastic bone resorption via Toll-like receptor-2 (TLR-2) during estrogen deficiency (Koh et al. 2009) that suggests that HSP60 and TLR-2 may be novel mediators of estrogen deficiency-induced bone loss.
However, controversial effects of HSP60 in bone resorption have been reported. Cpn60.1, one of two homologous chaperonin (Cpn)60 proteins produced by Mycobacterium tuberculosis, inhibits bone breakdown both in vitro, in murine calvaria and in vivo, in experimental arthritis (Winrow et al. 2008).
The HSP70 family and osteoclast/bone resorption
HSP70s are a family of conserved, ubiquitously expressed HSPs. The differences between several HSP70 proteins expressed by eukaryotic organisms are slight (Tavaria et al. 1996). HSP70 can suppress aggregation, remodel folding pathways, and regulate activity by protecting partially folded structures as well as unfolded protein chains (Mashaghi et al. 2016). In addition to improving overall protein integrity, HSP70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome (Beere et al. 2000).
Heat shock 70-kDa protein 8 binds to MNSFb (a ubiquitin-like protein) via noncovalent association, and double knockdown of MNSFb and HSPA8 strongly inhibits RANKL-induced osteoclastogenesis of Raw264.7 macrophage-like cells. ERK1/2 and p38 phosphorylation and TNF-α production induced by RANKL are also inhibited by the same treatment, which suggests that the association between MNSFb and HSPA8 may play an important role in RANKL-induced osteoclastogenesis (Notsu et al. 2016).
The HSP90 family and osteoclast/bone resorption
HSP90 is a chaperone protein that assists other proteins to fold properly, protects proteins from heat stress, and aids in the degradation of proteins. HSP90 has been a target for cancer therapy, because of its ability to stabilize numerous proteins, including growth factor receptors, PI3K, and AKT, that are required for tumor growth (Sawai et al. 2008; Lurje and Lenz 2009).
The role of HSP90 in osteoclastogenesis is also controversial. In one study, SNX-2112, a selective HSP90 inhibitor, potently inhibited osteoclast formation via downregulation of ERK/c-fos and PU.1 in multiple myeloma and other hematological tumors (Okawa et al. 2009). Multiple myeloma overexpressed RANKL that activates osteoclasts and Dickkopf WNT signaling pathway inhibitor 1 (Dkk1) that is an antagonistic inhibitor of the WNT signaling pathway that is crucial for a correct bone mass achievement. Six hundred nanomolars of another HSP90 inhibitor, 17-allylamino-17-demethoxygeldanamycin (17-AAG), induced inhibition of osteoclasts in human marrow mononuclear cells (Francis et al. 2006). However, in studies on human breast cancer cell lines and osteoclast progenitors, 17-AAG has been found to enhance osteoclastogenesis (Price et al. 2005; van der Kraan et al. 2013). 17-AAG-enhanced osteoclast formation was Hsf1 (heat shock factor 1, the major regulator of heat shock protein transcription in eukaryotes)-dependent (et al. 2014). HSP90 also functions as a chaperone of C-terminal Src kinase, and inhibition of HSP90 promotes osteoclastogenesis through Src kinase activation (et al. 2008). Furthermore, inhibition of HSP90 activity by 17-AAG rescues glucocorticoid-induced bone loss by enhancing osteogenesis (Chen et al. 2017).
HSP families and bone formation
HSP families, including the HSP70 and HSP90 families, the collagen-specific chaperone HSP47, and cytosolic chaperones, are differentially expressed during the process of endochondral bone formation in a stage-specific pattern that reaches very high levels during specific stages (Loones and Morange 1998). Immunohistochemical analyses of the expressions of HSP27 in craniofacial development and osteogenesis have revealed that HSP27 is involved in specific regional and temporal expressions during tooth development (Leonardi et al. 2004).
The small HSP family and osteoblast/bone formation
Small HSPs have a subunit molecular weight of 12–43 kDa and are characterized by highly conserved C-terminal domains called the α-crystallin domain (Bakthisaran et al. 2015). They are classified into class I and class II (Taylor and Benjamin 2005). Class I (HSP27, αB-crystallin, HSP20, and HSP22) proteins display ubiquitous expression, whereas class II (HSPB2, HSPB3, HSPB7, HSPB9, HSPB10, and αA-crystallin) proteins exhibit a tissue-restricted pattern of expression. Among all sHSPs, HSP27 is the most widely studied protein in bone metabolism. Like other sHSPs, HSP27, also known as HSP beta-1 (HSPB1), is involved in chaperone activity, thermotolerance, inhibition of apoptosis, and regulation of cell development differentiation. High expression levels of different phosphorylated HSP27 species may be correlated with muscle/neurodegenerative diseases and various cancers (Sarto et al. 2000), and negatively correlated with cell proliferation, metastasis, and resistance to chemotherapy (Vargas-Roig et al. 1997).
In osteoblasts, heat treatment induces the expression of HSP27, facilitated by extrogen (Shakoori et al. 1992; Cooper and Uoshima 1994). Chemical stimulations such as sodium arsenite (arsenite) and physiological regulators of bone metabolism such as endothelin-1 (ET-1), prostaglandin F2 alpha (PGF2 alpha), prostaglandin D2 (PGD2), prostaglandin E2, glucocorticoids, transforming growth factor-β (TGF-β), and basic fibroblast growth factor may induce HSP27 expression (Suzuki et al. 1996; Kawamura et al. 1999; Kozawa et al. 1999a; Kozawa et al. 1999b; Kozawa et al. 2001a; Kozawa et al. 2001b; Hatakeyama et al. 2002; Kozawa et al. 2002). Importantly, chemical stress is negatively regulated by activation of protein kinase C (PKC) (Kozawa and Tokuda 2002; Tokuda et al. 2002). In addition to p38 MAP kinase and p44/p42 MAP kinase, stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) may also play a role in ET-1- and PGF2 alpha-induced HSP27 in osteoblasts (Tokuda et al. 2003; Tokuda et al. 2004), and methotrexate may enhance PGD2-stimulated HSP27 induction downstream from MAP kinases in osteoblasts (Yoshida et al. 2004).
TGF-β-stimulated HSP27 induction can be regulated by Rho kinase via SAPK/JNK activation in osteoblasts (Natsume et al. 2009; Kato et al. 2010). Rho kinase-regulated PGD2 stimulates HSP27 induction in osteoblasts via activation of both SAPK/JNK and p38 MAP kinase (Kato et al. 2010). In addition, HSP27 induction is critical for TGF-β-induced vascular endothelial growth factor (VEGF) release in osteoblasts (Kato et al. 2011a). By contrast, unphosphorylated HSP27 attenuates fibroblast growth factor-2-stimulated VEGF synthesis in osteoblasts (Kondo et al. 2013). HSP70 inhibits TGF-β-stimulated VEGF synthesis in osteoblasts, and this inhibitory effect occurs upstream of p38 MAP kinase (Sakai et al. 2017). Regarding bone metabolism, VEGF is necessary for the coupling of blood vessel invasion and impaired trabecular bone formation, promoting the expansion of the hypertrophic chondrocyte zone in the mouse tibial epiphyseal growth plate (Gerber et al. 1999).
Unphosphorylated, but not phosphorylated, HSP27 has a suppressive role in osteocalcin (OC) synthesis induced by recombinant BMP-4 or T3 in osteoblast-like MC3T3-E1 cells (Kato et al. 2011b). Osteocalcin is a hormone affecting reproduction and energy/insulin metabolism; however, its effect on bone mineral is relatively minor. The activation of p38 MAPK or the cAMP-dependent protein kinase induces the phosphorylation of HSP27 in MC3T3-E1 cells, so p38 MAPK and cAMP-dependent protein kinase may act as regulators of OC synthesis by fine-tuning HSP27-induced suppression in osteoblasts.
Mineralization in osteoblast cells is enhanced by decreased expression of OC induced by unphosphorylated HSP27. OC is secreted solely by osteoblasts and is the most abundant osteoblast-specific non-collagenous protein. OC has dual roles in bone metabolism: it regulates bone remodeling by modulating osteoblasts and osteoclast activity, and acts as a regulator of bone mineralization (Neve et al. 2013). The inhibition of OC in the regulation of bone matrix mineralization has been reported in several studies (Menanteau et al. 1982; Boskey et al. 1985; Romberg et al. 1986; Hoang et al. 2003; Hunter et al. 1996). In a similar manner as a steady-state gel system, OC inhibits the nucleation of hydroxyapatite in an early stage of mineralization (Hunter et al. 1996). However, there is controversy concerning the role of OC in bone formation. OC-deficient mice developed a phenotype with higher bone mass and bones of improved functional quality, suggesting that OC inhibits osteoblast bone formation (Ducy et al. 1996). However, OC also accelerates bone formation and regeneration, based on the earlier and increased expression of bone-specific matrix proteins and multifunctional adhesion proteins such as osteopontin, bone sialoprotein, and CD44. In addition, OC activates both osteoclasts and osteoblasts during early bone formation, supporting the above observations (Rammelt et al. 2005).
In addition to HSP27, other sHSP families also play an important role in osteogenesis. HSP22 (HSPB8), interacts with HSP27, negatively regulates the TGF-β-induced migration of osteoblasts by suppressing the activation of R-Smads resulting from the downregulation of TGF-β receptor II protein expression in osteoblast-like MC3T3-E1 cells (Yamamoto et al. 2016).
However, HSP22 has a close connection with the osteogenic differentiation of cells. The osteogenic differentiation capability of dental pulp stem cells (DPSCs) is gradually reduced during in vitro proliferation (Flanagan et al. 2017). However, the expression of HSPB8 is significantly reduced during the in vitro expansion of DPSCs when the cells lose osteogenic differentiation potential. Furthermore, knockdown of HSPB8 in early-passage DPSCs results in a decrease in the osteogenic differentiation potential of DPSCs.
Platelet-derived growth factor-BB (PDGF-BB) stimulates the migration of osteoblast-like MC3T3-E1 cells, HSP27 functions as a negative regulator in the PDGF-BB-stimulated migration of osteoblasts, and its suppressive effect is amplified by the phosphorylation of HSP27 (Kainuma et al. 2017).
HSP22, HSP27, TGF-β, and PDGF-BB may be part of the negative feedback system that controls the metabolism and migration in the development and differentiation of osteoblasts.
The HSP60 family and osteoblast/bone formation
In postmenopausal women, a series of changes occurs as a result of decreased plasma estrogen levels. Plasma HSP60 levels are approximately 3.5-fold higher in postmenopausal women (median, 1152.4 ng/mL; range, 724.7–2123.4 ng/mL) than in premenopausal women (median, 316.3 ng/mL; range, 164.6–638.4 ng/mL) (Kim et al. 2009). In vitro, exogenous HSP60 binds to cells via specific cell surface receptors, including CD14 and TLRs (Ranford and Henderson 2002), and that human HSP60 activates the Toll receptor-interleukin-1 receptor signaling pathways that ultimately leads to activation of the NF-kappaB signaling pathway by binding TLR-2 and TLR-4 (Vabulas et al. 2001). In this system, HSP60 significantly reduces cell viability and increases apoptosis in osteoblast lineages. On a molecular level, HSP60 increases TLR-2 and TLR-4 expression, activates caspase-3 and caspase-9 in the HS-5 hBMSC cell line, and increases the release of mitochondrial cytochrome c into the cytosol. P38 and NF-kappaB are activated at the same time. Pretreatment with blocking antibodies for TLR-2 and TLR-4 almost completely eliminates the effects of HSP60 on apoptosis, caspase-3, and caspase-9 activation, and the activation of NF-kappaB and p38 MAPK. These results suggest that HSP60 induces TLR-dependent apoptosis in osteoblast lineages via activation of p38 MAPK and NF-kappaB pathways. In estrogen-deprived patients, osteoblast apoptosis has been observed (Kousteni et al. 2002), and the degree of osteoblastic apoptosis is an important determinant of bone formation in postmenopausal osteoporosis (Manolagas and Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis 2000).
However, the link between estrogen deficiency and HSP60 is still not definitive. Under conditions of estrogen deficiency, the production of IL-1 and TNF-α increase (Cappello et al. 2006). Furthermore, in osteoclast lineages, IL-1 and TNF-α stimulate the production and secretion of HSP60 and proinflammatory cytokines, and enhance the expression of HSP60 (Chen et al. 1999).
HSP60 is expressed not only in mitochondria as a molecular chaperone that assists protein folding, transport, and secretion (Cappello et al. 2008), but also at the cell surface (Xu et al. 1994; Soltys and Gupta 1997). Anti-citrullinated protein antibodies (ACPAs) induce Saos-2 cells that are sarcoma osteogenic cells generated from human osteoblast apoptosis. Citrullinated HSP60 (citHSP60), but not HSP60, inhibits ACPA-mediated apoptosis (Lu et al. 2016). The HSP60 structure does not contain a significant stretch of hydrophobic residues that could act as a membrane-spanning domain (Jindal et al. 1989; Woodlock et al. 1997), while HSP60 could associate with other cell membrane proteins, such as TLR4 or α3β1 integrin, to facilitate cell signaling (Barazi et al. 2002; Ohashi et al. 2000). HSP60 binds to TLR4 (Ohashi et al. 2000), and antibody blocking of TLR4 inhibits ACPA-mediated Saos-2 cell apoptosis, suggesting that ACPA induces Saos-2 cell apoptosis via binding to surface-expressed citHSP60 through TLR signaling (Lu et al. 2016).
The HSP70 family and osteoblast/bone formation
HSP70 (200 ng/mL) increases alkaline phosphatase activity and promotes human mesenchymal stem cell (hMSC) mineralization (Chen et al. 2015). Under osteogenic induction conditions, HSP70 significantly upregulates the expression of osteo-specific genes, such as the runt family transcription factors Runx2 and osterix. Microarray and pathway analyses have revealed that HSP70 promotes osteogenesis of hMSCs through activation of the ERK signaling pathway. Downregulation of HSP70 impairs the osteogenic and chondrogenic differentiation of hMSCs, as well as the enhancement of these processes by thermal treatment (Li et al. 2018). Overexpression of HSP family A member 1A (HSPA1A), which encodes cognate HSP70, enhances osteogenic differentiation of BMSCs, partly through the Wnt/β-catenin signaling pathway (Zhang et al. 2016).
The HSP90 family and osteoblast/bone formation
Mouse osteoblasts at day 3 of cell culture exposed to low-intensity pulsed ultrasound (LIPUS) at a frequency of 3.0 MHz by 30 mW/cm2 for 15 min or to 42 °C heat shock for 20 min upregulated expression of HSP90 and phosphorylation of Smad1 and Smad5 at 24 h. These treatments enhanced cell viability and proliferation at 24 h, while 10 days after LIPUS (but not heat shock) stimulation, osteoblasts had stronger mineralized nodule formation (Miyasaka et al. 2016).
HSPs and bone diseases
HSPs have been studied widely in bone diseases. In osteosarcomas, HSPs or HSFs have already been targeted clinically to treat solid tumors, based on their strong anti-tumor effects. Recently, the discussion of type I collagen folding and related diseases has been reshaped due to discoveries of severe bone disorders in patients with deficiencies in several endoplasmic reticulum chaperones (Makareeva et al. 2011). HSPs may also become a promising therapeutic target in osteoarthritis.
HSPs and osteosarcomas
HSP72 was significantly overexpressed in osteosarcomas compared to nonmalignant bone tumors, and HSP72-positive osteosarcoma patients responded better to neoadjuvant chemotherapy than HSP72-negative patients (Trieb et al. 1998). HSP72 may regulate the interaction between T lymphocytes and osteosarcoma cells in a specific group of osteosarcoma patients expressing HSP72. Thus, induction of HSP72 in osteosarcomas might lead to an increased immune response and rejection of the osteosarcoma (Trieb et al. 2000a).
As mentioned before, HSP90 has been a target for cancer therapy, because of its ability to stabilize numerous proteins. For examples, sera samples from 20 high-grade osteosarcoma patients were tested using an enzyme-linked immunosorbent assay, and anti-HSP90 antibodies were found to correlate with a better response to neoadjuvant chemotherapy (p < 0.01) (Trieb et al. 2000b). 17-AAG, an HSP90 inhibitor, is quite toxic and will cause severe bone loss. Other HSP90 inhibitors, including AUY922 and STA-9090 (Ganetespib), also cause bone-related adverse events such as bone pain and fractures in clinical trials. HSF-1 limited the HSP90 inhibitor’s anti-cancer activity by a feedback mechanism (Chen et al. 2013), so HSF1 may be a new target to enhance HSP90 inhibitors’ activity in human cancers. IHSF115, a new inhibitor targeting human transcription factor HSF1, is cytotoxic for a variety of human cancer cell lines, multiple myeloma lines consistently exhibiting high sensitivity (Vilaboa et al. 2017). An increase in anti-HSP60 antibodies is also seen at the time of the first diagnosis of osteosarcoma (Trieb et al. 2000c).
In a drug-resistant cell line developed by repeatedly treating the HOS human osteosarcoma cell line with zoledronic acid, the expression of HSP27 was upregulated and its resistance was inhibited by HSP27 silencing (Morii et al. 2010). Because of their important role in osteosarcoma, various HSPs or anti-HSP products have been developed. Numerous HSP products for treating solid tumors have progressed to ongoing or completed clinical trials. These include HSPPC-96 (NCT00293423, phase II, brain and central nervous system tumors), OGX-427 (NCT01120470, phase II, castration-resistant prostate cancer), Vitespen (NCT00003025, phase I, pancreatic cancer), IPI-504 (NCT00627627, phase II, breast cancer), SNX-5422 (NCT01892046, phase I, cancer), and vitespen (NCT00005628, phase II, soft tissue sarcoma).
HSPS and osteoarthritis
Anti-HSP60 IgA is increased in the sera of OA patients (Watanabe et al. 2003). Chondrocytes are more resistant to cell death induced by monoiodoacetate, by overexpressing HSP70 (Grossin et al. 2006). Intra-articular injections of a reversible proteasome inhibitor (MG132) induce local induction of HSP70 that protects articular chondrocytes from cellular death in a rat OA knee model induced by an anterior cruciate ligament transection (Etienne et al. 2008). A combination of microwave (MW) exposure and glutamine significantly enhances the expression of HSP70, and OA severity is significantly milder in rat OA models (Fujita et al. 2012). Inhibition of HSP90 expression results in increased HSP70 synthesis, suggesting that HSP90 limits HSP70 expression. In addition, an HSP90 inhibitor increases cartilage sulfated glycosaminoglycan levels even beyond baseline, stimulates subchondral bone thickness, protects against cartilage degradation, and suppresses macrophage activation (Siebelt et al. 2013).
Conclusions
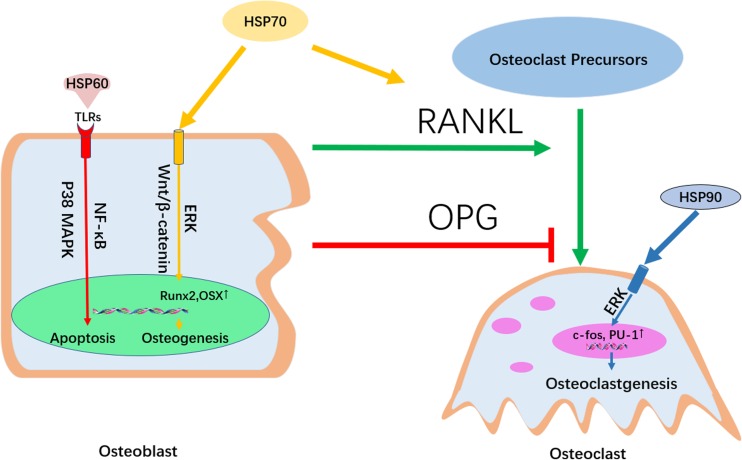
HSP60 promotes osteoclast formation and induces TLR-dependent apoptosis in osteoblast lineages via activation of p38 MAPK and NF-kappaB pathways, so it is highly possible that HSP60 also regulates proliferation by affecting the state of osteoblasts. HSP70 both positively regulates osteoclastic bone resorption via the RANKL/RANK pathway and promotes osteogenesis through activation of the ERK and Wnt/β-catenin signaling pathways. HSP90 induces the production of osteoclast-associated genes such as PU.1 and c-fos to promote the formation of osteoclasts. A series of chemical and physiological stimulations affect the production of sHSPs in osteoblasts such as HSP27, regulating the differentiation of osteoblasts (see Fig. 3).
Fig. 3.
The signaling pathways involved in the regulation of HSPs in bone metabolism. HSP60 induces TLR-dependent apoptosis in osteoblast lineages via activation of p38 MAPK and NF-kappaB pathways. HSP70 both positively regulates osteoclastic bone resorption via the RANKL/RANK pathway and promotes osteogenesis through activation of the ERK and Wnt/β-catenin signaling pathways. HSP90 induces the production of osteoclast-associated genes such as PU.1 and c-fos to promote the formation of osteoclasts
Increasing numbers of HSP-based drugs such as HSP90 inhibitors have been clinically used to treat solid tumors, based on their strong anti-tumor effects. In view of their extensive roles in bone metabolism, HSP-based drugs are promising treatments for bone diseases such as OA and osteoporosis. HSP90 inhibitors are most widely studied in clinical trials due to their ability of stabilizing numerous proteins that are important in many diseases. However, the large number of client proteins of HSPs means that compounds that bind them will have complex effects. For example, HSP90 blockers usually induce a strong stress response that limits their anti-cancer actions by HSF1.
Although numerous HSPs are involved in bone metabolism and bone-related diseases, the actions of the various HSP families are different. HSP60 is most important for bone cell function, while sHSPs are the most common HSPs induced in bone cells by drugs, hormones, or other stimuli. In spite of significant progress in the field of HSPs, there are still important issues that need to be resolved. The exact signaling pathways that mediate the promoting function of HSP60 in osteoclasts via osteoblasts are still not definitively known; neither are the reasons why HSP90 has the opposite effects on osteoclasts. Additional studies need to better characterize the mechanisms discussed in this review, to provide more effective, precise, and rational therapeutic strategies using HSPs.
Author contributions
ZJP and DTX: conception and design; KH, CY, and EC: literature research and review. WZ: data collecting; KH, CY, and EC: article writing with contributions from other authors.
Funding
This work was supported by grants from the National Natural Science Foundation of China (nos. 81672147, 81401011, 81201397, and 81271973); Zhejiang Provincial Natural Science Foundation of China (nos. LY15H060001, LY15H060002, and LY16H060003); the Education Department of Zhejiang Province (nos. Y201328201 and Y201534637); Public welfare projects of Zhejiang Science and Technology Department (no. 2012C23079); and Zhejiang medical and health science and technology plan project (nos. 2015KYB182, 2017KY382, 2015104766, and 2014KYA092).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Contributor Information
Kai Hang, Email: 21718242@zju.edu.cn.
Chenyi Ye, Email: yechenyi@zju.edu.cn.
Erman Chen, Email: 11618316@zju.edu.cn.
Wei Zhang, Email: zhangweilook@zju.edu.cn.
Deting Xue, Phone: +86-0571-87783567, Email: blueskine@zju.edu.cn.
Zhijun Pan, Phone: +86-0571-87783567, Email: zrpzj@zju.edu.cn.
References
- Antonova G, Lichtenbeld H, Xia T, Chatterjee A, Dimitropoulou C, Catravas JD. Functional significance of hsp90 complexes with NOS and sGC in endothelial cells. Clin Hemorheol Microcirc. 2007;37(1–2):19–35. [PubMed] [Google Scholar]
- Bakthisaran R, Tangirala R, Rao Ch M. Small heat shock proteins: role in cellular functions and pathology. Biochim Biophys Acta. 2015;1854(4):291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Barazi HO, Zhou L, Templeton NS, Krutzsch HC, Roberts DD. Identification of heat shock protein 60 as a molecular mediator of alpha 3 beta 1 integrin activation. Cancer Res. 2002;62(5):1541–1548. [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2(8):469–75.10.1038/35019501. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Wians FH, Jr, Hauschka PV. The effect of osteocalcin on in vitro lipid-induced hydroxyapatite formation and seeded hydroxyapatite growth. Calcif Tissue Int. 1985;37(1):57–62. doi: 10.1007/BF02557680. [DOI] [PubMed] [Google Scholar]
- Cao Y, Ohwatari N, Matsumoto T, Kosaka M, Ohtsuru A, Yamashita S. TGF-beta1 mediates 70-kDa heat shock protein induction due to ultraviolet irradiation in human skin fibroblasts. Pflugers Arch. 1999;438(3):239–244. doi: 10.1007/s004240050905. [DOI] [PubMed] [Google Scholar]
- Cappello F, et al. Hsp60 and Hsp10 down-regulation predicts bronchial epithelial carcinogenesis in smokers with chronic obstructive pulmonary disease. Cancer. 2006;107(10):2417–2424. doi: 10.1002/cncr.22265. [DOI] [PubMed] [Google Scholar]
- Cappello F, Conway de Macario E, Marasà L, Zummo G, Macario AJL. Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol Ther. 2008;7(6):801–809. doi: 10.4161/cbt.7.6.6281. [DOI] [PubMed] [Google Scholar]
- Capulli M, Paone R, Rucci N. Osteoblast and osteocyte: games without frontiers. Arch Biochem Biophys. 2014;561:3–12. doi: 10.1016/j.abb.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Chai RC, et al. Molecular stress-inducing compounds increase osteoclast formation in a heat shock factor 1 protein-dependent manner. J Biol Chem. 2014;289(19):13602–13614. doi: 10.1074/jbc.M113.530626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, et al. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162(6):3212–3219. [PubMed] [Google Scholar]
- Chen Y, et al. Targeting HSF1 sensitizes cancer cells to HSP90 inhibition. Oncotarget. 2013;4(6):816–829. doi: 10.18632/oncotarget.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, et al. Extracellular heat shock protein 70 promotes osteogenesis of human mesenchymal stem cells through activation of the ERK signaling pathway. FEBS Lett. 2015;589(24 Pt B):4088–4096. doi: 10.1016/j.febslet.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Chen H, et al. Inhibition of heat shock protein 90 rescues glucocorticoid-induced bone loss through enhancing bone formation. J Steroid Biochem Mol Biol. 2017;171:236–246. doi: 10.1016/j.jsbmb.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Cayado-Gutierrez N, Maccioni M, Cuello-Carrion FD. Heat shock proteins (HSPs) based anti-cancer vaccines. Curr Mol Med. 2012;12(9):1183–1197. doi: 10.2174/156652412803306684. [DOI] [PubMed] [Google Scholar]
- Cooper LF, Uoshima K. Differential estrogenic regulation of small M(r) heat shock protein expression in osteoblasts. J Biol Chem. 1994;269(11):7869–7873. [PubMed] [Google Scholar]
- Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Ann N Y Acad Sci. 2010;1192:437–443. doi: 10.1111/j.1749-6632.2009.05246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didelot C, Lanneau D, Brunet M, Joly AL, Thonel A, Chiosis G, Garrido C. Anti-cancer therapeutic approaches based on intracellular and extracellular heat shock proteins. Curr Med Chem. 2007;14(27):2839–2847. doi: 10.2174/092986707782360079. [DOI] [PubMed] [Google Scholar]
- Ducy P, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382(6590):448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- Etienne S, Gaborit N, Henrionnet C, Pinzano A, Galois L, Netter P, Gillet P, Grossin L. Local induction of heat shock protein 70 (Hsp70) by proteasome inhibition confers chondroprotection during surgically induced osteoarthritis in the rat knee. Biomed Mater Eng. 2008;18(4–5):253–260. [PubMed] [Google Scholar]
- Flanagan M., Li C., Dietrich M. A., Richard M., Yao S. Downregulation of heat shock protein B8 decreases osteogenic differentiation potential of dental pulp stem cells during in vitro proliferation. Cell Proliferation. 2017;51(2):e12420. doi: 10.1111/cpr.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis LK, et al. Combination mammalian target of rapamycin inhibitor rapamycin and HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin has synergistic activity in multiple myeloma. Clin Cancer Res. 2006;12(22):6826–6835. doi: 10.1158/1078-0432.ccr-06-1331. [DOI] [PubMed] [Google Scholar]
- Fujita S, et al. Combined microwave irradiation and intraarticular glutamine administration-induced HSP70 expression therapy prevents cartilage degradation in a rat osteoarthritis model. J Orthop Res. 2012;30(3):401–407. doi: 10.1002/jor.21535. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Grossin L, et al. Gene transfer with HSP 70 in rat chondrocytes confers cytoprotection in vitro and during experimental osteoarthritis. Faseb J. 2006;20(1):65–75. doi: 10.1096/fj.04-2889com. [DOI] [PubMed] [Google Scholar]
- Hatakeyama D, Kozawa O, Niwa M, Matsuno H, Ito H, Kato K, Tatematsu N, Shibata T, Uematsu T. Upregulation by retinoic acid of transforming growth factor-beta-stimulated heat shock protein 27 induction in osteoblasts: involvement of mitogen-activated protein kinases. Biochim Biophys Acta. 2002;1589(1):15–30. doi: 10.1016/S0167-4889(01)00183-5. [DOI] [PubMed] [Google Scholar]
- Hoang QQ, et al. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003;425(6961):977–980. doi: 10.1038/nature02079. [DOI] [PubMed] [Google Scholar]
- Hunter GK, et al. Nucleation and inhibition of hydroxyapatite formation by mineralized tissue proteins. Biochem J. 1996;317(Pt 1):59–64. doi: 10.1042/bj3170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal S, Dudani AK, Singh B, Harley CB, Gupta RS. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989;9(5):2279–2283. doi: 10.1128/MCB.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julio CB, Carlos HIR. Protein folding assisted by chaperones. Protein Pept Lett. 2005;12(3):257–261. doi: 10.2174/0929866053587165. [DOI] [PubMed] [Google Scholar]
- Kainuma S, et al. Heat shock protein 27 (HSPB1) suppresses the PDGF-BB-induced migration of osteoblasts. Int J Mol Med. 2017;40(4):1057–1066. doi: 10.3892/ijmm.2017.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, et al. Rho-kinase regulates prostaglandin D(2)-stimulated heat shock protein 27 induction in osteoblasts. Exp Ther Med. 2010;1(4):579–583. doi: 10.3892/etm_00000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, et al. Role of heat shock protein 27 in transforming growth factor-beta-stimulated vascular endothelial growth factor release in osteoblasts. Int J Mol Med. 2011;27(3):423–428. doi: 10.3892/ijmm.2011.595. [DOI] [PubMed] [Google Scholar]
- Kato K, et al. Regulation by heat shock protein 27 of osteocalcin synthesis in osteoblasts. Endocrinology. 2011;152(5):1872–1882. doi: 10.1210/en.2010-1062. [DOI] [PubMed] [Google Scholar]
- Kawamura H, et al. Endothelin-1 stimulates heat shock protein 27 induction in osteoblasts: involvement of p38 MAP kinase. Am J Phys. 1999;277(6 Pt 1):E1046–E1054. doi: 10.1152/ajpendo.1999.277.6.E1046. [DOI] [PubMed] [Google Scholar]
- Kim YS, et al. Increased circulating heat shock protein 60 induced by menopause, stimulates apoptosis of osteoblast-lineage cells via up-regulation of toll-like receptors. Bone. 2009;45(1):68–76. doi: 10.1016/j.bone.2009.03.658. [DOI] [PubMed] [Google Scholar]
- Kirby AC, et al. The potent bone-resorbing mediator of Actinobacillus actinomycetemcomitans is homologous to the molecular chaperone GroEL. J Clin Invest. 1995;96(3):1185–1194. doi: 10.1172/jci118150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JM, et al. Heat shock protein 60 causes osteoclastic bone resorption via toll-like receptor-2 in estrogen deficiency. Bone. 2009;45(4):650–660. doi: 10.1016/j.bone.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Kondo A, et al. Unphosphorylated heat shock protein 27 suppresses fibroblast growth factor2stimulated vascular endothelial growth factor release in osteoblasts. Mol Med Rep. 2013;8(2):691–695. doi: 10.3892/mmr.2013.1533. [DOI] [PubMed] [Google Scholar]
- Kousteni S, et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298(5594):843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- Kozawa O, Tokuda H. Heat shock protein 27 in osteoblasts. Nihon Yakurigaku Zasshi. 2002;119(2):89–94. doi: 10.1254/fpj.119.89. [DOI] [PubMed] [Google Scholar]
- Kozawa O, et al. Sphingosine 1-phosphate induces heat shock protein 27 via p38 mitogen-activated protein kinase activation in osteoblasts. J Bone Miner Res. 1999;14(10):1761–1767. doi: 10.1359/jbmr.1999.14.10.1761. [DOI] [PubMed] [Google Scholar]
- Kozawa O, Tokuda H, Miwa M, Ito H, Matsuno H, Niwa M, Kato K, Uematsu T. Involvement of p42/p44 mitogen-activated protein kinase in prostaglandin f(2alpha)-stimulated induction of heat shock protein 27 in osteoblasts. J Cell Biochem. 1999;75(4):610–619. doi: 10.1002/(SICI)1097-4644(19991215)75:4<610::AID-JCB7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kozawa O, et al. Stimulatory effect of basic fibroblast growth factor on induction of heat shock protein 27 in osteoblasts: role of protein kinase C. Arch Biochem Biophys. 2001;388(2):237–242. doi: 10.1006/abbi.2000.2290. [DOI] [PubMed] [Google Scholar]
- Kozawa O, Otsuka T, Hatakeyama D, Niwa M, Matsuno H, Ito H, Kato K, Matsui N, Uematsu T. Mechanism of prostaglandin D(2)-stimulated heat shock protein 27 induction in osteoblasts. Cell Signal. 2001;13(8):535–541. doi: 10.1016/S0898-6568(01)00180-2. [DOI] [PubMed] [Google Scholar]
- Kozawa O, et al. Specific induction of heat shock protein 27 by glucocorticoid in osteoblasts. J Cell Biochem. 2002;86(2):357–364. doi: 10.1002/jcb.10221. [DOI] [PubMed] [Google Scholar]
- Laplante AF, Moulin V, Auger FA, Landry J, Li H, Morrow G, Tanguay RM, Germain L. Expression of heat shock proteins in mouse skin during wound healing. Journal of Histochemistry & Cytochemistry. 1998;46(11):1291–1301.10.1177/002215549804601109. doi: 10.1177/002215549804601109. [DOI] [PubMed] [Google Scholar]
- Leonardi R, et al. Immunolocalization of heat shock protein 27 in developing jaw bones and tooth germs of human fetuses. Calcif Tissue Int. 2004;75(6):509–516. doi: 10.1007/s00223-004-0077-1. [DOI] [PubMed] [Google Scholar]
- Li C, et al. Downregulation of heat shock protein 70 impairs osteogenic and chondrogenic differentiation in human mesenchymal stem cells. Sci Rep. 2018;8(1):553. doi: 10.1038/s41598-017-18541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loones MT, Morange M. Hsp and chaperone distribution during endochondral bone development in mouse embryo. Cell Stress Chaperones. 1998;3(4):237–244. doi: 10.1379/1466-1268(1998)003<0237:HACDDE>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MC, et al. Anti-citrullinated protein antibodies promote apoptosis of mature human Saos-2 osteoblasts via cell-surface binding to citrullinated heat shock protein 60. Immunobiology. 2016;221(1):76–83. doi: 10.1016/j.imbio.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77(6):400–10.10.1159/000279388. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- Makareeva E, Aviles NA, Leikin S. Chaperoning osteogenesis: new protein-folding-disease paradigms. Trends Cell Biology. 2011;21(3):168–176. doi: 10.1016/j.tcb.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC, et al. Endocr Rev. 2000;21(2):115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- Mashaghi A, et al. Alternative modes of client binding enable functional plasticity of Hsp70. Nature. 2016;539(7629):448–451. doi: 10.1038/nature20137. [DOI] [PubMed] [Google Scholar]
- Matz JM, et al. Characterization and regulation of cold-induced heat shock protein expression in mouse brown adipose tissue. Am J Physiol. 1995;269(1 Pt 2):R38–R47. doi: 10.1152/ajpregu.1995.269.1.R38. [DOI] [PubMed] [Google Scholar]
- Meghji S, Lillicrap M, Maguire M, Tabona P, Gaston JSH, Poole S, Henderson B. Human chaperonin 60 (Hsp60) stimulates bone resorption: structure/function relationships. Bone. 2003;33(3):419–425. doi: 10.1016/S8756-3282(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Menanteau J, Neuman WF, Neuman MW. A study of bone proteins which can prevent hydroxyapatite formation. Metab Bone Dis Relat Res. 1982;4(2):157–162. doi: 10.1016/0221-8747(82)90030-3. [DOI] [PubMed] [Google Scholar]
- Miyasaka M, et al. Low-intensity pulsed ultrasound stimulation enhances heat-shock protein 90 and mineralized nodule formation in mouse calvaria-derived osteoblasts. Tissue Eng Part A. 2016;22(9–10):827–828. doi: 10.1089/ten.tea.2015.0234.correx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morii T, Ohtsuka K, Ohnishi H, Mochizuki K, Satomi K. Inhibition of heat-shock protein 27 expression eliminates drug resistance of osteosarcoma to zoledronic acid. Anticancer Res. 2010;30(9):3565–3571. [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259(5100):1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Nakashima T. Bone and Calcium Research Update 2015. Regulation of bone remodeling by osteocytes. Clin Calcium. 2015;25(1):21–8.CliCa15012128. [PubMed] [Google Scholar]
- Nakashima T, Hayash M, Takayanagi H. Regulation of bone resorption by osteocytes. Clin Calcium. 2012;22(5):685–96.CliCa1205685696. [PubMed] [Google Scholar]
- Natsume H, et al. Involvement of Rho-kinase in TGF-beta-stimulated heat shock protein 27 induction in osteoblasts. Mol Med Rep. 2009;2(5):687–691. doi: 10.3892/mmr_00000157. [DOI] [PubMed] [Google Scholar]
- Neve A, Corrado A, Cantatore FP. Osteocalcin: skeletal and extra-skeletal effects. J Cell Physiol. 2013;228(6):1149–1153. doi: 10.1002/jcp.24278. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Takemoto S, Takakura Y. Heat shock protein derivatives for delivery of antigens to antigen presenting cells. Int J Pharm. 2008;354(1–2):23–27. doi: 10.1016/j.ijpharm.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Notsu K, Nakagawa M, Nakamura M. Ubiquitin-like protein MNSFbeta noncovalently binds to molecular chaperone HSPA8 and regulates osteoclastogenesis. Mol Cell Biochem. 2016;421(1–2):149–156. doi: 10.1007/s11010-016-2795-x. [DOI] [PubMed] [Google Scholar]
- Ohashi K, et al. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J Immunol. 2000;164(2):558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Okawa Y, et al. SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in multiple myeloma and other hematologic tumors by abrogating signaling via Akt and ERK. Blood. 2009;113(4):846–855. doi: 10.1182/blood-2008-04-151928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JT, et al. The heat shock protein 90 inhibitor, 17-allylamino-17-demethoxygeldanamycin, enhances osteoclast formation and potentiates bone metastasis of a human breast cancer cell line. Cancer Res. 2005;65(11):4929–4938. doi: 10.1158/0008-5472.can-04-4458. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Cohen IR. The HSP60 immune system network. Trends Immunol. 2011;32(2):89–95. doi: 10.1016/j.it.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285(33):25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammelt S, et al. Osteocalcin enhances bone remodeling around hydroxyapatite/collagen composites. J Biomed Mater Res A. 2005;73(3):284–294. doi: 10.1002/jbm.a.30263. [DOI] [PubMed] [Google Scholar]
- Ranford JC, Henderson B. Chaperonins in disease: mechanisms, models, and treatments. Mol Pathol. 2002;55(4):209–213. doi: 10.1136/mp.55.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranford JC, Coates AR, Henderson B. Chaperonins are cell-signalling proteins: the unfolding biology of molecular chaperones. Expert Rev Mol Med. 2000;2(8):1–17. doi: 10.1017/S1462399400002015. [DOI] [PubMed] [Google Scholar]
- Reddi K, et al. The Escherichia coli chaperonin 60 (groEL) is a potent stimulator of osteoclast formation. J Bone Miner Res. 1998;13(8):1260–1266. doi: 10.1359/jbmr.1998.13.8.1260. [DOI] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia. 1962;18(12):571–573. doi: 10.1007/bf02172188. [DOI] [Google Scholar]
- Romberg RW, Werness PG, Riggs BL, Mann KG. Inhibition of hydroxyapatite crystal growth by bone-specific and other calcium-binding proteins. Biochemistry. 1986;25(5):1176–1180. doi: 10.1021/bi00353a035. [DOI] [PubMed] [Google Scholar]
- Sakai G, Tokuda H, Fujita K, Kainuma S, Kawabata T, Matsushima-Nishiwaki R, Kozawa O, Otsuka T. Heat shock protein 70 negatively regulates TGF-beta-stimulated VEGF synthesis via p38 MAP kinase in osteoblasts. Cell Physiol Biochem. 2017;44(3):1133–1145.10.1159/000485418. doi: 10.1159/000485418. [DOI] [PubMed] [Google Scholar]
- Sarto C, Binz PA, Mocarelli P. Heat shock proteins in human cancer. ELECTROPHORESIS. 2000;21(6):1218–1226. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1218::AID-ELPS1218>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Sawai A, et al. Inhibition of Hsp90 down-regulates mutant epidermal growth factor receptor (EGFR) expression and sensitizes EGFR mutant tumors to paclitaxel. Cancer Res. 2008;68(2):589–596. doi: 10.1158/0008-5472.can-07-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger MJ. Heat shock proteins. J Biol Chem. 1990;265(21):12111–12114. [PubMed] [Google Scholar]
- Shakoori AR, et al. Expression of heat shock genes during differentiation of mammalian osteoblasts and promyelocytic leukemia cells. J Cell Biochem. 1992;48(3):277–287. doi: 10.1002/jcb.240480308. [DOI] [PubMed] [Google Scholar]
- Siebelt M, et al. Hsp90 inhibition protects against biomechanically induced osteoarthritis in rats. Arthritis Rheum. 2013;65(8):2102–2112. doi: 10.1002/art.38000. [DOI] [PubMed] [Google Scholar]
- Sims NA and Martin TJ (2014) Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. BoneKEy Rep 3. 10.1038/bonekey.2013.215 [DOI] [PMC free article] [PubMed]
- Soltys BJ, Gupta RS. Cell surface localization of the 60 kDa heat shock chaperonin protein (hsp60) in mammalian cells. Cell Biol Int. 1997;21(5):315–320. doi: 10.1006/cbir.1997.0144. [DOI] [PubMed] [Google Scholar]
- Sorger PK. Heat shock factor and the heat shock response. Cell. 1991;65(3):363–366. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- Suzuki A et al (1996) Protein kinase C activation inhibits stress-induced synthesis of heat shock protein 27 in osteoblast-like cells: function of arachidonic acid. J Cell Biochem 62(1):69–75. 10.1002/(SICI)1097-4644(199607)62:1<69::AID-JCB8>3.0.CO;2-# [DOI] [PubMed]
- Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1(1):23–28. doi: 10.1379/1466-1268(1996)001<0023:AHSGTT>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RP, Benjamin IJ. Small heat shock proteins: a new classification scheme in mammals. J Mol Cell Cardiol. 2005;38(3):433–444. doi: 10.1016/j.yjmcc.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Tokuda H, Kozawa O, Niwa M, Matsuno H, Kato K, Uematsu T. Mechanism of prostaglandin E2-stimulated heat shock protein 27 induction in osteoblast-like MC3T3-E1 cells. J Endocrinol. 2002;172(2):271–281. doi: 10.1677/joe.0.1720271. [DOI] [PubMed] [Google Scholar]
- Tokuda H, Niwa M, Ito H, Oiso Y, Kato K, Kozawa O. Involvement of stress-activated protein kinase/c-Jun N-terminal kinase in endothelin-1-induced heat shock protein 27 in osteoblasts. Eur J Endocrinol. 2003;149(3):239–245. doi: 10.1530/eje.0.1490239. [DOI] [PubMed] [Google Scholar]
- Tokuda H, et al. Involvement of stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK) in prostaglandin F2alpha-induced heat shock protein 27 in osteoblasts. Prostaglandins Leukot Essent Fat Acids. 2004;70(5):441–447. doi: 10.1016/j.plefa.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Trieb K, Lechleitner T, Lang S, Windhager R, Kotz R, Dirnhofer S. Heat shock protein 72 expression in osteosarcomas correlates with good response to neoadjuvant chemotherapy. Hum Pathol. 1998;29(10):1050–1055. doi: 10.1016/S0046-8177(98)90412-9. [DOI] [PubMed] [Google Scholar]
- Trieb K, Lang S, Kotz R. Heat-shock protein 72 in human osteosarcoma: T-lymphocyte reactivity and cytotoxicity. Pediatr Hematol Oncol. 2000;17(5):355–364. doi: 10.1080/08880010050034283. [DOI] [PubMed] [Google Scholar]
- Trieb K, et al. Antibodies to heat shock protein 90 in osteosarcoma patients correlate with response to neoadjuvant chemotherapy. Br J Cancer. 2000;82(1):85–87. doi: 10.1054/bjoc.1999.0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieb K, et al. Serum antibodies against the heat shock protein 60 are elevated in patients with osteosarcoma. Immunobiology. 2000;201(3–4):368–376. doi: 10.1016/s0171-2985(00)80091-1. [DOI] [PubMed] [Google Scholar]
- Urushibara M, et al. HSP60 may predict good pathological response to neoadjuvant chemoradiotherapy in bladder cancer. Jpn J Clin Oncol. 2007;37(1):56–61. doi: 10.1093/jjco/hyl121. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276(33):31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- van der Kraan AG, et al. HSP90 inhibitors enhance differentiation and MITF (microphthalmia transcription factor) activity in osteoclast progenitors. Biochem J. 2013;451(2):235–244. doi: 10.1042/bj20121626. [DOI] [PubMed] [Google Scholar]
- Vargas-Roig LM, Fanelli MA, López LA, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock proteins and cell proliferation in human breast cancer biopsy samples. Cancer Detect Prev. 1997;21(5):441–451. [PubMed] [Google Scholar]
- Vilaboa N, et al. New inhibitor targeting human transcription factor HSF1: effects on the heat shock response and tumor cell survival. Nucleic Acids Res. 2017;45(10):5797–5817. doi: 10.1093/nar/gkx194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, et al. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12(1):17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Walter S, Buchner J. Molecular chaperones—cellular machines for protein folding. Angew Chem Int Ed Engl. 2002;41(7):1098–1113. doi: 10.1002/1521-3773(20020402)41:7<1098::AID-ANIE1098>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Watanabe S, et al. IgG and IgA antibody titers against human heat-shock protein (hsp60) in sera of rheumatoid arthritis and osteoarthritis patients. Mod Rheumatol. 2003;13(1):22–26. doi: 10.3109/s101650300003. [DOI] [PubMed] [Google Scholar]
- Winrow VR, et al. The two homologous chaperonin 60 proteins of Mycobacterium tuberculosis have distinct effects on monocyte differentiation into osteoclasts. Cell Microbiol. 2008;10(10):2091–2104. doi: 10.1111/j.1462-5822.2008.01193.x. [DOI] [PubMed] [Google Scholar]
- Woodlock TJ, et al. Association of HSP60-like proteins with the L-system amino acid transporter. Arch Biochem Biophys. 1997;338(1):50–56. doi: 10.1006/abbi.1996.9798. [DOI] [PubMed] [Google Scholar]
- Wyzewski Z, et al. Functional role of Hsp60 as a positive regulator of human viral infection progression. Acta Virol. 2018;62(1):33–40. doi: 10.4149/av_2018_104. [DOI] [PubMed] [Google Scholar]
- Xu Q, et al. Surface staining and cytotoxic activity of heat-shock protein 60 antibody in stressed aortic endothelial cells. Circ Res. 1994;75(6):1078–1085. doi: 10.1161/01.res.75.6.1078. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, et al. Heat shock protein 22 (HSPB8) limits TGF-beta-stimulated migration of osteoblasts. Mol Cell Endocrinol. 2016;436:1–9. doi: 10.1016/j.mce.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Yano A, et al. Inhibition of Hsp90 activates osteoclast c-Src signaling and promotes growth of prostate carcinoma cells in bone. Proc Natl Acad Sci U S A. 2008;105(40):15541–15546. doi: 10.1073/pnas.0805354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, et al. Methotrexate enhances prostaglandin D2-stimulated heat shock protein 27 induction in osteoblasts. Prostaglandins Leukot Essent Fat Acids. 2004;71(6):351–362. doi: 10.1016/j.plefa.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Zhang W, et al. Overexpression of HSPA1A enhances the osteogenic differentiation of bone marrow mesenchymal stem cells via activation of the Wnt/beta-catenin signaling pathway. Sci Rep. 2016;6:27622. doi: 10.1038/srep27622. [DOI] [PMC free article] [PubMed] [Google Scholar]