Abstract
Calcific tendinopathy is characterized by the deposition of calcium, mostly hydroxyapatite crystals, in tendons. Women are more frequently affected than men, with prevalence in the fourth and fifth decades of life. This condition has been reported between 2.5 and 7.5% of healthy shoulders in adults, but other tendons may also be affected. A complication of this condition is the migration of calcium deposits from tendons, usually the supraspinatus, into the subacromial–subdeltoid bursa. We reported a rare complication of calcific tendinopathy, that is, calcium deposits migrated from the long head of the biceps tendon over the proximal tract of the biceps brachii muscle.
Keywords: Shoulder, Ultrasound, Tendon, Calcific tendinopathy
Sommario
La tendinopatia calcifica è caratterizzata dalla deposizione di calcio, per lo più cristalli di idrossiapatite, nei tendini. Le donne sono più frequentemente colpite rispetto agli uomini, con una prevalenza nella quarta e quinta decade di vita. Questa condizione è stata riportata tra il 2,5% e il 7,5% delle spalle sane negli adulti, ma può interessare anche altri tendini. Una complicanza di questa condizione è la migrazione dei depositi di calcio dai tendini, di solito dal tendine sovraspinato alla borsa sub-acromion-deltoidea. Abbiamo riportato un caso di tendinopatia calcifica migrata dal tendine capo lungo del bicipite brachiale sopra al tratto prossimale del muscolo bicipite brachiale, rara complicanza della tendinopatia calcifica.
Introduction
Calcific tendinopathy is characterized by the deposition of calcium, mostly hydroxyapatite crystals, in tendons [1–3]. It has been extensively described in the literature over the years in the rotator cuff [1], but also in other tendons. This condition has been reported in 2.5–7.5% of healthy shoulders in adults, occurring in women in about 70% of cases, especially during the fourth and fifth decades of life [4, 5]. It does not seem to be related to physical activity [6, 7].
The pathogenesis is not completely understood, but it seems to be related to areas of hypoxia in tendons that lead to fibrocartilaginous metaplasia and cellular necrosis, followed by the formation of a calcium deposit, typically in an intact tendon. Although considered a self-healing condition, it can cause acute/chronic pain and functional disability depending on the stage of the disease [1, 2].
Four stages of disease have been described in the Uhthoff cycle: precalcific, in which fibrocartilaginous transformation occurs within tendon fibers, usually asymptomatic (stage 1); formative, in which calcifications are formed, usually poorly symptomatic, including subacute low grade pain increasing at night (stage 2); resorptive, in which the tendon develops increased vascularization and calcium deposits are usually removed by phagocytes, but calcifications may migrate into the adjacent structures (stage 3); and postcalcific, in which self-healing and repair of the tendon fibers occur, lasting for several months, and may be associated with pain and restricted function (stage 4) [1].
It is common for hydroxyapatite deposits in tendons to migrate to adjacent tissues, such as bursae, and less frequently bones. However, migration of hydroxyapatite crystals along the tendon toward the myotendinous junction has been described in only few cases, and no report is available on the long head of the biceps tendon [8, 9].
Case description
A 57-year-old male patient was seen due to pain and functional limitations in his right arm for the last 3 months. The pain was severe (VAS 7/10) mostly during the night and was partially responsive to conservative treatments, such as cryotherapy and oral NSAID (Diclofenac 150 mg/day). The physical examination revealed a palpable and painful mass on the anterior surface of the patient’s right shoulder. The swelling was red and warm, with palm-up and Yergason’s tests turning positive.
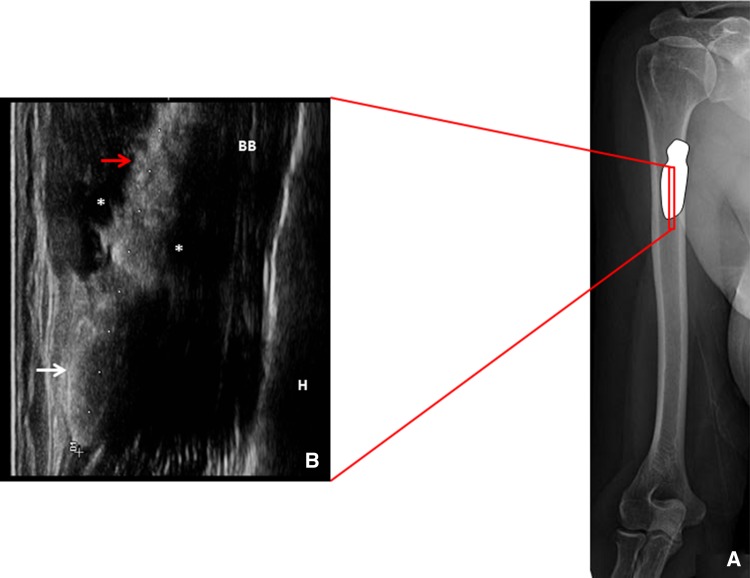
Ultrasound examination (US) was performed with a high-frequency linear probe, and demonstrated a hyperechoic and inhomogeneous mass of 40 mm, with an irregular posterior acoustic shadow, located along the anterior aspect of the proximal third of the biceps brachii muscle (Fig. 1).
Fig. 1.
Position of the probe at the proximal third of the biceps brachii muscle in the long axis view (a). The corresponding ultrasound image shows the calcium deposit located over the superficial fascia of the biceps brachii muscle with a caudal crystalline portion elliptical in shape (white arrow), and a cranial hydrated portion, more irregular in shape, slipped between the fasciae (red arrow) with peri-calcific edema (*) (b)
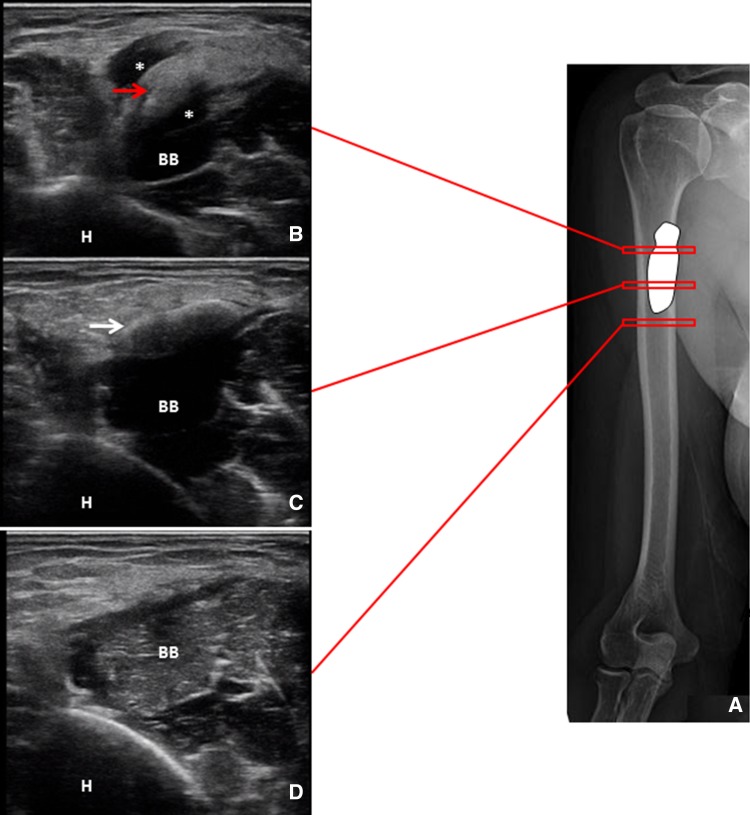
The ultrasonographic finding was compatible with a calcific deposition with two different components: a hard portion with a complete acoustic shadow (Fig. 1a, b white arrows) and a hydrated soft portion, with a partial acoustic shadow, slipped between muscular fasciae (Fig. 1a, b red arrow). Color power Doppler showed peri-calcific hyperemia correlating with neoangiogenesis and capillary proliferation typical of the resorptive phase (stage 3) (Fig. 2).
Fig. 2.
Positions of the probe at the proximal third of the biceps brachii muscle in the short axis view (a). The corresponding ultrasound images confirm the superficial localization of both the hydrated calcium deposit (red arrow) and the crystalline portion (white arrow). Of note, the peri-calcific edema (*) surrounding the hydrated component is not visible in the distal segment of the biceps brachii muscle (b–d). H humerus, BB biceps brachii
Due to the clinical and ultrasonographic findings, we prescribed a pharmacological therapy with analgesic drugs and planned a US-guided percutaneous aspiration of calcific tendinopathy (US-PICT) with a double-needle technique to fragment the solid component of the deposition and then to drain the calcium material.
After 4 days, the patient reported a remarkable clinical improvement with conservative management. Thus, the mini-invasive procedure was not performed. After 1 month, the painful shoulder disorder was completely resolved.
Discussion
Calcific tendinopathy is characterized by the deposition of calcium, mostly hydroxyapatite crystals, in tendons [1–3]. It is common for the deposits to migrate to adjacent tissues with pain and functional limitations. This condition is usually self-limited. Thus, the initial management of pain is typically conservative and involves rest, physical therapy, and oral administration of NSAIDs.
For cases in which conservative treatment has failed, non-surgical therapeutic options may be used, such as extracorporeal shock wave therapy (ESWT), steroid injection (US guided or not guided), and US-PICT.
ESWT is based on the application of repetitive pulses over the affected site. The results are alternate, and the exact underlying mechanism of the therapeutic effect on calcific tendinopathy is still debated. It seems to be related to the phagocytosis of calcium deposition induced by neovascularization response and leukocyte chemotaxis [10]. Furthermore, ESWT is painful, expensive, and not widely available.
The use of conservative treatments or ESWT in patients with acute pain given by calcific tendinopathy in the resorption phase seems to be suboptimal, and often fails because the symptoms significantly affect the quality of life [11], and many patients may not be able to tolerate the duration of time to resolution [12]. Therefore, invasive interventional techniques, such as steroid injections of the SAD bursa and/or glenohumeral joint (US guided or not guided) and US-PICT, may be pursued minimally [13].
De Witta et al. report that US-PICT is superior to steroid injections in the calcific tendinitis of the rotator cuff [14]. It is always indicated in the acute phase of the pathology with soft or fluid calcifications. In cases of hard calcifications and mildly symptomatic patients, the elective treatment should be considered [15]. The percutaneous treatment is not indicated when patients are asymptomatic and/or the calcification is very small (≤ 5 mm) [16].
Different approaches have been reported in recent studies, and all include the use of a fluid (local anesthetic or saline solution) to dissolve the calcium deposits, and one or two needles are used to inject and retrieve the fluid to dissolve the deposits.
Recent evidence showed that a double-needle approach might be more appropriate to treat harder deposits because two needles can fragment the deposits, while one needle may be more useful in treating fluid calcifications [17]. The main advantages of US-PICT are that the procedure does not require any hospitalization, the procedure is performed under local anesthesia, the patient can return home about 30 min after the procedure, there is no need for postprocedural immobilization, and the patient can return to work early [18, 19].
Arthroscopic treatment of calcific tendinitis can be applied to selected cases. In fact, it is the last option in chronic conditions in which conservative or less invasive approaches have failed. Calcification removal techniques vary regarding the type of tendon incision and the instrumentation used to remove the calcium deposit [20]. The surgery allows to remove the calcification and, at the same time, to clean thoroughly the joint of interest, but requires hospitalization, general anesthesia or sedation, and quite a long rehabilitation period after invasive treatment [21].
In conclusion, calcific tendinopathy is a common pathology but with multiple and complex complications, such as bone, muscle, and inter-fascial migrations. The conservative, mini-invasive, or surgical treatment depends on the Uhthoff stages, the anatomical localizations of the calcium deposits, and the clinical conditions of the patient.
In our case, the red and warm swelling on the anterior surface of the shoulder could have mimed a muscle injury or an infected mass. Thus, the integration of clinical and ultrasonographic findings was fundamental for the diagnosis. Moreover, the double nature of the calcific deposit (partially hard and partially soft) encouraged us to plan a double-needle US-PICT, but the spontaneous resolution of the pathology changed the therapeutic route of the patient.
Last, but not least, the inter-fascial migration of calcium seems to be a prognostically favorable condition with quite a rapid spontaneous resolution so that it is possible to wait for a minimally invasive procedure and opt for a conservative strategy as a first-level approach.
Funding
This study was not funded.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the patient included in the case report.
References
- 1.Serafini G, Sconfienza M, Lacelli F, et al. Rotator cuff calcific tendonitis: short-term and 10-year outcomes after two needle US-guided percutaneous treatment—nonrandomized controlled trial. Radiology. 2009;252(1):157–164. doi: 10.1148/radiol.2521081816. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi S, Martinoli C. Ultrasound of the musculoskeletal system. Berlin: Springer; 2007. pp. 198–332. [Google Scholar]
- 3.Tagliafico A, Russo G, Boccalini S, et al. Ultrasound-guided interventional procedures around the shoulder. Radiol Med. 2014;119(5):318–326. doi: 10.1007/s11547-013-0351-2. [DOI] [PubMed] [Google Scholar]
- 4.Clavert P, Sirveaux F. Societe francaise da [Shoulder calcifying tendinitis] Rev Chir Orthop Reparatrice Appar Mot. 2008;94:336–355. doi: 10.1016/j.rco.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Barile A, Bruno F, Mariani S, et al. Follow-up of surgical and minimally invasive treatment of Achilles tendon pathology: a brief diagnostic imaging review. Musculoskelet Surg. 2017;101:51–61. doi: 10.1007/s12306-017-0456-1. [DOI] [PubMed] [Google Scholar]
- 6.Uhthoff HK, Sarkar K. Calcifying tendinitis. Baillieres Clin Rheumatol. 1989;3(567–81):7. doi: 10.1016/s0950-3579(89)80009-3. [DOI] [PubMed] [Google Scholar]
- 7.Barile A, La Marra A, Arrigoni F, et al. Anaesthetics, steroids and platelet-rich plasma (PRP) in ultrasound-guided musculoskeletal procedures. Br J Radiol. 2016;89:1065. doi: 10.1259/bjr.20150355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pereira BP, Chang EY, Resnick DL, Pathria MN. Intramuscular migration of calcium hydroxyapatite crystal deposits involving the rotator cuff tendons of the shoulder: report of 11 patients. Skelet Radiol. 2016;45(1):97–103. doi: 10.1007/s00256-015-2255-9. [DOI] [PubMed] [Google Scholar]
- 9.Mileto A, Gaeta M. Calcific tendonitis of supraspinatus simulating acute brachial neuritis (Parsonage–Turner syndrome) Clin Radiol. 2011;66:578–581. doi: 10.1016/j.crad.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Kim YS, Lee HJ, Kim YV, Kong CG. Which method is more effective in treatment of calcific tendinitis in the shoulder? Prospective randomized comparison between ultrasound-guided needling and extracorporeal shock wave therapy. J Shoulder Elbow Surg. 2014;23:1640–1646. doi: 10.1016/j.jse.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 11.Robotti G, Canepa MG, Bortolotto C, Draghi F. Interventional musculoskeletal US: an update on materials and methods. J Ultrasound. 2013;16(2):45–55. doi: 10.1007/s40477-013-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cura JL, et al. A sonographically guided percutaneous needle lavage in calcific tendinitis of the shoulder: short- and long-term results. Am J Roentgenol. 2007;189(3):128–134. doi: 10.2214/AJR.07.2254. [DOI] [PubMed] [Google Scholar]
- 13.De Zordo T, Ahmad N, Ødegaard F, et al. US-guided therapy of calcific tendinopathy: clinical and radiological outcome assessment in shoulder and non-shoulder tendons. Ultraschall Med. 2011;32(Suppl 1):S117–S123. doi: 10.1055/s-0029-1245333. [DOI] [PubMed] [Google Scholar]
- 14.Witte PB, Selten JW, Navas A, et al. Calcific tendinitis of the rotator cuff: a randomized controlled trial of ultrasound-guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41(7):1665–1673. doi: 10.1177/0363546513487066. [DOI] [PubMed] [Google Scholar]
- 15.Serafini G, Sconfienza LM, Lacelli F, et al. Rotator cuff calcific tendonitis: short-term and 10-year outcomes after two-needle us-guided percutaneous treatment–nonrandomized controlled trial. Radiology. 2009;252:157–164. doi: 10.1148/radiol.2521081816. [DOI] [PubMed] [Google Scholar]
- 16.Sconfienza LM, Albano D, Messina C, et al. How, when, why in magnetic resonance arthrography: an international survey by the european society of musculoskeletal radiology (ESSR) Eur Radiol. 2018;28(6):2356–2368. doi: 10.1007/s00330-017-5208-y. [DOI] [PubMed] [Google Scholar]
- 17.Orlandi D, Mauri G, Lacelli F, et al. Rotator cuff calcific tendinopathy: randomized comparison of US-guided percutaneous treatments by using one or two needles. Radiology. 2017;285:518–527. doi: 10.1148/radiol.2017162888. [DOI] [PubMed] [Google Scholar]
- 18.Draghi F, Robotti G, Jacob D, et al. Interventional musculoskeletal ultrasonography: precautions and contraindications. J Ultrasound. 2010;13(3):126–133. doi: 10.1016/j.jus.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cocco G, Draghi F, Schiavone C. Ultrasonographic diagnosis and percutaneous treatment of insertional calcific tendinopathy of iliotibial band: case report. Euro Rad. 2018 doi: 10.1594/EURORAD/CASE.15881. [DOI] [Google Scholar]
- 20.Rebuzzi E, Coletti N, Schiavetti S, Giusto F. Arthroscopy surgery versus shock wave therapy for chronic calcifying tendinitis of the shoulder. J Orthop Traumatol. 2008;9:179–185. doi: 10.1007/s10195-008-0024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fields LK, Muxlow CJ, Caldwell PE., 3rd Arthroscopic treatment of subscapularis calcific tendonitis. Arthrosc Tech. 2014;3:e571–e573. doi: 10.1016/j.eats.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]