Abstract
Purpose
To investigate the frequency of thickening of pulleys for flexor tendons in patients with early arthritis in their hands, and to evaluate it as a predictive sign of PsA.
Methods
A prospective observational study involving 228 consecutive patients presenting with recent onset of arthritis in their hands was conducted at rheumatology outpatient clinics in the Veneto region of Italy between October 2014 and September 2017. Diabetic patients were excluded because of the high frequency of trigger finger. The final diagnosis of the rheumatologist delivered after 12 months of follow-up, was considered as the gold standard for the analysis of diagnostic accuracy.
Results
Twenty-two patients were excluded from the study because of diabetes. A total of 86 patients with thickening of A1 pulleys in flexor tendons and 120 without were evaluated. Pulley thickness was significantly associated with a family history of psoriasis (18/86 vs 3/120, p ˂ 0.001) and diabetes (9/86 vs 4/120, p = 0.036), and with a personal history of cutaneous psoriasis (25/86 vs 10/120, p ˂ 0.001), psoriatic onychopathy (7/86 vs 2/120, p = 0.028), lower back pain (22/86 vs 11/120, p = 0.001), Dupuytren’s disease (7/86 vs 2/120 p = 0.028) and De Quervain tenosynovitis (4/86 vs 0/120, p = 0.028). In isolation, this sign had a good sensitivity rate (80%). The specificity rate for the disease was barely significative (71%), with an LR+ of 2.71 for PsA.
Conclusions
The thickening of the pulleys in the flexor tendons is an easy-to-detect sign with good sensitivity for the diagnosis of PsA. Its specificity and positive predictive value are not very high; however, if it is included in a complete classification process, sonographers should report it during hand evaluations of patients with arthritis.
Keywords: Psoriatic arthritis, Ultrasonography, Tendon pulleys
Sommario
Scopo
Studiare la frequenza di ispessimento delle pulegge tendinee A1 (A1P) dei muscoli flessori delle dita in pazienti affetti da artrite della mano in fase precoce e valutarlo come un segno predittivo di Artrite Psoriasica (AP).
Metodi
Uno studio prospettico osservazionale multicentrico su 228 pazienti ambulatoriali consecutivi che presentavano recente insorgenza di artrite alle mani tra ottobre 2014 e settembre 2017. I pazienti diabetici sono stati esclusi a causa dell’alta frequenza di dito a scatto. La diagnosi finale del Reumatologo Curante dopo un follow-up di 12 mesi è stata confrontata come Gold Standard.
Risultati
Un totale di 86 pazienti con ispessimento delle A1P e 120 senza sono stati arruolati. L’ispessimento delle A1P era significativamente associato a storia familiare di psoriasi (18/86 vs 3/120, p ˂ 0.001) e di diabete (9/86 vs 4/120, p = 0.036), con storia personale di psoriasi cutanea (25/86 vs 10/120, p ˂ 0.001), di onicopatia psoriasica (7/86 vs 2/120, p = 0,028), di lombalgia (22/86 vs 11/120, p = 0,001), di malattia di Dupuytren (7/86 vs 2/120 p = 0,028) e di tenosinovite di De Quervain (4/86 vs 0/120, p = 0,028). Isolato, questo segno ha dimostrato una buona sensibilità (80%) per la diagnosi di AP; la specificità per AP era appena significativa (71%) con una LR+ di 2,71.
Conclusioni
L’ispessimento delle A1P è facile da individuare con una buona sensibilità per la diagnosi di AP. La specificità e il VPP non erano molto elevati. Tuttavia, lo studio ecografico delle pulegge tendinee dovrebbe essere incluso nel processo diagnostico di pazienti con artrite acrale all’esordio.
Introduction
Clinical recognition of early-stage psoriatic arthritis (PsA) is especially challenging when the disease predominantly affects peripheral joint. The Assessment of SpondyloArthritis International Society (ASAS) criteria for peripheral spondyloarthritis (SpA) [1] are to be applied in patients with peripheral arthritis. Usually, however, peripheral arthritis is asymmetric, and predominantly involves the lower limbs or entheses. Even after the introduction of the Classification Criteria for Psoriatic Arthritis (CASPAR) [2], peripheral arthritis still presents one of the most difficult differential diagnosis scenarios in rheumatology.
Pulley thickening has been described with MRI in PsA patients with dactylitis. Tan et al. noted that the small entheses associated with the tendon pulleys (A2, A3, and A4, but not A1) and sheaths could be sites of enthesitis relevant to the tenosynovitis pathology [3]. It is unclear if this observation is specific to dactylitis in PsA, as it had been previously noted that there was also an absence of flexor tendon insertion enthesitis in osteoarthritis of the finger joints, although the tendon pulleys and sheaths were not examined [4]. In all these studies, there is neither evidence relating to the frequency of these aspects in other forms of arthritis nor correlation with the degree of disease activity.
For these reasons, we decided to confirm the previous data with a high spatial resolution method like ultrasound, focusing on the more explorable pulleys (A1) to confirm the evidence of thickening in patients with early arthritis in their hands and to investigate it as a predictive sign of PsA.
Methods
This prospective observational study was approved by the ethics committee at the University Hospital of Padua (Italy). In accordance with the principles outlined in the Declaration of Helsinki, every patient provided informed consent before enrollment.
Patients at rheumatology outpatient clinics in the Veneto region of Italy who presented with recent onset of arthritis in their hands between October 2014 and September 2017 were enrolled. Demographic, clinical and laboratory findings were recorded for every patient. The disease duration was defined as the interval between the onset of symptoms and the rheumatologist’s evaluation.
Since tendon pulley thickness is often the result of mechanical stress with stenosing tenosynovitis (for example, in patients that make heavy use of their hands for work or athletic purposes), we excluded patients with this specific risk. As previously noted, diabetic patients were also excluded because of the high frequency of trigger finger already reported in this disease [5–7].
In previous anatomical and sonographic studies, the mean ± standard deviation (M ± SD) of pulley thickness in healthy subject/controls was 0.6 ± 0.14 mm [8, 9]. We defined A1 pulley thickening as any value above two times the SD, prudentially increased to 1.0 mm.
Imaging
All ultrasound examinations were recorded using a Hitachi Ascendus Model EZU-MT28-SI and Linear 4 cm Probe 18 MHz Ref. EUP-L75, both of which were manufactured in April 2013 (Hitachi, Tokyo, Japan).
A radiologist (RS) and a rheumatologist (AF), both with more than 5 years’ experience in hand-ultrasound (US), evaluated the study participants. To ensure interobserver agreement, the evaluations of the last 100 patients enrolled were independently repeated by a second sonographer. Finally, the US findings were recorded, and the patients were then followed by the treating rheumatologists for 1 year before final diagnoses were provided. We assumed this final diagnosis as the gold standard for the evaluation of the sensitivity and specificity of the sign.
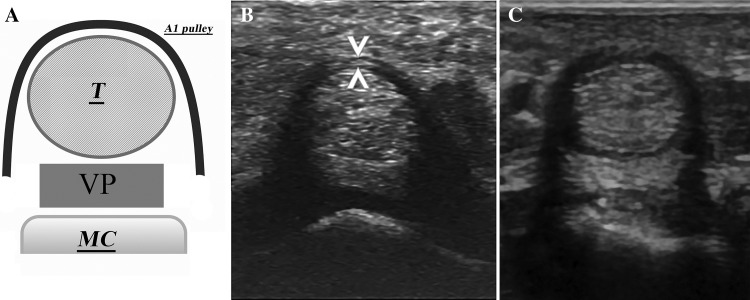
The following structures were bilaterally evaluated with a multiplanar technique in the dorsal view according to the Backhaus et al. guidelines for musculoskeletal US in rheumatology (sections 4.3 wrist and 4.4 hand) [10] and with the addition of a pulley assessment: extensor digital tendons, sheaths and retinaculum, radiocarpal joint, ulnar styloid and surrounding soft tissues, carpal tunnel and median nerve, palmar plate, A1 pulleys of digital flexor tendons, metacarpophalangeal joints (MCP), proximal interphalangeal joints (PIP), distal interphalangeal joints (DIP), and trapezium-metacarpal joint (TMC). The limits of the pulley and other components were defined by dynamic examination. Thickening synovial membranes and the degree of power of Doppler signals were recorded. The former were also scored using the semi-quantitative scale developed by Filipucci (0–3) [11]. Hand digit volar aspects were studied in longitudinal and transverse planes. Measurement on the transverse plane was done for the A1 pulley at the thickest point of the pulley arcs, as shown in Fig. 1.
Fig. 1.
The A1 pulley. a The A1 pulley scheme. T: flexor tendons (superficial and deep), VP: volar plate, MC metacarpal bone. b A normal A1 pulley, note the homogeneous and thin thickness of the arc and the transverse measurement of the thinness (arrowheads). c a thickened pulley, the arc thickness is more than 1 mm
Statistical methods
Based on the findings from epidemiological Italian studies [12], where the prevalence of PsA was about 0.42%, a total of 206 enrolled patients was necessary to observe a difference of at least 30% in pulleys’ thickening frequency between PsA and other diagnoses, with a power of 90% and an alpha error of 5%.
Baseline characteristics of and differences between the two groups were estimated using Student’s t test. Comparison of categorical variables was conducted by Fisher’s exact test or Pearson Chi-square test, as applicable. Only patients with final follow-up diagnoses of inflammatory arthropathy (PsA, rheumatoid arthritis [RA], or undifferentiated arthritis [UnA]) have been included in disease activity scoring (DAS28-CRP).
Furthermore, the diagnostic performance of the pulley sign was determined by calculating sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−), positive predictive value (PPV), and negative predictive value (NPV). Finally, interobserver concordance between the two sonographers in the thickening assessment was indicated using Cohen’s κ. The κ values were interpreted as follows: 0–0.2 = poor agreement, 0.2–0.4 = fair agreement, 0.4–0.6 = moderate agreement, 0.6–0.8 = good agreement, and 0.8–1 = excellent agreement. All analyses were performed using MedCalc Statistical Software version 15.8 (MedCalc Software, Ostend, Belgium; https://www.medcalc.org; 2015); p values < 0.05 were considered significant.
Results
A total of 228 patients were examined, but data were analyzed for only 206 of them because of the exclusion criteria. The study included 86 patients with thickening A1 pulleys (cases) and 120 without (controls). The details of demography and baseline clinical characteristics are reported in Table 1. The case and control populations had comparable age ranges, M/F ratios, and disease durations. Pulley thickening was significantly associated with a family history of psoriasis (18/86 vs 3/120, p ˂ 0.001) and diabetes (9/86 vs 4/120, p = 0.036). However, there was no significant association with ischemic heart disease, hypertension, endocrinopathies or cancer (data not reported). An analysis of comorbidity among examinees also showed a significant association between the thickening of pulleys and cutaneous psoriasis (25/86 vs 10/120, p ˂ 0.001), psoriatic onychopathy (7/86 vs 2/120, p = 0.028), lower back pain (22/86 vs 11/120, p = 0.001), Dupuytren’s disease (7/86 vs 2/120 p = 0.028), and De Quervain tenosynovitis (4/86 vs 0/120, p = 0.028). Table 2 shows the relation of the US findings to the clinical disease activity. Even after the data were stratified for a specific inflammatory condition (PsA, RA or UnA), there was no association between the US pulleys findings and the disease activity indexes, including the joint counts (tenderness and swollenness) and the DAS28-CRP.
Table 1.
Clinical and demographic characteristics of the patients
| With thickness (cases) (N = 86) | Without thickness (ctrls) (N = 120) | p | |
|---|---|---|---|
| Age, years | 56.9 ± 9.6 | 55.0 ± 12.9 | 0.249 |
| M/F | 64/22 | 96/24 | 0.397 |
| Disease duration (months) | 7.8 ± 7.2 | 8.2 ± 7.3 | 0.868 |
| Familiarity | |||
| Psoriasis | 18 | 3 | <0.001 |
| Ischemic cardiopathy | 5 | 9 | 0.438 |
| Diabetes | 9 | 4 | 0.036 |
| Thyroiditis | 3 | 5 | 0.561 |
| Comorbidity | |||
| Psoriasis | 25 | 10 | <0.001 |
| Psoriatic onychopathy | 7 | 2 | 0.028 |
| Low back pain | 22 | 11 | 0.001 |
| Dupuytren’s dis. | 7 | 2 | 0.028 |
| De Quervain’s dis. | 4 | 0 | 0.028 |
| Carpal tunnel synd. | 11 | 7 | 0.065 |
| Raynaud’s phen. | 1 | 1 | 0.659 |
| Thyroiditis | 7 | 9 | 0.533 |
| Laboratory | |||
| Anemia | 8 | 12 | 0.538 |
| Thrombocytopenia | 4 | 9 | 0.029 |
| Leucopenia | 7 | 4 | 0.487 |
| ESR (mm) | 19.2 | 20.9 | 0.580 |
| CRP (mg/l) | 6.4 | 7.8 | 0.622 |
| RF | 6 | 13 | 0.258 |
| ACCP | 3 | 6 | 0.433 |
| Anti-thyroid antibodies | 3 | 5 | 0.624 |
| ANA | 12 | 21 | 0.368 |
Data presented as mean ± SD unless stated otherwise
CRP C-reactive protein assessment, ESR eritrosedimentation rate after 1 h, RF rheumatoid factor, ACCP anti-citrullinated peptides antibodies, ANA anti nuclear antibodies
Table 2.
Disease activity and thickening of the pulleys
| With thickening (N = 86) | Without thickening (N = 120) | p | |
|---|---|---|---|
| TJC | 7.4 ± 5.2 | 6.4 ± 4.5 | 0.123 |
| SJC | 3.5 ± 3.5 | 3.0 ± 2.9 | 0.288 |
| DAS28 | 4.0 ± .83 | 3.7 ± 0.92 | 0.046 |
| Dactylitis | 33/86 | 20/120 | 0.001 |
| DAS28 | |||
| PsA | 4.28 ± 0.70 | 3.53 ± 0.76 | 0.092 |
| RA | 4.19 ± 0.97 | 4.61 ± 0.67 | 0.246 |
| UnA | 3.69 ± 0.72 | 3.16 ± 0.77 | 0.087 |
| Filippucci’s score | |||
| MCP | 0.56 ± 0.796 | 0.79 ± 0.898 | 0.251 |
| PIP | 0.39 ± 0.698 | 0.41 ± 0.712 | 0.362 |
| DIP | 0.55 ± 0.688 | 0.10 ± 0.316 | 0.076 |
Data reported as M ± SD
TJC tender joints count, SJC swollen joint count, DAS28 disease activity score computed with CRP, PsA psoriatic arthritis, RA rheumatoid arthritis, UnA undifferentiated arthritis, MCP metacarpophalangeal joints, PIP proximal interphalangeal joints, DIP distal interphalangeal joints
PsA patients presented cystic degeneration of the pulleys in 16/50 cases, vs 21/156 for all the other diseases (p = 0.004) but the number of fingers with cystic lesions was not different (1.43 ± 0.87 vs 1.75 ± 1.18, p = 0.369). There was also no association between the final diagnosis and the number of fingers affected by the thickening (M ± SD, PsA: 3.60 ± 2.56 vs other diagnosis 2.80 ± 1.77, p = 0.103).
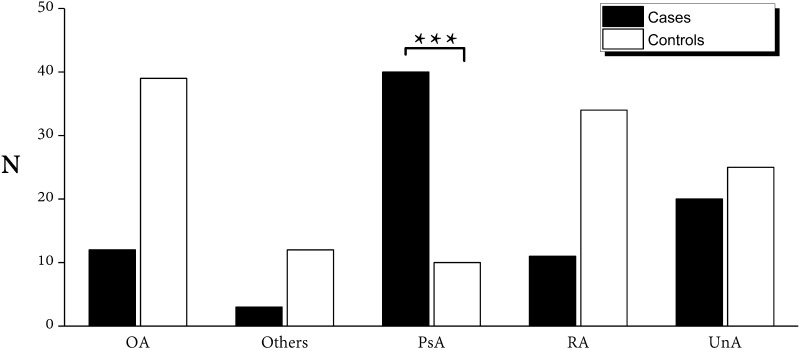
The final diagnoses of the treating rheumatologist were compared with the US findings and the results are summarized in Table 3 and Fig. 2. The sensitivity, specificity, LR+, and LR− for each diagnosis are reported. The pulley thickening was statistically associated with a final diagnosis of PsA (40/50, p < 0.001), with a sensitivity of 80.0% (CI 66.3–90.0%) and specificity of 71.0% (CI 62.3–77.5%). The sensitivity for the diagnosis of RA was 24.4% (CI 12.9–39.5%) and the specificity 53.4% (45.4–61.3%). For OA, the sensitivity was 23.5% (CI 12.8–37.5%) and the specificity 49.0% (CI 40.6–57.4%). Finally, interobserver concordance between the two sonographers, assessed by Cohen’s κ, showed a value of 0.763, or f “good agreement” in the evaluation of the thickening of the pulleys.
Table 3.
Diagnostic power of the pulleys thickening vs the final diagnosis provided by the treating rheumatologist after 12 months
| Cases (%) (N = 86) | Controls (%) (N = 120) | Sensitivity % | Specificity % | LR+ | LR− | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| Psoriatic arthritis | 40 (46.5) | 10 (8.3) | 80.0 | 71.0 | 2.71 | 0.28 | 46.5 | 91.7 |
| Rheumatoid arthritis | 11 (12.8) | 34 (28.3) | 24.4 | 53.4 | 0.52 | 1.41 | 12.8 | 71.7 |
| Osteoarthritis | 12 (14.0) | 39 (32.5) | 23.5 | 48.9 | 0.46 | 1.56 | 14.0 | 64.6 |
| Undifferentiated arthritis | 20 (23.3) | 25 (20.8) | – | – | – | – | – | – |
| Other/negative | 3 (3.5) | 12 (10.0) | – | – | – | – | – | – |
LR+ positive likelihood ratio, LR− negative likelihood ratio, PPV positive predictive value, NPV negative predictive value
Fig. 2.
Frequency of the thickening of the pulleys in different final diagnosis provided by the treating Rheumatologist after 12 months of follow-up. OA osteoarthritis, UnA undifferentiated arthritis, RA rheumatoid arthritis, PsA psoriatic arthritis, Other other diagnosis (connectivitis, reactive arthritis, viral arthritis, rheumatic polymyalgia)
Discussion
This study used the US to explore whether the thickening of the pulleys in flexor tendons could be a useful sign in differential diagnoses of early arthritis.
There is a growing interest in specific findings relating to flexor tendons’ involvement in PsA. After the end of this study, we read with interest the recent article published by Tinazzi et al. [13], who reported thickening of accessory pulleys in patients with established PsA compared with other arthropathies. Thickening was especially pronounced in subjects with a history of dactylitis. Their results support the idea of a “deep” Koebner phenomenon in dactylitis and sites of high physical stress. While we are glad to note the two studies have similar results, the aim of our work was different. The population studied in our research was selected from early arthritis outpatient clinics. Our subjects included those with a mean low disease activity and a poor clinical differentiation rate. All were without a confirmed diagnosis. In this scenario, it was interesting to observe that the thickening of pulleys can be used as a sign to differentiate arthritis regardless of the disease activity assessed with the DAS28.
Even when we excluded diabetic patients, our analysis showed a higher frequency of pulleys thickening in subjects with at least a diabetic relative; other thickenings of connective tissue structures in the hand, such as those in the first extensor compartment of the wrist (De Quervain’s tenosynovitis) or the volar plate (Dupuytren’s disease) followed a similar trend.
This study had several limitations. First, we did not record the absolute thickness of the pulley, but only recorded the presence or absence of thickening. The choice of 1 mm as the limit of thickness was prudentially high, but not a validated value, as there are no relevant data published, and the mean results obtained by Tinazzi et al. were thinner (0.46–0.68 mm in PsA). This discrepancy may affect the sensitivity of our method. We also did not distinguish between the frequency of involvement of the dominant hand and that of individual fingers. Even if the focus of the study was on differential diagnoses of hand arthropathies, it would have been interesting to know if pulley thickening is present in patients with another kind of joint involvement. The patients of this study may not be representative to the PsA population in general.
In conclusion, this sign has good sensitivity (80%) as an indicator for a PsA diagnosis in the difficult scenario of early arthritis in the hand. The specificity for the disease is also high (71%). Additionally, the presence of cysts on the pulleys is frequently recorded in the same group. These results support the involvement of extra synovial structures as a key factor in differential diagnoses between PsA and other kinds of arthritis. However, taken alone, they do not provide enough PPV to suspect the disease if not associated with other classification criteria. For this reason, the presence of thickening in the pulleys of flexor tendons cannot be used as a single diagnostic factor, regardless of its usefulness in clinical assessment. That said, we suggest that sonographers should search for and report it during sonographic hand evaluations.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the ethics committee at the University Hospital of Padua (Italy).
Informed consent
Every patient provided informed consent before enrollment.
References
- 1.Rudwaleit M. New approaches to diagnosis and classification of axial and peripheral spondyloarthritis. Curr Opin Rheumatol. 2010;22:375–380. doi: 10.1097/BOR.0b013e32833ac5cc. [DOI] [PubMed] [Google Scholar]
- 2.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 3.Tan AL, Fukuba E, Halliday NA, Tanner SF, Emery P, McGonagle D. High-resolution MRI assessment of dactylitis in psoriatic arthritis shows flexor tendon pulley and sheath-related enthesitis. Ann Rheum Dis. 2015;74:185–189. doi: 10.1136/annrheumdis-2014-205839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan AL. Combined high-resolution magnetic resonance imaging and histological examination to explore the role of ligaments and tendons in the phenotypic expression of early hand osteoarthritis. Ann Rheum Dis. 2006;65:1267–1272. doi: 10.1136/ard.2005.050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebiedz-Odrobina D, Kay J. Rheumatic manifestations of diabetes mellitus. Rheum Dis Clin North Am. 2010;36:681–699. doi: 10.1016/j.rdc.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Kameyama M, Meguro S, Funae O, Atsumi Y, Ikegami H. The presence of limited joint mobility is significantly associated with multiple digit involvement by stenosing flexor tenosynovitis in diabetics. J Rheumatol. 2009;36:1686–1690. doi: 10.3899/jrheum.081024. [DOI] [PubMed] [Google Scholar]
- 7.Arkkila PET, Gautier J-F. Musculoskeletal disorders in diabetes mellitus: an update. Best Pract Res Clin Rheumatol. 2003;17:945–970. doi: 10.1016/j.berh.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto H, Miura T, Isayama H, Masuzaki R, Koike K, Ohe T. Stiffness of the first annular pulley in normal and trigger fingers. J Hand Surg Am. 2011;36:1486–1491. doi: 10.1016/j.jhsa.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 9.Sato J, Ishii Y, Noguchi H, Takeda M. Sonographic appearance of the flexor tendon, volar plate, and A1 pulley with respect to the severity of trigger finger. J Hand Surg Am. 2012;37:2012–2020. doi: 10.1016/j.jhsa.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Backhaus M, Burmester GR, Gerber T, Grassi W, Machold KP, Swen WA, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001;60:641–649. doi: 10.1136/ard.60.7.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippucci E, Iagnocco A, Salaffi F, Cerioni A, Valesini G, Grassi W. Power Doppler sonography monitoring of synovial perfusion at the wrist joints in patients with rheumatoid arthritis treated with adalimumab. Ann Rheum Dis. 2006;65:1433–1437. doi: 10.1136/ard.2005.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salaffi F, De Angelis R, Grassi W, Prevalence MAP, Study ING Prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community-based study. I. The MAPPING study. Clin Exp Rheumatol. 2005;23:819–828. [PubMed] [Google Scholar]
- 13.Tinazzi I, McGonagle D, Aydin SZ, Chessa D, Marchetta A, Macchioni P. ‘Deep Koebner’ phenomenon of the flexor tendon-associated accessory pulleys as a novel factor in tenosynovitis and dactylitis in psoriatic arthritis. Ann Rheum Dis. 2018;77:922–925. doi: 10.1136/annrheumdis-2017-212681. [DOI] [PubMed] [Google Scholar]