Abstract
Splenic injuries are common emergencies in the setting of abdominal trauma, as the spleen is the second most frequently injured abdominal organ after the liver. The treatment of splenic injuries underwent a severe shift from operative to non-operative due to an increased awareness of the double physiological function, both immunological and hematological, of the spleen. This, in turn, led to an increased application of splenic preservation techniques. The non-operative approach has been strengthened through radiological imaging and interventional radiology. While multidetector computed tomography is mandatory in the evaluation of hemodynamically stable patients after high-energy trauma, one ultrasound (US) can be used as a first-line technique to examine patients in cases of low-energy trauma. Unfortunately, baseline US has low sensitivity in the detection of traumatic injuries. With the introduction of contrast-enhanced ultrasound (CEUS) as a reliable alternative to baseline ultrasound for low-grade abdominal trauma, the sensitivity of the US technique in recognizing traumatic abdominal lesions has strongly increased, reaching levels of accuracy similar to those of the CT. It has also been strongly recommended for use with children, as it allows for the performance of imaging techniques with the lowest dose of radiation possible. In this review, the authors aim to present the typical appearance of traumatic splenic injuries, using enhanced CEUS capability to overcome baseline US limits, and to describe the different techniques applied according to the hemodynamic stability of the patient.
Keywords: Trauma, Trauma imaging, Traumatic splenic injuries, Splenic trauma, Blunt abdominal trauma, BAT, Contrast-enhanced ultrasound, CEUS, Multidetector computed tomography, MDCT, Non-operative management
Sommario
La milza è il secondo organo più comunemente sede di trauma nel contesto dei traumi chiusi dell’addome. Nel corso degli ultimi decenni il trattamento dei traumi splenici ha subito un rapido shift verso un approccio sempre meno aggressivo. Il razionale alla base di questo trend sta nel duplice ruolo svolto da quest’organo dal punto di vista fisiologico, sia immunologico che ematologico, che ha portato allo sviluppo di manovre terapeutiche sempre più conservative. Questo approccio nonoperativo è stato fortemente sostenuto dalle nuove metodiche di imaging e dal rapido evolversi delle tecniche di radiologia interventistica. Dal punto di vista dell’imaging, mentre la TC Multidetector è mandatoria nella valutazione del paziente che ha subito un trauma ad elevata energia e stabile emodinamicamente, nei traumi a bassa energia l’ecografia può essere utilizzata come metodica di primo approccio. Sfortunatamente essa è caratterizzata da una bassa sensibilità nell’ individuazione delle lesioni traumatiche a carico degli organi addominali. Con l’introduzione dell’ecografia con mezzo di contrasto (CEUS) come alternativa alla sola ecografia nella valutazione dei traumi a bassa energia, la sensibilità di tale metodica è notevolmente aumentata, raggiungendo livelli di accuratezza molto vicini a quelli della TC. Il suo utilizzo è stato fortemente sostenuto nella valutazione iniziale del paziente pediatrico al fine di ridurre il più possibile l’esposizione a radiazioni ionizzanti. In questo articolo gli autori si propongono di presentare una revisione dei traumi splenici, enfatizzando il ruolo della CEUS rispetto alla sola ecografia e di descrivere quali sono le metodiche da utilizzare in base alla stabilità emodinamica del paziente.
Introduction
Splenic injury is a common event after a blunt abdominal trauma (BAT), and since clinical examinations do not always provide sufficient information regarding the presence and the extension of injuries, the use of reliable imaging techniques is becoming crucial to accurately stage the condition. In BAT settings, the spleen is the second most frequently injured organ after the liver, with road traffic collisions, falls from heights, motorcycle accidents, and sports being the most common mechanisms of trauma. Some anatomical or pathologic conditions, such as splenomegaly, can predispose an individual to lesions after a relatively trivial trauma. Penetrating injuries are less common.
Isolated involvement of the spleen accounts for 46% of cases. In the remaining cases, splenic injury presents in association with other abdominal organ injuries. While in adults, the spleen is partially protected by the rib cage, in children it is bigger and protrudes from the chest wall. In 25% of BAT cases, children present both isolated and multiorgan lesions [1, 2].
The spleen is the most vascularized organ in the body: about 350 L of blood flows through it daily, which is why its injury is a potentially life-threatening condition, exposing the patient to a massive hemoperitoneum.
Splenectomy was considered the unique treatment for splenic trauma until the 1960s, regardless of the injury grading. Increased awareness over time of the spleen’s double physiological function, both immunological and hematological, has lead to an increased application of preservation techniques. In 1968, Upudhyaya and Simpson proposed an alternative approach, non-operative management (NOM), in a cohort of 52 pediatric patients who had sustained a blunt splenic trauma [3]. Since then, a trend towards splenic conservation therapies has spread throughout many trauma centers. The main purpose of NOM is to preserve the spleen through medical treatment and splenic embolization, without access to the peritoneal cavity. While, according to the American Association for the Surgery of Trauma (AAST), this is a standardized approach in mild or low-energy trauma, with a grade I–II lesion, and in highly select cases involving grade III injuries, its role is still under debate in cases of more complex injuries (grade IV and V) [4, 5]. However, the management of splenic high-grade injuries lies outside the scope of this assessment.
This shift towards a non-operative approach requires an increased number of imaging studies to monitor the healing of lesions through contrast-enhanced computed tomography (CE-CT). However, we have to consider that the high number of children and women of reproductive age involved in blunt abdominal trauma, and the use of ionizing radiation and contrast media on the one hand, has lead to an increased use of CEUS in approaching low-grade blunt abdominal injury; this shift was also strengthened by the improved imaging provided by this technique with respect to CE-CT.
The aim of this article is to describe the role of CEUS in the diagnosis and management of traumatic splenic injuries, giving an update on the current literature.
Imaging techniques
US-CEUS
Ultrasonography (US) is often the first technique employed in the minor trauma field, as it is non-invasive, repeatable and inexpensive, and characterized by high sensitivity in detecting free abdominal fluid (63–99%) [6–8]. However, US sensitivity in the identification of solid organ injuries, especially in the absence of free abdominal fluid, is quite low (< 50%), even with a highly experienced operator [9]. In recent years, some authors have strongly supported contrast-enhanced ultrasonography (CEUS) as an alternative means of evaluating low-grade abdominal trauma. After being tested in trauma practice for more than a decade, this technique may now have an important role to play in the prompt evaluation of stable patients who have sustained a blunt abdominal trauma.
Its employment did increase the sensitivity of US techniques for recognizing traumatic abdominal lesions. These reached levels of accuracy are quite similar to those obtained with CT (up to 95%) [10–12].
The contrast agents consist of perfluorocarbon or sulfur hexafluoride, encapsulated by a very resistant phospholipid shell composed of stabilized gas microbubbles (1–7 micron), which are blood-pool agents with a non-linear reverberation. They remain intravascular and produce a non-linear harmonic response, which can be separated from the tissue signal using contrast harmonic US.
SonovueR is the USCA currently used in Europe. It is a lyophilized powder to be dissolved in water, and, once in solution, it is immediately ready to be used. It does not require fasting or laboratory tests.
The contrast agent is administered through an antecubital vein. The arterial phase starts after 10–20 s, and can continue up to 30–40 s. During the venous and late phase, the contrast agent is distributed to the whole capillary bed, and the concentration slowly decreases until the agent is excreted through the lungs. The venous and late phase lasts in the range of 2–6 min, varying in each abdominal parenchyma.
The low solubility of SonovueR, associated with the high resistance of its shell to the mechanical effect of ultrasound beam, gives it a long duration, so that it is possible to explore all the vascular phases in real time.
Contrast imaging requires specific software that operates at a low mechanical index, analyzing the resonance signals originated by second-generation contrast agents without bubble destruction, thus allowing the whole vascular phase to be performed in real time. The software suppresses the signals coming from stationary tissue, thereby improving contrast resolution and differentiating the signals produced by background tissue and gas-filled microbubbles. The microbubbles emit harmonics at twice the insonation frequency through the reflection of ultrasound beams. Thereafter, the transducer separates the fundamental frequency from the second harmonic using an inverted phase pulse, and then acquires the signal [13].
Based on the authors’ experience, a total of 4.8 mL of Sonovue, divided into two 2.4 mL doses, is administered through an 18-gauge needle in an antecubital vein, followed by 5–10 mL of saline solution. After the first bolus, the right-side organs are explored for 1–3 min. After the administration of the second bolus, the left-side organs are studied for 3–4 min.
Spleen evaluation by CEUS is usually limited by the interposition of the rib cage and the colonic splenic flexure, especially with regard to the upper pole and the subphrenic region. The arterial phase starts at 12–20 s and has a long duration. It produces the so-called “zebra” enhancement of the organ due to the movement of the contrast media within the red and the white pulp. In this phase, it is difficult to recognize any tissue lesion. The venous phase, starting at 40–60 s after contrast media injection, is the most reliable one for the detection of organ injury because the healthy parenchyma appears as homogeneous enhancing tissue with a long duration (about 5–7 min) [14, 15]. A negative factor affecting this technique is the quick decrease of enhancement in splenic veins, because the spleen acts as an active filter of microbubbles, thus resulting in a mild perfusion of splenic vessels. This effect may create a problem of differential diagnosis, as the veins can be mistaken for lacerations. When in doubt, a reinjection of a second bolus of USCA, as recommended by Valentino et al. [10], can help.
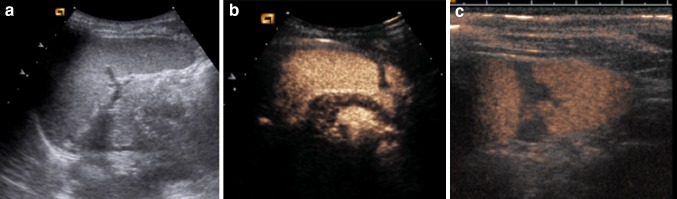
CEUS seems very useful when performing imaging in children (Fig. 1) according to the ALARA principles, which aim to reduce exposure to ionizing radiation using the imaging technique without any or with the lowest possible radiation dose. Children are particularly susceptible to radio exposure, which is associated with an increased risk of developing malignancy later in life [16–18].
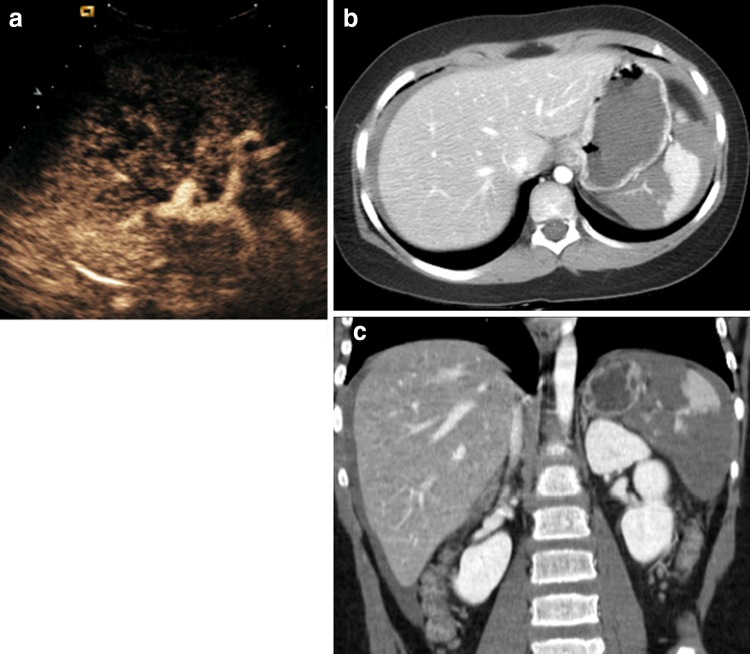
Fig. 1.
Direct blunt abdominal trauma in a 10-year-old boy. a US depicts a subtle inhomogeneity of the middle spleen without evidence of a real injury. b CEUS shows a deep splenic lesion without perisplenic fluid
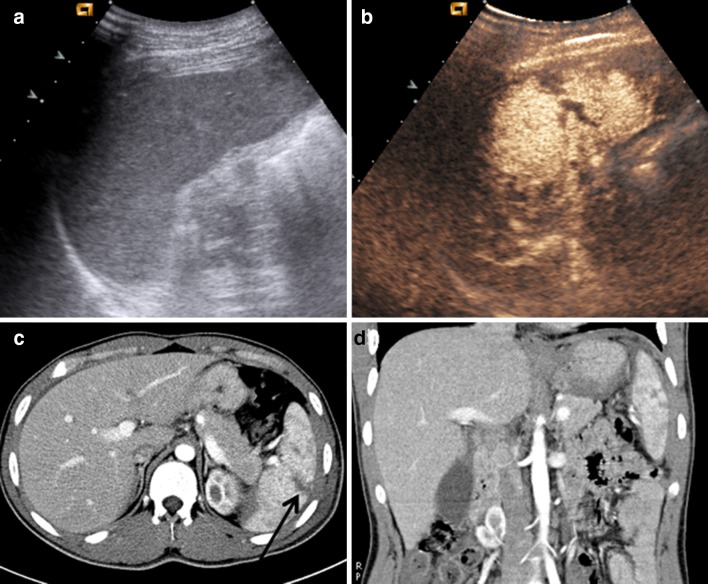
CEUS performance has been investigated in the follow-up of low-energy hepatic and splenic injuries [19]. Studies demonstrate that in this setting, the technique achieves a detection power similar to that of CT or magnetic resonance (MR) imaging [20], while allowing real-time study of lesions (Fig. 2).
Fig. 2.
a CEUS shows a double splenic lesion, at the upper and lower pole, respectively. b CE-CT on axial plane and c CE-CT on coronal plane confirm CEUS findings
CE-MDCT
Multidetector computed tomography (MDCT) is the standard imaging technique performed to evaluate hemodynamically stable patients after BAT [21, 22].
The authors usually perform CE-MDCT examination within 1 h after CEUS, with a biphasic protocol consisting of an arterial and a portal venous phase. A volume of 100–150 mL of intravenous non-ionic contrast agent is administered at a rate of 2–4 mL/s through an 18–20 gauge angiographic catheter; the arterial phase starts with a delay of about 40 s, and the portal one with about 70–80 s. CT sections are obtained at a pitch of 1.5 and reconstructed in 1-mm-thick sections; coronal multiplayer reformatted images are reviewed on a picture archiving and communication system.
The added value of the arterial phase with regard to the standard portal venous one is still under debate; a retrospective review of 3525 patients who underwent a dual-phase CT after a blunt abdominal injury carried out by Corwin et al. [23] demonstrated that the routine use of the arterial phase did not improve the clinical benefit, while increasing the radiation dose by 62%. In this study, the active extravasation was identified on the portal phase in 25% of patients with splenic injuries, and a focal splenic blush was identified in only five patients. The authors suggest that in this case, the arterial phase may positively contribute to the final diagnosis, since the size stability and enhancement of the lesion that follows blood through multiple phases assists in discerning between a contained vascular injury and an active extravasation. Nevertheless, the authors stress that a delayed scan could be more helpful, because it could be performed after the identification of the splenic injury on the portal phase, and therefore be used only in patients with splenic injury.
On the other hand, some authors highlighted the importance of performing the arterial phase so as to identify non-bleeding vascular injuries, which can be missed if only portal and excretory phases are performed [24–26]. According to these authors, the complete knowledge of splenic active bleeding and non-bleeding vascular injuries is a key point in achieving an accurate grading of the trauma to evaluate the best treatment options.
A study performed by Saksobhavivat et al. [27] presented the MDCT-based splenic grading scale as the best individual variable for decision-making between observation of and intervention with hemodynamically stable patients with blunt splenic injuries. MDCT and the abbreviated injury scale (AIS) were revealed to be the best combination of tools in the selection of patients for observation or splenic intervention.
Traumatic lesions
Splenic injuries have different shapes. They can be linear, branched or complex, and associated with the presence of hemoperitoneum in case of splenic capsular rupture, or with subcapsular or intraparenchymal hematoma, if the capsule is undamaged (around 25% of cases).
As demonstrated by Sivit et al. [28], hemoperitoneum linked to capsular lesion is most frequent when the hilum region is involved and the blood flows along the splenorenal ligament at first, then reaches the retroperitoneal zone in the anterior left pararenal space and around the pancreatic tail. These authors studied 1744 children who had undergone blunt trauma. Of the 96 patients with splenic blunt trauma, extraperitoneal fluid, confined to the anterior pararenal space, was observed in 8 (8%) of them; the fluid separated the splenic vein and pancreas in 25% of cases. This fluid was also observed in 38 of the 1608 patients with blunt trauma that did not cause splenic injury. In 45% of these patients (i.e., 17/38), it was associated with pancreatic injury. Sivit et al. aimed to highlight that fluid in the anterior pararenal space and between the splenic vein and the pancreas could be observed in isolated splenic injuries, a feature useful for reducing errors in interpretation.
Often, the most evident sign of splenic lesion is the presence of a hypo-anechoic fluid collection in the subcapsular or perisplenic space [29].
Parenchymal lesions can be very difficult to recognize, especially when perisplenic fluid is not visualized. In addition, fresh blood echogenicity is very similar to normal parenchyma. For this reason, the use of baseline US can cause even the largest traumatic lesions to be overlooked. CEUS overcomes this limitation. Parenchymal lesion evaluation must be performed during the venous phase, 120–240 s after contrast medium injection, when normal parenchyma shows high homogeneous echogenicity.
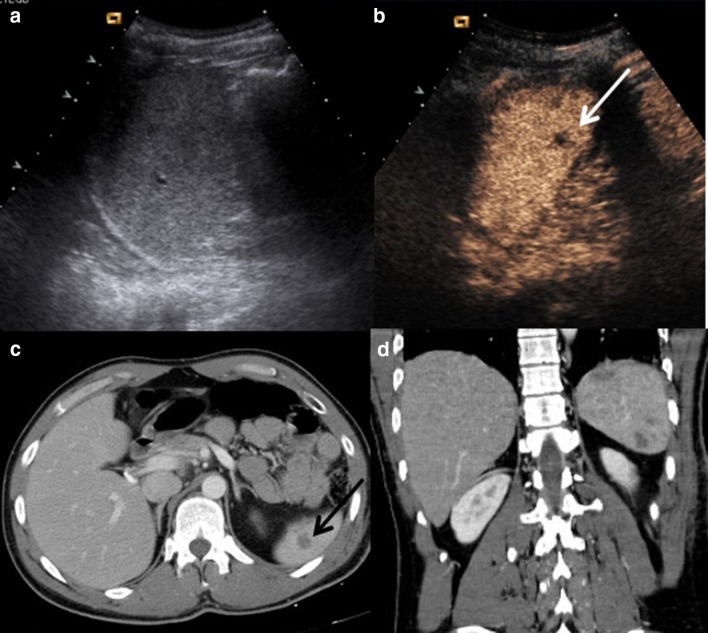
CEUS sensitivity in depicting splenic parenchymal lesions is very similar to that afforded by CT, and also allows for detailed follow-up even during the bed rest phase, without radiation exposure (Fig. 3) [30].
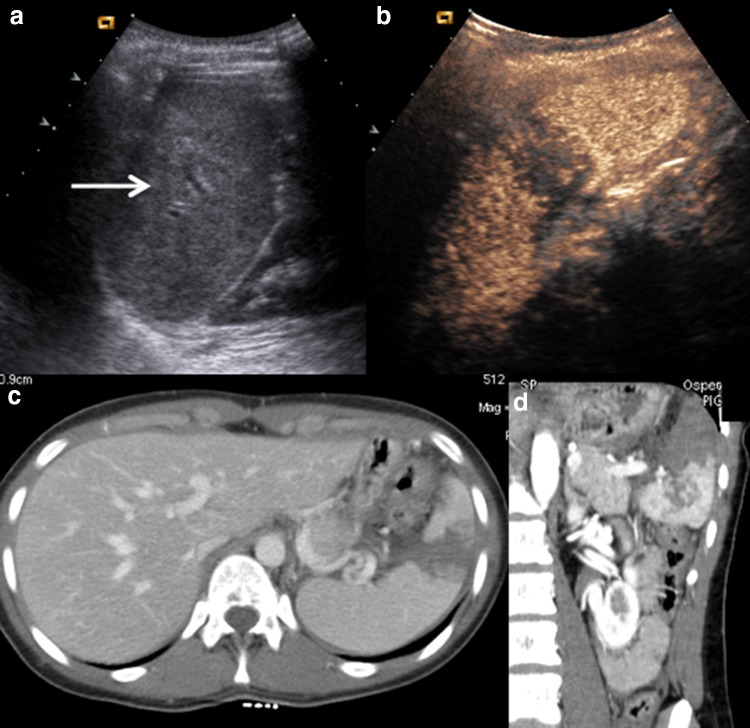
Fig. 3.
a US shows inhomogeneity at upper-middle splenic pole. No evidence of any parenchymal injury. b CEUS fully depicts a complete splenic laceration. c CE-CT on axial plane and d CE-CT on coronal plane confirm CEUS findings
In a recent study undertaken by the authors [31], in which 256 patients with a history of low-energy blunt abdominal trauma were retrospectively evaluated, CEUS identified 34/35 splenic injuries, using CT as the standard of reference; the false negative result was due to a splenic lesion measuring < 1 cm, which had no relevant consequences for patient management and prognosis. This study demonstrated that CEUS was as accurate as CT in detecting and staging traumatic splenic injuries.
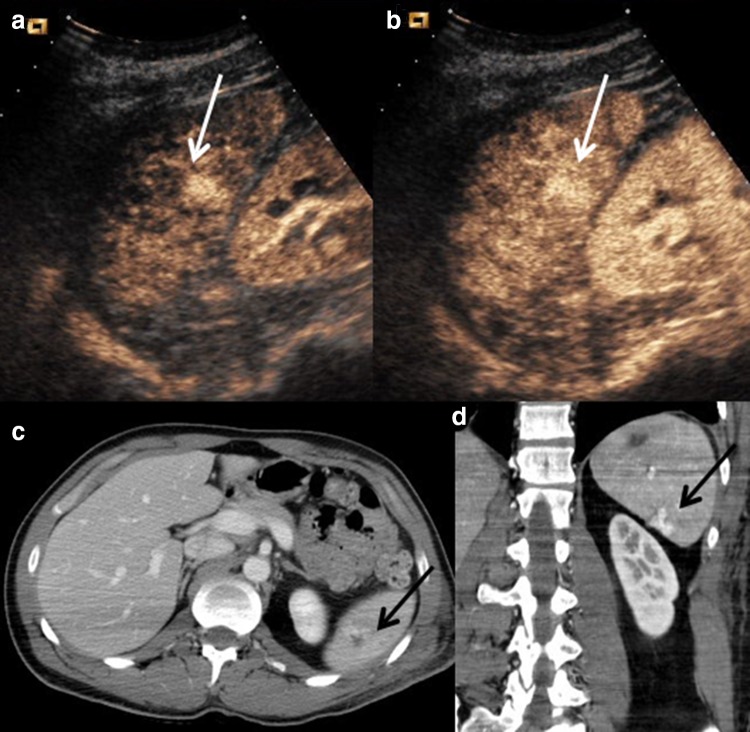
CEUS findings can be defined within the context of contrast media distribution as homogeneous, heterogeneous or absent. The normal parenchyma appears as homogeneous and hyperechoic without any distortion of the echotexture, and the vascular structures are clearly recognizable. The CEUS aspect of splenic lesions is anechoic, clearly highlighted as normal hyperechoic parenchyma (Fig. 4), especially during the venous phase (Fig. 5).
Fig. 4.
Direct blow on the left hypchondrium. a US shows a mild inhomogeneity at splenic upper pole. b CEUS clearly depicts a deep and complete laceration of the upper pole. c CE-CT on axial plane and d CE-CT on coronal plane confirm CEUS findings, along with the presence of mild perisplenic fluid
Fig. 5.
a With US, the upper pole is difficult to study because of lung air interposition. b CEUS clearly depicts the deep laceration (white calipers) at the upper pole. c CE-CT on axial plane and d CE-CT on coronal plane confirm CEUS findings
Specifically, bruises have different aspects, appearing as simple and ill-defined oedematous areas in hypoechoic images, with reduced perfusions.
Lacerations can involve the parenchyma, either partially or as a full thickness tear causing the complete fracture of the organ; they appear as linear or branched hypoechoic bands, perpendicular to the organ capsule, and they can be associated with capsular discontinuity (Fig. 6).
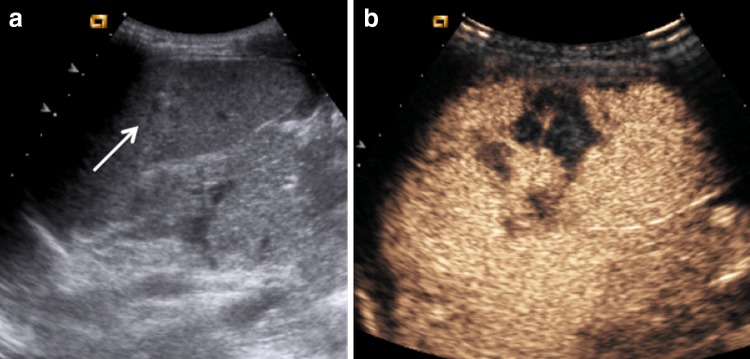
Fig. 6.
Direct blunt abdominal trauma in a 5-year-old girl. a US does not show any alterations. b CEUS performed with convex and c with high-frequency linear probe depicts a deep laceration at splenic lower pole
Contusive lesions have lower severity; they are a bruising of the parenchyma caused by a direct contact with small blood vessels, and cause laceration openings in the nearby tissues. They can appear in the absence of subcapsular and perisplenic fluid, and as hypoechoic lesions with respect to the normal perfused parenchyma.
The intraparenchymal haematoma appears as a heterogeneously hypoechoic area with ill-defined contours and a poor definition of vascular structures; subcapsular haematoma is usually identified as a non-enhancing lenticular area surrounding parenchyma, in which it can be possible to recognize the active extravasation of contrast media [32].
The extension of the lesion to the splenic capsula and the capsular interruption is easily recognizable. Furthermore, subcapsular or perisplenic fluid is more visible due to the parenchymal contrast enhancement (Fig. 7).
Fig. 7.
a US shows a mild inhomogeneity at splenic lower pole; no evidence of traumatic injury or perisplenic fluid. b CEUS clearly depicts a huge lower pole laceration with capsular involvement and a mild perisplenic fluid. c CE-CT on axial plane and d CE-CT on coronal plane confirm splenic injury which involves the capsule with perisplenic fluid (black arrow in c)
Pseudoaneurysms (PAs) are rare entities which develop following injury to the arterial wall, with leakage of blood into a contained cavity. Communication with the arterial lumen is maintained, but this creates a high-pressure cavity with the risk of a life-threatening rupture.
Arteriovenous fistulae are characterized by an abnormal connection between arteries and veins. If the fistula is large enough, it becomes hemodynamically relevant, causing a decrease in peripheral resistance. Fistulae show a tendency to increase in size, leading to surgical treatment in many cases. In some cases, the arteriovenous fistula occludes spontaneously, or can be compressed via ultrasonography.
By means of CEUS, they are often undistinguishable, appearing as hyperechoic circumscribed areas (Figs. 8, 9), thus requiring an angiographic study to obtain the correct diagnosis.
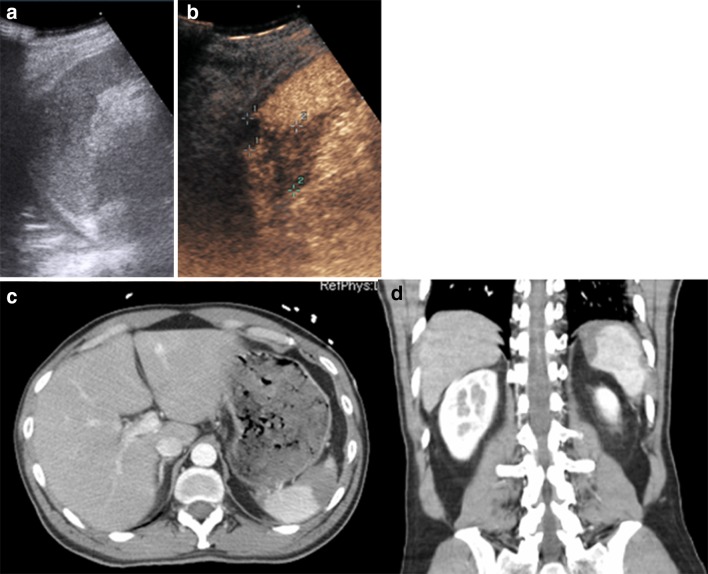
Fig. 8.
a US shows a mild inhomogeneity at the lower pole of the spleen, without evidence of traumatic injury. b CEUS depicts a little splenic lesion (white arrow) as a non-enhancing defect, sharply demarcated from the well-enhanced normal healthy tissue. c CE-CT, axial plane, d CE-CT, coronal plane confirm the traumatic lesion at the lower pole (black arrow in c) without any signs of active bleeding
Fig. 9.
Same patient as in Fig. 2, CEUS follow-up at 72 h from trauma. a CEUS in arterial phase and b CEUS in portal venous phase depict a hyperechoic focus (white arrows) within the well-established traumatic injury, suggesting the diagnosis of AVF. c CE-CT on axial plane and d CE-CT on coronal plane confirm the AVF finding as a well-demarcated area of contrast enhancement within the injury zone (black arrows). The patient underwent a splenic embolization that was not successful, and a splenectomy was subsequently performed
Active bleeding is shown in the early stage as microbubble extravasation within the peritoneal or retroperitoneal space. Some studies reported the tangible role of CEUS in detecting contrast medium extravasation, as Lv et al. demonstrated in a series of 392 patients with liver and/or splenic trauma [33]. These authors established that CEUS detection rate for active bleeding was not different from that of CE-CT, with a sensitivity of 72.4%. It also predicted the need for surgical or non-operative management. In CEUS, active bleeding was described in various terms: as a spring or fountain with a high velocity; or, in presence of a trauma involving the organ capsule, as a drip with a low velocity when there was effusion around the organ; or as active bleeding within the lesion as an isolated, hyperechoic or isoechoic region or a spot of variable size and shape.
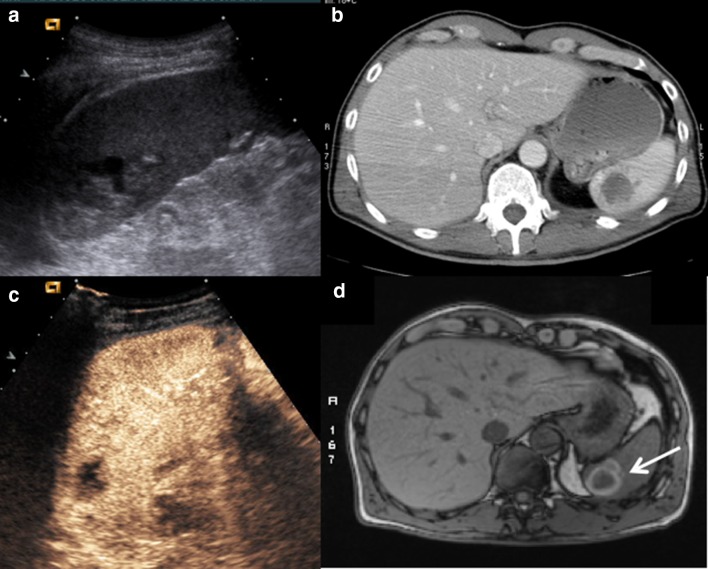
The absence of organ perfusion is indicative of complete avulsion of the vascular pedicle. In some cases, it appears in the absence of perisplenic effusion (Fig. 10).
Fig. 10.
a US performed at admission shows an intraparenchymal laceration of the upper pole. b CE-CT on axial plane confirms the wide laceration of the upper pole. c CEUS performed at 1 month after non-operative management (splenic embolization) shows a hypoechoic lesion within the area of the previous injury. d MR performed after 1 month confirms the presence of the injury, appearing as an organizing hematoma because of a hyperintense edge, expression of extracellular methaemoglobin content (white arrow)
The life-threatening condition of a shattered spleen, which consists in the breaking of the organ into three or more fragments, together with a large hemoperitoneum, can be clinically related to hemodynamic instability. It requires urgent surgery, as it represents a high death risk as a consequence of hemorrhagic shock.
In children, the presence of congenital clefts can lead to misinterpretation and false positive patients. Congenital clefts have regular and homogeneous well-defined profiles compared to the lacerations, which appear more irregular and are often linked to subcapsular and/or perisplenic fluid.
Management of splenic injuries
CEUS and CT gradings are based on the American Association for the Surgery of Trauma (AAST) classification (Table 1), which is not directly linked to the need for operative management. In fact, such studies demonstrated that this is a poor predictor for the success of NOM, since it does not include contained vascular injuries responsible for the failure of non-operative management. To overcome these limits, a new grading system was developed. This is the so-called “Baltimore” grading system (Table 2), which underlines that the evidence of a vascular injury or contrast blush strongly indicates the need for arterial embolization or surgery.
Table 1.
AAST grading of splenic trauma
| Grade | I | II | III | IV | V |
|---|---|---|---|---|---|
| Subcapsular hematoma (< % of total surface area) | < 10% | 10–50% | > 50% or expanding or ruptured | ||
| Capsular laceration (depth) | < 1 cm | 1–3 cm | > 3 cm | ||
| Parenchymal hematoma (diameter) | < 5 cm | > 5 cm or ruptured | |||
| Vessels | Not trabecular | Trabecular | Segmental or hilar (25% devascularization) | Hilar (shattered, devascularized) |
Table 2.
Baltimore classification of splenic trauma according to CT findings: CT Severity Index
| Grade | I (cm) | II (cm) | III (cm) | IVa | IVb |
|---|---|---|---|---|---|
| Subcapsular hematoma (thick) | < 1 | 1–3 | > 3 | Ruptured | |
| Capsular laceration (depth) | < 1 | 1–3 | > 3 | ||
| Parenchymal hematoma (diameter) | < 1 | 1–3 | > 3 | ||
| Bleeding | Parenchymal and subcapsular; shattered spleen | Peritoneal | |||
| Vessels | PSA; AVF |
In cases of low-energy trauma where the patient is hemodynamically stable, despite hemoperitoneum or self-limited bleeding, conservative management is recommended, both because the immunitary function of the organ is thereby preserved and because early and late complications caused by surgery are prevented, subsequently shortening hospitalization. Hemodynamic instability or the possibility thereof is the main condition leading to a splenectomy.
CEUS is able to help direct the management of the patient, which can lead to the patient being discharged if the result is negative. A positive result requires that a CE-CT be performed to stage the disease and to evaluate the prognostic factors (e.g., vascular injuries and active bleeding), which can dramatically change the clinical outcome. Low-grade injuries (I–II), such as superficial lacerations or hematoma, well-enhanced by CEUS, can be managed conservatively. Non-bleeding vascular injuries and deep lacerations, or lacerations with intrasplenic active bleeding, meanwhile, can be managed by means of embolization, although there is no general consensus on this topic; high-grade injuries (active intraperitoneal bleeding, shattered spleen, total devascularization) require an urgent laparotomy.
CEUS may also be useful in the follow-up of low-grade injuries, although until now no standardized protocol has been validated. Some authors focused on the role of CEUS in the follow-up of hepatic and splenic injuries [20], showing an excellent correlation with CT with regard to the number, location and extension of the lesions. The authors aimed to encourage its use at this stage in place of the CT, especially with young patients. In their experience [22], the protocol for the follow-up of blunt abdominal trauma, conservatively treated, includes a CEUS at 24 h and 72 h after trauma, a CEUS and MRI at 1 month, and only MRI thereafter, until the complete resolution or the demonstration of stabilized residual scarring. Only in case of clinical worsening should a CE-MDCT be performed. This approach is supported by strong evidence in favor of CEUS, and also by the fact that clinical parameters are always monitored to quickly recognize those patients who need a prompt evaluation or a change in therapeutic approach.
Discussion
A diagnostic evaluation of a polytraumatized patient has to take into account the hemodynamic conditions, since the management changes dramatically depending on hemodynamic stability or instability.
In a hemodynamically unstable patient who sustained a high-energy trauma, the use of US as a life-saving method in the intensive care unit is now well-established under the focused assessment with sonography for trauma (FAST) protocol. FAST is a very reliable diagnostic tool. Because of its rapidity and non-invasivity, it can be used at a patient’s bedside, and does not interfere with resuscitation procedures. Its aim is to identify a free fluid effusion through the ultrasound exploration of four regions (subxiphoid region, right and left hypochondriac regions, and pelvic cavity).
Several studies demonstrated its high sensitivity for the detection of free abdominal fluid, which is the first indication for an abdominal laparoscopy [34]. However, it has very poor accuracy for the assessment of parenchymal injuries, which depend on lesion location. Its accuracy in these cases ranges from 27% to 68.6% [35].
In hemodynamic stable patients sustaining a high-energy trauma, the most used and sensitive imaging technique is CE-CT, because it not only provides a comprehensive assessment of the disease, but also grades trauma severity and assists with the selection of the most appropriate patient management procedure [35]. Nevertheless, it is characterized by some drawbacks, including the potential for contrast agent allergy, nephrotoxicity, and costs. Another problem is that it uses ionizing radiation, which is its main limitation, especially in cases involving fertile females and pediatric patients.
The authors usually perform CEUS instead of baseline US in hemodynamically stable patients undergoing a low-energy trauma, because of its aforementioned advantages, which are well-known. When CEUS does not identify any lesions, the patient can be discharged; when lesions are identified, a CE-CT is always performed to stage the disease.
CEUS has also been demonstrated to be more feasible in children, as it is more sensitive and accurate than baseline US and almost as sensitive as CT when it comes to the identification and characterization of blunt abdominal trauma [36–39]. These results are reflected in the experience of Valentino et al. [40], who suggest that CEUS can be considered for the triage of hemodynamically stable children with a history of abdominal trauma.
Conclusions
The hemodynamic stability of patients sustaining a BAT is essential to deciding the diagnostic work-up and the subsequent management.
In hemodynamic stable patients who have undergone a high-energy trauma, CE-CT is the main imaging technique performed to stage the disease.
In hemodynamic stable patients who have undergone a low-energy trauma, the diagnostic work-up may start with CEUS, which may also be used in the follow-up of patients managed conservatively until discharge (Fig. 10). CEUS is used both to reduce unnecessary CT examinations and to overcome poorly visible traumatic injuries at baseline US, which are better revealed using USCAs. CEUS has been demonstrated to be a reliable technique for use in such conditions, and could be considered as a replacement for the CE-CT in monitoring these patients. This issue is of outmost importance when dealing with fertile females and children, in which cases an accurate risk–benefit ratio must be calculated before any imaging technique is performed.
Conflict of interest
The authors declare they have no conflict of interest.
Ethical statements
The manuscript has not been submitted elsewhere. The manuscript has not been published previously (partly or in full). It has not been split up into several parts and submitted to various journals or to one journal over time. No data have been fabricated or manipulated (including images) to support the conclusions. No data, text, or theories by others are presented as if they are the author’s own. All co-authors have contributed sufficiently to the scientific work.
Informed consent
Informed consent was obtained from all individual participants and from parents of children included in the study.
Contributor Information
Claudia Lucia Piccolo, Email: clapiccolo@libero.it.
Margherita Trinci, Email: margherita.trinci@gmail.com.
Antonio Pinto, Email: antopin1968@libero.it.
Luca Brunese, Email: lucabrunese@libero.it.
Vittorio Miele, Email: vmiele@sirm.org.
References
- 1.Williamson J. Splenic injury: diagnosis and management. Br J Hosp Med (Lond) 2015;76(204–206):227–229. doi: 10.12968/hmed.2015.76.4.204. [DOI] [PubMed] [Google Scholar]
- 2.Poletti PA, Wintermark M, Schnyder P, et al. Traumatic injuries: role of imaging in the management of the polytrauma victim (conservative expectation) Eur Radiol. 2002;12:969–978. doi: 10.1007/s00330-002-1353-y. [DOI] [PubMed] [Google Scholar]
- 3.Upadhyaya P. Conservative management of splenic trauma: history and current trends. Pediatr Surg Int. 2003;19:617–627. doi: 10.1007/s00383-003-0972-y. [DOI] [PubMed] [Google Scholar]
- 4.Sanders MN, Civil I. Adult splenic injuries: treatment patterns and predictive indicators. Aust NZ J Surg. 1999;69:430–432. doi: 10.1046/j.1440-1622.1999.01594.x. [DOI] [PubMed] [Google Scholar]
- 5.Peitzman AB, Heil B, Rivera L, et al. Blunt splenic injury in adults: multiinstitutional study of the Eastern Association for the Surgery of Trauma. J Trauma. 2000;49:177–187. doi: 10.1097/00005373-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Brown MA, Sirlin CB, Hoyt DB, et al. Screening ultrasound in blunt abdominal trauma. J Intensive Care Med. 2003;18:253–260. doi: 10.1177/0885066603256103. [DOI] [PubMed] [Google Scholar]
- 7.Branney SW, Wolfe RE, Moore EE, et al. Quantitative sensitivity of ultrasound in detecting free intraperitoneal fluid. J Trauma. 1995;39:375–380. doi: 10.1097/00005373-199508000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Latteri S, Malaguarnera G, Mannino M, et al. Ultrasound as point of care in management of polytrauma and its complication. J Ultrasound. 2017;20:171–177. doi: 10.1007/s40477-017-0252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu WC, Cushing BM, Rodriguez A, et al. Abdominal injuries without hemoperitoneum: a potential limitation focused abdominal sonography trauma (FAST) J Trauma. 1997;42:617–623. doi: 10.1097/00005373-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Valentino M, Serra C, Zironi G, et al. Blunt abdominal trauma: emergency contrast-enhanced sonography for detection of solid organ injuries. AJR Am J Roentgenol. 2006;186:1361–1367. doi: 10.2214/AJR.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Miele V, Piccolo CL, Trinci M, et al. Diagnostic imaging of blunt abdominal trauma in pediatric patients. Radiol Med. 2016;121:409–430. doi: 10.1007/s11547-016-0637-2. [DOI] [PubMed] [Google Scholar]
- 12.Zavariz JD, Konstantatou E, Deganello A, et al. Common and uncommon features of focal splenic lesions on contrast-enhanced ultrasound: a pictorial review. Radiol Bras. 2017;50(6):395–404. doi: 10.1590/0100-3984.2015.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns PN, Wilson SR, Simpson DH. Pulse inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast. Invest Radiol. 2000;35:58–71. doi: 10.1097/00004424-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Chiavaroli R, Grima P, Tundo P. Characterization of nontraumatic focal splenic lesions using contrast-enhanced sonography. J Clin Ultrasound. 2011;39:310–315. doi: 10.1002/jcu.20831. [DOI] [PubMed] [Google Scholar]
- 15.Frush DP, Frush KS. The ALARA concept in pediatric imaging: building bridges between radiology and emergency medicine: consensus conference on imaging safety and quality for children in the emergency setting, Feb. 23–24, 2008, Orlando, FL—executive summary. Pediatr Radiol. 2008;38(Suppl 4):S629–S632. doi: 10.1007/s00247-008-1006-7. [DOI] [PubMed] [Google Scholar]
- 16.Brody AS, Frush DP, Huda W, et al. Radiation risk to children from computed tomography. Pediatrics. 2007;120:677–682. doi: 10.1542/peds.2007-1910. [DOI] [PubMed] [Google Scholar]
- 17.Baker N, Woolridge D. Emerging concepts in pediatric emergency radiology. Pediatr Clin North Am. 2013;60:1139–1151. doi: 10.1016/j.pcl.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Huda W. Radiation doses and risks in chest computed tomography examinations. Proc Am Thorac Soc. 2007;4:316–320. doi: 10.1513/pats.200611-172HT. [DOI] [PubMed] [Google Scholar]
- 19.Pinto F, Valentino M, Romanini L, et al. The role of CEUS in the assessment of haemodynamically stable patients with blunt abdominal trauma. Radiol Med. 2015;120:3–11. doi: 10.1007/s11547-014-0455-3. [DOI] [PubMed] [Google Scholar]
- 20.Manetta R, Pistoia ML, Bultrini C, et al. Ultrasound enhanced with sulphur-hexafluoride-filled microbubbles agent (SonoVue) in the follow-up of mild liver and spleen trauma. Radiol Med. 2009;114:771–779. doi: 10.1007/s11547-009-0406-6. [DOI] [PubMed] [Google Scholar]
- 21.Marovic P, Beech PA, Koukounaras J, Kavnoudias H, Goh GS. Accuracy of dual bolus single acquisition computed tomography in the diagnosis and grading of adult traumatic splenic parenchymal and vascular injury. J Med Imaging Radiat Oncol. 2017;61(6):725–731. doi: 10.1111/1754-9485.12619. [DOI] [PubMed] [Google Scholar]
- 22.Miele V, Piccolo CL, Sessa B, et al. Comparison between MRI and CEUS in the follow-up of patients with blunt abdominal trauma managed conservatively. Radiol Med. 2016;121:27–37. doi: 10.1007/s11547-015-0578-1. [DOI] [PubMed] [Google Scholar]
- 23.Valentino M, De Luca C, Galloni SS, et al. Contrast-enhanced US evaluation in patients with blunt abdominal trauma. J Ultrasound. 2010;3(1):22–27. doi: 10.1016/j.jus.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corwin MT, Fananapazir G, Lamba R, et al. Arterial phase CT for the detection of splenic injuries in blunt trauma: would it improve clinical outcomes? Clin Imaging. 2016;40:212–216. doi: 10.1016/j.clinimag.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Boscak AR, Shanmuganathan K, Mirvis SE, et al. Optimizing trauma multidetector CT protocol for blunt splenic injury: need for arterial and portal venous phase scans. Radiology. 2013;268:79–88. doi: 10.1148/radiol.13121370. [DOI] [PubMed] [Google Scholar]
- 26.Uyeda JW, LeBedis CA, Penn DR, et al. Active hemorrhage and vascular injuries in splenic trauma: utility of the arterial phase in multidetector CT. Radiology. 2014;270:99–106. doi: 10.1148/radiol.13121242. [DOI] [PubMed] [Google Scholar]
- 27.Saksobhavivat N, Shanmuganathan K, Chen HH, et al. Blunt splenic injury: use of a Multidetector CT–based splenic injury grading system and clinical parameters for triage of patients at admission. Radiology. 2015;274:702–711. doi: 10.1148/radiol.14141060. [DOI] [PubMed] [Google Scholar]
- 28.Sivit C. Imaging children with abdominal trauma. AJR Am J Roentgenol. 2009;192:1179–1189. doi: 10.2214/AJR.08.2163. [DOI] [PubMed] [Google Scholar]
- 29.Marmery H, Shanmuganathan K, Mirvis SE, et al. Correlation of multidetector CT findings with splenic arteriography and surgery: prospective study in 392 patients. J Am Coll Surg. 2008;206:685–693. doi: 10.1016/j.jamcollsurg.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Cokkinos D, Antypa E, Stefanidis K, et al. Contrast enhanced ultrasound for imaging blunt abdominal trauma—indications, description of the technique and imaging review. Ultraschall Med. 2012;33:60–67. doi: 10.1055/s-0031-1273442. [DOI] [PubMed] [Google Scholar]
- 31.Sessa B, Trinci M, Ianniello S, et al. Blunt abdominal trauma: role of contrast enhanced ultrasound (CEUS) in the detection and staging of abdominal traumatic lesions compared to US and CE-MDCT. Radiol Med. 2015;120:180–189. doi: 10.1007/s11547-014-0425-9. [DOI] [PubMed] [Google Scholar]
- 32.Afaq A, Harvey C, Aldin Z, Leen E, et al. Contrast-enhanced ultrasound in abdominal trauma. Eur J Emerg Med. 2012;19:140–145. doi: 10.1097/MEJ.0b013e328348c980. [DOI] [PubMed] [Google Scholar]
- 33.Lv F, Tang J, Luo Y, et al. Contrast-enhanced ultrasound imaging of active bleeding associated with hepatic and splenic trauma. Radiol Med. 2011;116:1076–1082. doi: 10.1007/s11547-011-0680-y. [DOI] [PubMed] [Google Scholar]
- 34.Pinto F, Miele V, Scaglione M, et al. The use of contrast-enhanced ultrasound in blunt abdominal trauma: advantages and limitations. Acta Radiol. 2014;55:776–784. doi: 10.1177/0284185113505517. [DOI] [PubMed] [Google Scholar]
- 35.Poletti PA, Kinkel K, Vermeulen B, et al. Blunt abdominal trauma: should US be used to detect both free fluid and organ injuries? Radiology. 2003;227:95–103. doi: 10.1148/radiol.2271020139. [DOI] [PubMed] [Google Scholar]
- 36.Cantisani V, Wilson SR. CEUS: where are we in 2015? Eur J Radiol. 2015;84(9):1621–1622. doi: 10.1016/j.ejrad.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 37.Poletti PA, Mirvis SE, Shanmuganathan K, et al. Blunt abdominal trauma patients: can organ injury be excluded without performing computed tomography? J Trauma. 2004;57:1072–1081. doi: 10.1097/01.TA.0000092680.73274.E1. [DOI] [PubMed] [Google Scholar]
- 38.Catalano O, Sandomenico F, Raso MM, et al. Real time, contrast- enhanced sonography: a new tool for detecting active bleeding. J Trauma. 2005;59:933–939. doi: 10.1097/01.ta.0000188129.91271.ab. [DOI] [PubMed] [Google Scholar]
- 39.Menichini G, Sessa B, Trinci M, et al. Accuracy of contrast-enhanced ultrasound (CEUS) in the identification and characterization of traumatic solid organ lesions in children: a retrospective comparison with baseline US and CE-MDCT. Radiol Med. 2015;120:989–1001. doi: 10.1007/s11547-015-0535-z. [DOI] [PubMed] [Google Scholar]
- 40.Valentino M, Serra C, Pavlica P, et al. Blunt abdominal trauma: diagnostic performance of contrast enhanced US in children—initial experience. Radiology. 2008;246:903–909. doi: 10.1148/radiol.2463070652. [DOI] [PubMed] [Google Scholar]