Abstract
Purpose
To assess the diagnostic effectiveness of Multiparametric ultrasound (MPUS), which includes color Doppler ultrasound (CDUS), CEUS and Shear wave elastography (SWE), for evaluating carotid plaque as compared with CT-angiography (CTA) and histology.
Materials and methods
Forty-three consecutive patients scheduled to undergo carotid endarterectomy underwent MPUS. Then, after periods ranging from 2 days to 2 weeks, all underwent CTA. Each plaque was classified by means of dedicated scores for CEUS and SWE as compared with CTA features. At surgery, each plaque was removed in a single fragment to facilitate histological analysis, which evaluated 4 features: extension of the lipid core, thickness of the fibrous cap, inflammatory infiltrate (CD68 + and CD3 + markers) and the presence of intraplaque microvessels. For the CEUS, SWE and CTA, the following values for identifying plaque vulnerability were evaluated: sensitivity, specificity, accuracy, negative predictive value (NPV), positive predictive value (PPV) and Area under the curve (AUC). Cohen’s kappa was used to evaluate the concordance between measurements in the different imaging methods. A p < 0.05 was considered statistically significant.
Results
At histology, 31 out of 43 plaques were identified as vulnerable because of the presence of at least one of the following criteria: fibrous cap < 200 μm, lipid core, intraplaque hemorrhage, inflammatory infiltrate or intraplaque neovascularization. CTA showed a sensitivity of 87.1%, a specificity of 100%, a PPV of 100%, an NPV of 75% and an AUC of 93.5%. SWE showed a sensitivity of 87.1%, a specificity of 66.7%, a PPV of 87.1%, an NPV of 66.7% and an AUC of 76.9%. CEUS showed a sensitivity of 87.1%, a specificity of 58.3%, a PPV of 84.4%, an NPV of 63.6% and an AUC of 72.7%.
Conclusions
Multiparametric ultrasound is an effective modality to obtain comprehensive information on carotid plaques. Further studies are needed to determine whether it can be considered a diagnostic standard.
Keywords: Carotid artery, Plaque, Stenosis, CEUS, US elastography, CDUS
Sommario
Scopo
Valutare l’efficacia diagnostica della CEUS e dell’elastografia Shear Wave (SWE), nella valutazione della placca carotidea in comparazione con l’angiografia-TC (CTA) e la valutazione istologica.
Materiali e Metodi
Quarantatre pazienti consecutivi candidati ad endoarteriectomia carotidea sono stati sottoposti ad esame ecografico completo di valutazione CEUS e SWE e quindi, in un periodo compreso tra due giorni e due settimane, a CTA.Ogni placca è stata classificata mediante scale di valutazione dedicate per la CEUS e la SWE in comparativa con la CTA. In fase di intervento ogni placca è stata rimossa come singolo frammento in modo da facilitarne l’analisi istologica, che ha preso in considerazione quattro caratteristiche: estensione del nucleo lipidico, spessore del cappuccio fibroso, presenza di infiltrato infiammatorio (tramite markers per i CD68 + ed i CD3 +) e presenza di vascolarizzazione intraplacca. Per la CEUS, la SWE e la CTA sono stati calcolati la sensibilità, la specificità, l’accuratezza, i valori predittivi positivo e negativo (PPV e NPV rispettivamente) e l’area sottesa alla curva (AUC) per l’identificazione della vulnerabilità di placca. Per valutare la concordanza tra i valori delle differenti metodiche di imaging è stato utilizzato il K di Cohen. Un valore di p < 0.05 è stato considerato statisticamente significativo.
Risultati
Alla valutazione istologica 31 delle 43 placche sono state idetificate come vulnerabili per la presenza di almeno una delle seguenti caratteristiche: cappuccio fibroso di spessore < 200 µm, presenza di nucleo lipidico, di infiltrato infiammatorio o di vascolarizzazione intraplacca. La CTA ha dimostrato una sensibilità del 87.1%, una specificità del 100%, un PPV of 100%, un NPV of 75% e una AUC del 93.5%. La SWE ha dimostrato una sensibilità del 87.1%, una specificità del 66.7%, un PPV del 87.1%, un NPV del 66.7% e una AUC del 76.9%. La CEUS ha dimostrato una sensibilità del 87.1%, una specificità del 58.3%, un PPV del 84.4%, un NPV del 63.6% e una AUC del 72.7%.
Conclusioni
La CEUS e la SWE sono metodiche efficaci per ottenere informazioni sia qualitative che quantitative sulle placche carotidee. Ulteriori studi sono necessari per determinare se possano essere accettate come metodiche diagnostiche standard.
Introduction
Stroke remains the second leading cause of death in Europe, accounting for 405,000 deaths (9%) in men and 583,000 (13%) deaths in women each year [1], despite the recent reduction in incidence and mortality. Approximately, 7% of ischemic strokes are associated with extracranial carotid stenosis [2]. Many of these may be prevented by revascularization [3].
The main parameter for determining which patients undergo surgical carotid revascularization is the percentage of luminal stenosis [3], but evidence has shown a lack of information on plaque characteristics to be a major limitation [4]. In fact, some plaques are more prone to progression or rupture [5], with a consequent increase in risk of ischemic symptoms. The so-called “vulnerability” of the plaque is linked to numerous histological patterns, such as the lipid core, the thickness of the fibrous cap and the presence of inflammatory infiltrate, intraplaque hemorrhage, ulcerations and intraplaque neovascularization (IPN) [5].
Ultrasound is usually the first imaging modality for assessing the presence of carotid plaques and is used as well for the follow-up of known atheromas. Nevertheless, despite the recent development of several ultrasonographic tools, none of these are currently used in the clinical routine. These new techniques, such as contrast-enhanced ultrasound (CEUS) [6–8] and elastography [9, 10], may help to characterize plaques as “stable” or “vulnerable”. This would add a new type of clinical information to ultrasound examination that is still focused only on establishing the grade of stenosis. The aim of this study was to assess the diagnostic effectiveness of Multiparametric Ultrasound, which includes CEUS and Shear wave elastography (SWE), for evaluating carotid plaque as compared with CT-angiography (CTA) and histology.
Materials and methods
Forty-three consecutive patients scheduled to undergo carotid endarterectomy between June 2016 and September 2017 at the Department of Surgery “Pietro Valdoni”, Sapienza University, Rome, Italy were included in the study. It was approved by the Ethical Committee of the “Sapienza” University of Rome, in accordance with the Helsinki Declaration and the Guideline for Good Clinical Practice. Before beginning the study, all participants provided written informed consent for intervention.
All patients underwent Multiparametric Ultrasound, then, after periods ranging from 2 days to 2 weeks, underwent CTA.
Multiparametric Ultrasounds were performed with high-end equipment (Toshiba Aplio 500, Japan) by one radiologist, with 14 years of experience with CEUS and 8 years of experience with US-elastography, using a 5–14 MHz linear array transducer. Another radiologist, blinded to clinical information and Ultrasound reports, reviewed the CTA imaging.
Diagnostic modality
US and CDUS Patients were laid down in a supine position with a pillow under their shoulders to allow neck hyper-extension. Axial and longitudinal sonograms were acquired in order to judge the grade of stenosis and to characterize the morphology of the plaque.
Elastography SWE was performed immediately after US with the same US unit and linear array probe; to obtain a quantitative evaluation (in kPa), proprietary elasticity software was used. For SWE, the operator placed the transducer perpendicular to the plaque without pressure, maintaining only slight contact with the skin in order to minimize compression artifacts, and kept it stable and motionless for about 3 s to allow measurements.
In this technique, the probe produces push pulse that generates downward displacement, which can be tracked by color Doppler to measure shear wave propagation speed. The ROI for measurement was positioned within the plaque. This system represents in real time the elasticity and speed by means of colorimetric map within the elastographic box. Then, the measurement of the absolute stiffness of the ROI in kPa is available. The system also features a quality control map that shows the shear wave propagation as wave-front lines. Putting the ROI when the lines are parallel to each other may help to achieve more reliable measurement; however, if the lines are distorted or lacking due to artifacts, measurement may need to be repeated.
US images, significant SWE frames and cine loops were saved to the local picture archive and communication system (PACS).
Hardness of the plaque was expressed by a three-grade scale: (1) soft plaques with values between 11 and 25 kPa; (2) mixed plaques with values between 26 and 65 kPa; (3) hard plaques with values over 65 kPa. Mixed plaques were considered vulnerable (Figs. 1, 2).
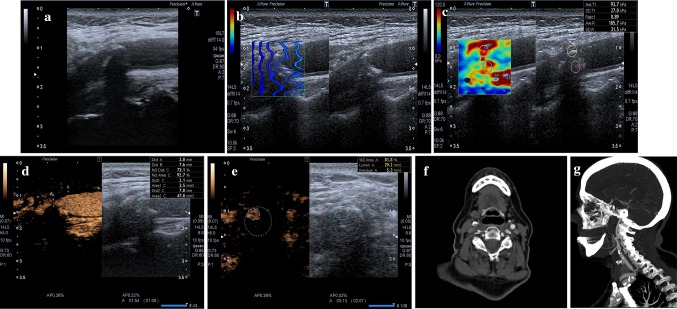
Fig. 1.
a At baseline US, a mainly calcified plaque was detected at right carotid bifurcation. b SWE quality control shows that the sampling was done correctly. c SWE confirms that it is a hard plaque (93.7 kPa). d, e CEUS in axial and longitudinal view allows a clear evaluation of the grade of stenosis; no significant focus of contrast enhancement is visible within the plaque; an ulceration is visible (better seen in longitudinal sonogram). f, g CTA in axial and sagittal (MIP) view; plaque is mixed, ulceration is confirmed; no significant contrast enhancement is detected
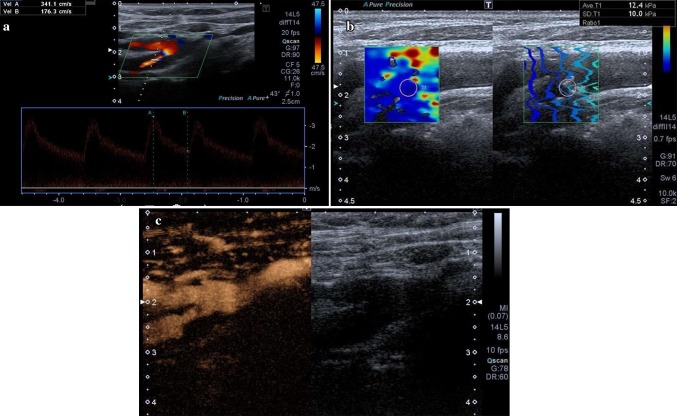
Fig. 2.
a At CDUS and pulsed wave Doppler ultrasound a soft plaque is detected at right carotid bifurcation, extending to the proximal tract of internal carotid artery; it is an hemodynamically significant stenosis causing a strong increase of peak systolic velocity. b SWE confirms it is a soft plaque (12.4 kPa); quality control confirms that the sampling was done correctly. c CEUS in longitudinal view confirms the high-grade stenosis; no significant focus of contrast enhancement is visible within the plaque; no ulcerations are seen along the border of the plaque
Contrast-enhanced ultrasound (CEUS) CEUS was performed after the bolus injection of 1.2 ml of SonoVue (Bracco, Milan, Italy) through a 20-gauge cannula into an antecubital vein, followed by a 10-ml saline flush. The carotid plaque was scanned to portray the whole plaque and surrounding arterial walls. The evaluation continued for at least 2 min, using a non-destructive US mode with low MI (MI 0.05–0.07). A video clip of the procedure was digitally recorded for further analysis.
Both quantitative and qualitative analyses were performed. For quantitative analysis, the curve of increased signal was analyzed after contrast bolus and expressed as a ratio compared with the enhancement within the adjacent normal carotid wall. For qualitative analysis, a scale from 1 to 3 was used: (1) absence of contrast enhancement, (2) enhancement confined to the adventitial or peripheral region of the plaque, and (3) diffuse intraplaque contrast enhancement. The percentage of lipid core and the possible presence of ulcerations and adventitial angiogenesis were evaluated as well.
CTA The exam was carried out with Somatom 64 (Siemens, Erlangen, Germany). A two-phase CT protocol was used, with a pre-contrast phase and an arterial phase (started with bolus tracking), using 130 mL of the non-ionic contrast agent Iomeron (Bracco, Milan, Italy) at 4 mL/s. The other scanning parameters were: 1.2 mm acquisition; reconstruction with a soft-margin kernel algorithm (B30) at 1.5 and 3 mm; pre-contrast scans at a low-power tube (120 mAs); the arterial phase at 120 kVp and 200 mAs. Coronal and oblique reconstructions along the longitudinal axis of carotids were obtained. CT images were analyzed on a dedicated workstation (Aquarius, TeraRecon, San Mateo, Ca) using traditional post-processing techniques.
We considered as vulnerable those plaques that showed at least one of the following criteria: (1) absence of calcifications accounting for > 50% of the plaque; (2) negative HU values at pre-contrast CT scan; (3) > 20 HU enhancement in post-contrast CT scan.
Histological exam The atherosclerotic plaques removed during carotid surgery were sent for histological analyses. Particular care was given, during surgery, to remove the plaque as a single fragment to facilitate the analysis. We evaluated 4 aspects: extension of the lipid core, thickness of the fibrous cap, inflammatory infiltrate (CD68 + or CD3 + markers) and the presence of intraplaque microvessels. Lipid core was categorized with a 4-grade scale: (1) absent; (2) minimal; (3) moderate; (4) extensive. For the presence of inflammatory cellular lines (CD68 + and CD3 +), we also used a 4-grade scale: (1) absence; (2) minimally represented; (3) moderately represented; (4) extensively represented. In this scale, grades 1 and 2 define a low inflammatory infiltrate, while grades 3 and 4 define a high inflammatory infiltrate. A fibrous cap thickness of less than 200 μm was considered thin. Microvessels were evaluated as area and percentage in 5 fields occupied by CD34 +.
Statistical analysis
Statistical analysis was performed to evaluate the accuracy of the different techniques to identify patterns of vulnerability of the atherosclerotic plaque. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and area under the curve (AUC) were calculated for this purpose. Differences between AUCs of different imaging methods were evaluated using the Bonferroni test. Cohen’s kappa was used to evaluate the concordance between the measurements in the different imaging methods. A pV < 0.05 was considered statistically significant. Statistical analysis was carried out with Stata software (Stata v. 15, Statacorp LLC, College Station TX, USA).
Results
Thirty-one out of 43 surgically removed plaques were found to be vulnerable: 13 because of the presence of a lipid core (nine moderate; four extensive); 11 because of the presence of high-grade inflammatory infiltrate (seven moderate; four extensive); ten because of significant amounts of microvessels; and nine for the presence of intraplaque hemorrhage.
No side effects due to the diagnostic methods were registered.
Accuracy of CTA
Twenty-seven out of 43 plaques were considered vulnerable at CTA and all of these specimens were confirmed at histology. Four vulnerable plaques were not detected by CTA. There were no false-positive cases.
These results determined a sensitivity of 87.1% (95% CI 70.2–96.4%), a specificity of 100% (95% CI 73.5–100%), a PPV of 100% (95% CI 87.2–100%) and an NPV of 75.0% (95% CI 47.6–92.7%). AUC was 93.5% (87.6–99.5%).
Accuracy of SWE
Histology confirmed as vulnerable 27 of the 31 soft- and mixed-classified plaques at SWE. Of the 12 labeled as not vulnerable by histology, four cases were identified as positive at SWE.
These results determined a sensitivity of 87.1% (95% CI 70.2–96.4%), a specificity of 66.7% (95% CI 34.9–90.1%), a PPV of 87.1% (95% CI 70.2–96.4%) and an NPV of 66.7% (95% CI 34.9–90.1%), thus giving an AUC of 76.9% (95% CI 61.7–92.0%).
Accuracy of CEUS
Thirty-two out of the 43 plaques demonstrated contrast enhancement and histologic vulnerability criteria; out of these, 31 were confirmed at Histology. Conversely, in the absence of plaque enhancement, only four plaques were found to be vulnerable at histology.
These results determined a sensitivity of 87.1% (95% CI 70.2–96.4%), a specificity of 58.3% (95% CI 27.7–84.8%), a PPV of 84.4% (95% CI 67.2–94.7%) and an NPV of 63.6% (95% CI 30.8–89.1%), with a total AUC of 72.7% (95% CI 57.0–88.5%).
Table 1 presents the results.
Table 1.
CTA, CEUS and SWE diagnostic performance
| Vulnerable plaques | Stable plaques | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | AUC (95% CI) | |
|---|---|---|---|---|---|---|---|
| CTA | |||||||
| Positive | 27 | 0 | 87.1% (70.2–96.4%) | 100% (73.5–100%) | 100% (87.2–100%) | 75% (47.6–92.7%) | 93.5% (87.6–99.5%) |
| Negative | 4 | 12 | |||||
| SWE | |||||||
| Positive | 27 | 4 | 87.1% (70.2–96.4%) | 66.7% (34.9–90.1%) | 87.1% (70.2–96.4%) | 66.7% (34.9–90.1%) | 76.9% (61.7–92.0%) |
| Negative | 4 | 8 | |||||
| CEUS | |||||||
| Positive | 27 | 5 | 87.1% (70.2–96.4%) | 58.3% (27.7–84.8%) | 84.4% (67.2–94.7%) | 63.6% (30.8–89.1%) | 72.7% (57.0–88.5%) |
| Negative | 4 | 7 | |||||
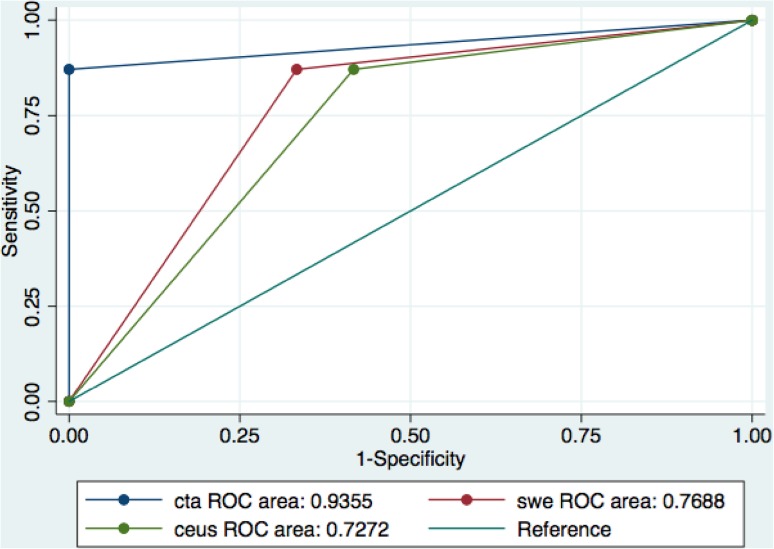
Comparing the AUCs of the different imaging techniques revealed a statistically significant difference between CTA and CEUS (93.5% vs. 72.7%, pV 0.027), but not between CTA and SWE (93.5% vs. 76.8%, 0.066).
A higher level of agreement was found between CTA and SWE (81.4%, k = 0.58; pV < 0.001), while a lower level was found between CTA and CEUS (74.4%, k = 0.42; pV = 0.002). Figure 3 presents the results.
Fig. 3.
CTA, CEUS and SWE ROC curves
Discussion
In recent years, the vulnerability features of atherosclerotic plaque have been extensively studied [11–13]. These have been proved to represent reliable markers of intraplaque events that possibly lead to stroke and myocardial infarction. Neovascularization, inflammation, thin fibrous cap, lipid core and intraplaque hemorrhage are all considered causes of plaque vulnerability [12, 14].
This research led to the development of non-invasive systems aimed at detecting and stratifying the risk of plaque instability.
SWE is an elastographic dynamic method that applies a directional force to the tissue to cause shear deformation that propagates as a shear wave [9, 10].
Shear wave speed measurement, calculated through modulus G, allows judging a tissue’s stiffness. Plaque composition, such as fibrous cap and lipid core, affects the elastic properties of the plaque itself, implying that elasticity plays an important role in determining vulnerability [15]. In our study, we used SWE to measure plaque stiffness to evaluate a possible correlation between soft plaques and the presence of a lipid core at CTA and histology. Our results show a high concordance of 81.4% between SWE and CTA.
Thus, although SWE has been proven efficient in solid lesion evaluation, such as for thyroid nodules [16], it may be heavily affected by artifacts when the shear wave transits through a liquid medium such as arterial blood.
CEUS is a well-established method for assessing vascularization, with several clinical applications (e.g., neoplastic lesion, blunt trauma, inflammation, aortic endoleaks after EVAR [17–19]). Since plaque neovascularization is a consistent feature of vulnerable plaques [13, 20–24], we assessed the correlation between contrastographic enhancement in atheromas with neovascularization and inflammatory processes. CEUS-defined neovascularization was obtained comparing enhancement intensity with the number of microvessels per field at histology. A high grade of contrast enhancement also seems to be related to a significant inflammatory infiltrate. Even if SonoVue is a blood pool agent, meaning that microbubbles do not diffuse in the extravascular space [25, 26], Hoogi [27, 28] demonstrated an indirect correlation between contrast enhancement and the degree of inflammatory infiltrate. However, other studies, such as Li’s [29], failed to show a correlation between contrast enhancement and inflammatory infiltration (CD68). In our study, 71% of plaques with a high-grade CD3 + infiltrate and 63.6% of plaques with a high-grade CD68 + infiltrate showed significant contrast enhancement. Nevertheless, we also found that 28.6% of plaques with CD3 + high-grade infiltrate and 19% of plaques with CD 68 + high grade infiltrate did not show contrast enhancement, resulting in false-negative patients when using CEUS.
Our study confirms the presence of a significant correlation between contrast enhancement grade and neovascularization in plaques, although a higher level of agreement was found between CTA and SWE.
The visual discrete scoring system, used to determine CEUS contrast, correlates well with histologic examination for plaque neovascularization [27]. It is easy to learn and apply, and does not require any special equipment or software. However, because of its subjective nature, this technique is obviously prone to inter-observer variability, so it needs to be further assessed.
Our results show that SWE and CEUS are sensitive (87.1% and 87.1%, respectively) at detecting vulnerable plaques, which is comparable to CTA (87.1%); nevertheless, the diagnostic performances of both SWE and CEUS have a higher number of false positive cases, which leads to a lower specificity (66.7% and 58.3%, respectively) in comparison to CTA (100%).
Conclusions
Multiparametric ultrasound is a safe and effective modality to obtain comprehensive information on carotid plaques. Further studies are needed to determine whether it can be considered a diagnostic standard in cases where the evaluation of plaque stability can have a pivotal role in deciding if a patient must undergo surgery or not.
Conflict of interest
Vito Cantisani has been lecturer for Toshiba, Samsung and Bracco. The other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical standard statement
The authors declare that this study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Informed consent
Informed consent was obtained from all patients for being included in the study.
References
- 1.Wilkins E, Wilson L, Wickramasinghe K, Bhatnagar P, Leal J, Luengo-Fernandez R, Burns R, Rayner M, Townsend N. European cardiovascular disease statistics 2017. Brussels: European Heart Network; 2017. [Google Scholar]
- 2.Methawasin K, Suwanwela NC, Phanthumchinda K. The 2-year outcomes comparison between ischemic stroke patients with intracranial arterial stenosis without significant extracranial carotid stenosis and patients with extracranial carotid stenosis. J Med Assoc Thai. 2015;98(Suppl 9):S98–S105. [PubMed] [Google Scholar]
- 3.Naylor AR, Ricco JB, de Borst GJ, Debus S, de Haro J, Halliday A, Hamilton G, Kakisis J, Kakkos S, Lepidi S, Markus HS, McCabe DJ, Roy J, Sillesen H, Van den Berg JC, Vermassen F, Kolh P, Chakfe N, Hinchliffe RJ, Koncar I, Lindholt JS, de Vega Ceniga M, Verzini F, Archie J, Bellmunt S, Chaudhuri A, Koelemay M, Lindahl AK, Padberg F, Venermo M, Esvs Guidelines Committee. Esvs Guideline Reviewers Editor’s Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS) Eur J Vasc Endovasc Surg. 2018;55:3–81. doi: 10.1016/j.ejvs.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Sun J, Hatsukami TS. Plaque imaging to decide on optimal treatment: medical versus carotid endarterectomy versus carotid artery stenting. Neuroimaging Clin N Am. 2016;26:165–173. doi: 10.1016/j.nic.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 6.Iezzi R, Petrone G, Ferrante A, Lauriola L, Vincenzoni C, la Torre MF, Snider F, Rindi G, Bonomo L. The role of contrast-enhanced ultrasound (CEUS) in visualizing atherosclerotic carotid plaque vulnerability: which injection protocol? Which scanning technique? Eur J Radiol. 2015;84:865–871. doi: 10.1016/j.ejrad.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, Bertolotto M, Calliada F, Clevert DA, Cosgrove D, Deganello A, D’Onofrio M, Drudi FM, Freeman S, Harvey C, Jenssen C, Jung EM, Klauser AS, Lassau N, Meloni MF, Leen E, Nicolau C, Nolsoe C, Piscaglia F, Prada F, Prosch H, Radzina M, Savelli L, Weskott HP, Wijkstra H. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (Short Version) Ultraschall Med. 2018
- 8.Rafailidis V, Charitanti A, Tegos T, Destanis E, Chryssogonidis I. Contrast-enhanced ultrasound of the carotid system: a review of the current literature. J Ultrasound. 2017;20(2):97–109. doi: 10.1007/s40477-017-0239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D’Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: basic principles and technology. Ultraschall Med. 2013;34:169–184. doi: 10.1055/s-0033-1335205. [DOI] [PubMed] [Google Scholar]
- 10.Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V, D’Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Fromageau J, Havre RF, Jenssen C, Ohlinger R, Săftoiu A, Schaefer F. Dietrich CF; EFSUMB. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: clinical applications. Ultraschall Med. 2013;34:238–253. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]
- 11.Redgrave JN, Gallagher P, Lovett JK, Rothwell PM. Critical cap thickness and rupture in symptomatic carotid plaques: the oxford plaque study. Stroke. 2008;39:1722–1729. doi: 10.1161/STROKEAHA.107.507988. [DOI] [PubMed] [Google Scholar]
- 12.Kolodgie FD, Yahagi K, Mori H, Romero ME, Trout HH, Finn AV, Virmani R. High-risk carotid plaque: lessons learned from histopathology. Semin Vasc Surg. 2017;30:31–43. doi: 10.1053/j.semvascsurg.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Dunmore BJ, McCarthy MJ, Naylor AR, Brindle NP. Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J Vasc Surg. 2007;45:155–159. doi: 10.1016/j.jvs.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 14.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 Suppl):C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 15.Naim C, Cloutier G, Mercure E, Destrempes F, Qin Z, El-Abyad W, Lanthier S, Giroux MF, Soulez G. Characterisation of carotid plaques with ultrasound elastography: feasibility and correlation with high-resolution magnetic resonance imaging. Eur Radiol. 2013;23:2030–2041. doi: 10.1007/s00330-013-2772-7. [DOI] [PubMed] [Google Scholar]
- 16.Cosgrove D, Barr R, Bojunga J, Cantisani V, Chammas MC, Dighe M, Vinayak S, Xu JM, Dietrich CF. WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: part 4. Thyroid. Ultrasound Med Biol. 2017;43(1):4–26. doi: 10.1016/j.ultrasmedbio.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 17.David E, Cantisani V, Grazhdani H, et al. What is the role of contrast-enhanced ultrasound in the evaluation of the endoleak of aortic endoprostheses? A comparison between CEUS and CT on a widespread scale. J Ultrasound. 2016;19(4):281–287. doi: 10.1007/s40477-016-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantisani V, Ricci P, Erturk M, Pagliara E, Drudi F, Calliada F, Mortele K, D’Ambrosio U, Marigliano C, Catalano C, Marin D, Di Seri M, Longo F, Passariello R. Detection of hepatic metastases from colorectal cancer: prospective evaluation of gray scale US versus SonoVue® low mechanical index real time-enhanced US as compared with multidetector-CT or Gd-BOPTA-MRI. Ultraschall Med. 2010;31(5):500–505. doi: 10.1055/s-0028-1109751. [DOI] [PubMed] [Google Scholar]
- 19.Cantisani V, Grazhdani H, Clevert DA, Iezzi R, Aiani L, Martegani A, Fanelli F, Di Marzo L, Wlderk A, Cirelli C, Catalano C, Di Leo N, Di Segni M, Malpassini F, D’Ambrosio F. EVAR: benefits of CEUS for monitoring stent-graft status. Eur J Radiol. 2015;84(9):1658–1665. doi: 10.1016/j.ejrad.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Cantisani V, Consorti F, Guerrisi A, Guerrisi I, Ricci P, Di Segni M, Mancuso E, Scardella L, Milazzo F, D’Ambrosio F, Antonaci A. Prospective comparative evaluation of quantitative-elastosonography (Q-elastography) and contrast-enhanced ultrasound for the evaluation of thyroid nodules: preliminary experience. Eur J Radiol. 2013;82(11):1892–1898. doi: 10.1016/j.ejrad.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Cantisani V, Bertolotto M, Weskott HP, Romanini L, Grazhdani H, Passamonti M, Drudi FM, Malpassini F, Isidori A, Meloni FM, Calliada F, D’Ambrosio F. Growing indications for CEUS: the kidney, testis, lymph nodes, thyroid, prostate, and small bowel. Eur J Radiol. 2015;84(9):1675–1684. doi: 10.1016/j.ejrad.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Cantisani V, Grazhdani H, Fioravanti C, Rosignuolo M, Calliada F, Messineo D, Bernieri MG, Redler A, Catalano C, D’Ambrosio F. Liver metastases: contrast-enhanced ultrasound compared with computed tomography and magnetic resonance. World J Gastroenterol. 2014;20(29):9998–10007. doi: 10.3748/wjg.v20.i29.9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy MJ, Loftus IM, Thompson MM, Jones L, London NJ, Bell PR, Naylor AR, Brindle NP. Angiogenesis and the atherosclerotic carotid plaque: an association between symptomatology and plaque morphology. J Vasc Surg. 1999;30:261–268. doi: 10.1016/S0741-5214(99)70136-9. [DOI] [PubMed] [Google Scholar]
- 24.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O’Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 25.Feinstein SB. The powerful microbubble: from bench to bedside, from intravascular indicator to therapeutic delivery system, and beyond. Am J Physiol Heart Circ Physiol. 2004;287:H450–H457. doi: 10.1152/ajpheart.00134.2004. [DOI] [PubMed] [Google Scholar]
- 26.Feinstein SB. Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J Am Coll Cardiol. 2006;48:236–243. doi: 10.1016/j.jacc.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 27.Hoogi A, Akkus Z, van den Oord SC, ten Kate GL, Schinkel AF, Bosch JG, de Jong N, Adam D, van der Steen AF. Quantitative analysis of ultrasound contrast flow behavior in carotid plaque neovasculature. Ultrasound Med Biol. 2012;38:2072–2083. doi: 10.1016/j.ultrasmedbio.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 28.van den Oord SC, Akkus Z, Bosch JG, Hoogi A, ten Kate GL, Renaud G, Sijbrands EJ, Verhagen HJ, van der Lugt A, Adam D, de Jong N, van der Steen AF, Schinkel AF. Quantitative contrast enhanced ultrasound of intraplaque neovascularization in patients with carotid atherosclerosis. Ultraschall Med. 2015;36:154–161. doi: 10.1055/s-0034-1366410. [DOI] [PubMed] [Google Scholar]
- 29.Li C, He W, Guo D, Chen L, Jin X, Wang W, Huang B, Wang W. Quantification of carotid plaque neovascularization using contrast-enhanced ultrasound with histopathologic validation. Ultrasound Med Biol. 2014;40:1827–1833. doi: 10.1016/j.ultrasmedbio.2014.02.010. [DOI] [PubMed] [Google Scholar]