Abstract
Dectin-2 is a C-type lectin involved in the recognition of several pathogens such as Aspergillus fumigatus, Candida albicans, Schistosoma mansonii, and Mycobacterium tuberculosis that triggers Th17 immune responses. Identifying pathogen ligands and understanding the molecular basis of their recognition is one of the current challenges. Purified M. tuberculosis mannose-capped lipoarabinomannan (ManLAM) was shown to induce signaling via Dectin-2, an activity that requires the (α1 → 2)-linked mannosides forming the caps. Here, using isogenic M. tuberculosis mutant strains, we demonstrate that ManLAM is a bona fide and actually the sole ligand mediating bacilli recognition by Dectin-2, although M. tuberculosis produces a variety of cell envelope mannoconjugates, such as phosphatidyl-myo-inositol hexamannosides, lipomannan or manno(lipo)proteins, that bear (α1 → 2)-linked mannosides. In addition, we found that Dectin-2 can recognize lipoglycans from other bacterial species, such as Saccharotrix aerocolonigenes or the human opportunistic pathogen Tsukamurella paurometabola, suggesting that lipoglycans are prototypical Dectin-2 ligands. Finally, from a structure/function relationship perspective, we show, using lipoglycan variants and synthetic mannodendrimers, that dimannoside caps and multivalent interaction are required for ligand binding to and signaling via Dectin-2. Better understanding of the molecular basis of ligand recognition by Dectin-2 will pave the way for the rational design of potent adjuvants targeting this receptor.
Introduction
Innate immune recognition is based on the detection of microbial molecular structures by host pattern recognition receptors (PRRs)1. PRRs belong to several families, among which C-type lectins are specialized in the binding of sugar moieties, via carbohydrate recognition domains (CRD) that contain one or more calcium-dependent carbohydrate-binding sites2. Dectin-2 (Dendritic-Associated C-type lectin-2) is a C-type lectin that was initially identified on murine dendritic cells3, but was subsequently shown to be expressed on several cell types, including macrophages4,5. It is constituted of one CRD, a transmembrane domain and a short intracellular domain devoid of signaling motif3. Association of Dectin-2 with a FcRγ chain triggers the recruitment and phosphorylation of the tyrosine kinase Syk4, formation of the Card9/Malt1/Bcl10 complex6 and translocation of NF-κB to the nucleus, leading to the production of cytokines and chemokines, such as TNF-α7. Syk also activates the MAPK pathway through the phospholipase Cγ2 and drives the production of Th17 polarizing molecules8,9.
Dectin-2 is involved in the recognition of several pathogens, such as Aspergillus fumigatus, Candida albicans and Schistosoma mansonii, driving the production of pro-inflammatory cytokines and inducing protective immunity7,10,11. Indeed, the CRD of Dectin-2 exhibits specificity toward high mannose glycoconjugates containing Manα1-2Man motifs12, mainly found in fungi. The recognition of the yeast Malassezia furfur by Dectin-2 was shown to be mediated through an O-linked mannobiose-rich mannoprotein13. More recently, glycan array experiments indicated that the presence of Manα1-2Man motifs increased the binding to Dectin-214 and, accordingly, the crystal structure of human Dectin-2 CRD complexed with Man9GlcNAc2 oligosaccharide revealed a canonical C-type primary monosaccharide binding site centered on a Ca2+ ion as well as a secondary binding site for a second mannose residue15.
In addition to fungi and yeasts, Dectin-2 has been shown to play a key role in the detection of mycobacteria and induction of a protective immune response in mice16. Indeed, Dectin-2 knockout mice infected by the opportunistic pathogen Mycobacterium avium show a higher bacterial load than the wild-type mice. The lipoglycan mannose-capped lipoarabinomannan (ManLAM) produced by the slow-growing mycobacterial species, such as the human pathogen Mycobacterium tuberculosis, the vaccine strain Mycobacterium bovis BCG, or M. avium, but not by the non-pathogenic fast-growing species, such as Mycobacterium smegmatis17,18, was identified as a ligand of Dectin-2 when used as a purified molecule16. Recognition of purified ManLAM by Dectin-2 required the mannose caps16, which are mono-, (α1 → 2)-di- or (α1 → 2)-tri-mannosyl units present on the non-reducing ends of its arabinan domain17. However, the mycobacterial cell envelope contains a variety of complex glycoconjugates that bear similar structures, such as the phosphatidyl-myo-inositol hexamannosides (PIM6), lipomannan (LM) and manno(lipo)proteins (M(L)P) (Fig. 1), ubiquitously found in the envelope of mycobacteria18–21. Altogether, several questions remain unanswered. Is ManLAM a bona fide Dectin-2 ligand in the context of M. tuberculosis bacilli recognition? Are there other mycobacterial ligands of Dectin-2? Are bacterial lipoglycans prototypical ligands of this receptor? What is the precise molecular basis of ligand recognition?
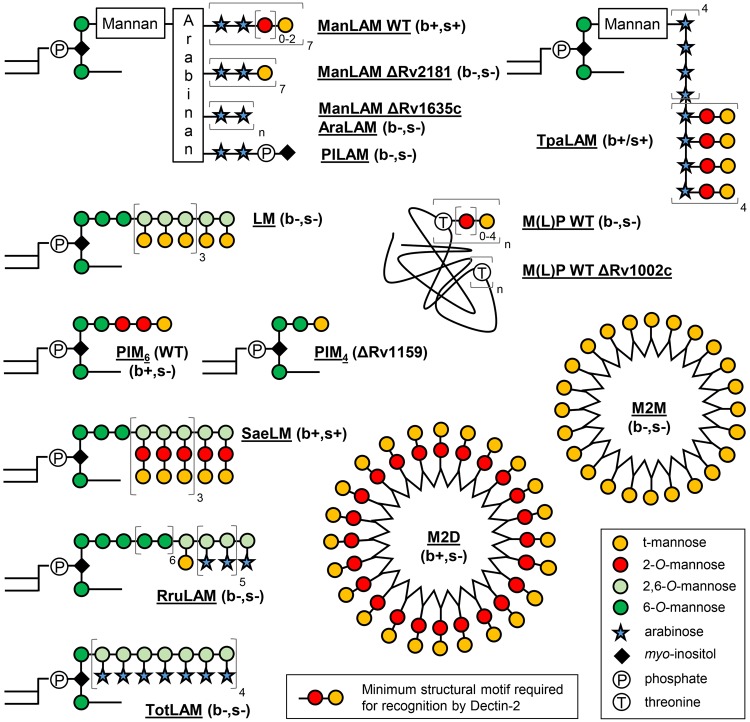
Figure 1.
Chemical structure of the natural and synthetic mannoconjugates evaluated. AraLAM, lipoarabinomannan devoid of caps; LM: lipomannan; ManLAM, mannose-capped lipoarabinomannan; M(L)P, manno(lipo)proteins; M2M, M2D, second-generation mannodendrimers capped with mono- or di-mannosides respectively; PILAM, phospho-myo-inositol-capped lipoarabinomannan; PIM4, phosphatidyl-myo-inositol tetramannosides; PIM6: phosphatidyl-myo-inositol hexamannosides; RruLAM; LAM from R. ruber; SaeLM, LM from S. aerocolonigenes; TotLAM, LAM from T. otitidis; TpaLAM, LAM from Ts. paurometabola. Detailed structures are shown in Fig. S1. (b+/−, s+/−) indicates the ability (+) or not (−) of the mannoconjugates to bind (b) or induce signaling (s) via Dectin-2.
Here, using on the one hand a set of M. tuberculosis isogenic mutant strains as well as purified and synthetic mannoconjugates, and on the other hand a combination of biochemical and cellular assays, we investigated: (i) the contribution of ManLAM and other mannoconjugates in M. tuberculosis recognition by Dectin-2, and (ii) the structure/function relationships and the molecular basis of ligand recognition by the receptor.
Results
ManLAM is the sole mycobacterial ligand triggering signaling via and mediating M. tuberculosis recognition by Dectin-2
In order to first evaluate whether LM, PIM6 or M(L)P might constitute additional mycobacterial ligands of Dectin-2, we investigated the capacity of these compounds, purified from different strains, to (i) bind a soluble form of human Dectin-2 receptor (Dectin-2-Fc), (ii) induce NF-κB activation in HEK cells expressing murine Dectin-2 (HEK-Dectin-2) and a NF-κB-inducible reporter system (secreted alkaline phosphatase), and (iii) induce Dectin-2-dependent production of cytokines by murine bone marrow-derived dendritic cells (BMDCs). In agreement with published data16, ManLAM purified from M. tuberculosis efficiently bound Dectin-2-Fc (Fig. 2A), and induced NF-κB activation in HEK-Dectin-2 cells (Figs 2B and S2A) as well as a Dectin-2-dependent TNF-α production by BMDCs, as demonstrated by antibody blocking experiments (Fig. 2C). In contrast, but as expected, phosphoinositol-capped (PILAM) from Mycobacterium fortuitum22 and AraLAM from Mycobacterium chelonae23, which are devoid of mannose caps (Fig. 1)17–19, failed to do so (Fig. 2). M. tuberculosis LM bound and induced signaling via Dectin-2, although to a much weaker extent than ManLAM from the same strain (Figs 1 and S2A), while surprisingly M. smegmatis LM was completely inactive (Fig. 2). Only subtle differences in the mannan core ramification and acylation degrees between the structure of M. tuberculosis and M. smegmatis LM have been described to date19 and are unlikely to explain the difference in activity. However, the LAM and LM purification procedure involves a gel permeation chromatographic step to separate both lipoglycans24,25, which is not highly resolving, and yields preparation with a slight cross-contamination between the compound fractions. Whereas a contamination of M. smegmatis LM with PILAM from the same species would have no impact since PILAM is not a ligand of Dectin-2, a contamination of M. tuberculosis LM by ~1% of the strong agonist ManLAM would explain the activity observed for the former (Fig. S2A). Biochemical analysis of the M. tuberculosis LM batch used indeed confirmed a slight contamination by ManLAM, as demonstrated by the detection of arabinose after total acid hydrolysis (Fig. S2B). Accordingly, degradation of contaminating ManLAM by selective mild acid hydrolysis of its arabinan domain completely abrogated M. tuberculosis LM binding to and signaling via Dectin-2 (Fig. 2), while it did not affect LM ability to activate TLR2 (Fig. S2C). PIM6 bound weakly to Dectin-2-Fc (Fig. 2A), but failed to induce signaling in HEK-Dectin-2 cells or BMDCs (Fig. 2B and C). We finally tested two of the most abundant M(L)P purified from M. tuberculosis: the 19 kDa MLP (LpqH) and 45 kDa MP (Apa)20,26,27. The 19 kDa MLP, but not 45 kDa MP, bound and slightly induced signalling via Dectin-2 (Fig. 2). However again, depletion of ManLAM by anti-LAM antibody treatment completely abrogated the 19 kDa MLP Dectin-2-dependant activity (Fig. 2), while 19 kDa MLP remained potent at stimulating TLR2 (Fig. S2D). Altogether, although PIM6 weakly bound to Dectin-2, among all the mannoconjugates tested, ManLAM appears to be the sole mycobacterial ligand able to induce signaling via this receptor.
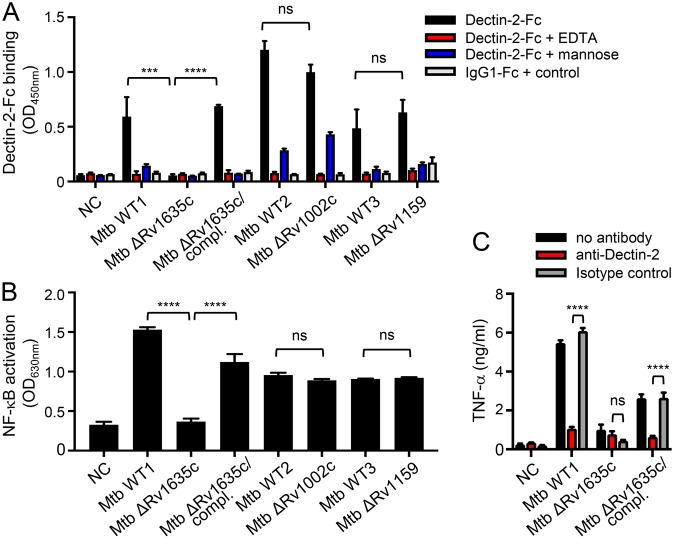
Figure 2.
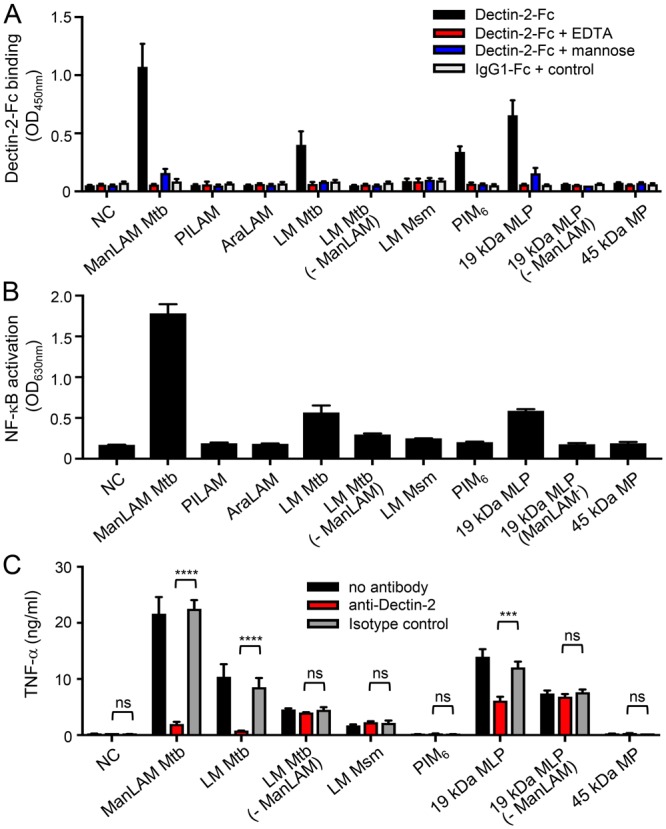
ManLAM, but not the other mycobacterial cell envelope mannoconjugates tested, induce cell signaling via Dectin-2. Mannoconjugates (1 µg in (A,B) 0.1 µg in (C)) were coated in 96-well plates and tested for their capacity to bind Dectin-2-Fc (A), and to induce NF-κB activation in HEK-Dectin-2 cells (B) and TNF-α production by BMDCs. (A) Dectin-2-Fc or IgG1-Fc control proteins (1 μg/ml) were pre-incubated or not with 20 mM EDTA or 40 mM mannose and allowed to react with the mannoconjugates for 2 h at RT. Bound proteins were detected using a biotin-conjugated anti-IgG Fcγ specific antibody and avidin-HRP, and reading O.D. at 450 nm. (B) HEK-Dectin-2 cells were stimulated for 24 h and NF-κB activation was determined by measuring alkaline phosphatase activity and reading O.D. at 630 nm. (C) BMDCs were stimulated for 24 h and TNF-α release in the supernatant was quantified by ELISA. Dectin-2 dependence was investigated by pre-incubating cells for 30 min at 37 °C with 5 μg/ml of anti-Dectin-2 or rIgG2a isotype control antibodies. Data show mean ± SEM. Mtb, M. tuberculosis; Msm, M. smegmatis; NC, non-coated; - ManLAM, ManLAM-depleted.
In order to evaluate the contribution of ManLAM, and other possible ligands, to M. tuberculosis recognition by Dectin-2, we investigated the capacity of different mutant strains to bind or induce signaling via the receptor. A M. tuberculosis ΔRv1635c/CapA mutant strain, which produces a LAM devoid of mannose caps (Fig. 1) while other mannoconjugates remain intact28,29, completely failed, in contrast to the wild-type and complemented strains, to bind Dectin-2-Fc (Fig. 3A). However, a M. tuberculosis ΔRv1159/PimE mutant strain, which is impaired for the production of PIM6 and accumulates PIM glycoforms devoid of (α1 → 2)-linked units30 (Figs 1 and S3), and a M. tuberculosis ΔRv1002c/PMT mutant strain, which is deficient for protein O-mannosylation20 (Fig. 1), bound Dectin-2-Fc as efficiently as their wild-type counterparts (Fig. 3A). Finally, the capacity of a bacterial lysate to induce NF-κB activation in HEK-Dectin-2 cells, as well as a Dectin-2-dependent TNF-α production by BMDCs, was fully abrogated in the ΔRv1635c/CapA mutant strain (Fig. 3B and C).
Figure 3.
ManLAM is the sole ligand mediating M. tuberculosis recognition by Dectin-2. The indicated M. tuberculosis (Mtb) strains (106 bacilli in (A) 1 µg lysate in (B and C)) were tested for their capacity to bind Dectin-2-Fc (A), and to induce NF-κB activation in HEK-Dectin-2 cells (B) and TNF-α production by BMDCs (C). Conditions are the same as in Fig. 2. Data show mean ± SEM. compl., complemented; NC, non-coated.
Thus, ManLAM is concluded to be the sole ligand mediating M. tuberculosis recognition by Dectin-2.
Dimannoside caps are required for ManLAM, and related lipoglycans, binding to and signaling via Dectin-2
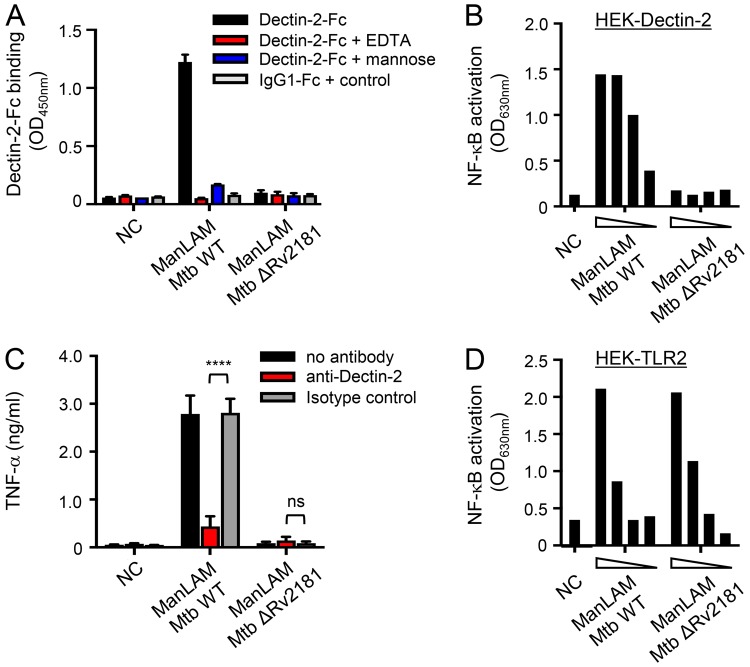
The mannose caps are necessary for ManLAM recognition by Dectin-216. However, these motifs are heterogeneous and consist of mono-, (α1 → 2)-di- or (α1 → 2)-tri-mannosyl units17. In order to assess the impact of the mannose cap length, we used ManLAM purified from a M. tuberculosis ΔRv2181 mutant strain, which harbours single mannose residues at the non-reducing arabinan termini instead of (α1 → 2)-linked oligomannosides31 (Fig. 1). In contrast to wild-type ManLAM, ManLAM capped with single mannose units failed to bind or induce signaling via Dectin-2, while, as expected32,33, it was still able to activate TLR2 (Fig. 4). Thus, at least dimannoside caps are required for ManLAM recognition by Dectin-2, in agreement with the reported i) glycan array analyses showing selective binding of the Dectin-2 CRD to glycans containing (α1 → 2)-linked dimannoside epitopes14,15, and ii) the crystal structure of the CRD in complex with a mammalian-type high-mannose Man9GlcNAc2 revealing two monosaccharide binding sites that allow the interaction of dimannosides15.
Figure 4.
Dimannoside caps are required for ManLAM binding to and signaling via Dectin-2. ManLAM (1 µg in (A) from 300 to 10 ng in (B and D) 0.1 µg in (C)) purified from M. tuberculosis wild-type or ΔRv2181 mutant strains were tested for their capacity to bind Dectin-2-Fc (A), to induce NF-κB activation in HEK-Dectin-2 cells (B) and HEK-TLR2 cells (D), and to induce TNF-α production by BMDCs (C). Conditions are the same as in Fig. 2. Data show mean ± SEM. NC, non-coated.
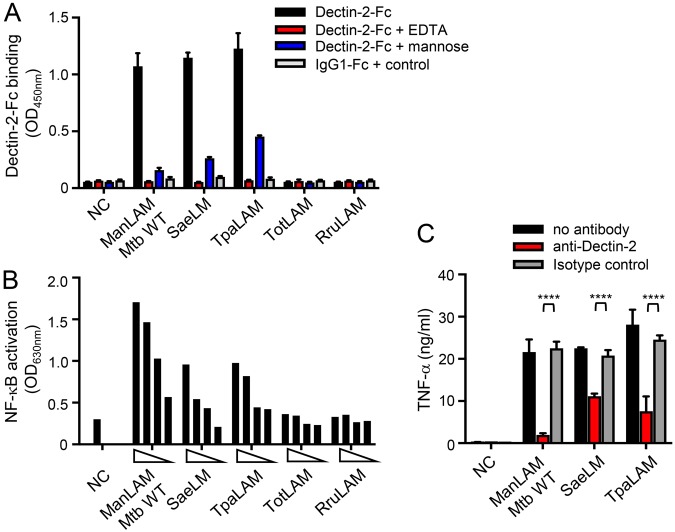
Interestingly, bacteria belonging to genera phylogenetically close to mycobacteria produce lipoglycans with structures related to LAM32. LAM from Tsukamurella paurometabola (TpaLAM)34 and LM from Saccharotrix aerocolonigenes (SaeLM)35 contain (α1 → 2)-linked dimannoside side chains, whereas LAM from Turicella otitidis (TotLAM)36 or Rhodococcus ruber (RruLAM)37 show single terminal mannose units only as in mycobacterial LM (Fig. 1). SaeLM and TpaLAM, but not TotLAM or RruLAM, were able to bind and induce signaling via Dectin-2 (Fig. 5), further supporting the structure/function relationship conclusions drawn with mycobacterial lipoglycans. However, if (α1 → 2)-linked dimannosides are required for the recognition by the CRD, they are not sufficient since both PIM6 and M(L)P fail to induce signaling via Dectin-2.
Figure 5.
Actinobacteria lipoglycans bearing (α1 → 2)-linked dimannoside caps bind and induce signaling via Dectin-2. Lipoglycans (1 µg in (A) from 300 to 10 ng in (B) 0.1 µg in (C)) were tested for their capacity to bind Dectin-2-Fc (A), and to induce NF-κB activation in HEK-Dectin-2 cells (B) and TNF-α production by BMDCs (C). Conditions are the same as in Fig. 2. Data show mean ± SEM. NC, non-coated.
Signaling via Dectin-2 relies on multivalent interaction
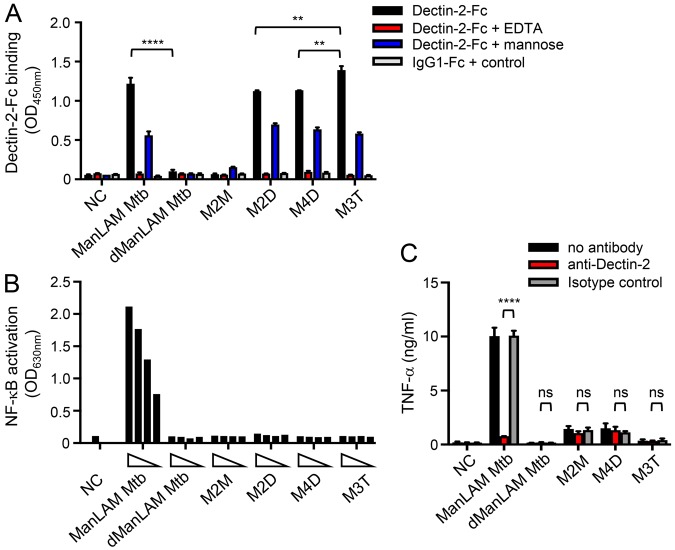
High avidity recognition by C-type lectins relies on multivalent binding that accumulates the strength of the multiple low affinities of the interaction between individual CRDs and oligosaccharides38,39. We previously found that high avidity recognition of purified ManLAM by the C-type lectins Mannose Receptor or DC-SIGN requires a ManLAM supramolecular organization induced by the aggregation of fatty acids in aqueous solution21,40,41. Indeed, although fatty acids do not directly interact with the receptor, they are involved in the 3D conformational presentation of the mannose caps and are required for high avidity binding of ManLAM. Accordingly, deacylation of ManLAM completely abrogated its ability to bind and induce signaling via Dectin-2 (Fig. 6). With the aim to mimic the bioactive supramolecular structure of ManLAM, we recently designed and chemically synthesized a set of mannodendrimers, made of poly(phosphorhydrazone) dendrimers grafted with (α1 → 2)-linked mannose caps (Figs 1 and S1), that bind and induce signaling via DC-SIGN as efficiently as the natural M. tuberculosis molecule42. Whatever the dendrimer generation, mannodendrimers grafted with dimmanoside or trimannoside caps bound Dectin-2-Fc as efficiently as ManLAM, whereas mannodendrimer with monomannoside caps did not (Fig. 6A), confirming again with the use of synthetic compounds that (α1 → 2)-linked dimannosides are required for the recognition by the CRD. However, none of the mannodendrimers was able to activate Dectin-2 signaling in reporter cells (Fig. 6B) or BMDCs (Fig. 6C), indicating that the ligand 3D conformational requirements for high avidity recognition by DC-SIGN and Dectin-2 differ.
Figure 6.
Mannodendrimers bind but do not induce signaling via Dectin-2. Mannoconjugates (1 µg in (A) from 300 to 10 ng in (B) 0.1 µg in (C)) were tested for their capacity to bind Dectin-2-Fc (A), and to induce NF-κB activation in HEK-Dectin-2 cells (B) and TNF-α production by BMDCs (C). Conditions are the same as in Fig. 2. Data show mean ± SEM. dManLAM, deacylated ManLAM; M2M, M2D, second-generation mannodendrimers capped with mono- or di-mannosides respectively; M3T, third-generation mannodendrimer capped with trimannosides; M4D, fourth-generation mannodendrimer capped with dimannosides; NC, non-coated.
Discussion
C-type lectins play a key role in the immune system with functions including cell adhesion, glycoprotein turnover or pathogen recognition based either on recognition of endogenous mammalian glycans or on binding to glycans on micro-organisms2. The spatial arrangement of CRDs in C-type lectin oligomers, although still poorly understood, has long been recognized as important in determining specificity for pathogen glycans, especially as the selectivity of the CRDs was initially thought to be low, by binding only the terminal monosaccharides of glycans2,43. However, recent studies have revealed that several C-type lectin CRDs comprise extended binding sites that can interact with disaccharide or trisaccharide units in terminal, but also possibly in internal, positions15,39,44. The geometry, organization and amino-acid composition of the extended binding site lead to a selectivity towards, or preclude an efficient binding of, specific classes of oligosaccharides (containing given monosaccharides and linkages: position, anomeric configuration)15. However, if the mechanisms of glycans binding to CRDs are increasingly well understood, how it leads to initiation of C-type lectin-associated signaling pathways remains almost unknown2,45.
The recently published crystal structure of human Dectin-2 CRD complexed with Man9GlcNAc2 oligosaccharide has revealed an extended binding site providing the molecular basis for binding of Manα1-2Man in external or internal positions of glycans15, structures that are found in several pathogens, such as fungal mannans, certain bacterial lipopolysaccharides, or mycobacterial mannose-capped LAM. Accordingly, we show here that effective binding of Dectin-2 to ManLAM or synthetic mannodendrimers requires (α1 → 2)-linked dimannoside caps. Interestingly, binding was increased by addition of a third (α1 → 2)-linked mannosyl unit, as observed for mannodendrimers (M3T vs M4D and M2D), in agreement with the crystal structure that shows space to model an additional α-linked mannose residue attached to the 2-OH group of the secondary site mannose residue (at the non-reducing end)15. However, substitution of the 6-OH group of the mannosyl unit bearing the (α1 → 2)-linked mannosyl unit at the non-reducing end impairs the binding, most probably because of a steric hindrance, as demonstrated by the inactivity of mycobacterial LM in contrast to SaeLM. We wish here to highlight the observation that traces amount of the highly potent Dectin-2 ligand ManLAM were sufficient to confer activity to other compounds purified from M. tuberculosis cell envelope (LM, 19 kDa MLP). When working with ManLAM-producing bacteria, one has thus to be cautious regarding compound purity. Surprisingly, M(L)P did not bind Dectin-2 although they bear (α1 → 2)-linked oligomannosides. In addition, PIM6, which weakly binds, but also mannodendrimers, which strongly bind Dectin-2-Fc, at least as efficiently as ManLAM, failed to induce signaling. This probably results from their inability to establish adequate multivalent interactions with Dectin-2, which are required for efficient high avidity binding to and triggering of intracellular signaling via the membrane-expressed receptor. Mannodendrimers were previously designed and chemically synthesized to mimic the bioactive supramolecular structure of ManLAM, and to bind and induce signaling via DC-SIGN42. Third-generation poly(phosphorhydrazone) mannodendrimers were sufficient to induce signaling via DC-SIGN as efficiently as ManLAM42. However, in the present study, even a fourth-generation poly(phosphorhydrazone) mannodendrimer was not able to trigger signaling via Dectin-2. Increasing the generation is not likely to make any improvement. Other dendrimer scaffolds, with a different geometry, should rather be tested to try to obtain an adequate mutivalency with Dectin-2. How ligand binding initiates intracellular signaling remains arguably the most poorly understood mechanistic aspect of C-type lectin function2. Ligand avidity is certainly a key parameter, but apparently not sufficient. Mannodendrimer binding to DC-SIGN was evaluated in our previous study using a set of bioassays, including inhibition of HEK293 cells expressing wild-type DC-SIGN protein binding to mannan-coated microplates42. Interestingly, we observed that the mannodendrimer IC50 values were not completely sufficient for prediction of their capacity to induce DC-SIGN signaling in human monocyte-derived dendritic cells (i.e. inhibition of pro-inflammatory cytokine production by LPS-stimulated cells). Here again, binding to Dectin-2 was not predictive of the capacity to induce signaling. Clustering of receptors by ligand binding is likely to be important for signaling initiation, but so far little is known about the oligomeric state of Dectin-2 or stoichiometry of the complexes that form with FcRγ2.
To determine the Dectin-2 ligand(s) involved in M. tuberculosis recognition, we used a set of knockout mutant strains. Indeed, ligands identified using purified molecules may not be accessible or relevant in the bacterial envelope46–48. In contrast, physiological ligands of C-type lectins might poorly bind the receptor in a solid phase binding assay because of a different clustering. Finally, unsuspected Dectin-2 ligand(s) might be involved in M. tuberculosis recognition. However, a M. tuberculosis ΔRv1635c/CapA mutant strain, which produces a LAM devoid of mannose caps, completely failed, in contrast to the other mutant strains tested, to bind Dectin-2, indicating that ManLAM is the sole ligand mediating M. tuberculosis recognition by Dectin-2. PIM6, which binds Dectin-2-Fc but does not induce signaling via the receptor, is not involved in whole bacilli recognition by Dectin-2 in reporter cells or BMDCs. Although we cannot completely exclude that PIM6-Dectin-2 interaction may play a role in other cellular contexts, this means that Dectin-2 can sense slow-growing mycobacteria (which are mostly the pathogenic ones) but not fast-growing mycobacteria, which produce LAM devoid of mannose caps. This property is unique so far among C-type lectin receptors, which show different specificities towards mycobacteria. Indeed, Mannose Receptor can recognize several purified mycobacterial mannoconjugates, such as ManLAM, LM, PIM and the 19 kDa MLP and 45 kDa MP, some of them (LM, PIM and glycoproteins in general) being also produced by fast-growing mycobacteria. Accordingly, Mannose Receptor binds both slow- and fast-growing mycobacteria. DC-SIGN recognizes the same purified mycobacterial mannoconjugates. However, surprisingly, it binds species of the M. tuberculosis complex only46. The molecular basis of this selective binding is not yet fully understood46–48.
Beyond mycobacteria, our data also suggest that Dectin-2 might be involved in the detection of the human opportunistic pathogen Ts. paurometabola, with some strains of the species reported to cause lung infection, lethal meningitis, and necrotizing tenosynovitis49.
Knowledge of the mechanism of carbohydrate recognition by C-type CRDs is now becoming sufficient that glycomimetic drugs can be envisaged2. The recent discovery that some C-type lectins can induce intracellular signaling and elicit cell-mediated immune responses has attracted interest in these receptors and their ligands in the field of adjuvants and immunomodulation45,50. Indeed, there is a growing interest in the development of vaccine adjuvants that direct robust Th1 and Th17 responses to subunit vaccines. An analogue of the mycobacterial trehalose-6,6′-dimycolate ligand of Mincle, trehalose-6,6′-dibehenate (TDB), formulated with dimethyldioctadecylammonium was reported to promote long-lived M. tuberculosis-specific T-cell responses in humans51 and completed phase I clinical trial52. Addition of a newly identified Dectin-2 ligand, the glycoprotein Blastomyces Eng2, to a pan-fungal subunit vaccine was recently shown to prime large numbers of Ag-specific Th17 and Th1 cells, augment activation and killing of fungi, and protect mice from lethal fungal challenge53. Moreover, Dectin-2 activation by ManLAM was found to trigger limited inflammatory responses that could be beneficial for the adjuvantation of therapeutic vaccines for infectious diseases or cancer16. Therefore, better understanding the molecular basis of ligand recognition by Dectin-2 will pave the way for the rational design of potent adjuvants targeting this receptor54.
Materials and Methods
Mannoconjugates and mycobacterial strains
Lipoglycans and lipoproteins were purified as previously described; ManLAM Mtb, LM Mtb and PIM6 from M. tuberculosis H37Rv, AraLAM from M. chelonae, PILAM from M. fortuitum, LM Msm from M. smegmatis46,55–57; TpaLAM from Ts. paurometabola34; SaeLM from S. aerocolonigenes35; TotLAM from T. otitidis36; RruLAM from R. ruber37; the native 19 kDa lipoprotein58 and 45 kDa mannoprotein59 from M. tuberculosis H37Rv; ManLAM Mtb ΔRv2181 from M. tuberculosis H37RvΔRv218131. Bacterial strains were grown under the following biosafety conditions: M. tuberculosis strains (level 3), M. chelonae, M. fortuitum and T. otitidis (level 2), other bacterial species (level 1). Mannodendrimers were chemically synthesized as previously reported42.
Traces of ManLAM were removed from the purified 19 kDa mannolipoprotein solution by immunoprecipitation, using magnetic beads coated with an anti-LAM antibody60. Beads (500 µg; ~10 µg antibody) were washed twice with PBS, added to 100 µl of the mannolipoprotein solution (at 1 mg/mL) and incubated for 1 h at room temperature. Using a magnet, the LAM-free supernatant was removed and used for subsequent experiments. ManLAM present in M. tuberculosis LM preparation was degraded by mild acid hydrolysis (0.1 M HCl for 20 min at 110 °C) that selectively depolymerizes the arabinan domain while keeping intact the lipomannan core34.
M. tuberculosis H37RvΔRv1635c28, M. tuberculosis H37RvΔRv1002c20, M. tuberculosis H37RvΔRv1159 (see below), and corresponding wild-type strains were grown as surface pellicle in 7H9 medium supplemented with ADC. For binding experiments to Dectin-2-Fc (see below), bacteria were dissociated by gentle shaking for 30 s with 4-mm glass beads and numbered with a Thoma cell counting chamber. To prepare a bacterial lysate containing lipoglycans (LAM, LM and PIM6) and lipoproteins, mycobacteria were delipidated by several extractions with CHCl3/CH3OH (1:1, v/v). Delipidated cells were then disrupted by sonication and further extracted by refluxing in 50% ethanol at 65 °C46. Ethanol/water extract was dried and used in subsequent experiments.
Construction of a M. tuberculosis H37RvΔRv1159 mutant
The Ts/sacB method was used to achieve allelic replacement at the pimE (Rv1159) locus of M. tuberculosis H37Rv (ATCC 25618)61. pimE and flanking regions were PCR-amplified using the pair of primers Rv1159.5 (5′-ggcggcgggtgcgggttccgc-3′)/Rv1159.6 (5′-ccaagttgacggcggccaccg-3′) and a disrupted allele was obtained by replacing 964 bp of the coding sequence of this gene bracketed between two SmaI sites by the Kan cassette from pUC4K (Amersham Pharmacia Biotech). Mutant clones were confirmed by PCR using the set of primers Rv1159.1 (5′-CCCGGCCCATATGTGCCGCACCCTGATCGAC-3′) and Rv1159.2 (5′-CCCAAGCTTATTGGCCATGCGCCGCGGCC-3′). Allelic replacement at the pimE locus of eight candidate mutant clones was confirmed by PCR (Fig. S3A). Negative ion mode MALDI-TOF-MS analyses of the PIM content of one of the mutants, performed as previously described62, revealed a complete absence of PIM6 acyl-forms with a concomitant increase in PIM4 acyl-forms (Fig. S3B), indicating that the disruption of pimE had the same effects on polar PIM synthesis in M. tuberculosis as the inactivation of the orthologous gene (MSMEG_5149) in M. smegmatis30.
Monosaccharide analysis
Mannoconjugates were submitted to strong acid hydrolysis with 2 M trifluoroacetic acid at 110 °C for 2 h and then dried under speed-vacuum. The resulting monosaccharides were derivatized for 90 min at 55 °C using a solution of 0.2 M 1-aminopyrene-3,6,8-trisulfonate (APTS) in 15% acetic acid and 1 M sodium cyanoborohydride solution dissolved in tetrahydrofuran. The APTS-labelled monosaccharides were suspended in water and subjected to analysis by capillary electrophoresis monitored by laser-induced fluorescence, as previously described63.
Binding of Dectin-2-Fc
Mannoconjugates (1 μg/well in isopropanol) or mycobacteria (heat-inactivated, 106/well in isopropanol) were coated on 96-wells Maxisorp plates (Nunc). Dectin-2-Fc, a soluble form of the human Dectin-2 receptor, was constructed by fusing the C-terminal extracellular domain of human Dectin-2 (aa 42–209) to the C-terminus of an engineered human IgG1 Fc domain. A soluble form of the murine Dectin-1 receptor fused to the same human IgG1-Fc domain was used as a non-relevant protein control (IgG1 Fc control). hDectin-2-Fc and IgG1-Fc control proteins were expressed in CHO cells and purified by G protein affinity chromatography. Human IgG1-Fc control or Dectin-2-Fc fusion proteins (1 μg/ml in PBS, 1 mM CaCl2, 1% BSA) were pre-incubated or not with 20 mM EDTA or 40 mM mannose (Sigma) and were allowed to react with mannoconjugates or bacterial cells for 2 h at RT (in 50 μl). Wells were washed once with PBS and the bound Fc fusion proteins were detected using biotin-conjugated anti-human IgG Fcγ specific antibodies (eBioscience) and avidin-horseradish peroxidase (eBioscience).
Dectin-2 and TLR2 reporter cell lines experiments
The HEK-BlueTM mDectin-2 and HEK-BlueTM hTLR2 (InvivoGen), derivatives of HEK293 cells that stably express the murine Dectin-2 or human TLR2 genes respectively, along with a NF-κB-inducible reporter system (secreted alkaline phosphatase) were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 10% Fetal Bovine Serum (FBS, Gibco) 4.5 g/l glucose, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (Sigma) and 100 μg/ml zeocin, 200 μg/ml hygromycin, 10 μg/ml blasticidin, 1 μg/ml puromycin and 50 μg/ml mofetil (all from InvivoGen). Mannoconjugates (1 μg/well in isopropanol, except in Figs 4B,D, 5B, 6B and S2C, from 300 ng to 10 ng/well in isopropanol, in Fig. S2D from 20 to 0.2 ng/well) or bacterial cells lysate (1 µg/well in isopropanol) were added to 96-well plates, followed by evaporation of the solvents as previously described. Reporter cells (5 × 104/well) were stimulated for 24 h, after which alkaline phosphatase activity was measured by mixing 20 μl of the culture supernatant and 180 μl of Quanti-BlueTM (InvivoGen), and reading O.D. at 630 nm.
Generation and activation of murine bone marrow-derived dendritic cells
All methods were carried out in accordance with the Centre National de la Recherche Scientifique guidelines and regulations for housing and care of laboratory animals. All experimental protocols were approved by the Structure chargée du bien-être animal (no. 2015.Ni.15). Bone marrow cells were flushed from the tibias and femurs of C57BL/6 mice (Janvier) with 5 ml of cold DMEM. The cell suspension was cultured at a density of 106 cells/ml in Iscove’s modified Dulbecco’s medium (IMDM, Lonza) supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-mercaptoethanol and 10% J558 cell conditioned medium (as a source of GM-CSF). On day 3, fresh medium containing GM-CSF was added and on day 6 one half of the medium was renewed. BMDCs were harvested and used on day 8. They were distributed in 96-well plates at 2 × 105 cells/well to wells previously coated with mannoconjugates (0.1 µg/well) or bacterial cells lysate (1 µg/well) as described above. After 18 h, TNF-α was assayed in the culture supernatant using a commercially available kit (eBioscience). To investigate Dectin-2 dependence, BMDCs were pre-incubated for 30 min at 37 °C with 5 μg/ml of anti-mDectin-2 antibody (clone 11E4, Life Technology) or isotype control (rIgG2a, eBiosciences).
Statistical analysis
Data are expressed as mean ± SEM and were analyzed using Two-way analysis of variance followed by Tukey test to determine significant differences between samples.
Electronic supplementary material
Acknowledgements
This work was supported by Association Nationale de la Recherche et de la Technologie (A.D. was the recipient of a CIFRE PhD fellowship from ANRT and Invivogen), Centre National de la Recherche Scientifique, Université Paul Sabatier, Fondation pour la Recherche Médicale (fellowship to S.G.), and the National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH) grant AI064798 (to M.J.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions
A.D., S.S.-G., D.D., M.G., M.J., A.V., G.T. and J.N. designed research; A.D., S.S.-G., D.D., M.G., G.L.-M., M.J. and A.V. performed research; E.B., M.R., J.P., A.-M.C., B.H., G.K., D.K., K.M.D., M.L., I.C.S., G.S.B. and B.J.A. contributed reagents; A.D., S.S.-G., D.D., M.G., G.L.-M., M.J., A.V., G.T. and J.N. analyzed data; A.D., S.S.-G. and J.N. wrote the manuscript with input from all authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alexiane Decout and Sandro Silva-Gomes contributed equally.
Gérard Tiraby is Deceased.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35393-5.
References
- 1.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Drickamer K, Taylor ME. Recent insights into structures and functions of C-type lectins in the immune system. Curr. Opin. Struct. Biol. 2015;34:26–34. doi: 10.1016/j.sbi.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariizumi K, et al. Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J. Biol. Chem. 2000;275:11957–11963. doi: 10.1074/jbc.275.16.11957. [DOI] [PubMed] [Google Scholar]
- 4.Kanazawa N, Tashiro K, Inaba K, Lutz MB, Miyachi Y. Molecular cloning of human dectin-2. J. Invest. Dermatol. 2004;122:1522–1524. doi: 10.1111/j.0022-202X.2004.22602.x. [DOI] [PubMed] [Google Scholar]
- 5.Gavino ACP, Chung J-S, Sato K, Ariizumi K, Cruz PD. Identification and expression profiling of a human C-type lectin, structurally homologous to mouse dectin-2. Exp. Dermatol. 2005;14:281–288. doi: 10.1111/j.0906-6705.2005.00312.x. [DOI] [PubMed] [Google Scholar]
- 6.Robinson MJ, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med. 2009;206:2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi L, et al. CARD9 mediates dectin-2-induced IkappaBalpha kinase ubiquitination leading to activation of NF-kappaB in response to stimulation by the hyphal form of Candida albicans. J. Biol. Chem. 2010;285:25969–25977. doi: 10.1074/jbc.M110.131300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorjestani S, et al. Phospholipase Cγ2 (PLCγ2) is key component in Dectin-2 signaling pathway, mediating anti-fungal innate immune responses. J. Biol. Chem. 2011;286:43651–43659. doi: 10.1074/jbc.M111.307389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saijo S, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Loures FV, et al. Recognition of Aspergillus fumigatus hyphae by human plasmacytoid dendritic cells is mediated by dectin-2 and results in formation of extracellular traps. Plos Pathog. 2015;11:e1004643. doi: 10.1371/journal.ppat.1004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritter M, et al. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc. Natl. Acad. Sci. USA. 2010;107:20459–20464. doi: 10.1073/pnas.1010337107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGreal EP, et al. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology. 2006;16:422–430. doi: 10.1093/glycob/cwj077. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa T, et al. Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe. 2013;13:477–488. doi: 10.1016/j.chom.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Zheng RB, et al. Insights into Interactions of Mycobacteria with the Host Innate Immune System from a Novel Array of Synthetic Mycobacterial Glycans. ACS Chem. Biol. 2017;12:2990–3002. doi: 10.1021/acschembio.7b00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feinberg H, et al. Mechanism of pathogen recognition by human dectin-2. J. Biol. Chem. 2017;292:13402–13414. doi: 10.1074/jbc.M117.799080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yonekawa A, et al. Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity. 2014;41:402–413. doi: 10.1016/j.immuni.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Nigou J, Gilleron M, Puzo G. Lipoarabinomannans: from structure to biosynthesis. Biochimie. 2003;85:153–166. doi: 10.1016/S0300-9084(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 18.Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol. Microbiol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- 19.Gilleron, M., Jackson, M., Nigou, J. & Puzo, G. Structure, activities and biosynthesis of the phosphatidyl-myo-inositol-based lipoglycans. In The mycobacterial cell envelope (Daffé, M. & Reyrat, J. M., 2008).
- 20.Liu C-F, et al. Bacterial protein-O-mannosylating enzyme is crucial for virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 2013;110:6560–6565. doi: 10.1073/pnas.1219704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vergne, I., Gilleron, M. & Nigou, J. Manipulation of the endocytic pathway and phagocyte functions by Mycobacterium tuberculosis lipoarabinomannan. Front. Cell. Infect. Microbiol. 4 (2015). [DOI] [PMC free article] [PubMed]
- 22.Gilleron M, et al. Mycobacterium smegmatis phosphoinositols-glyceroarabinomannans. Structure and localization of alkali-labile and alkali-stable phosphoinositides. J. Biol. Chem. 1997;272:117–124. doi: 10.1074/jbc.272.1.117. [DOI] [PubMed] [Google Scholar]
- 23.Guerardel Y, et al. Structural study of lipomannan and lipoarabinomannan from Mycobacterium chelonae. Presence of unusual components with alpha 1,3-mannopyranose side chains. J. Biol. Chem. 2002;277:30635–30648. doi: 10.1074/jbc.M204398200. [DOI] [PubMed] [Google Scholar]
- 24.Nigou J, et al. The phosphatidyl-myo-inositol anchor of the lipoarabinomannans from Mycobacterium bovis bacillus Calmette Guérin. Heterogeneity, structure, and role in the regulation of cytokine secretion. J. Biol. Chem. 1997;272:23094–23103. doi: 10.1074/jbc.272.37.23094. [DOI] [PubMed] [Google Scholar]
- 25.Nigou J, Gilleron M, Brando T, Puzo G. Structural analysis of mycobacterial lipoglycans. Appl. Biochem. Biotechnol. 2004;118:253–267. doi: 10.1385/ABAB:118:1-3:253. [DOI] [PubMed] [Google Scholar]
- 26.Dobos KM, Swiderek K, Khoo KH, Brennan PJ, Belisle JT. Evidence for glycosylation sites on the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. Infect. Immun. 1995;63:2846–2853. doi: 10.1128/iai.63.8.2846-2853.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrmann JL, O’Gaora P, Gallagher A, Thole JE, Young DB. Bacterial glycoproteins: a link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J. 1996;15:3547–3554. doi: 10.1002/j.1460-2075.1996.tb00724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afonso-Barroso A, et al. Lipoarabinomannan mannose caps do not affect mycobacterial virulence or the induction of protective immunity in experimental animal models of infection and have minimal impact on in vitro inflammatory responses: Lipoarabinomannan and mycobacterial virulence. Cell. Microbiol. 2013;15:660–674. doi: 10.1111/cmi.12065. [DOI] [PubMed] [Google Scholar]
- 29.Dinadayala P, et al. Genetic basis for the synthesis of the immunomodulatory mannose caps of lipoarabinomannan in Mycobacterium tuberculosis. J. Biol. Chem. 2006;281:20027–20035. doi: 10.1074/jbc.M603395200. [DOI] [PubMed] [Google Scholar]
- 30.Morita YS, et al. PimE Is a Polyprenol-phosphate-mannose-dependent Mannosyltransferase That Transfers the Fifth Mannose of Phosphatidylinositol Mannoside in Mycobacteria. J. Biol. Chem. 2006;281:25143–25155. doi: 10.1074/jbc.M604214200. [DOI] [PubMed] [Google Scholar]
- 31.Kaur D, et al. Lipoarabinomannan of Mycobacterium: mannose capping by a multifunctional terminal mannosyltransferase. Proc. Natl. Acad. Sci. USA. 2008;105:17973–17977. doi: 10.1073/pnas.0807761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ray A, Cot M, Puzo G, Gilleron M, Nigou J. Bacterial cell wall macroamphiphiles: pathogen-/microbe-associated molecular patterns detected by mammalian innate immune system. Biochimie. 2013;95:33–42. doi: 10.1016/j.biochi.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Nigou J, et al. Mannan chain length controls lipoglycans signaling via and binding to TLR2. J. Immunol. 2008;180:6696–6702. doi: 10.4049/jimmunol.180.10.6696. [DOI] [PubMed] [Google Scholar]
- 34.Gibson KJC, et al. Tsukamurella paurometabola lipoglycan, a new lipoarabinomannan variant with pro-inflammatory activity. J. Biol. Chem. 2004;279:22973–22982. doi: 10.1074/jbc.M310906200. [DOI] [PubMed] [Google Scholar]
- 35.Gibson KJC, et al. A lipomannan variant with strong TLR-2-dependent pro-inflammatory activity in Saccharothrix aerocolonigenes. J. Biol. Chem. 2005;280:28347–28356. doi: 10.1074/jbc.M505498200. [DOI] [PubMed] [Google Scholar]
- 36.Gilleron M, et al. Characterization of a Truncated Lipoarabinomannan from the Actinomycete Turicella otitidis. J. Bacteriol. 2005;187:854–861. doi: 10.1128/JB.187.3.854-861.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibson KJC, et al. Structural and functional features of Rhodococcus ruber lipoarabinomannan. Microbiology. 2003;149:1437–1445. doi: 10.1099/mic.0.26161-0. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell DA, Fadden AJ, Drickamer K. A Novel Mechanism of Carbohydrate Recognition by the C-type Lectins DC-SIGN and DC-SIGNR: Subunit Organization and Binding to Multivalent Ligands. J. Biol. Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 39.Feinberg H. Structural Basis for Selective Recognition of Oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294:2163–2166. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- 40.Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- 41.Rivière M, Moisand A, Lopez A, Puzo G. Highly Ordered Supra-Molecular Organization of the Mycobacterial Lipoarabinomannans in Solution. Evidence of a Relationship Between Supra-Molecular Organization and Biological Activity. J. Mol. Biol. 2004;344:907–918. doi: 10.1016/j.jmb.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 42.Blattes E, et al. Mannodendrimers prevent acute lung inflammation by inhibiting neutrophil recruitment. Proc. Natl. Acad. Sci. USA. 2013;110:8795–8800. doi: 10.1073/pnas.1221708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunol. Rev. 1998;163:19–34. doi: 10.1111/j.1600-065X.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 44.Feinberg H, et al. Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle. J. Biol. Chem. 2013;288:28457–28465. doi: 10.1074/jbc.M113.497149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geijtenbeek TBH, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitarque S, et al. Deciphering the molecular bases of Mycobacterium tuberculosis binding to the lectin DC-SIGN reveals an underestimated complexity. Biochem. J. 2005;392:615–624. doi: 10.1042/BJ20050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Appelmelk BJ, et al. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium-host interaction. Cell. Microbiol. 2008;10:930–944. doi: 10.1111/j.1462-5822.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 48.Driessen NN, et al. Role of phosphatidylinositol mannosides in the interaction between mycobacteria and DC-SIGN. Infect. Immun. 2009;77:4538–4547. doi: 10.1128/IAI.01256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munk AC, et al. Complete genome sequence of Tsukamurella paurometabola type strain (no. 33T) Stand. Genomic Sci. 2011;4:342–351. doi: 10.4056/sigs.1894556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lang R, Schoenen H, Desel C. Targeting Syk-Card9-activating C-type lectin receptors by vaccine adjuvants: Findings, implications and open questions. Immunobiology. 2011;216:1184–1191. doi: 10.1016/j.imbio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Lindenstrøm T, et al. Vaccine-Induced Th17 Cells Are Maintained Long-Term Postvaccination as a Distinct and Phenotypically Stable Memory Subset. Infect. Immun. 2012;80:3533–3544. doi: 10.1128/IAI.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Dissel JT, et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 2014;32:7098–7107. doi: 10.1016/j.vaccine.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, et al. Ligation of Dectin-2 with a novel microbial ligand promotes adjuvant activity for vaccination. Plos Pathog. 2017;13:e1006568. doi: 10.1371/journal.ppat.1006568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Decout A, et al. Rational design of adjuvants targeting the C-type lectin Mincle. Proc. Natl. Acad. Sci. 2017;114:2675–2680. doi: 10.1073/pnas.1612421114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilleron M, Bala L, Brando T, Vercellone A, Puzo G. Mycobacterium tuberculosis H37Rv Parietal and Cellular Lipoarabinomannans: Characterization of the Acyl- and Glyco-forms. J. Biol. Chem. 2000;275:677–684. doi: 10.1074/jbc.275.1.677. [DOI] [PubMed] [Google Scholar]
- 56.Gilleron M, Nigou J, Cahuzac B, Puzo G. Structural study of the lipomannans from Mycobacterium bovis BCG: characterisation of multiacylated forms of the phosphatidyl-myo-inositol anchor. J. Mol. Biol. 1999;285:2147–2160. doi: 10.1006/jmbi.1998.2438. [DOI] [PubMed] [Google Scholar]
- 57.Gilleron M, Quesniaux VFJ, Puzo G. Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis bacillus Calmette Guerin and mycobacterium tuberculosis H37Rv and its implication in Toll-like receptor response. J. Biol. Chem. 2003;278:29880–29889. doi: 10.1074/jbc.M303446200. [DOI] [PubMed] [Google Scholar]
- 58.Yu C-H, et al. RP105 Engages Phosphatidylinositol 3-Kinase p110δ To Facilitate the Trafficking and Secretion of Cytokines in Macrophages during Mycobacterial Infection. J. Immunol. 2015;195:3890–3900. doi: 10.4049/jimmunol.1500017. [DOI] [PubMed] [Google Scholar]
- 59.Harriff, M. J. et al. HLA-E Presents Glycopeptides from the Mycobacterium tuberculosis Protein MPT32 to Human CD8+ T cells. Sci. Rep. 7 (2017). [DOI] [PMC free article] [PubMed]
- 60.Hamasur B, et al. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab’)2 fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin. Exp. Immunol. 2004;138:30–38. doi: 10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pelicic V, et al. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 1997;94:10955–10960. doi: 10.1073/pnas.94.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korduláková J, et al. Definition of the first mannosylation step in phosphatidylinositol mannoside synthesis. PimA is essential for growth of mycobacteria. J. Biol. Chem. 2002;277:31335–31344. doi: 10.1074/jbc.M204060200. [DOI] [PubMed] [Google Scholar]
- 63.Nigou J, Vercellone A, Puzo G. New structural insights into the molecular deciphering of mycobacterial lipoglycan binding to C-type lectins: lipoarabinomannan glycoform characterization and quantification by capillary electrophoresis at the subnanomole level. J. Mol. Biol. 2000;299:1353–1362. doi: 10.1006/jmbi.2000.3821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.