Abstract
Aromatase, an enzyme that converts androgens to estrogens, has been reported to be involved in several brain functions, including synaptic plasticity, neurogenesis, neuroprotection, and regulation of sexual and emotional behaviours in rodents, pathophysiology of Alzheimer’s disease and autism spectrum disorders in humans. Aromatase has been reported to be involved in aggressive behaviours in genetically modified mice and in personality traits by genotyping studies on humans. However, no study has investigated the relationship between aromatase in living brains and personality traits including aggression. We performed a positron emission tomography (PET) study in 21 healthy subjects using 11C-cetrozole, which has high selectivity and affinity for aromatase. Before performing PET scans, subjects answered the Buss-Perry Aggression Questionnaire and Temperament and Character Inventory to measure their aggression and personality traits, respectively. A strong accumulation of 11C-cetrozole was detected in the thalamus, hypothalamus, amygdala, and medulla. Females showed associations between aromatase levels in subcortical regions, such as the amygdala and supraoptic nucleus of the hypothalamus, and personality traits such as aggression, novelty seeking, and self-transcendence. In contrast, males exhibited associations between aromatase levels in the cortices and harm avoidance, persistence, and self-transcendence. The association of aromatase levels in the thalamus with cooperativeness was common to both sexes. The present study suggests that there might exist associations between aromatase in the brain and personality traits. Some of these associations may differ between sexes, while others are likely common to both.
Introduction
Aromatase is an enzyme that converts androgens to estrogens and is localized not only in the gonads but also in the brain1. In mammals such as rodents and non-human primates, the regions rich in aromatase are the hypothalamus and amygdala2–4. In addition, the thalamus also contains high concentrations of aromatase in humans5–7. Aromatase in the brain has been suggested to be related to several brain functions. Aromatase knockout mice display altered aggressive behaviours8–11, disrupted sexual behaviour9,11, and displayed depressive-like behaviour12. Human postmortem brain studies demonstrated lower aromatase expression in the hypothalamus of Alzheimer’s disease13 and depression patients14, and in the frontal cortex of autism spectrum disorder (ASD) patients15,16, as compared with the same brain regions of control subjects.
The association between aromatase and aggression in animals has been reviewed by Trainor et al.17. Rodent studies suggested that the medial amygdala has been related to aggression18–20. In human studies, fMRI studies showed increased amygdala activation related to aggression21,22 and MRI morphometric study demonstrated reduced amygdala volume in healthy volunteers with higher aggression trait23. The lines of evidence indicate that the amygdala may be a key region in aggression. Besides aggression, there is a study indicating a relation between aromatase gene polymorphism and personality trait, harm avoidance24. So far, however, there was no study on the association between aromatase in living brain and personality traits including aggression. For assessment of personality traits, a questionnaire method is often used. In this study, we employed the Buss-Perry Aggression Questionnaire (BAQ)25,26 for aggression assessment and the Temperament and Character Inventory (TCI)27,28, which can evaluate personality traits, namely, novelty seeking, harm avoidance, reward dependence, persistence, self-directedness, cooperativeness, and self-transcendence.
To investigate molecular dynamics in the living body, positron emission tomography (PET) is a suitable technique, which allows quantitative analysis of compound accumulation in tissues. Previously, a PET study using 11C-vorozole, which was developed as the first PET probe for aromatase imaging, was performed to image aromatase in the brain of healthy men and women6,7. In that study, the authors demonstrated a unique distribution of aromatase in living human brains for the first time. However, a certain amount of nonspecific signals was observed in 11C-vorozole PET images probably caused by unintended reuptake of the radioactive metabolite into the brain.
We have developed a novel PET probe for aromatase imaging to overcome the disadvantages of 11C-vorozole, 11C-cetrozole29. Our previous study showed that 11C-cetrozole had a higher signal-to-noise ratio than 11C-vorozole since almost no radioactive metabolite of 11C-cetrozole was not taken up into the brain, that is, 11C-cetrozole is superior to 11C-vorozole in terms of specificity and metabolic stability29. In the present study, we performed a 11C-cetrozole PET study in healthy subjects to examine the association between aromatase in the brain and personality traits.
Results
Distribution of aromatase in human brain
The binding potential (BPND) values of 11C-cetrozole, which are an index of aromatase concentration, were calculated in 21 healthy individuals (10 females and 11 males). High BPNDs were found in the thalamus with heterogeneous distribution among the medulla, hypothalamus, and amygdala in both sexes (Figs 1 and 2). Males had higher BPND values in most of these regions than females, except for in the right hypothalamus; however, a significant sex difference was found only in the left hypothalamus (P = 0.005; Fig. 2). There were no regions which BPNDs depended on sex hormone levels in plasma (estradiol for females, and free testosterone for males).
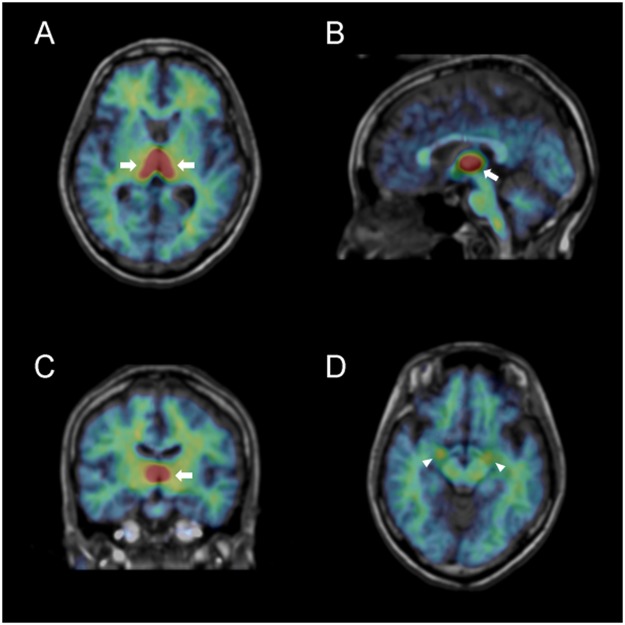
Figure 1.
Representative distribution volume (BPND + 1) images of 11C-cetrozole in a female human brain (rainbow colour scale) superimposed on the structural MR image (gray scale) of the same subject. (A) transaxial slice at level of the thalamus; (B) sagittal slice at the midline; (C) coronal slice at the level of the thalamus; (D) transaxial slice at the level of the amygdala. Arrows and arrow heads indicate the thalamus and amygdala, respectively.
Figure 2.
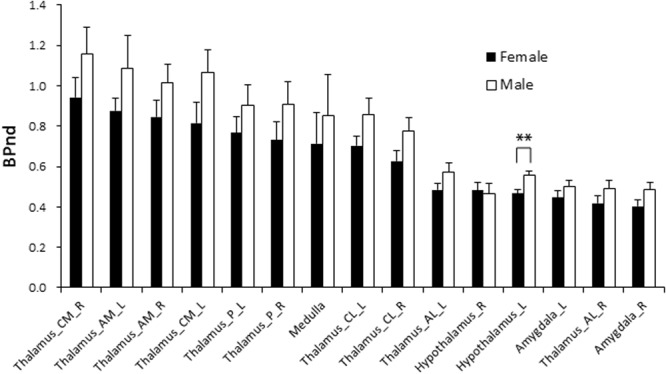
BPND values for the subregions of thalamus, amygdala, hypothalamus, and medulla. In all regions except for the right hypothalamus, males had higher BPND values than females. A significant sex difference was observed only in the left hypothalamus (**P = 0.005). AM, anterior medial; AL, anterior lateral; CM, central medial; CL, central lateral; P, posterior; L, left; R, right.
Association between aromatase and personality traits
We assessed associations between BPND images of 11C-cetrozole and scores of BAQ. Given that earlier studies showed that the amygdala is implicated in aggression18–23, we focused on the amygdala as a volume of interest (VOI). Using Statistical Parametric Mapping 8 software (SPM8, Wellcome Department of Imaging Neuroscience), a voxel-wise analysis corrected by family-wise error rate for aggression scores on the VOI was performed. Apparently, region-specific differences among individuals are evident and the associations between personality traits and region-specific level of aromatase were also observed (Figs 3 and 4). In females, aggression scores were positively associated with BPND in the left amygdala (PFWE-corr < 0.05, R2 = 0.83, Fig. 3). In contrast, data from male and combined data from male and female did not exhibit a significant association between aggression scores and BPND in the amygdala. For an evaluation of individuals’ personality traits, subjects answered TCI. Associations between traits and brain regions analysed in each sex group are listed in Table 1. Concerning other traits, sex-specific associations were observed in the amygdala and supraoptic nucleus of hypothalamus and in the inferior parietal gyrus in females. On the other hand, males-specific associations were observed in the anterior cingulate gyrus, supramarginal gyrus, caudate nucleus, pons, and midbrain. When the data of female and male were combined, almost no significant associations were found, except for cooperativeness. Cooperativeness scores were negatively associated with BPND in the bilateral thalamus of females and males (R2 = 0.71, Fig. 4). The subregions that associated with cooperativeness were localized to the ventral lateral and ventral posterior parts of the thalamus.
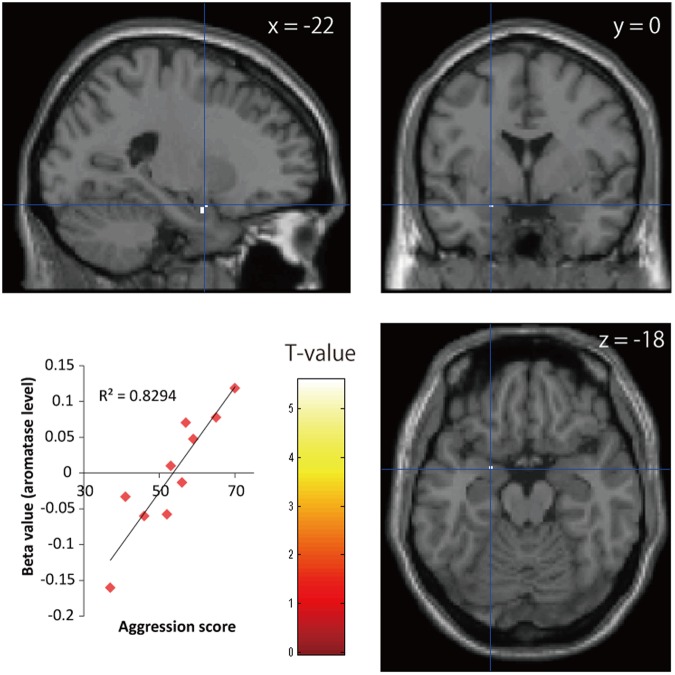
Figure 3.
Statistical parametric maps of associations between 11C-cetrozole BPND values in the amygdala of females and aggression scores (PFWE-corr < 0.05, 40 mm3). Peak coordinates (x = −22, y = 0, z = −18) are mapped on the template brain.
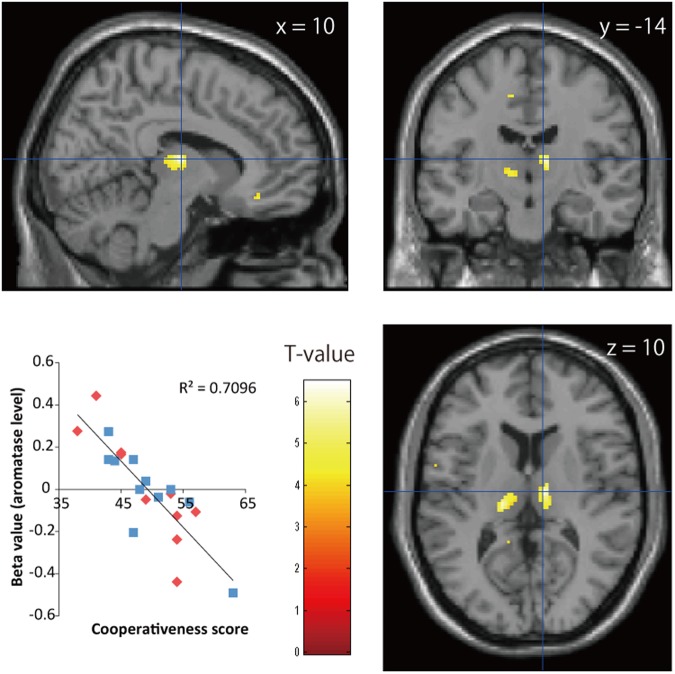
Figure 4.
Statistical parametric maps of associations between 11C-cetrozole BPND values in the thalamus and cooperativeness scores in females and males (P < 0.001, uncorrected, 640 mm3). Peak coordinates (x = 10, y = −14, z = 10) are mapped on the template brain. Red diamonds and blue squares represent the data from females and males, respectively.
Table 1.
The association between 11C-cetrozole BPND and traits (scores on TCI; P < 0.001, uncorrected, cluster size ≥ 80 mm3).
| Traits | Association | Sex | Region | Side | MNI Coordinates | P value | Z score | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| Novelty Seeking | Negative Negative Positive |
F F M |
Hypothalamus(SON) Amygdala Caudate nucleus |
R R R |
8 24 8 |
2 −6 18 |
−14 −20 −4 |
<0.001 <0.001 <0.001 |
4.04 3.71 3.96 |
136 80 104 |
| Harm Avoidance | Negative Negative |
M M |
Pons Supramarginal gyrus |
R R |
10 60 |
−22 −44 |
−30 34 |
<0.001 <0.001 |
3.55 3.38 |
88 88 |
| Reward Dependence | Positive | M | Thalamus | L | −18 | −26 | 6 | <0.001 | 3.79 | 152 |
| Persistence | Negative Negative Negative |
M M F & M |
Anterior cingulate gyrus Supramarginal gyrus Lingual gyrus |
L R L |
−12 58 −16 |
42 −46 −76 |
14 38 −12 |
<0.001 <0.001 <0.001 |
3.76 3.43 3.54 |
96 96 144 |
| Self-Directedness | Positive | F | Inferior parietal gyrus | L | −36 | −56 | 46 | <0.001 | 3.58 | 96 |
| Cooperativeness | Positive Negative Negative Negative Negative Negative |
M F & M F & M F & M F & M F & M |
Anterior cingulate gyrus Thalamus Thalamus Superior frontal gyrus Inferior frontal gyrus, triangular part Inferior frontal gyrus, orbital part |
L R L L L L |
−12 10 −16 −22 −48 −44 |
40 −14 −24 34 28 24 |
20 10 8 38 24 −6 |
<0.001 <0.001 <0.001 <0.001 <0.001 <0.001 |
3.94 4.17 3.99 3.87 3.76 3.76 |
104 640 1296 96 168 160 |
| Self-Transcendence | Positive Negative Negative |
F M M |
Hypothalamus (SON) Anterior cingulate gyrus Midbrain |
R L R |
8 −12 8 |
2 40 −26 |
−14 20 −18 |
<0.001 <0.001 <0.001 |
4.01 4.02 3.70 |
120 152 80 |
There were 2 regions that exhibited associations between BPND of 11C-cetrozole and female traits, namely, the right supraoptic nucleus of the hypothalamus (SON; MNI: x = 8, y = 2, z = −14) and the right amygdala (x = 24, y = −6, z = −20), although several associations were present in small clusters (Tables 2 and 3, Fig. 5). The traits that associated with BPND in the SON were novelty seeking (negative, R2 = 0.998), harm avoidance (positive, R2 = 0.97), reward dependence (negative, R2 = 0.997), persistence (negative, R2 = 0.98), cooperativeness (positive, R2 = 0.995), and self-transcendence (positive, R2 = 0.997; Table 2). The traits associated with BPND in the right amygdala were novelty seeking (negative, R2 = 0.999), persistence (negative, R2 = 0.994), cooperativeness (positive, R2 = 0.999), and self-transcendence (positive, R2 = 0.999; Table 3).
Table 2.
Traits in females that associated with BPND of 11C-cetrozole in the SON (x = 8, y = 2, z = −14). No traits in males were associated with BPND in this region.
| Traits | Association | P value | Z score | Cluster size (mm3) |
|---|---|---|---|---|
| Novelty Seeking | Negative | <0.001 | 4.04 | 136 |
| Harm Avoidance | Positive | <0.001 | 3.43 | 16 |
| Reward Dependence | Negative | <0.001 | 3.06 | 56 |
| Persistence | Negative | <0.001 | 3.58 | 24 |
| Cooperativeness | Positive | <0.001 | 3.62 | 32 |
| Self-Transcendence | Positive | <0.001 | 4.01 | 120 |
Table 3.
Traits in females that associated with the BPND of 11C-cetrozole in the right amygdala (x = 24, y = −6, z = −20). No traits in males were associated with this region.
| Traits | Association | P value | Z score | Cluster size (mm3) |
|---|---|---|---|---|
| Novelty Seeking | Negative | <0.001 | 3.71 | 80 |
| Persistence | Negative | <0.001 | 3.40 | 24 |
| Cooperativeness | Positive | <0.001 | 3.44 | 32 |
| Self-Transcendence | Positive | <0.001 | 3.50 | 40 |
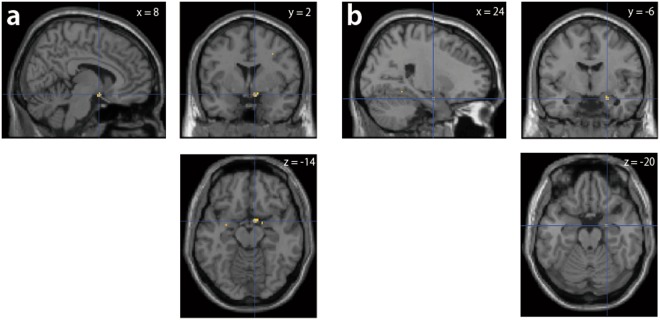
Figure 5.
Two regions that exhibited associations between BPND of 11C-cetrozole and female traits are the right supraoptic nucleus of the hypothalamus ((a) SON; MNI: x = 8, y = 2, z = −14) and the right amygdala ((b) x = 24, y = −6, z = −20).
Discussion
In this study, we demonstrated the distribution of aromatase in living human brains using our originally developed PET probe, 11C-cetrozole, and suggested that aromatase levels in the brain may relate to personality traits. The first PET scan of brain aromatase using 11C-vorozole was performed by Biegon et al.6,7. They demonstrated high levels of aromatase in the thalamus, amygdala, preoptic area, medulla, etc. 11C-Vorozole had high specificity and affinity for aromatase; however, the metabolites of 11C-vorozole were taken up into the brain with radiolabelling29. Thus, the measurements were less quantitative. Aiming for a more quantitative measurement of aromatase, we developed 11C-cetrozole as a novel PET probe for aromatase imaging29.
In the present study, we performed PET scans with 11C-cetrozole in 21 healthy human subjects. Approximately 50% of parental compound was intact 60 min after injection, indicating that this PET probe was suitable to measure aromatase in a living body (Supplemental Fig. S1). High BPNDs of 11C-cetrozole were found in the thalamus with a heterogeneous distribution among the amygdala, hypothalamus, and medulla in both sexes (Figs 1 and 2). The distribution pattern of 11C-cetrozole binding was consistent with that of 11C-vorozole7 and with immunohistochemistry in the postmortem human brain5, suggesting that considerable aromatase enzyme is expressed in the thalamus, hypothalamus, amygdala, and medulla in the human brain. Unlike other mammals that have more aromatase enzyme in male brains, such as rats and monkeys2–4, there was no distinct sex difference in aromatase levels in our human cohort, except for in the left hypothalamus (P = 0.005). However, males showed a tendency towards relatively higher aromatase expression in all brain regions than females, except for the right hypothalamus. The reason why males have more aromatase in the brain than females do is considered to compensate lower circulating estrogens since estrogen is an important hormone related to regulation of sexual behaviour and emotions, neural plasticity, neuroprotection, etc. in the brain30–32. In the present study we showed that BPND of 11C-cetrozole varied between individuals, especially in the thalamic subregions. This variability was associated with personality trait variability.
Both females and males showed a negative association between BPND of 11C-cetrozole and cooperative scores in the thalamus region in this study. The subregions that associated with cooperativeness were localized to the ventral lateral and ventral posterior parts of the thalamus. The ventral lateral nucleus of the thalamus contains estrogen receptor β and is known to project to the primary motor cortex33. Diffusion-weighted imaging studies have segmented the thalamus on a connectivity basis and reported individual variations in segmentation34. Johansen-Berg et al.34 reported that the ventral lateral nucleus primarily connected to the prefrontal cortex in some subjects and to the primary motor cortex in others. This individual variation may provide cause of different personality. High aromatase in the thalamus is unique to humans, while monkeys, baboons, and rats have high amount of aromatase in the amygdala and hypothalamus3,4,7,29,35,36. The characteristically high social abilities of humans such as cooperativeness may be processed in the thalamus through the regulation of estrogens.
In addition to cooperativeness and the thalamus, the traits and associated regions were different between sexes (Table 1). In females, sex-specific associations were observed in the amygdala and supraoptic nucleus of hypothalamus and in the inferior parietal gyrus. On the other hand, males-specific associations were observed in the anterior cingulate gyrus, supramarginal gyrus, caudate nucleus, pons, and midbrain.
The right SON and the right amygdala showed associations between BPNDs of 11C-cetrozole and female traits (Fig. 5). The localization of aromatase in the SON was demonstrated previously via immunohistochemistry13. Further, the SON is known to be a region in which oxytocin is synthesized37. Oxytocin production is regulated by estradiol38. Regulation of oxytocin by estradiol may affect personality traits given that oxytocin is implicated in stress susceptibility, emotion, memory, and social interaction39. The traits that associated with BPND in the SON were novelty seeking (negative), harm avoidance (positive), reward dependence (negative), persistence (negative), cooperativeness (positive), and self-transcendence (positive; Table 2). The traits associated with BPND in the right amygdala were novelty seeking (negative), persistence (negative), cooperativeness (positive), and self-transcendence (positive; Table 3). These 4 traits are consistent with the traits that associated with BPND in the SON, which suggests functional connectivity between the right amygdala and SON in females.
Since animal and human studies suggested that the amygdala is involved in aggression18–23, the VOI was drawn on the amygdala, and a regression analysis was performed between BPND of 11C-cetrozole in the amygdala and aggression score. There was a positive association between aggression scores and BPND in the left amygdala in females. Earlier studies found a relationship between aggression and reactivity in the left amygdala, although one of the studies combined female and male data21,22. Another study reported that lower amygdala volume was correlated with more aggression in healthy females23. Animal studies have demonstrated that aromatase neurons in the medial amygdala regulate male aggression and maternal aggression20. Our results show that aromatase in the female left amygdala is associated with aggression, which indicates that estrogen synthesis in the left amygdala may induce aggression in females.
Furthermore, polymorphisms in the aromatase and estrogen receptor genes have been reported to be associated with harm-avoidance traits24,40. Although we did not perform genetic analyses, gene polymorphisms or DNA methylation may affect the expression of aromatase or hormone receptors. Multi-disciplinary studies that consider gene polymorphisms, epigenetic changes, and imaging are needed in the future.
Our results showed that there may be associations between aromatase in the brain and personality traits, and some of the associations may differ between sexes, while others are likely common to both. Whether sex differences exist in the brain has long been contentious and remains controversial. Classical differences in brain structure, e.g., greater corpus callosum volume in females or enlarged cortical language regions in females, have been dismissed via meta-analysis41. However, neurochemistry suggests tendencies for females to have higher activity in serotonergic (5-HT transporter, 5-HT1A and 5-HT2A receptors), dopaminergic (dopamine transporter), and GABAergic (neurotransmitter level) systems42, which are involved in mood, emotions, and personality traits. An animal study revealed that exogenous estrogen treatments increased the mRNA and protein levels of tryptophan hydroxylase, a rate-limiting enzyme in the production of a serotonin precursor, in the raphe nucleus of spayed rhesus monkeys43. Further studies are needed to clarify the differences and commonalities between sexes.
Previously the results between monoamine oxidase (MAO) level measured with PET and mood/personality disorders were reported44,45. Our present results also show that the aromatase level in different brain sub-regions measured with PET is associated with a variety of personality traits. The dynamics of interaction between sex hormone and monoaminergic neurotransmitter systems are partly regulated by both enzyme levels. The PET studies in combination of both enzymes may be an interesting target in the future study.
There are several limitations of the present study. The associations between TCI scores and BPND did not survive a family-wise error correction, then were analysed with a significance threshold of P < 0.001, uncorrected, k ≥ 10 voxels. As for the SON and the right amygdala of females, the associations with TCI scores were discussed even though the criteria were not fulfilled (k < 10 voxels). When we could increase the number of subjects, the results might become clearer. As regards sex hormone in plasma, we measured testosterone, free testosterone, estradiol, and progesterone in both males and females, however, free testosterone in female (N = 2), estradiol in male (N = 7) were too little to be quantified. Thus we tested the association between aromatase level and free testosterone in male or estradiol in female.
Although the subjects in this study were all healthy, there were associations between aromatase levels in the brain and personality traits, which suggests that regulation of estrogens might affect personality. Further PET studies that include patients with personality disorders using other PET tracers for estrogen and androgen receptors would help clarify the association between sex hormone systems and personality traits.
Methods
Subjects
Subjects were recruited by advertisements at Osaka City University and RIKEN. Twenty-four healthy adults (11 females and 13 males) participated in the present study. Participants were excluded if they had past or current serious medical illnesses and/or organic brain diseases or if they took drugs actin on the central nervous system. All females were not taking oral contraceptives and had regular menstrual cycle. Two females (35 and 45 yrs old) and 2 males (36 and 35 yrs old) received a whole-body PET scan to measure their radiation exposure, and 10 females and 11 males (average age of 34.7 ± 6.4 and 31.7 ± 8.1 yrs, mean ± SD, respectively) received a brain PET scan. Each subject completed the validated Japanese version of BAQ25 and TCI27,28 before PET scanning. All experiments were conducted in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association (the Declaration of Helsinki) and registered to the UMIN Clinical Trials Registry (No. UMIN000006586). This study was approved by the Ethics Committee of the Kobe Institute of RIKEN and the Osaka City University Graduate School of Medicine. All participants provided written informed consent for participation in the study.
Positron emission tomography
11C-Cetrozole was synthesized at Osaka City University Hospital according to the previously published procedure29. The desired compound was dissolved in a mixture of polysorbate 80 (0.1 ml), propylene glycol (0.9 ml), and saline (9 ml). The identity and concentration of 11C-cetrozole were assessed using high-performance liquid chromatography. The specific activity of 11C-cetrozole was 81.2 ± 26.0 GBq/μmol (mean ± SD) at administration. The radiochemical purity was greater than 99.5%. The dose of 11C-cetrozole was 4.7 ± 1.0 MBq/kg bodyweight. Subjects were positioned in the PET scanner (Biograph-16, Siemens, Knoxville, TN, USA) with their heads lightly tied with bandages to minimize movement during the scans. The left and right median cubital veins were cannulated for blood sampling and radiotracer administration, respectively. Four males received cannulation in the left radial artery instead of the median cubital vein for arterial blood sampling. Before the emission scans, CT scans were performed for head positioning and attenuation correction. At the start of the emission scan, 11C-cetrozole was intravenously administered for approximately 30 sec, and the catheter line was flushed with 15–20 ml saline to prevent radiotracer retention. Serial PET scanning of the brain was performed for 60 min in the 3-dimensional dynamic mode in the following frames: 6 × 10 sec, 6 × 30 sec, 11 × 60 sec, and 15 × 180 sec. Blood samples were taken 5, 10, 20, 30, 45, and 60 min after administration of 11C-cetrozole from the venous line and at 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, 160, 170, and 180 sec, and 4, 5, 10, 20, 30, 45, and 60 min after administration from the arterial line. Venous and arterial blood samples taken after 5 min and later were used for radiometabolite analyses.
Magnetic resonance (MR) image
Fine structural whole-brain T1-weighted magnetic resonance anatomical images were acquired using the Philips Achieva 3.0 TX (Royal Philips Electronics, Eindhoven, The Netherlands) with the following parameters: Repetition time = 5.9 msec, echo time = 2.7 msec, flip angle = 12 degrees, slice gap = 0 mm, matrix size = 256, field of view = 220 mm, voxel size = 0.86 × 0.86 × 0.90 mm.
PET image processing
Brain PET images were reconstructed by Fourier rebinning and 2-dimensional filtered backprojection without additional smoothing filters. For quantitative image analyses, PMOD software (PMOD Technologies Ltd., Zurich, Switzerland) was used. VOIs were delineated in the thalamus, amygdala, hypothalamus, and medulla, which are structures known to contain a rich supply of aromatase enzyme5–7, and in the cerebellar lobules on the same individual’s MR images and transferred to PET images. Decay-corrected time-activity curves were generated for each brain region, arterial blood plasma, and parent unchanged compound, as measured by thin-layer chromatography (Supplemental Fig. S1). The time-activity curves for plasma and parent unchanged fraction were fitted to a 3-exponential model and a Hill function, respectively. The data with arterial blood sampling were analysed with a Logan plot46, and the total distribution volume (Vt) in each brain region was calculated. The data without arterial blood sampling were analysed with a Logan reference tissue model based on average k2’47 using the cerebellum as a reference. Nondisplaceable binding potential (BPND) and distribution volume ratio (DVR), which are linear functions of enzyme availability, were calculated (DVR = BPND + 1). Since no arterial blood sampling is preferable because of possible intense pain caused by arterial puncture, we compared the data with or without arterial blood sampling, that is, the data calculated by Logan plot or by Logan reference tissue model. Vt values of all examined brain regions were divided by the Vt value of the cerebellum and were compared with BPND values. Since the difference between normalized Vt values and BPND was 4 ± 1% (mean ± SD, N = 4), we decided to employ the Logan reference tissue model to analyse the remainder of the data. Then, BPND images were generated by model fitting. After co-registration of PET and MR images, whole-head structural images were normalized to the Montréal Neurological Institute (MNI) T1 image template, with the same parameters applied to the BPND images. BPND images were resampled to a voxel size of 2.0 × 2.0 × 2.0 mm using SPM8.
Quantification of aromatase in the rich regions
For measurements of the aromatase level in the brain, VOIs of the amygdala, hypothalamus, thalamus, and medulla were superimposed on BPND images. Because 11C-cetrozole binding was heterogeneously distributed in the thalamus, the thalamus was further divided into 5 subregions, namely, anterior medial, anterior lateral, central medial, central lateral, and posterior parts, consistent with a prior study48. BPND values were extracted from BPND images. The difference between sexes in each VOI was analysed by a t-test. All P-values were two-tailed, and P values less than 0.05 were considered significant. These analyses were performed with the GraphPad PRISM 5.0 software package (GraphPad Software, Inc., La Jolla, CA).
Statistical analysis of PET images
For aggression, we focused on the amygdala since earlier studies showed that this region is implicated in aggression18–23. A VOI of the amygdala was delineated using the WFU-Pickatlas SPM tool49. Using SPM8, voxel-wise analysis was performed on the amygdala of BPND images by applying the score of BAQ as a covariate. A family-wise error corrected significance threshold was set at P < 0.05 in the amygdala. The associations between scores of TCI and BPND were analysed as whole-brain analyses with a significance threshold of P < 0.001, uncorrected, k ≥ 10 voxels (=80 mm3). Voxel-wise analyses were performed on the BPND images using the general linear model in SPM8, with covariates of 7 traits of TCI. Although the criteria were not fulfilled (k < 10 voxels), 6 and 4 traits of TCI showed the associations in the identical coordinates (x = 8, y = 2, z = −14 and x = 24, y = −6, z = −20, respectively), thus these 2 regions were discussed separately.
Electronic supplementary material
Acknowledgements
We thank Ms. Yumiko Katayama and Ms. Kanako Tajima of the RIKEN Center for Life Science Technologies and Dr. Naohiro Tsuyuguchi, Mr. Takashi Yamanaga, Mr. Hideki Kawahata, and Dr. Hisako Kobata of Osaka City University for their expert technical assistance and Prof. Sanae Fukuda of Kansai University of Welfare Sciences for her support in participant recruitment. This work was supported in part by a consignment expense for molecular imaging research programs entitled “Research Base for Exploring New Drugs” from the Japanese Ministry of Education, Culture, Sports, Science and Technology; and JSPS KAKENHI Grant Numbers 22791155, 25830024. No other potential conflicts of interest relevant to this article exist.
Author Contributions
Kayo T., T.H. and Y. Watanabe designed this research and wrote the paper. K.O., T.T., M.T., A.I., Y.N., S.T. and Y. Wada performed experiments and analysed the data. Kazuhiro T., H.D. provided critical advice and discussion. All authors commented on the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35065-4.
References
- 1.Simpson ER, et al. Aromatase–a brief overview. Annu. Rev. Physiol. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 2.Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res. Bull. 1997;44:351–357. doi: 10.1016/S0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 3.Abdelgadir SE, Roselli CE, Choate JV, Resko JA. Distribution of aromatase cytochrome P450 messenger ribonucleic acid in adult rhesus monkey brains. Biol. Reprod. 1997;57:772–777. doi: 10.1095/biolreprod57.4.772. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, et al. Imaging of aromatase distribution in rat and rhesus monkey brains with [11C]vorozole. Nucl. Med. Biol. 2006;33:599–605. doi: 10.1016/j.nucmedbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Sasano H, Takashashi K, Satoh F, Nagura H, Harada N. Aromatase in the human central nervous system. Clin. Endocrinol. (Oxf). 1998;48:325–329. doi: 10.1046/j.1365-2265.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- 6.Biegon A, et al. Aromatase imaging with [N-methyl-11C]vorozole PET in healthy men and women. J. Nucl. Med. 2015;56:580–585. doi: 10.2967/jnumed.114.150383. [DOI] [PubMed] [Google Scholar]
- 7.Biegon A, et al. Unique distribution of aromatase in the human brain: in vivo studies with PET and [N-methyl-11C]vorozole. Synapse. 2010;64:801–807. doi: 10.1002/syn.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm. Behav. 2004;46:1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem. Biophys. Res. Commun. 1998;252:445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- 10.Honda S, Wakatsuki T, Harada N. Behavioral analysis of genetically modified mice indicates essential roles of neurosteroidal estrogen. Frontiers in endocrinology. 2011;2:1–8. doi: 10.3389/fendo.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato T, et al. Brain masculinization requires androgen receptor function. Proc. Natl. Acad. Sci. USA. 2004;101:1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalla C, Antoniou K, Papadopoulou-Daifoti Z, Balthazart J, Bakker J. Oestrogen-deficient female aromatase knockout (ArKO) mice exhibit depressive-like symptomatology. Eur. J. Neurosci. 2004;20:217–228. doi: 10.1111/j.1460-9568.2004.03443.x. [DOI] [PubMed] [Google Scholar]
- 13.Ishunina TA, et al. Diminished aromatase immunoreactivity in the hypothalamus, but not in the basal forebrain nuclei in Alzheimer’s disease. Neurobiol. Aging. 2005;26:173–194. doi: 10.1016/j.neurobiolaging.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Wu JL, et al. Aromatase changes in depression: A postmortem and animal experimental study. Psychoneuroendocrinology. 2017;77:56–62. doi: 10.1016/j.psyneuen.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Sarachana T, Xu M, Wu RC, Hu VW. Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PloS one. 2011;6:e17116. doi: 10.1371/journal.pone.0017116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crider A, Thakkar R, Ahmed AO, Pillai A. Dysregulation of estrogen receptor beta (ERbeta), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Molecular autism. 2014;5:46. doi: 10.1186/2040-2392-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: how interactions between aromatase and the environment modulate aggression. Front. Neuroendocrinol. 2006;27:170–179. doi: 10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halasz J, Liposits Z, Meelis W, Kruk MR, Haller J. Hypothalamic attack area-mediated activation of the forebrain in aggression. Neuroreport. 2002;13:1267–1270. doi: 10.1097/00001756-200207190-00010. [DOI] [PubMed] [Google Scholar]
- 19.Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-K. [DOI] [PubMed] [Google Scholar]
- 20.Unger EK, et al. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell reports. 2015;10:453–462. doi: 10.1016/j.celrep.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaver JD, Lawrence AD, Passamonti L, Calder AJ. Appetitive motivation predicts the neural response to facial signals of aggression. J. Neurosci. 2008;28:2719–2725. doi: 10.1523/JNEUROSCI.0033-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol. Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Matthies S, et al. Small amygdala-high aggression? The role of the amygdala in modulating aggression in healthy subjects. The world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry. 2012;13:75–81. doi: 10.3109/15622975.2010.541282. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto Y, et al. Effect of the cytochrome P450 19 (aromatase) gene polymorphism on personality traits in healthy subjects. Behav. Brain Res. 2009;205:234–237. doi: 10.1016/j.bbr.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 25.Ando A, et al. Development of the Japanese version of the Buss-Perry Aggression Questionnaire (BAQ) Shinrigaku Kenkyu. 1999;70:384–392. doi: 10.4992/jjpsy.70.384. [DOI] [PubMed] [Google Scholar]
- 26.Buss AH, Perry M. The aggression questionnaire. J. Pers. Soc. Psychol. 1992;63:452–459. doi: 10.1037/0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 27.Kijima N, Tanaka E, Suzuki N, Higuchi H, Kitamura T. Reliability and validity of the Japanese version of the Temperament and Character Inventory. Psychol. Rep. 2000;86:1050–1058. doi: 10.2466/pr0.2000.86.3.1050. [DOI] [PubMed] [Google Scholar]
- 28.Cloninger, C. R., Svrakic, D. M., Przybeck, T. R. & Wetzel, R. D. The Temperament and Character Inventory (TCI): a guide to its development and use. (ed. Cloninger, C. R.)(Center for Psychobiology of Personality, Washington University, St. Louis, Missouri 1994).
- 29.Takahashi K, et al. 11C-Cetrozole: An Improved C-11C-Methylated PET Probe for Aromatase Imaging in the Brain. J. Nucl. Med. 2014;55:852–857. doi: 10.2967/jnumed.113.131474. [DOI] [PubMed] [Google Scholar]
- 30.Azcoitia I, Arevalo MA, Garcia-Segura LM. Neural-derived estradiol regulates brain plasticity. J. Chem. Neuroanat. 2018;89:53–59. doi: 10.1016/j.jchemneu.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Azcoitia I, et al. Brain aromatase is neuroprotective. J. Neurobiol. 2001;47:318–329. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- 32.Blakemore J, Naftolin F. Aromatase: Contributions to Physiology and Disease in Women and Men. Physiology (Bethesda) 2016;31:258–269. doi: 10.1152/physiol.00054.2015. [DOI] [PubMed] [Google Scholar]
- 33.Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann. N. Y. Acad. Sci. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- 34.Johansen-Berg H, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb. Cortex. 2005;15:31–39. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- 35.Lephart ED. A review of brain aromatase cytochrome P450. Brain research reviews. 1996;22:1–26. doi: 10.1016/0165-0173(96)00002-1. [DOI] [PubMed] [Google Scholar]
- 36.Biegon A, et al. Nicotine blocks brain estrogen synthase (aromatase): in vivo positron emission tomography studies in female baboons. Biol. Psychiatry. 2010;67:774–777. doi: 10.1016/j.biopsych.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onaka T. Neural pathways controlling central and peripheral oxytocin release during stress. J. Neuroendocrinol. 2004;16:308–312. doi: 10.1111/j.0953-8194.2004.01186.x. [DOI] [PubMed] [Google Scholar]
- 38.Acevedo-Rodriguez A, Mani SK, Handa RJ. Oxytocin and Estrogen Receptor beta in the Brain: An Overview. Frontiers in endocrinology. 2015;6:160. doi: 10.3389/fendo.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature reviews. Neuroscience. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 40.Gade-Andavolu R, et al. Association between the estrogen receptor TA polymorphism and Harm avoidance. Neurosci. Lett. 2009;467:155–158. doi: 10.1016/j.neulet.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 41.Eliot L. The trouble with sex differences. Neuron. 2011;72:895–898. doi: 10.1016/j.neuron.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front. Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- 44.Kolla NJ, Vinette SA. Monoamine Oxidase A in Antisocial Personality Disorder and Borderline Personality Disorder. Curr Behav Neurosci Rep. 2017;4:41–48. doi: 10.1007/s40473-017-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer J. Novel Phenotypes Detectable with PET in Mood Disorders: Elevated Monoamine Oxidase A and Translocator Protein Level. PET Clin. 2017;12:361–371. doi: 10.1016/j.cpet.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Logan J, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J. Cereb. Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 47.Logan J, et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J. Cereb. Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Yasuno F, et al. Low dopamine d(2) receptor binding in subregions of the thalamus in schizophrenia. Am. J. Psychiatry. 2004;161:1016–1022. doi: 10.1176/appi.ajp.161.6.1016. [DOI] [PubMed] [Google Scholar]
- 49.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.