Abstract
Consumption of coffee, tea and alcohol might be shaped by individual differences in bitter taste perception but inconsistent observational findings provide little insight regarding causality. We conducted Mendelian randomization analyses using genetic variants associated with the perception of bitter substances (rs1726866 for propylthiouracil [PROP], rs10772420 for quinine and rs2597979 for caffeine) to evaluate the intake of coffee, tea and alcohol among up to 438,870 UK Biobank participants. A standard deviation (SD) higher in genetically predicted bitterness of caffeine was associated with increased coffee intake (0.146 [95%CI: 0.103, 0.189] cups/day), whereas a SD higher in those of PROP and quinine was associated with decreased coffee intake (−0.021 [−0.031, −0.011] and −0.081 [−0.108, −0.054] cups/day respectively). Higher caffeine perception was also associated with increased risk of being a heavy (>4 cups/day) coffee drinker (OR 1.207 [1.126, 1.294]). Opposite pattern of associations was observed for tea possibly due to the inverse relationship between both beverages. Alcohol intake was only negatively associated with PROP perception (−0.141 [−1.88, −0.94] times/month per SD increase in PROP bitterness). Our results reveal that bitter perception is causally associated with intake of coffee, tea and alcohol, suggesting a role of bitter taste in the development of bitter beverage consumption.
Introduction
Coffee, tea and alcohol are widely consumed beverages with bitter taste1 and have been implicated in both beneficial and adverse health effects2,3. Individual differences in metabolizing caffeine4,5 and ethanol6–10 present in these beverages determine their consumption level, whereas the influence of taste factors remains unclear.
The relationship between the perception of bitter compounds, such as propylthiouracil (PROP), quinine, and caffeine, and the consumption level of these bitter beverages have been inconsistent across observational studies11–22. As performing taste tests is a resource-intensive process, these investigations likely lacked sufficient power to convincingly rule out moderate effects. Potential confounders or illnesses that both affect taste perception and consumption in these studies limits their ability to provide unbiased causal effects. Fortunately, these issues can be overcome by recent advances in taste genetics and statistical methodology.
Heritability for the perceived intensity of PROP, quinine, and caffeine have been estimated to be 0.73, 0.40, and 0.36 in classical twin studies23,24. Furthermore, candidate gene and genome-wide association studies (GWAS) have pinpointed precise genetic factors25–27, including SNPs within the bitter taste receptor gene TAS2R38 for PROP and SNPs within the cluster of bitter taste receptors genes on chromosome 12 for quinine and caffeine. Identification of these genetic variants provides an opportunity to apply Mendelian randomization (MR), a technique commonly used in disease epidemiology, to test the causal relationship between bitter taste and an outcome of interest using genetic variants (e.g. SNPs) as instrumental variables (IVs). The fundamental principle of MR is based on Mendel’s Law of Independent Assortment whereby genetic variants are shuffled at meiosis, conceptually mimicking a “natural” randomized trial28–30. Since genetic variants are randomized at conception, they can be used as risk factor instrumental variables that are generally free from confounding and reverse causality to evaluate the relationship of a risk factor on a specific outcome of interest31,32.
In this study, we investigate the causal relationship between bitter taste perception and the consumption of coffee, tea and alcohol through a MR framework. We use confirmed genetic markers for the perception of PROP (rs1726866), quinine (rs10772420) and caffeine (rs2597979) separately as genetic proxies for bitter taste perception and test their associations with the consumption of coffee, tea and alcohol among more than 400,000 participants in the UK Biobank cohort33.
Methods
Study design
We employed a two-sample Mendelian randomization design30, wherein the IV-taste perception association (ratio denominator) and IV-consumption association (ratio numerator) were obtained from two independent sample sets. The SNP-taste perception estimates were derived from a GWAS of taste perception27 described below (sample 1), while the SNP-beverage consumption association was examined in the UK Biobank (sample 2)33.
Genetic instruments for bitter taste (sample 1)
Genetic instruments for perceived intensity of PROP, quinine and caffeine were obtained from the largest GWAS of these traits to-date27, which included 1757 twins and their siblings (aged 12–25; 54% females; all European ancestry) from a subset of the Brisbane Adolescent Twin study34. All three taste-associated SNPs were genome-wide significant (rs1726866 in TAS2R38 on chromosome 7 accounted for approximately 46% variance in PROP (P = 5.6e-198); rs10772420 and rs2597979 in the cluster of bitter taste receptor genes on chromosome 12 respectively accounted for 6% of the variance in quinine (P = 7.8e-23) and 2% of the variance in caffeine (P = 4.2e-8)) and replicated findings from previous smaller GWAS25,26,35. The corresponding genetic instrument F-statistics were 901.1 (PROP), 96.8 (Quinine) and 30.0 (Caffeine) respectively, indicating all of the SNPs used were strong instruments (Supplementary Table 1). In the GWAS, taste intensity ratings were square root transformed and then converted into standard deviation units. Here, we assigned the effect allele (EA) to represent the allele associated with higher intensity ratings on bitterness. The EA for rs1726866 (G allele), rs10772420 (A allele), and rs2597979 (G allele) increased the intensity rating of PROP, quinine, and caffeine by 0.965, 0.337, and 0.264 standard deviations (SD), respectively.
UK Biobank (sample 2)
The UK Biobank recruited 502,650 participants (5% of those invited) aged 37–73 years at 21 centres across England, Wales and Scotland in 2006–201033. Written informed consent was obtained from participants and the study has been approved by the UK National Health Service National Research Ethics Service. Full details of participant recruitment and data collection have been previously published36 as have details of the genome-wide data collection and its quality control33.
UK Biobank Genetic data
All UK Biobank participants have been genotyped using the Affymetrix UK BiLEVE Axiom array or Affymetrix UK Biobank Axiom® array comprising 805,426 markers in the official release. Imputations were performed using IMPUTE2 and UK10K haplotype and Haplotype Reference Consortium (HRC) reference panels, as described elsewhere33. For the current analysis, the three SNPs of interest were genotyped (rs1726866, rs10772420) or imputed (rs2597979) with high quality (INFO score >0.99) based on the HRC reference panel samples. Our present analyses focused on the population of white-British ancestry, as determined by similarity of ancestral principal component values (PC1, PC2) to those who self-reported and were classified as white-British37. Using this criterion, we identified 438,870 individuals of white-British ancestry.
UK Biobank Coffee, tea and alcohol consumption
Self-reported data on tea, coffee and alcohol consumption were collected using the touch-screen questionnaire completed by UK Biobank participants at the assessment centre. Amounts of coffee and tea consumption were quantified separately e.g. “How many cups of coffee do you drink each day? (including decaffeinated coffee)” and “How many cups of tea do you drink each day? (including black and green tea)”. For alcohol, participants were asked “About how often do you drink alcohol?” and to report on their drinking behaviour on a 6-point frequency scale (Never, Special occasions only, 1–3 times a month, 1–2 times a week, 3-4 times a week, Daily or almost daily). Participants who preferred not to answer were excluded from the analyses. We converted the alcohol intake scores into monthly frequencies (frequency of alcohol consumption per month) from the 6-point frequency scale to 0, 1, 2, 6, 15, 30 times per month. For participants completing the assessment at multiple times, we computed their average consumption.
To assess risks of heavy-consumption, the cut-off for light/non-drinkers and heavy drinkers are set at 20th and 80th percentile of the consumption distribution. For coffee, light/non-drinkers were classified as participants with <2 cups per day and heavy drinkers as >4 cups per day of coffee intake. The cut-offs were <2 cups/day and >5 cups/day for tea-drinking. For alcohol, non-drinkers were defined as individuals reporting no consumption of alcohol, while heavy-drinkers were individuals who consume more frequently than 3-4 times weekly.
Statistical Analysis
Association between each bitter-taste SNP and bitter beverage consumption were estimated through linear and logistic additive models using BOLT-LMM38 (linear and logistic) and again with plink239 (logistic). Covariates included age, sex and the first 10 principal components (PC). The use of BOLT-LMM allows the use of a linear mixed model to adjust for cryptic relatedness, hence maximizing power as related individuals can be retained in the analyses. We reported estimates derived from BOLT-LMM for the main analyses. For binary traits, such as drinker status, we additionally compute the SNP-associations using software plink239 after excluding related individuals (n ~ 36,000), with the results retained as sensitivity analyses.
The MR estimate of the effect of bitter taste perception on consumption was calculated using the wald-type ratio estimator28,40. The causal magnitude of association is estimated through , where βbev refers to the magnitude of association on quantity of bitter beverages for each perception-increasing allele and βbitter refers to the magnitude of association for per-SD change in square-rooted perception score for each perception-increasing allele. For the analyses of logistic traits (e.g. non- vs heavy-drinkers), the equivalent causal odds ratio were estimated using log where log ORdrinkstatus refers to the (log) increase in heavy-drinking risk per perception-decreasing allele. The standard errors of our MR estimates were derived using the delta method41. Estimates hence reflect the change in consumption quantity per SD change in bitter perception score in PROP, quinine and caffeine. All statistical analyses were performed using in-house scripts written in the statistical package R. As we investigated 3 different bitter stimuli against 3 different beverages of interest in our analyses, we conservatively set our Bonferroni-corrected P-value to be 0.00556 (0.05/9).
We further assessed evidence for SNP associations with potential confounders through a look-up effort on publicly available GWAS summary results. Databases such as the GENEATLAS UK Biobank GWAS repository42 and the PhenoScanner web interface43 were used. Here potential confounders are selected based on prior evidence of association with coffee intake or traits which might potentially associate coffee intake in a reverse causality fashion. These include smoking behaviour, socio-economic status, BMI, diet, sleep pattern and gastrointestinal disorders.
Disclaimer
All authors did not attempt to de-identify participants from the UKB across the use of two UKB application data (performed separately).
Results
Table 1 summarizes the baseline characteristics including self-reported measurements of bitter beverage consumption among 438,870 individuals that were included in our analyses. The genotype frequencies for each of the bitter SNPs are shown in Supplementary Table 2. Power calculation for our MR analyses reveal that we had very good power to detect even very small effect sizes (Supplementary Table 3).
Table 1.
Baseline characteristics of 438,870 individuals from the UK Biobank cohort included in the analyses.
| Variable | Statistics | Value based on 438, 870 participants |
|---|---|---|
| Age | Mean (SD) | 56.5 (8.1) |
| Sex | N of males (%) | 223,040 (45.8%) |
| Height (cm) | Mean (SD) | 168.5 (9.3) |
| BMI | Mean (SD) | 27.42 (4.8) |
| Smoking | N (%) | |
| Ever smoked | 194,764 (40.2%) | |
| Self-reported alcohol drinking frequency | N (%) | |
| Never | 39,434 (8.1%) | |
| Special occasions only | 55,197 (11.4%) | |
| 1–3 times/month | 56,299 (11.6%) | |
| 1–2 times/ week | 123,195 (25.3%) | |
| 3–4 times/week | 115,327 (23.7%) | |
| Daily or almost daily | 96,870 (19.9%) | |
| Coffee consumption | Mean (SD) | |
| Coffee (cups per day) | 2.13 (2.1) | |
| Tea consumption | Mean (SD) | |
| Tea (cups per day) | 3.51 (2.9) |
BMI is the body mass index. N refers to number (Sample size). SD refers to standard deviation.
Mendelian randomization estimates of bitter perception on coffee and tea consumption
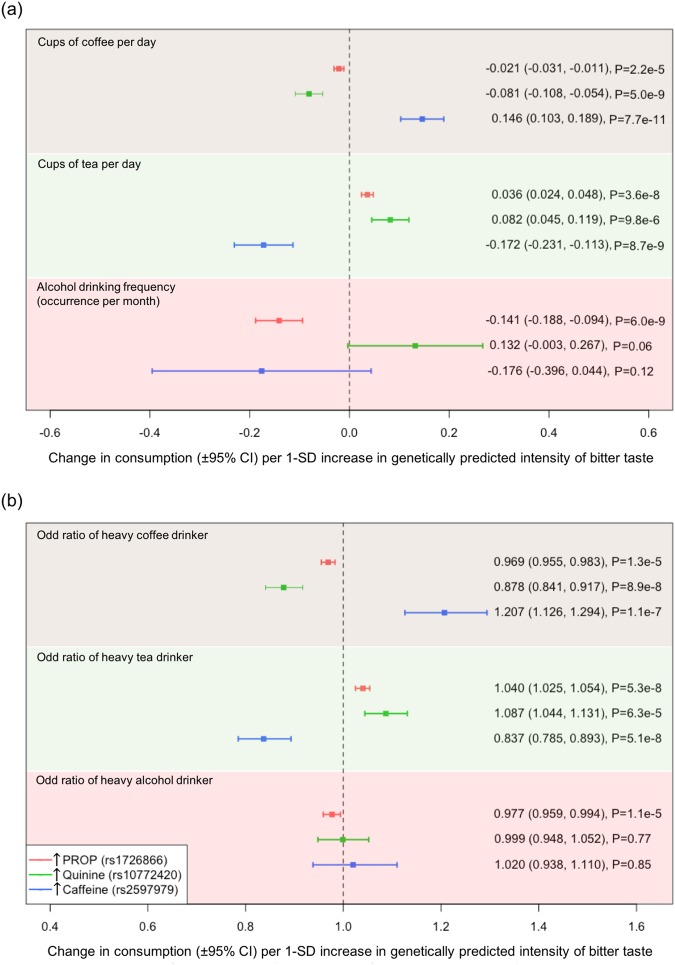
Power calculation for our MR analyses indicated that we had very good power to detect even very small effect sizes on changes in consumption due to differences in bitter perception. Associations between SNPs associated with perception of bitterness and the consumption of bitter beverages are summarized in Supplementary Tables 4 (linear) and 5 (logistic). The approximation of SNP-beverage estimates derived from BOLT-LMM to log(OR) values can be found in Supplementary Table 5. Mendelian randomization causal effect estimates of tea/coffee/alcohol consumption per SD increase in perceived bitterness are shown in Fig. 1a. We identified robust associations between genetically predicted higher perception for each bitter taste and coffee consumption (P < 1e-4); however the effect sizes were relatively weak. The magnitude of association for higher caffeine perception (rs2597979) is the largest (beta = 0.039; 95% CI: 0.027,0.050) and was consistently in the opposite direction compared to perception of PROP (rs1726866) and quinine (rs10772420). Higher perception of caffeine is causally associated with increased risk of being a heavy coffee drinker (Causal OR = 1.207; 95% CI: 1.126, 1.294), see Fig. 1b), with similar evidence for association with higher cups per day, but higher perception of PROP and quinine results in lower coffee consumption.
Figure 1.
Causal effects of perceived bitterness of PROP, quinine, and caffeine on (a) the intake of coffee, tea, and alcohol and (b) the odds ratio of being a heavy coffee, tea, and alcohol drinker. The bar around the box represents the 95% confidence intervals around the point estimate.
Genetically predicted higher PROP and quinine intensity was associated with increased tea consumption, with a change of 0.034 (95% CI: 0.022,0.047) and 0.028 (95% CI: 0.015, 0.040) cups per day per SD increase in perception score. In contrast, higher caffeine perception leads to a decrease in tea consumption (beta = −0.045, 95% CI: −0.061, −0.030). Similar patterns of association were observed when comparing non-tea drinkers against heavy tea drinkers (see Fig. 1b).
Conditional Mendelian randomization analyses to address inverse correlation between coffee and tea
Given the opposite pattern of results for coffee and tea, we investigated whether this was driven by the negative correlation between coffee and tea intake in the UK Biobank (r = −0.3). We performed conditional analyses by adjusting for cups per day of tea consumption when estimating the MR causal estimates on bitter perception of coffee, and vice versa. Our conditional analyses (Supplementary Table 6) revealed that the magnitude of association between predicted quinine and caffeine perception with coffee intake were stronger after conditioning for tea intake, as well as the association between predicted PROP and caffeine perception and tea intake after adjusting for coffee intake, although there is limited evidence for statistical heterogeneity between these and the original estimates.
When stratified by non-drinker status for tea (or coffee) for the association between bitter taste and coffee (or tea) (Supplementary Tables 7 and 8), the direction of effect was largely consistent with the unstratified model - with larger standard errors due to a loss of effective sample size. However, the magnitude of positive (negative) association for caffeine perception on coffee (tea) among non-tea (coffee) drinkers was larger than the unstratified model (e.g. beta_coffee among non-tea drinkers = 0.124; as compared to 0.039 from the original model), suggesting a potential beverage preference based on caffeine perception. Complete results for the conditional and stratified analyses can be found in Supplementary Tables 6–8.
Mendelian randomization estimates for alcohol intake
The MR analysis of bitter taste perception on alcohol varied by stimuli (Fig. 1). For PROP, increased predicted intensity was associated with lower alcohol consumption (beta = −0.136 times/month (95% CI: −0.182, −0.090) for every SD increase in PROP intensity). Our MR analyses revealed weak evidence that genetically predicted increased quinine perception is associated with higher alcohol consumption as the confidence interval overlaps zero. Genetically predicted caffeine perception was weakly associated with alcohol consumption, with wide confidence intervals that included the null value (beta = −0.047, 95% CI: −0.105, 0.011). For drinker status, direction of association was largely consistent (Fig. 1b), but none of the associations between predicted bitter taste and drinking status were statistically significant at the conventional p-value of 0.05 after correcting for multiple testing. Through the stratified MR analyses by types of alcohol, we further showed the association between PROP and alcohol intake is mainly driven by red wine (Supplementary Table 9).
Investigating sex differences on the causal association between bitter taste and bitter beverage consumption
In light of previously suggested interaction of sex on the association between bitter perception and these beverages14,16,19, we attempted to evaluate whether these differences were observed in our data, by performing our MR analyses separately for each sex (Supplementary Table 10). We found no consistent evidence to support a sex difference, except for the association between caffeine perception and tea intake (p = 0.007) which appeared to be much stronger in females (for every SD increase in caffeine perception score, beta = −0.261 cups per day (95% CI: −0.341, −0.182) in females and beta = −0.091 cups per day (95% CI: −0.184, 0.004) in males).
Discussion
We investigated the effect of perception of different bitter compounds on tea, coffee and alcohol consumption in a large population-based cohort (UK Biobank) using a MR approach. Our findings showed that a higher perceived intensity of PROP and quinine decreased coffee consumption and that a higher perceived intensity of caffeine increased coffee consumption; opposite relationships were observed for tea consumption. For alcohol, a higher predicted intensity of PROP resulted in lower consumption but the perception of quinine and caffeine had no clear influence.
Increased predicted perceived intensity of PROP and quinine leads to a lower coffee intake and a lower risk of being a heavy coffee drinker. The direction of association could be attributed to the inborn aversion towards bitterness, which is a defensive mechanism that prevents ingestion of poisonous food44. Previous studies investigating the effect of PROP taster status on coffee consumption have reported null findings12,13,17,18,22 (except for one study of Polish elderly women (n = 118) showed that tasters consume coffee more frequently)16, but with sample sizes between 8 and 3328, these studies may have been underpowered. In the current analysis of over 438,870 cohort participants, the TAS2R38 genotype corresponding to increased perceived PROP intensity was associated with a lower consumption of coffee. The effect size is notably small, thus warranting a large sample size to detect. For the association with the perception of quinine, our finding can be supported by an earlier report that coffee drinkers tend to be less sensitive to quinine15. The effect of quinine on coffee is stronger than that of PROP, presumably because the quinine naturally occurs in coffee45 whereas PROP does not.
While the perception of caffeine demonstrated the largest magnitude of association, its direction of effect was opposite to the other two bitter substances PROP and quinine, i.e. an increased predicted perceived intensity of caffeine leads to a higher intake of coffee. Caffeine is a non-volatile component of coffee and it contributes to the perceived strength, body and bitterness of coffee46, which has been related to its intake11,15. It is possible that coffee consumers acquire a taste for (or an ability to detect) caffeine given the learned positive reinforcement (i.e. stimulation) elicited by caffeine.
For the relationship between bitter taste perception and tea intake, we showed that a higher predicted perceived intensity of PROP and quinine increased tea intake and a higher predicted perceived intensity of caffeine decreased intake. These effects of bitter taste on tea consumption were in the opposite direction compared with those on coffee consumption, consistent with the negative correlation between coffee and tea intake in the UK Biobank cohort. A negative relationship of similar magnitude has also been reported in an Australian population (N = 3908)47. Nevertheless, our sensitivity analyses, which used the IV estimate on tea conditional on coffee (and vice versa), showed the same direction of association and revealed a robust association between tea intake and the perception of PROP and caffeine (and between coffee intake and the perception of quinine and caffeine).
For alcohol, our MR results indicated that increased predicted perceived intensity of PROP led to a lower intake of alcohol. It has been reported that alcohol elicits bitterness in humans48,49 and such bitter sensation can mediate consumption behaviour50. Findings from this study could be supported by previous observations that a higher perceived intensity of PROP is associated with a stronger perceived intensity of alcohol and a lower intake14,19,50,51. While inconsistencies have been reported between sexes14,16,19, here we found no strong evidence of a difference between males and females in the large UK Biobank cohort.
The strengths of our study include the use of a two-sample approach in MR to avoid biased estimates40. Given that each of these SNPs are functionally related to taste biology, each of the SNP used were strong instruments backed with statistical evidence as well as biological plausibility. Taste perception can change overtime, whereas MR analyses evaluate the impact of average differences in taste perception across the life course on bitter beverage consumption. Thus, if there were a sensitive period for the impact (e.g. if bitter taste perception in early childhood determined drinking habits that persisted into later life) our results would be an underestimate of that effect. Additionally, the use of per SD change in taste perception allows us to assess the effect of changing from perception scale of strong/moderate to moderate/weak, without worrying about within-individual variation in taste perception as observed in earlier test-retest validations52. The use of a mixed model approach also allows us to leverage the relatedness in the UKB cohort to gain higher statistical power, resulting in slightly narrower confidence intervals than conventional regression models that exclude related individuals (Supplementary Table 11). Although our choice of a cut-off for high coffee and tea drinkers is arbitrary (based on a percentile), adopting alternative cut-offs made no meaningful difference to the results (Supplementary Table 12).
A major limitation for our study is the relatively small sample size (n = 1757) used to estimate the SNP-bitter taste associations. However, this limitation is unlikely to have substantially biased our finding as all of the SNPs used in this study have been replicated in other independent GWAS26,35. As our experiments only consist of one genetic instrument per trait, we were unable to perform sensitivity analyses that explore the validity of IVs through multiple SNPs-based approaches (e.g. MR-Egger40 and weighted median53 analyses) to evaluate potential bias due to horizontal pleiotropy and therefore we could not exclude the possibility that the SNPs are associated with bitter beverage intake via independent pathways. However, given the functional role of these SNPs in bitter taste receptor genes27 and the evidence showing the association of the expression of bitter taste receptor (i.e. TAS2R38) with bitter taste perception and intake of caffeine54, these SNPs are more likely to act on the intake of bitter beverage via taste perception. Applying multiple instruments for each trait can potentially allow better assessment of pleiotropy through the use of statistical models, although this will unlikely translate to better precision on our causal estimates given our individual SNP instruments already explain reasonably high amount of variance on bitter tastes. Additionally, our pleiotropic tests using publicly available GWAS summary results did not show evidence of effect of these SNPs on potential confounders (Supplementary Tables 13 and 14). Nevertheless, further research is still required to validate the causal effects detected in the present study.
The response amongst those invited to UK Biobank was ~5% and participants differ considerably from the underlying population with respect to socioeconomic position, health and survival55. This could potentially introduce some level of selection bias influencing our MR associations56. For example, if non-responsiveness is related to genetic predictors of a number of mental health and lifestyle/behavioural outcomes which happen to also confound drinking behaviour57. However, sensitivity analyses (Supplementary Table 13) evaluating the association between these bitter taste SNPs and proxies of socio-economic status (i.e. UK Biobank townsend deprivation index and number of vehicle in household) found no evidence of association. It is possible that behavioural and disease traits correlated to coffee consumption (e.g. diet behaviour, smoking, anorexia, and insomnia) influence bitter taste perception. But using publicly available GWAS summary data42,43 we found no strong evidence for this (Supplementary Table 14).
The use of the wald-type estimator to evaluate the causal effect has an intrinsic assumption that the exposure-outcome relationship is linear. For behavioural traits like coffee consumption, this assumption can be violated when we assess the effect of bitter taste on individuals at the extreme end of the trait distribution (i.e. non-tasters, or extremely sensitive tasters). We examined associations both as continuous and binary traits, in general the inference drawn for the continuous measure (e.g. cups per day) was similar to those estimated on drinker status.
Finally, beverage consumption data in the UK Biobank were based on self-reporting which will inevitably contain recall bias and measurement error, but these are unlikely to have inflated Type I error rates in MR analyses. Our findings were based on the intake of black and green tea, and the effects may differ for other tea types with different taste profiles. It is also important to note that our findings do not necessarily generalise to non-European populations.
Overall, our findings demonstrate that differences in bitter taste perception are causally associated with specific bitter beverage consumption behaviour. The magnitudes of causal effects that we see for coffee and tea are small (i.e. 0.02 to 0.15 cups per day for a SD greater bitter taste perception). Given the popularity of these bitter beverages at a population level their consumption could have significant impact on health outcomes, but this requires further investigation.
Electronic supplementary material
Acknowledgements
The authors thank Danielle Reed from the Monell Chemical Senses Center for providing critical feedback on the manuscript. This work is funded by National Institute on Deafness and Other Communication Disorders grant R03DC01337301A1 to MCC. This research has been conducted using the UK Biobank Resource (application numbers 14829, 25331, 21394). D.A.L. works in a Unit that receives funding from the University of Bristol and UK Medical Research Council (MC_UU_12013/5); she is a UK National Institute of Health Research Senior Investigator (NF-SI-0611-10196). LDH is supported by an NHMRC project grant GNT1125200. V.W.Z. is supported by a postdoctoral fellowship from the American Heart Association Strategically Focused Research Networks (14SFRN20480260). SM acknowledges Australian NHMRC project grant APP1123248 and an ARC Future Fellowship. This work was also supported by the National Institute of Health [DC02995 to PASB], the Australian National Health and Medical Research Council [241944 and 1031119 to NGM], and the Australian Research Council [DP1093900 and DP0664638 to NGM and MJW].
Author Contributions
J.S.O., L.D.H. and M.C.C. designed the study. J.S.O., L.D.H. and M.C.C. wrote the first draft of the manuscript. M.C.C. and S.M. obtained funding. Preliminary analyses using Phase 1 UKB data (App ID 14829) were performed by V.W.Z. and M.C. Complete analyses were performed using Phase 2 UKB release (App ID 25331) by J.S.O., L.D.H. and J.A. V.W.Z., J.W., D.L. and N.G.M. evaluated and provided critical feedback on methodology. J.S.O., L.D.H., P.G., P.A.S.B., M.J.W., J.A., V.W.Z., D.L., J.W., N.G.M., S.M. and M.C.C. contributed to the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jue-Sheng Ong and Daniel Liang-Dar Hwang contributed equally.
Contributor Information
Jue-Sheng Ong, Email: juesheng.ong@qimrberghofer.edu.au.
Daniel Liang-Dar Hwang, Email: d.hwang@uq.edu.au.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34713-z.
References
- 1.Cornelis MC, Tordoff MG, El-Sohemy A, van Dam RM. Recalled taste intensity, liking and habitual intake of commonly consumed foods. Appetite. 2017;109:182–189. doi: 10.1016/j.appet.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee Y, Son J, Jang J, Park K. Coffee and metabolic syndrome: A systematic review and meta-analysis. Journal of Nutrition and Health. 2016;49:213–222. doi: 10.4163/jnh.2016.49.4.213. [DOI] [Google Scholar]
- 3.Grosso G, Godos J, Galvano F, Giovannucci EL. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017;37:131–156. doi: 10.1146/annurev-nutr-071816-064941. [DOI] [PubMed] [Google Scholar]
- 4.Coffee and Caffeine Genetics Consortium et al. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol. Psychiatry20, 647–656 (2015). [DOI] [PMC free article] [PubMed]
- 5.Masi C, Dinnella C, Pirastu N, Prescott J, Monteleone E. Caffeine metabolism rate influences coffee perception, preferences and intake. Food Qual. Prefer. 2016;53:97–104. doi: 10.1016/j.foodqual.2016.06.002. [DOI] [Google Scholar]
- 6.Bosron WF, Li TK. Genetic polymorphism of human liver alcohol and aldehyde dehydrogenases, and their relationship to alcohol metabolism and alcoholism. Hepatology. 1986;6:502–510. doi: 10.1002/hep.1840060330. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi S, Matsushita S, Muramatsu T, Murayama M, Hayashida M. Alcohol and aldehyde dehydrogenase genotypes and drinking behavior in Japanese. Alcohol. Clin. Exp. Res. 1996;20:493–497. doi: 10.1111/j.1530-0277.1996.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 8.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res. Health. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
- 9.Bierut LJ, et al. ADH1B is associated with alcohol dependence and alcohol consumption in populations of European and African ancestry. Mol. Psychiatry. 2012;17:445–450. doi: 10.1038/mp.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawlor DA, et al. Exploring causal associations between alcohol and coronary heart disease risk factors: findings from a Mendelian randomization study in the Copenhagen General Population Study. Eur. Heart J. 2013;34:2519–2528. doi: 10.1093/eurheartj/eht081. [DOI] [PubMed] [Google Scholar]
- 11.Lipchock SV, et al. Caffeine Bitterness is Related to Daily Caffeine Intake and Bitter Receptor mRNA Abundance in Human Taste Tissue. Perception. 2017;46:245–256. doi: 10.1177/0301006616686098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ly A, Drewnowski A. PROP (6-n-Propylthiouracil) tasting and sensory responses to caffeine,sucrose, neohesperidin dihydrochalcone and chocolate. Chem. Senses. 2001;26:41–47. doi: 10.1093/chemse/26.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Masi C, Dinnella C, Monteleone E, Prescott J. The impact of individual variations in taste sensitivity on coffee perceptions and preferences. Physiol. Behav. 2015;138:219–226. doi: 10.1016/j.physbeh.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Wang JC, et al. Functional variants in TAS2R38 and TAS2R16 influence alcohol consumption in high-risk families of African-American origin. Alcohol. Clin. Exp. Res. 2007;31:209–215. doi: 10.1111/j.1530-0277.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- 15.Tanimura S, Mattes RD. Relationships between bitter taste sensitivity and consumption of bitter substances. J. Sens. Stud. 1993;8:31–41. doi: 10.1111/j.1745-459X.1993.tb00200.x. [DOI] [Google Scholar]
- 16.Mikołajczyk-Stecyna J, Malinowska AM, Chmurzynska A. TAS2R38 and CA6 genetic polymorphisms, frequency of bitter food intake, and blood biomarkers among elderly woman. Appetite. 2017;116:57–64. doi: 10.1016/j.appet.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Keller M, et al. TAS2R38 and its influence on smoking behavior and glucose homeostasis in the German Sorbs. PLoS One. 2013;8:e80512. doi: 10.1371/journal.pone.0080512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorovic N, et al. Genetic variation in the hTAS2R38 taste receptor and brassica vegetable intake. Scand. J. Clin. Lab. Invest. 2011;71:274–279. doi: 10.3109/00365513.2011.559553. [DOI] [PubMed] [Google Scholar]
- 19.Duffy VB, et al. Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol. Clin. Exp. Res. 2004;28:1629–1637. doi: 10.1097/01.ALC.0000145789.55183.D4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gayathri Devi A, Henderson SA, Drewnowski A. Sensory acceptance of Japanese green tea and soy products is linked to genetic sensitivity to 6-n-propylthiouracil. Nutr. Cancer. 1997;29:146–151. doi: 10.1080/01635589709514616. [DOI] [PubMed] [Google Scholar]
- 21.Ooi SX, Lee PL, Law HY, Say YH. Bitter receptor gene (TAS2R38) P49A genotypes and their associations with aversion to vegetables and sweet/fat foods in Malaysian subjects. Asia Pac. J. Clin. Nutr. 2010;19:491–498. [PubMed] [Google Scholar]
- 22.Lambert, J. D. et al. Bitter taste sensitivity, food intake, and risk of malignant cancer in the UK Women’s Cohort Study. Eur. J. Nutr. (2018). [DOI] [PubMed]
- 23.Knaapila A, et al. Genetic analysis of chemosensory traits in human twins. Chem. Senses. 2012;37:869–881. doi: 10.1093/chemse/bjs070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang, L. D. et al. Is the Association Between Sweet and Bitter Perception due to Genetic Variation? Chem. Senses (2016). [DOI] [PMC free article] [PubMed]
- 25.Reed DR, et al. The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Hum. Mol. Genet. 2010;19:4278–4285. doi: 10.1093/hmg/ddq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ledda M, et al. GWAS of human bitter taste perception identifies new loci and reveals additional complexity of bitter taste genetics. Hum. Mol. Genet. 2014;23:259–267. doi: 10.1093/hmg/ddt404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang LD, et al. Bivariate genome-wide association analysis strengthens the role of bitter receptor clusters on chromosomes 7 and 12 in human bitter taste. BMC Genomics. 2018;19:678. doi: 10.1186/s12864-018-5058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans DM, Davey Smith G. Mendelian Randomization: New Applications in the Coming Age of Hypothesis-Free Causality. Annu. Rev. Genomics Hum. Genet. 2015;16:327–350. doi: 10.1146/annurev-genom-090314-050016. [DOI] [PubMed] [Google Scholar]
- 29.Katan MB. Commentary: Mendelian Randomization, 18 years on. Int. J. Epidemiol. 2004;33:10–11. doi: 10.1093/ije/dyh023. [DOI] [PubMed] [Google Scholar]
- 30.Lawlor DA. Commentary: Two-sample Mendelian randomization: opportunities and challenges. Int. J. Epidemiol. 2016;45:908–915. doi: 10.1093/ije/dyw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith GD, et al. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med. 2007;4:e352. doi: 10.1371/journal.pmed.0040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor, M. et al. Testing the principles of Mendelian randomization: Opportunities and complications on a genomewide scale. bioRxiv 124362, 10.1101/124362 (2017).
- 33.Bycroft, C. et al. Genome-wide genetic data on ~500,000 UK Biobank participants. bioRxiv 166298, 10.1101/166298 (2017).
- 34.Wright MJ, Martin NG. Brisbane Adolescent Twin Study: Outline of study methods and research projects. Aust. J. Psychol. 2004;56:65–78. doi: 10.1080/00049530410001734865. [DOI] [Google Scholar]
- 35.Kim UK, et al. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 36.Sudlow C, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacGregor S, et al. Genome-wide association study of intraocular pressure uncovers new pathways to glaucoma. Nat. Genet. 2018;50:1067–1071. doi: 10.1038/s41588-018-0176-y. [DOI] [PubMed] [Google Scholar]
- 38.Loh PR, et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat. Genet. 2015;47:284–290. doi: 10.1038/ng.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang CC, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canela-Xandri, O., Rawlik, K. & Tenesa, A. An atlas of genetic associations in UK Biobank. bioRxiv 176834, 10.1101/176834 (2017). [DOI] [PMC free article] [PubMed]
- 43.Staley JR, et al. PhenoScanner: a database of human genotype-phenotype associations. Bioinformatics. 2016;32:3207–3209. doi: 10.1093/bioinformatics/btw373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed DR, Knaapila A. Genetics of taste and smell: poisons and pleasures. Prog. Mol. Biol. Transl. Sci. 2010;94:213–240. doi: 10.1016/B978-0-12-375003-7.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rizzi, G. P., Boekley, L. J. & Ekanayake, A. The Influence of Roasting-Derived Polymeric Substances on the Bitter Taste of Coffee Brew. In Nutraceutical Beverages871, 229–236 (American Chemical Society, 2003).
- 46.Buffo RA, Cardelli-Freire C. Coffee flavour: an overview. Flavour Fragr. J. 2004;19:99–104. doi: 10.1002/ffj.1325. [DOI] [Google Scholar]
- 47.Luciano M, Kirk KM, Heath AC, Martin NG. The genetics of tea and coffee drinking and preference for source of caffeine in a large community sample of Australian twins. Addiction. 2005;100:1510–1517. doi: 10.1111/j.1360-0443.2005.01223.x. [DOI] [PubMed] [Google Scholar]
- 48.Noble AC. Bitterness in wine. Physiol. Behav. 1994;56:1251–1255. doi: 10.1016/0031-9384(94)90373-5. [DOI] [PubMed] [Google Scholar]
- 49.Scinska A, et al. Taste responses in sons of male alcoholics. Alcohol Alcohol. 2001;36:79–84. doi: 10.1093/alcalc/36.1.79. [DOI] [PubMed] [Google Scholar]
- 50.Lanier SA, Hayes JE, Duffy VB. Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Physiol. Behav. 2005;83:821–831. doi: 10.1016/j.physbeh.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Hayes JE, et al. Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chem. Senses. 2011;36:311–319. doi: 10.1093/chemse/bjq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansen JL, Reed DR, Wright MJ, Martin NG, Breslin PAS. Heritability and genetic covariation of sensitivity to PROP, SOA, quinine HCl, and caffeine. Chem. Senses. 2006;31:403–413. doi: 10.1093/chemse/bjj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipchock SV, Mennella JA, Spielman AI, Reed DR. Human bitter perception correlates with bitter receptor messenger RNA expression in taste cells. Am. J. Clin. Nutr. 2013;98:1136–1143. doi: 10.3945/ajcn.113.066688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fry A, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am. J. Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munafò MR, Tilling K, Taylor AE, Evans DM, Davey Smith G. Collider scope: when selection bias can substantially influence observed associations. Int. J. Epidemiol. 2018;47:226–235. doi: 10.1093/ije/dyx206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor, A. et al. The molecular genetics of participation in the Avon Longitudinal Study of Parents and Children. bioRxiv 206698, 10.1101/206698 (2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.