Abstract Abstract
Two new sinistral species of the genus Satsuma A. Adams, 1868, Satsumasquamigerasp. n. and Satsumaadiriensissp. n., from southern Taiwan are described. Satsumasquamigerasp. n. is characterized by a microsculpture comprising coarse, irregularly-spaced ridges and dense, easily-dislodged triangular scales on its sinistral shell, an angulated periphery, and partly-opened umbilicus. This species inhabits secondary forests in lowland hills. Satsumaadiriensissp. n. is characterized by a thin, fragile smooth shell with microsculpture of coarse, loose ridges, a rounded periphery, completely-opened umbilicus, and elongated penial verge formed by two main pilasters. This new species was collected in a mountainous, mid-elevation, broad-leafed forest.
Keywords: anatomy, Gastropoda , land snail, sinistral, Stylommatophora , taxonomy
Introduction
The family Camaenidae, which includes the confamilial Bradybaeninae, is widely distributed in Asia and Australasia (Wade et al. 2007). Recent studies have elucidated the systematics of this family by means of molecular tools (e.g., Wade et al. 2007, Hoso et al. 2010, Criscione and Köhler 2014), however significant gaps persist in the documentation of local faunas, such as in the genus Satsuma A. Adams, 1868. This genus is distributed in East Asia (Schileyko 2004), containing more than 100 species inhabiting Japan, China, Philippines, and Taiwan (Minato 1988, Wang et al. 2014, Adams and Reeve 1850). Some Vietnamese species, currently assigned to other genera, are likely part of genus Satsuma as well (Schileyko 2011). Species of Satsuma are characterized by conical, brownish shells varying in shape, size, color, chirality and banding (Schileyko 2004). The reproductive system of this genus features an epiphallic flagellum and a penial caecum, while dart sac, accessory sac, and mucous glands are absent (Kuroda and Habe 1949, Schileyko 2004).
To date, 46 species have been described from Taiwan; most of them are endemic to Taiwan and narrowly distributed (Hsieh et al. 2013, Wu and Tsai 2014, 2015, 2016, Wu and Wu 2017a, 2017b, Hwang et al. 2017). Previous studies have suggested that there are potentially undescribed species in Taiwan, especially in mountainous areas (Wu et al. 2007, 2008, Hwang et al. 2017). In this study, we describe two new Taiwanese species from mountainous areas of lowland and mid-elevation, based on shell morphology and genital anatomy.
Materials and methods
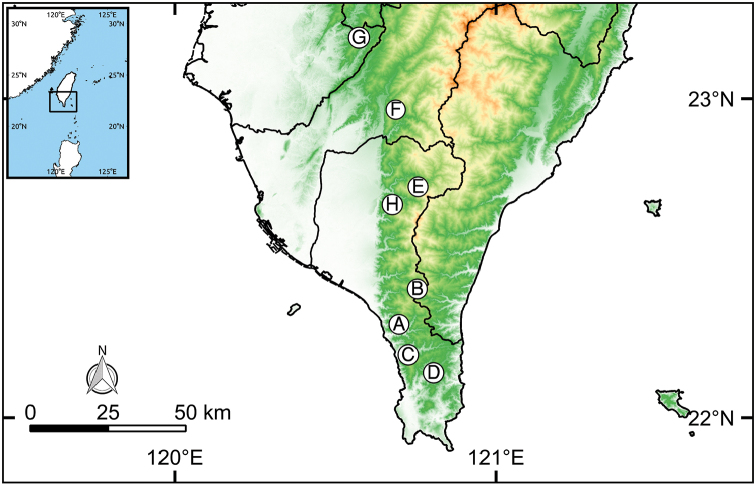
Specimens of the new species were collected in southern Taiwan (Figure 1). Live adults were drowned in water for 12 hours, then boiled briefly in hot water at 95 °C. Whole snails were fixed and preserved in 95% ethanol. Immediately before dissection, the snails’ tissues were softened with warm water, and the body was removed from the shell. Empty shells were then cleaned, oven-dried, and stored at room temperature. Reproductive systems were dissected under a stereomicroscope (Leica MZ7.5). Drawings were made using a camera lucida attachment. We used the methods described by Kerney and Cameron (1979) to measure shell characteristics to 0.1 mm and to count the number of whorls to 0.25 whorls. Measurements of genitalia were obtained from the digital images using ImageJ 1.48k (Schneider et al. 2012). We followed Gómez’s (2001) terminology in describing the reproductive system. The WGS84 coordinates of localities were recorded. A distribution map was created using the open-source software Quantum GIS 2.18.1 (QGIS Development Team 2016) with topographic databases ASTER GDEM V2 released by NASA and METI (downloadable from https://asterweb.jpl.nasa.gov) and GADM 2.8 released by Global Administrative Areas (downloadable from http://gadm.org/). The type specimens have been deposited in the National Museum of Natural Science, Taichung, Taiwan (NMNS).
Figure 1.
Distribution map of two Satsuma species in southern Taiwan. A–DS.squamigera sp. n.: A Ka-yo-fong waterfall, Shih-tze, Pingtung (type locality) B Da-han- shan forest road, Pingtung C Mt. Bei-li-long, Pingtung D Mu-dang, Pingtung; E–HS.adiriensis sp. n.: E A-li, Wu-tai, Pingtung (type locality) F Shan-ping, Liu-guei, Kaohsiung G Mt. Fan-bao-jian, Nan-xi, Tainan H Ma-jia, Pingtung.
Abbreviations
NMNS National Museum of Natural Science, Taichung, Taiwan.
Genitalia:
ag albumen gland;
at atrium;
bc bursa copulatrix;
ep epiphallus;
fl flagellum;
fod free oviduct;
p penis;
pc penial caecum;
pd pedunculus of the bursa copulatrix;
rm retractor muscle;
sod spermoviduct;
v verge;
va vagina;
vd vas deferens.
Shell measurements:
AH aperture height;
AW aperture width;
SH shell height;
SW shell width;
W# number of whorls
Systematics
Family Camaenidae Pilsbry, 1895
Satsuma A. Adams, 1868
Type species.Helixjaponica Pfeiffer, 1847, by subsequent designation (Kuroda and Habe 1949: 54)
Satsuma squamigera sp. n.
http://zoobank.org/DAAF8145-928F-4755-8132-B1FDF89A4509
Figure 2.
Shell of Satsumasquamigera sp. n. A shell of holotype (NMNS-7944-001) B scales on base of shell (paratype NMNS-794-002). Scale bar: 10 mm (A), 2 mm (B).
Figure 3.
Reproductive system showing whole genitalia and opened penis of Satsumasquamigera sp. n. (holotype NMNS-7944-001). Scale bar: 5 mm.
Type material.
HolotypeNMNS-7944-001, dry shell and dissected soft part in ethanol, coll. C. C. Hwang, 19 May 2016, collected from type locality; paratype NMNS- 7944-002, 1 specimen: dry shell and dissected soft part in ethanol, coll. S.P. Wu, 24 Jul 2014, collected from type locality; paratypes NMNS-7944-003, 5 specimens: 5 dry shells and 1 dissected soft part in ethanol, coll. S.T. Yang, 11 Feb. 2011, collected from type locality, paratypes NMNS-7944-004, 4 specimens: dry shells, coll. C. C. Hwang, 19 Aug 2014, collected from type locality, paratypes UTM2018001-5, 5 specimens: dry shells, coll. S. P. Wu, 11 May 2012, collected from type locality.
Type locality.
Taiwan: Pingtung County, Shih-tze, Ka-yo-fong waterfall (also named Nei-shih waterfall), 22°17.55'N; 120°41.88'E, alt. 170 m, secondary lowland broad-leafed forest (Figure 1A).
Diagnosis.
Shell sinistral with coarse and irregularly ridged and fine striations; surfaces with dense, fine, erected, triangular scales falling off easily; periphery angulated, umbilicus partly opened; penial caecum short, internally with elongated verge formed by two main pilasters.
Description.
Shell. Measurements (n = 11): SH 12.1–13.9 mm, SW 18.5–20.7 mm, AH 6.9–8.2 mm, AW 11.0–12.2 mm, W# 5.5–5.75, SH/SW 0.61–0.71; sinistral, with low conical spire, light brown to dark brown with red-brown peripheral band and umbilicus spot. Apex obtuse. Whorls regularly increasing, slightly convex. Periphery angulated. Base of shell convex. Surface completely covered with dense, fine, erected, curved, triangular, easily-dislodged scales and leaving crescent-shaped trace; upper surface with coarse, oblique axial ridges; spiral striation absent. Aperture roundly lunate. Peristome expanded; outer lip smoothly curved; columellar lip oblique, curve, joining curved basal lip smoothly or in an angle. Parietal callus smooth, thin, transparent. Umbilicus open, 2.6–3.2 mm in width, 1/5 covered by reflected columellar lip.
External morphology. Light brown with irregular, small, dark brown spots and a distinct yellowish line running from head between tentacles to collar. Tentacles dark brown.
Reproductive system. Bursa copulatrix oval with long pedunculus of 27–30 mm. Free oviduct short. Vagina muscular, furrowed externally corresponding to internal folds, 10–12 mm in length. Atrium short, finely wrinkled inside. Penis slender, 10–12 mm in length, evenly thickened, furrowed externally corresponding to 7–8 strong, straight, corrugated pilasters internally. Penial caecum short, protruding 2–3 mm. Verge extending along penial caecum, formed by two main pilasters, with wrinkled surface. Epiphallus slender, 15–17 mm in length, internally with 4 smooth pilasters. Penis retractor muscle attached at distal 1/4 of epiphallus. Flagellum short, tapering.
Etymology.
From squamigera (Latin, adjective in the nominative feminine singular case) meaning scale-bearing, for the scaly shell surface.
Distribution.
This species was found in southern Pingtung County, including the type locality, Da-han-shan forest road (22°24.20'N; 120°45.31'E, alt-1555 m), Mt. Bei-li-long (22°11.81'N; 120°43.63'E, alt-320 m) and Mu-dang (22°8.43'N; 120°48.34'E, alt-240 m) (Figure 1A–D).
Ecology.
All specimens were collected in mountainous, lowland, broad-leafed forest. Mature adults were collected in mid-May and February, from ground, rocks or fallen tree trunks. This species is sympatric with the congeners Satsumabacca (Pfeiffer, 1866), Satsumabatanicapancala (Schmacker & Boettger, 1891) and Satsumalongkiauwensis Wu, Lin & Hwang, 2007.
Remarks.
Satsumasquamigera sp. n. is distinguished from all other sinistral species by having dense and curved scales on the whole shell surface. When fully matured, the scales typically fall off, leaving crescent-shaped granules. Some intact scales may remain beside sutures, on the base of the last whorl or inside the umbilicus. The new species is similar to S.pekanensis (Rolle, 1911) and S.submeridionalis (Zilch, 1951) in shape of shell and angulated periphery. In comparison to S.pekanensis, the new species has a shortened spire and an extended flagellum (Chang 1989). The new species differs from S.submeridionalis in having a slender base of pedunculus of bursa copulatrix and a regularly thickened proximal vagina (Wang et al. 2014).
Satsuma adiriensis sp. n.
http://zoobank.org/676C67FF-BC84-4DE6-9D6E-12C30768DBB9
Figure 4.
Shell of Satsumaadiriensis sp. n. (holotype NMNS-7945-001). Scale bar: 10 mm.
Figure 5.
Reproductive system showing whole genitalia and opened penis of Satsumaadiriensis sp. n. (paratype NMNS-7945-002). Scale bar: 5 mm.
Type material.
Holotype NMNS-7945-001, dry shell, coll. C. C. Hwang, 24 Aug 1998, collected from type locality; paratype NMNS-7945-002, 1 specimen: dry shell and dissected soft part in ethanol, coll. S. C. Chang , 4 Jul 1997, Shan-ping, Liu-guei, Kaohsiung, 22°57.93'N; 120°41.28'E, alt. 850 m; paratype NMNS-7945-003, 1 dry shell, coll. C. C. Hwang, 25 May 1998, Mt. Fan-bao-jian, Nan-xi, Tainan, 22°71.48'N; 120°34.4'E, alt. 1000 m; paratype NMNS-7945-004, 1 dry subadult shell, coll. G. S. Hsiang, 29 Jun 1997, Ma-jia, Pingtung, 22°40.07'N; 120°40.65'E, alt. 1200 m.
Type locality.
Taiwan: Pingtung County, Wu-tai, A-li, 22°43.42'N; 120°45.44'E, alt. 1350 m, disturbed primary broad-leaf forest.
Diagnosis.
Shell sinistral, thin, fragile, smooth, with spaced, coarse ridges; periphery round, color band absent; umbilicus completely opened; penial caecum long, internally with elongated verge formed by two main pilasters.
Description.
Shell. Measurements (n = 3): SH 10.8–12.9 mm, SW 17.4–20.6 mm, AH 6.9–8.5 mm, AW 8.6–10.3 mm, W# 5.5, SH/SW 0.61–0.68; sinistral, thin, fragile, semi-translucent, with low conic spire, light brown, without color band. Apex obtuse. Whorls regularly increasing, slightly convex. Periphery bluntly angulated on the first 3/4 of last whorl, becoming rounded 1/4 whorl before peristome. Base of shell convex. Surface covered with loose, coarse, oblique axial ridges, becoming thin on base; spiral striation absent. Aperture roundly lunate. Peristome expanded; outer lip smoothly curved; columellar lip sub-vertical, not reflected, joining with basal lip in a weak angle. Parietal callus smooth, thin, transparent. Umbilicus completely opened, 3.3–3.6 mm in width.
External morphology. Light brown with dense, irregular, dark brown to black spots and a distinct yellowish line running from head between tentacles to collar. Tentacles dark brown.
Reproductive system. Bursa copulatrix oval; pedunculus long, 31 mm in length, with slightly expanded base. Free oviduct short. Vagina thickened, smooth externally, with eleven internal pilasters, 11 mm in length. Atrium obvious, finely wrinkled inside. Penis muscular, 13 mm in length, evenly thickened, furrowed externally; distal half internally supporting three main, finely wrinkled pilasters; proximal half supporting eleven strong, corrugated pilasters. Penial caecum thickened, with blunt apex, protruding 7 mm. Verge extending along penial caecum, formed by two main pilasters. Epiphallus slender, 16 mm in length, internally with three smooth pilasters. Penis retractor muscle attached at distal 1/6 of epiphallus. Flagellum long, tapering, slightly wavy at middle portion.
Etymology.
For Adiri, the indigenous Rukai name of the type locality, adjective of feminine gender.
Distribution.
Known from mid-elevation forest of Kaohsiung, Tainan and Pingtung (Figure 1E–H).
Ecology.
All specimens were collected in mountainous, mid-elevation, broad-leaf forest. The single live adult was collected in July, from a tree trunk. This species is sympatric with congeneric species S.albida (Adams, 1870) and S.friesiana (Moellendorff, 1884) at Shan-ping, S.amblytropis (Pilsbry, 1901) at Mt. Fan-bao-jian and an unknown Satsumaat the type locality A-li. Despite wide distribution in the mountainous areas of southwestern Taiwan, this species is quite rare.
Remarks.
Satsumaadiriensis sp. n. is similar to S.contraria (Pilsbry & Hirase, 1909), distributed in Kenting, Pingtung, in having a sinistral, semi-transparent shell with completely open umbilicus. The new species, however, has smaller shell width, round periphery on the final 1/4 of the last whorl, a sub-vertical columellar lip, a sinuous upper lip, coarse ridges on the surface, a slender pedunculus of bursa copulatrix, and a longer penial caecum and flagellum and shorter penis than the latter species (Hwang and Ger 2018).
The new species shares a sinistral and depressed conic shell with Satsumaformosensis (Pfeiffer, 1866) and S.yaeyamensis (Pilsbry, 1894), which are found in northern Taiwan and the Ryukyu Islands. Satsumaadiriensis differs from these two species by its thin, semi-transparent shell with loose, coarse surface ridges, a sub-vertical columellar lip joining basal lip in a weak angle, and a bluntly angulated periphery on the first 3/4 of the last whorl.
Discussion
In this study, two new species of sinistral Satsuma were described based on shell and reproductive system characteristics. This work has brought the number of known sinistral Satsuma species to seventeen. Among these seventeen species, eleven are distributed in Taiwan, three in the Ryukyu Islands, two in southern China, and one in Batan Island, Philippines. The diversification of Satsuma has been explained by allopatric speciation (Kameda et al. 2007), prey-predator coevolution and chirality (Hoso et al. 2010), and arboreal behavior (Wu et al. 2008).
Periostracal ornamentations such as granules and hairs are commonly seen in confamilial genera, e.g., Chloritis Beck, 1837, Moellendorffia Ancey, 1887, Aegista Albers, 1850 and many genera from Australia (Solem 1984, Hirano et al. 2014, Criscione and Köhler 2016). In the genus Satsuma, granules on embryonic whorls are commonly seen (personal observations), but rarely reported. This under-reporting may be due to the ease with which these granules wear off, or their simply being so small as to evade observation. Three sinistral species, S.perversa (Pilsbry, 1931), S.yaeyamensis and S.batanicapancala have been observed to have granulate embryonic whorls (Azuma 1995, personal observations), however these species do not have scales covering the whole shell surface, as does S.squamigera sp. n.
Short, hooked hairs have been observed over the entire shell surface of the sinistral species S.uncopila (Heude, 1882). Granules on the entire shell surface are also reported in some dextral species, e.g., S.ferruginea (Pilsbry, 1900), S.textilis (Pilsbry & Hirase, 1904), S.japonicagranulosa (Pilsbry, 1902), S.j.heteroglypta (Pilsbry, 1900), S.okiensis (Pilsbry & Hirase, 1908) and S.cristata (Pilsbry, 1902). The hairs are thought to promote the snails’ adherence to leaves when humidity levels are high (Pfenninger et al. 2005). The evolutionary significance of these varying ornamentations of size, shape, and position remains questionable. This question will not be adequately answered until more complete phylogeny and comparative studies of the Satsuma genus become available.
Author contributions
CC Hwang performed the anatomical studies, executed this study, and wrote the manuscript; SP Wu helped with the data collecting and paper writing.
Supplementary Material
Acknowledgements
We gratefully acknowledge the assistance of Dr. Hsueh-Wen Chang, Dr. Gao-Shih Hsiang, Ms. Su-Chin Chang, Mr. Chang-Yi Tsai (National Sun Yat-Sen University), Mr. Hsin-Te Yang (Da-Yeh University), Mr. Wei-Hsuan Tsai, Bo-An Lee, Wan-Bao Lee and Chi-Kai Liao (National University of Kaohsiung) and Mr. Chao-Ching Lee in the field works. Thanks are also due to Mr. James Bell for his editorial assistance. This study was partially supported by the Ministry of Science and Technology Grant (MOST 107-2321-B-845-001 -), We also wish to express our gratitude to Forestry Bureau and Taiwan Forestry Research Institute, Council of Agriculture for the permission for collection.
Citation
Hwang C-C, Wu S-P (2018) Two new species of Satsuma A. Adams, 1868 from Taiwan (Pulmonata, Camaenidae). ZooKeys 795: 115–126. https://doi.org/10.3897/zookeys.795.28958
References
- Adams A, Reeve L. (1850) Mollusca, Part III. In: Adams A. (Ed.) The Zoology of the Voyage of H.M.S. Samarang; Under the Command of Captain Sir Edward Belcher C.B., F.R.A.S., F.G.S., During the Years 1843–1846. Reeve, Benham & Reeve, London, 1–87.
- Azuma M. (1995) Colored Illustrations of the Land Snails of Japan. Hoikusha, 343 pp.
- Chang KM. (1989) Anatomy of Coniglobusnuxpaiwanis (Kuroda) and Coniglobuspekanensis (Rolle) from south Taiwan (Pulmonata: Camaenidae). Bulletin of Malacology 14: 1–8. [Google Scholar]
- Criscione F, Köhler F. (2014) Molecular phylogenetics and comparative anatomy of Kimberleytrachia Köhler, 2011 – a genus of land snail endemic to the coastal Kimberley, Western Australia with description of new taxa (Gastropoda, Camaenidae). Contributions to Zoology 83: 245–267. http://www.ctoz.nl/vol83/nr04/a03 [Google Scholar]
- Criscione F, Köhler F. (2016) Setobaudinianicolasi – a new species from Baudin Island, Kimberley, Western Australia (Stylommatophora, Camaenidae). Molluscan Research 36: 290–293. 10.1080/13235818.2016.1201037 [DOI]
- Gómez BJ. (2001) Structure and functioning of the reproductive system. In: Baker GM. (Ed.) The Biology of Terrestrial Molluscs.CABI Publishing, Oxon, 307–330. 10.1079/9780851993188.0307 [DOI]
- Hirano T, Kameda Y, Kimura K, Chiba S. (2014) Substantial incongruence among the morphology, taxonomy, and molecular phylogeny of the land snails Aegista, Landouria, Trishoplita, and Pseudobuliminus (Pulmonata: Bradybaenidae) occurring in East Asia. Molecular Phylogenetics and Evolution 70: 171–181. 10.1016/j.ympev.2013.09.020 [DOI] [PubMed] [Google Scholar]
- Hoso M, Kameda Y, Wu SP, Asami T, Kato M, Hori M. (2010) A speciation gene for left-right reversal in snails results in anti-predator adaptation. Nature Communications 1: 133. 10.1038/ncomms1133 [DOI] [PMC free article] [PubMed]
- Hsieh BC, Wu SP, Tsai CL. (2013) Land Snails of Taiwan (3rd edn). Forestry Bureau, Council of Agriculture, Executive Yuan, Taiwan, 381 pp. [Google Scholar]
- Hwang CC, Ger MJ. (2018) Reproductive system of land snail Satsumacontraria (Stylommatophora: Camaenidae). Bulletin of Malacology 41: 36–45. [Google Scholar]
- Hwang CC, Okubo K, Tada A. (2017) Satsumajinlunensis – a new species from Taiwan (Stylommatophora: Camaenidae). Molluscan Research. 10.1080/13235818.2017.1358340 [DOI]
- Kameda Y, Kawakita A, Kato M. (2007) Cryptic genetic divergence and associated morphological differentiation in the arboreal land snail Satsuma (Luchuhadra) largillierti (Camaenidae) endemic to the Ryukyu Archipelago, Japan. Molecular Phylogenetics and Evolution 45: 519–533. 10.1016/j.ympev.2007.03.021 [DOI] [PubMed] [Google Scholar]
- Kerney MP, Cameron RAD. (1979) Land Snails of Britain & North-west Europe. Harper & Collins, London, 288 pp. [Google Scholar]
- Kuroda T, Habe T. (1949) Helicacea. Sanmeisha, Tokyo, 129 pp. [Google Scholar]
- Minato H. (1988) A Systematic and Bibliographic List of the Japanese Land Snails. Shirahama, Japan, 294 pp. [Google Scholar]
- Pfenninger M, Hrabáková M, Steinke D, Dèpraz A. (2005) Why do snails have hairs? A Bayesian inference of character evolution. BMC Evolutionary Biology 5: 59. 10.1186/1471-2148-5-59 [DOI] [PMC free article] [PubMed]
- QGIS Development Team (2016) QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://www.qgis.org
- Schileyko AA. (2004) Treatise on Recent terrestrial pulmonate molluscs, Part 12: Bradybaenidae, Monadeniidae, Xanthonychidae, Epiphragmophoridae, Helminthoglyptidae, Elonidae, Humboldtianidae, Sphincterochilidae, Cochlicellidae. Ruthenica, supplement 2: 1627–1763. [Google Scholar]
- Schileyko AA. (2011) Check-list of land pulmonate molluscs of Vietnam (Gastropoda: Stylommatophora). Ruthenica 21: 1–68. https://biotaxa.org/Ruthenica/article/view/3603 [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solem A. (1984) Camaenid land snails from Western and central Australia (Mollusca: Pulmonata: Camaenidae). IV. Taxa from the Kimberley, Westraltrachia Iredale, 1933 and related genera. Records of the Western Australian Museum, Supplement 17: 427–705. [Google Scholar]
- Wade CM, Hudelot C, Davison A, Naggs F, Mordan PB. (2007) Molecular phylogeny of the helicoid land snails (Pulmonata: Stylommatophora: Helicoidea), with special emphasis on the Camaenidae. Journal of Molluscan Studies 73: 411–415. 10.1093/mollus/eym030 [DOI] [Google Scholar]
- Wang P, Xiao Q, Zhou WC, Hwang CC. (2014) Revision of three camaenid and one bradybaenid species (Gastropoda, Stylommatophora) from China based on morphological and molecular data, with description of a new bradybaenid subspecies from Inner Mongolia, China. ZooKeys 372: 1–16. 10.3897/zookeys.372.6581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SP, Hwang CC, Lin YS. (2008) Systematic revision of the arboreal snail Satsumaalbida species complex (Mollusca: Camaenidae) with descriptions of 14 new species from Taiwan. Zoological Journal of the Linnean Society 154: 437–493. 10.1111/j.1096-3642.2008.00415.x [DOI] [Google Scholar]
- Wu SP, Lin YS, Hwang CC. (2007) A new Satsuma species (Pulmonata: Camaenidae) endemic to Taiwan. Zootaxa 1608: 59–68. [Google Scholar]
- Wu SP, Tsai CL. (2014) A new sinistral Satsuma land snail (Pulmonata: Camaenidae) endemic to Taiwan. Bulletin of Malacology 37: 61–72. [Google Scholar]
- Wu SP, Tsai CL. (2015) A new endemic dextral Satsuma land snail (Pulmonata: Camaenidae) from Taiwan. Bulletin of Malacology 38: 41–48. [Google Scholar]
- Wu SP, Tsai CL. (2016) A new dextral species land snail of genus Satsuma (Pulmonata: Camaenidae) endemic to Taiwan. Bulletin of Malacology 39: 47–58. [Google Scholar]
- Wu SP, Wu CC. (2017a) A new and endemic sinistral Satsuma land snail (Pulmonata: Camaenidae) from South Taiwan. Bulletin of Malacology 40: 27–42. [Google Scholar]
- Wu SP, Wu CC. (2017b) A new dextral land snail of genus Satsuma (Pulmonata: Camaenidae) endemic to Taiwan. Bulletin of Malacology 40: 13–26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.