Abstract
Introduction:
Although hypovolemia remains the most relevant problem during acute de-compensated diabetes in its clinical manifestations (diabetic ketoacidosis, DKA, and hyperglycemic hy-perosmolar state, HHS), the electrolyte derangements caused by the global hydroelectrolytic imbalance usually complicate the clinical picture at presentation and may be worsened by the treatment itself.
Aim:
This review article is focused on the management of dysnatremias during hyperglycemic hyperos-molar state with the aim of providing clinicians a useful tool to early identify the sodium derangement in order to address properly its treatment.
Discussion:
The plasma sodium concentration is modified by most of the therapeutic measures common-ly required in such patients and the physician needs to consider these interactions when treating HHS. Moreover, an improper management of plasma sodium concentration (PNa+) and plasma osmolality dur-ing treatment has been associated with two rare potentially life-threatening complications (cerebral edema and osmotic demyelination syndrome). Identifying the correct composition of the fluids that need to be infused to restore volume losses is crucial to prevent complications.
Conclusion:
A quantitative approach based on the comparison between the measured PNa+ (PNa+M) and the PNa+ expected in the presence of an exclusive water shift (PNa+G) may provide more thorough infor-mation about the true hydroelectrolytic status of the patient and may therefore, guide the physician in the initial management of HHS. On the basis of data derived from our previous studies, we propose a 7-step algorithm to compute an accurate estimate of PNa
Keywords: Hyperglycemic hyperosmolar state, hyponatremia, hypernatremia, cerebral edema, osmotic demyelination syndrome, fluid therapy
1. INTRODUCTION
Hypovolemia is one of the most relevant clinical problems during acutely decompensated diabetes mellitus, irrespective of whether it is Diabetic Ketoacidosis (DKA) or Hyperglycemic Hyperosmolar State (HHS). Moreover, different electrolyte derangements may coexist with hypovolemia at the onset or may be caused by fluid replacement itself, if the composition of the infused saline solution is not appropriate.
Therefore, a careful evaluation of plasma osmolality (POsm), sodium (PNa+) and potassium concentrations (PK+) and of acid-base status is crucial to determine the correct composition of fluids to be administered.
This review is focused on the management of PNa+ during HHS. Particularly, we will discuss the potential role of a
quantitative approach to dysnatremias in identifying the proper amount of Na+ and K+ that need to be infused in order to prevent harmful derangements in POsm as well as two associated life-threatening complications: cerebral edema and Osmotic Demyelination Syndrome (ODS).
2. PATHOPHYSIOLOGY
In HHS the Extracellular Volume (ECV) effective osmolality (tonicity) is increased due to the elevation in the plasma glucose concentration (PG) according to the following formula:
Effective osmolality (mOsm/kgH2O) = 2 × PNa+ (mmol/L) + 2 × PK+ (mmol/L) + PG (mg/dL) / 18
Blood urea nitrogen is not included in this particular formula since it does not affect the osmotic gradient across cell membranes, being permeable through them [1, 2].
The restriction of glucose to the ECV generates an osmotic gradient which shifts water from the Intracellular Volume (ICV) to the ECV, leading to a dilutional fall in PNa+ [3]. In the absence of preexisting or associated electrolyte derangements, this process causes hypertonic hyponatremia since, despite a low PNa+, POsm rises, usually above 320 mOsm/kg due to the high PG.
Obviously, this is not a static condition and other variables affect the process.
First of all, hyperglycemia causes an osmotic diuresis which increases renal excretion of water in excess of that of Na+ and other ions. This large electrolyte-free water output tends to raise both PNa+ and POsm [4-6]. Water intake is consequently one of the main determinants of the clinical presentation and the laboratory findings of hyperglycemic crisis: in fact, in those patients who are able to compensate the volume lost through osmotic diuresis with oral water intake, the rise in POsm can be dampened, while PNa+ tends to be even lower. On the contrary, as HHS usually develops in elderly patients with an impaired thirst mechanism and/or a reduced access to water [7], volume depletion is often prevailing, such that the measured PNa+ can be normal or even increased (averaging 149 mEq/L in some studies [2, 8]).
A further crucial issue is K+ replacement. In the first phases of treatment, a high infusion rate of K+ salts may be required to prevent insulin-induced hypokalemia (even up to 10-20 mmol/h [9]). However, K+ administration leads to a rise in PNa+, since all millimoles of K+ infused should be considered as molar Na+ equivalents; this means that K+ administration induces an increase in PNa+ comparable to that observed when the same amount of Na+ is infused [10, 11]. Therefore, the amount of K+ administered has to be carefully considered during fluid infusion in order to avoid an overly rapid correction of hyponatremia or even the onset of hypernatremia [12].
The process is further enhanced by the simultaneous administration of insulin that promotes K+ entry into cells in several tissues (mainly liver, skeletal muscle, cardiac muscle and adipose tissue) [13, 14]. This effect is mediated by many transport pathways, particularly by the Na+/K+ ATPase [15], which pumps Na+ out of the ICV in exchange for K+, thus contributing to the increase in PNa+.
Other important elements that should be taken into account during fluid replacement are vomiting and nasogastric suction, which can increase water, solute and hydrogen ion losses.
3. QUANTITATIVE APPROACH
Over the years many correction factors have been proposed in order to help the physician to calculate PNa+ expected in the presence of hyperglycemia.
Until 1973, clinicians estimated a fall in PNa+ of 2.8 mmol/L for every 100 mg/dL increase in PG that occurred above a concentration of 100 mg/dL [16]. In 1973 Katz suggested that the water shift should stop before the normal ECV osmolality is restored, thus producing hyperosmolality in both compartments; on this basis, he mathematically calculated a correction factor of 1.6 mmol/L [17]. Later, in 1999, Hillier et al. demonstrated that the higher is PG, the more inaccurate is the correction factor proposed by Katz; a correction factor of 2.4 mmol/L was then introduced in clinical practice, although the Authors themselves admitted that it still underestimates the reduction in PNa+ for PG > 400 mg/dL and that, above this cut-off, a correction factor of 4 mmol/L is more reliable [18].
Although in the following years, a large number of studies have analyzed and reviewed the proposed correction factors [19], even now there is no consensus on which one should be considered the most accurate: for example, the American Diabetes Association (ADA) guidelines for the treatment of DKA and HHS [9] report the one proposed by Katz and then re-evaluated by Nguyen et al. (1.6 mmol/L) [20], while Spasovski et al. report in their clinical guidelines for the treatment of hyponatremia the value suggested by Hillier et al. (2.4 mmol/L) [21].
However, although the Literature seems deeply focused on this essentially mathematical aspect, it is important to highlight that the ΔPNa+/ΔPG ratio is affected by many variables that cannot be included in the simplified equations proposed, such that this ratio can widely vary depending on the magnitude of the concomitant solute and/or water depletion. We already demonstrated this effects in previous works using a computer simulation model [22-24].
Another challenging issue is the estimate of volume status; although these patients are almost always dehydrated, an accurate estimate of volume status is very important in order to avoid adverse events related to an incorrect treatment. Excluding invasive techniques (such as transpulmonary thermodilution [25]), that are not applicable to this kind of patients, non-invasive methods (based on echocardiography [26], changes in the pulse oximeter waveform [27] or biomarkers as copeptin [28]), although effective in identifying fluid overload, are not widely available and, at the moment, give unreliable results from the quantitative point of view. For these reasons we based our model on anthropometric measurements; we previously set up a mathematical model that allowed us to estimate both the ECV and the glucose excess [29]. We then computed the PNa+ expected on the basis of the water shift caused by hyperosmolality (PNa+G). The difference between the PNa+G and the measured PNa+ (PNa+M) stratified patients into 3 groups:
PNa+M ≈ PNa+G: in this situation, the measured PNa+ is comparable to that predicted by the water shift; this means that no changes in either water or Na+ content occurred, and that hyponatremia is completely explained by the dilution of Na+ content caused by water flows across cell membranes;
PNa+M < PNa+G: in this situation the measured PNa+ is lower than the expected one; it means that Na+ deficit is greater than water losses;
PNa+M > PNa+G: in this situation the measured PNa+ is higher than the expected one; it means that either an absolute water deficit is present or that it prevails over the associated Na+ loss.
This classification is useful in clinical practice and helps the physician to choose the correct initial fluid treatment.
4. TREATMENT
The first goal in treating HHS is to restore the water deficit, which averages 100-200 mL/kg (about 10.5 L in a typical 70-kg man) because of the massive losses due to the osmotic diuresis [30]. Water deficit is usually larger in HHS compared to DKA. In fact, while the relative lack of insulin allows the development of higher PG during the longer time course, the total lack of insulin in type 1 diabetes mellitus leads to earlier ion and acid-base derangements (euglycemic DKA also exists [31]) that call for earlier attention, as they are invariably fatal if left untreated. Fluid treatment is meant to expand the circulating volume, thus improving renal perfusion and function. It has been also demonstrated that this reduces the counter-regulatory hormone release and insulin resistance [32]. As already stated, selecting the proper fluid to be infused is important to avoid treatment complications. Isotonic saline (0.9% NaCl) is usually suggested as the first choice in emergency conditions: it is recommended to administer 1000-1500 mL in the first hour and then to reduce the infusion rate according to the hemodynamic and clinical response [9, 33]. Infusion rates should always be made compatible with the patients’ cardiac function, although the overall water deficit should be corrected within the first 24 hours of treatment [9]. However, as soon as hemodynamic stability has been achieved, it is important to switch to other solutions according to the corrected PNa+ (PNa+G) with the aim to avoid treatment-related dysnatremias. The classification we previously presented [22] can reasonably guide this choice:
If PNa+M < PNa+G, isotonic saline is the appropriate choice in order to compensate the Na+ deficit due to osmotic diuresis;
If PNa+M > PNa+G, a hypotonic fluid should be given in order to simultaneously administer a larger amount of water coupled to a lower amount of Na+, avoiding to worsen or to generate hypernatremia;
If PNa+M ≈ PNa+G, both 0.9% and 0.45% NaCl solutions can be appropriated, and their relative amounts are usually determined by the consideration that PNa+ will gradually rise along with insulin treatment (since the glucose shift from the ECV to the ICV reverses the osmotic gradient and drags water to the ICV, thus concentrating the ECV Na+ content).
As already stated, the molar equivalence existing between Na+ and K+ implies that 1 millimole of KCl will raise the PNa+ exactly as 1 mmol of NaCl will. Therefore, the need to give supplemental K+ together with fluid replacement must be considered in choosing the adequate solution. For example, in the last case presented above (PNa+M ≈ PNa+G), if K+ supplementation is required, hypotonic fluids should be preferred in order to avoid an overly rapid or even excessive increase in PNa+ and its associated risks. Particularly, ADA recommends to administer supplemental K+ when:
PK+ < 3.3 mmol/L before insulin infusion is started;
3.3 ≤ PK+ ≤ 5.2 mmol/L during insulin infusion.
K+ supplementation is not safe, and often unnecessary, when PK+ > 5.2 mmol/L, but PK+ has to be closely monitored (usually every 2 hours) during continuous insulin infusion [9]. As already described, this latter recommendation is explained by the K+ transport into cells promoted by the hormone, mainly through the activation of Na+/K+ ATPase [15]. Because of the constriction imposed by electrical neutrality, this molecular mechanism requires that Na+ shifts from cells to interstitium, such that a further rise in PNa+ must be expected.
On the other hand, it is important to be aware that the infusion of hypotonic solutions becomes necessary when PG falls below 300 mg/dL: in fact, glucose supplementation is required in order to keep PG within the 200-300 mg/dL range, so that glycogen stores can be gradually rebuilt [9]. The administration of such a large amount of electrolyte-free water has the obvious consequence to dampen the rise in PNa+ related to previous and simultaneous treatments (saline, insulin infusions, K+ supplementation).
Because of a large number of variables that affect the PNa+ variation in time (related both to the above mentioned treatments and to free water renal excretion), it is recommended to check electrolytes (together with venous pH, glucose and creatinine) at least every 2-4 hours in order to guide therapy [9].
To guide treatment, Table 1 reports the composition of the most common solutions available in clinical practice, including Ringer’s and glucose solutions.
4.1. Treatment Complications
It has been demonstrated that brain cells are partially permeable to glucose regardless of insulin action because of a selective expression of transport proteins [34]. Therefore, hypertonicity exclusively due to hyperglycemia is physiologically dampened in the Central Nervous System (CNS). On the other hand, hypertonicity due to hypernatremia has a greater impact on the CNS and it has been suggested that the PNa+ is a better predictor of neurological impairment than the PG itself, since severely hyperglycemic patients can be fully asymptomatic in the absence of hypernatremia [35].
However, when the development of hypernatremia-related hypertonicity is gradual, brain cells can activate an adaptation mechanism based on the accumulation of both inorganic osmolytes (basically electrolytes) and organic osmolytes (the so-called idiogenic osmoles) in order to reduce the osmotic transmembrane gradient, preventing an excessive water shift from cells and the related brain shrinking [36].
Treatment-related complications occur when these pathophysiological processes are ignored.
Clinically apparent cerebral edema causes a symptomatology characterized by headache, insisted vomiting, seizures, papilledema, abnormal pupillary response, cranial nerves palsies, altered mental status and impaired consciousness, sphincter incontinence, rising blood pressure with inappropriate bradycardia up to respiratory arrest [37-39]. Overt cerebral edema is uncommon in HHS and in adults [40], predominantly occurring in children with DKA (0.3-1.0% of DKA episodes in children [41], with a 21-24% mortality rate [42]). However, studies involving computerized tomography, electroencephalography and magnetic resonance diffusion-weighted imaging have demonstrated that the development of subclinical cerebral edema is frequent during the first 24 hours of treatment of hyperglycemic emergencies [4, 43-45]. The most accredited theory claims that cerebral edema is caused by an overly rapid reduction in POsm [46]: brain cells, which previously adapted to hypertonicity by accumulating idiogenic osmoles, become hypertonic when the POsm falls, because the elimination of organic osmolytes is a slow process compared to the quick changes in PG during treatment [4]: thus, the reverse osmotic gradient generates a water shift into brain cells. However, new evidences from recent clinical studies question the pivotal importance of this mechanism, as the pathogenesis of cerebral edema during hyperglycemic emergencies seems to be vasogenic rather than cytotoxic [47]: in fact, different rehydration protocols were not related to different rates of subclinical cerebral edema in a randomized clinical trial [48]. These findings may prove the role of other less reputed mechanisms, such as hypoxia, ischemia/reperfusion-like injury, inflammation and impaired ion transport [9]. Anyway, the only measure suggested to prevent cerebral edema in such patients is still a gradual reduction of POsm, that should be obtained by avoiding an excessive rehydration, by gradually decreasing the PG (the recommended rate is 50-75 mg/dL/h) and then by using glucose infusions to keep the PG within a 200-300 mg/dL range as long as the POsm is being normalized and the patient remains mentally alert [9].
ODS is a life-threatening complication typically associated with the overly rapid correction of a hypotonic state (typically, chronic hyponatremia) [49]. The clinical syndrome, characterized by the development of neurological impairment (confusion, drowsiness, altered mental status up to respiratory arrest or a so-called locked-in syndrome) even a few days after the correction of the hypotonic condition, is produced by an acute shrinking in brain cells with a secondary blood-brain barrier damage which triggers an inflammatory response, mainly complement-mediated [50]. This phenomenon, which is pathophysiologically related to adaptation mechanisms to hypotonicity, is more likely to occur in encephalic areas where cells are less prone to eliminate idiogenic osmoles, such as the central part of basal pons (central pontine myelinolysis) [51]. ODS is not typically associated to HHS: only a few case reports have been published since 1989, some of them reporting the neurological impairment at presentation (suggesting a possible role of hyperglycemia in the development of brain damage [52]), others as a complication of treatment [53]. Pathophysiologically, an overly rapid correction of Na+ deficit (in those with PNa+M < PNa+G) or a further increase in the PNa+ (in those with PNa+M > PNa+G or PNa+M ≈ PNa+G) can cause rapid fluctuations in POsm, essentially identical to the conditions that produce ODS while treating hyponatremia [54]. This complication explains why it is so important to evaluate the Na+ content of the infused fluids and to consider the effect of K+ and insulin on the PNa+. The occurrence of a catastrophic ODS when treating HHS remains infrequent, but an overly rapid development of iatrogenic hypernatremia may still worsen the patient’s neurological status and must be avoided.
5. EXAMPLES
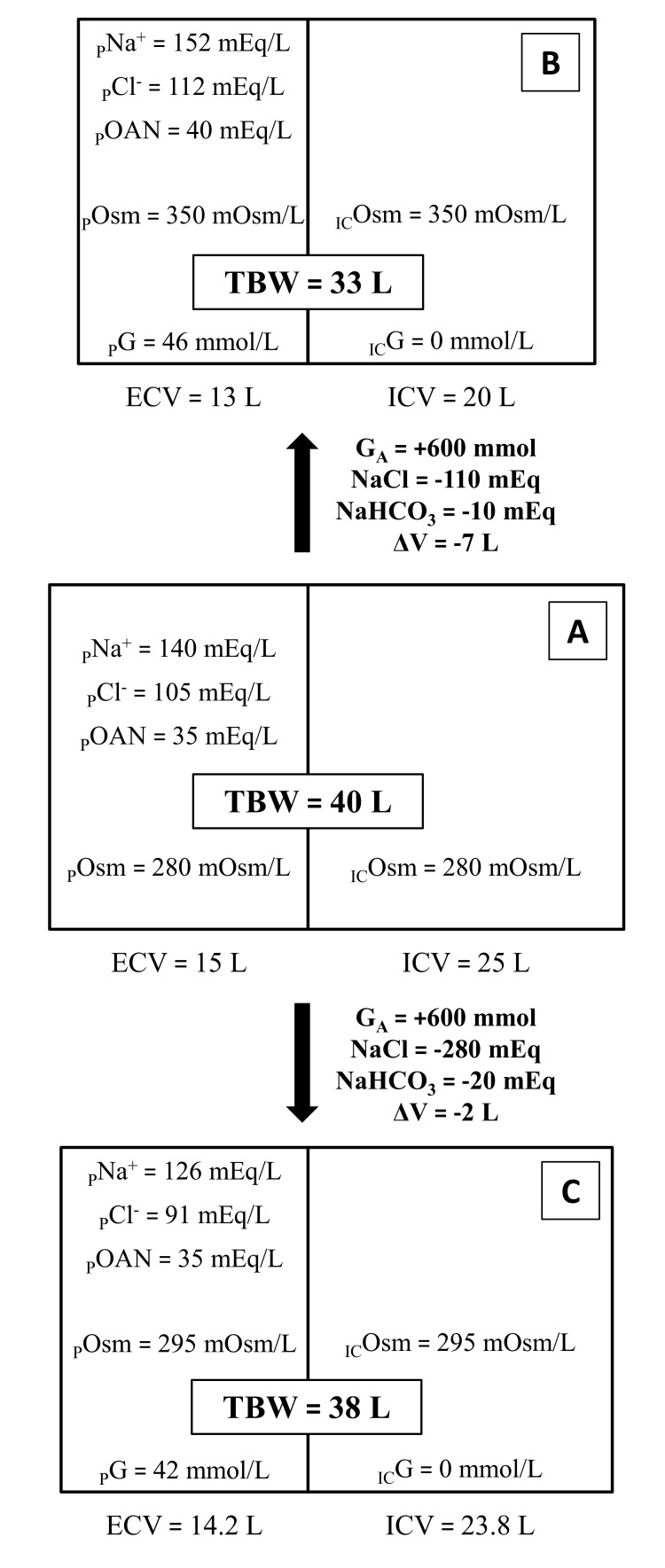
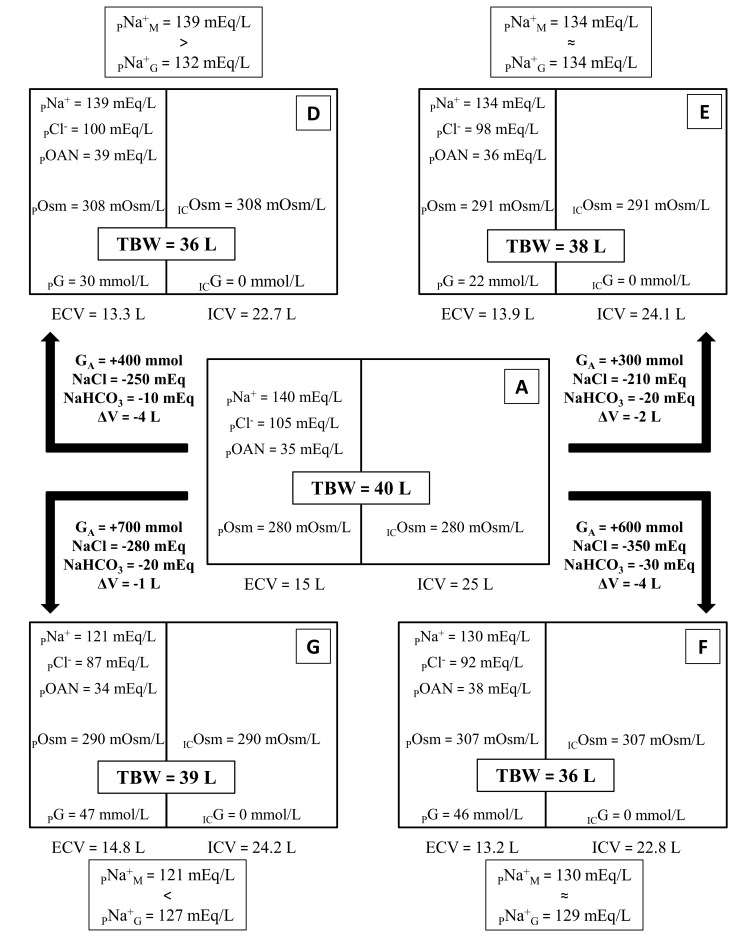
Figs. (1 and 2) give a schematic representation of the changes produced by hyperglycemia in total body water (TBW), ICV, ECV and their respective concentrations of Na+, Cl-, other (than chloride) anions (OAN) and osmolality. The same starting condition (A), modified by the same glucose addition, can evolve towards different clinical features according to different adaptive responses. Fig. (1) exemplifies the two extreme clinical patterns:
Fig. (1).
The same baseline condition (A), modified by the same amount of GA (600 mmol) can evolve towards two opposite clinical presentation depending on whether or not the patient compensate osmotic diuresis with water intake (respectively B and C).
Fig. (2).
The same baseline condition (A), modified by different combinations of GA, Na+ lost and volume lost, can produce different clinical presentations. The ratio between PNa+G and PNa+M can help the physician to estimate whether volume losses prevail over Na+ losses (D), Na+ losses prevail over volume losses (G) or the two actually balance and the reduction in PNa+ can be approximated to that exclusively produced by water shift (E, F). PNa+G has been calculated with the formulas reported in Table 2.
In B (the typical HHS presentation) osmotic diuresis produces massive water losses, which are larger than solute losses and are not completely compensated by water intake, thus producing a higher PG, a higher POsm and either normonatremia or hypernatremia;
In C free water intake is higher (mainly because thirst and consciousness are preserved) and the effects of the osmotic diuresis are partially compensated, thus producing a lower water deficit, a proportionally higher solute loss and, consequently, a lower PG, a lower POsm and either hyponatremia or normonatremia.
46 mmol/L = 830 mg/dL; 42 mmol/L = 758 mg/dL.
Fig. (1) reports two extreme examples in which the proper fluid to be infused would be easy to identify. However, in less striking cases, the correct choice may not be clearly evident. Therefore, once hemodynamic stability is ensured, it is mandatory to obtain information about the patient’s volume status through the physical examination (blood pressure, heart rate and their orthostatic changes; skin and mucous hydration, jugular or peripheral veins distension or collapse); additionally, body weight and its recent changes may give valuable information, but these data are hardly ever available, especially in the Emergency Department.
Laboratory tests needed to set the proper treatment include POsm, PG, PNa+, PK+ and PCl-. These data allow the clinician to compute the amount of water and cations to be administered in order to restore volume deficit and correct electrolyte derangements. The formulas we propose (reported in Table 2) are taken from our previous work, where the boundary conditions for their applicability were also identified: POsm1 > 280 mOsm/L (a necessary requirement in HHS) and PCl1/PNa1 ≠ 0.75 [24].
Fig. (2) presents laboratory data from four hypothetical example patients along with the respective computed PNa+G.
We think that these formulas can represent a valuable tool for the physician when fluids treatment of HHS needs to be started. However, since the computing algorithm includes different steps with a number of variables, in order to simplify its use in clinical practice, we are setting up an easily available calculation tool that instantly provides the PNa+G and other data that, together with a careful clinical examination, help the physician to choose the correct treatment.
30 mmol/L = 543 mg/dL; 22 mmol/L = 388 mg/dL; 46 mmol/L = 830 mg/dL; 47 mmol/L = 849 mg/dL.
CONCLUSION
Although hypovolemia remains the most relevant problem in HHS and requires immediate correction, electrolyte derangements and particularly dysnatremias usually complicate and worsen the global clinical picture. Most of the therapeutic measures routinely necessary in HHS (fluids infusion, insulin infusion, K+ supplementation) affect PNa+. The physician needs to be aware of this as well as of the underlying pathophysiological mechanisms in order to predict the change in PNa+ and prevent the potential complications of improper treatments. The quantitative approach we propose on the basis of our previous works can provide more accurate information about the patient’s hydroelectrolytic status and guide the physician in the choice of the proper composition of the fluids that need to be infused to restore volume losses.
Table 1.
Composition of the most common solutions available in clinical practice.
| Solution |
Na+
(mEq/L) |
K+
(mEq/L) |
Cl-
(mEq/L) |
Glucose
(g/L / mM) |
Osmolarity (mOsm/L) | pH |
|---|---|---|---|---|---|---|
| 0.9% NaCl (Normal saline) | 154 | 0 | 154 | 0 | 308 | 5 |
| 0.45% NaCl (½ Normal saline) | 77 | 0 | 77 | 0 | 154 | 5 |
| 0.22% NaCl (¼ Normal saline) | 39 | 0 | 39 | 0 | 78 | 5 |
| Ringer’s solutions * | 130 | 4 | 109 | 0 | 273 | 6.5 |
| 5% Glucose in water † | 0 | 0 | 0 | 50 / 278 | 278 † | 5 |
| 10% Glucose in water † | 0 | 0 | 0 | 100 / 556 | 556 † | 5 |
| 5% Glucose in 0.45% NaCl † | 77 | 0 | 77 | 50 / 278 | 406 † | 5 |
*The table reports the typical composition of Ringer’s solutions, but the content of each component may slightly vary among different producers.
†Since glucose is rapidly absorbed into the cells as a result of insulin action, the solutions of glucose in water can be considered hypotonic (although 5% is actually isotonic and 10% hypertonic); similarly, the final tonicity of the solutions of glucose in saline depends on the sodium chloride concentration (usually 0.45%).
Table 2.
Step-by-step algorithm to calculate PNa+G. The required variables are POsm0 and POsm1, normal body weight (needed to compute TBW0 and then ECV0), PNa+0, PNa+1 and PG1.
| - | Step-by-step Algorithm to Compute PNa+G |
|---|---|
| 1. Compute the first estimate of the variation in total body water | ΔVFE = (ΔPOsm × TBW0) / POsm1 |
| 2. Compute the first estimate of the variation in Na+ content | ΔNa+FE = (PNa+1 – PNa+0) × ΔVFE |
| 3. Compute the final extracellular volume | ECV1 = [(PNa+0 × ECV0) – ΔNa+FE] / PNa+1 |
| 4. Compute the amount of glucose in extracellular volume | GA = PG1 × ECV1 |
| 5. Compute the pOsmG (rise in POsm exclusively due to GA) | POsmG = [(POsm0 × TBW0) + GA] / TBW0 |
| 6. Compute the extracellular volume expected in the presence of exclusive osmotic water shift | ECVG = TBW0 – [(POsm0 × ICV0) / POsmG] |
| 7. Compute the PNa+ expected in the presence of exclusive osmotic water shift | PNa+G = (PNa+0 × ECV0) / ECVG |
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
list of Abbreviations
- DKA
Diabetic Ketoacidosis
- HHS
Hyperglycemic Hyperosmolar State
- POsm
Plasma Osmolality
- PNa+
Plasma Sodium Concentration
- PK+
Plasma Potassium Concentration
- ODS
Osmotic Demyelination Syndrome
- ECV
Extracellular Volume
- PG
Plasma Glucose Concentration
- ICV
Intracellular Volume
- PNa+G
Plasma Sodium Concentration Expected in the Presence of Exclusive Osmotic Water Shift
- PNa+M
Measured Plasma Sodium Concentration
- CNS
Central Nervous System
- TBW
Total Body Water
- PCl-
Plasma Chloride Concentration
- POAN
Plasma Concentration of Anions other than Chloride
- GA
Amount of Glucose in Extracellular Volume
- ΔVFE
First Estimate of the Variation in Total Body Water
- ΔNa+FE
First Estimate of the Variation in Na+ Content
- pOsmG
Rise in Plasma Osmolality Exclusively Due to the Amount of Glucose Diluted in Extracellular Volume
- ECVG
Extracellular Volume Expected in the Presence of Exclusive Osmotic Water Shift
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Lorber D. Nonketotic hypertonicity in diabetes mellitus. Med. Clin. North Am. 1995;79(1):39–52. doi: 10.1016/s0025-7125(16)30083-9. [DOI] [PubMed] [Google Scholar]
- 2.Ennis E.D., Stahl E.J.V.B., Kreisberg R.A. The hyperosmolar hyperglycemic syndrome. Diabetes Rev. (Alex.) 1994;2:115–126. [Google Scholar]
- 3.Seldin D.W., Tarail R. Effect of hypertonic solutions on metabolism and excretion of electrolytes. Am. J. Physiol. 1949;159:160–174. doi: 10.1152/ajplegacy.1949.159.1.160. [DOI] [PubMed] [Google Scholar]
- 4.Kitabchi A.E., Wall B.M. Diabetic ketoacidosis. Med. Clin. North Am. 1995;79(1):9–37. doi: 10.1016/s0025-7125(16)30082-7. [DOI] [PubMed] [Google Scholar]
- 5.Adrogué H.J., Wilson H., Boyd A.E., III, Suki W.N., Eknoyan G. Plasma acid-base patterns in diabetic ketoacidosis. N. Engl. J. Med. 1982;307(26):1603–1610. doi: 10.1056/NEJM198212233072603. [DOI] [PubMed] [Google Scholar]
- 6.Bartoli E., Bergamasco L., Sainaghi P.P., Guidetti F., Castello L. An improved method to compute the solute and water derangements of hyperglycemia. Eur. J. Appl. Physiol. 2007;102(1):97–105. doi: 10.1007/s00421-007-0561-1. [DOI] [PubMed] [Google Scholar]
- 7.Braaten J.T. Hyperosmolar nonketotic diabetic coma: diagnosis and management. Geriatrics. 1987;42(11):83–92. [PubMed] [Google Scholar]
- 8.Kitabchi A.E., Umpierrez G.E., Murphy M.B., et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131–153. doi: 10.2337/diacare.24.1.131. [DOI] [PubMed] [Google Scholar]
- 9.Kitabchi A.E., Umpierrez G.E., Miles J.M., Fisher J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelman I.S., Leibman J., O’Meara M.P., Birkenfeld L.W. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J. Clin. Invest. 1958;37(9):1236–1256. doi: 10.1172/JCI103712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laragh J.H. The effect of potassium chloride on hyponatremia. J. Clin. Invest. 1954;33(5):807–818. doi: 10.1172/JCI102952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pham P.C., Chen P.V., Pham P.T. Overcorrection of hyponatremia: where do we go wrong? Am. J. Kidney Dis. 2000;36(2):E12. doi: 10.1053/ajkd.2000.9013. [DOI] [PubMed] [Google Scholar]
- 13.Mount D.B., Zandi-Nejad K. Disorders of potassium balance. In: Brenner B.M., editor. Brenner and Rector’s The Kidney. Philadelphia: 2008. p. 547. [Google Scholar]
- 14.Ferrannini E., Taddei S., Santoro D., et al. Independent stimulation of glucose metabolism and Na+-K+ exchange by insulin in the human forearm. Am. J. Physiol. 1988;255(6 Pt 1):E953–E958. doi: 10.1152/ajpendo.1988.255.6.E953. [DOI] [PubMed] [Google Scholar]
- 15.Clause T., Everts M.E. Regulation of the Na, K-pump in skeletal muscle. Kidney Int. 1989;35(1):1–13. doi: 10.1038/ki.1989.1. [DOI] [PubMed] [Google Scholar]
- 16.Alpern R.J., Saxton C.R., Seldin D.W. Clinical interpretation of laboratory values. In: Kokko J.P., Tannen R.L., editors. Fluids and Electrolytes. Philadelphia: WB Saunders; 1990. pp. 3–69. [Google Scholar]
- 17.Katz M.A. Hyperglycemia-induced hyponatremia--calculation of expected serum sodium depression. N. Engl. J. Med. 1973;289(16):843–844. doi: 10.1056/NEJM197310182891607. [DOI] [PubMed] [Google Scholar]
- 18.Hillier T.A., Abbott R.D., Barrett E.J. Hyponatremia: evaluating the correction factor for hyperglycemia. Am. J. Med. 1999;106(4):399–403. doi: 10.1016/s0002-9343(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 19.Emmett M. 2013. [Google Scholar]
- 20.Nguyen M.K., Kurtz I. Are the total exchangeable sodium, total exchangeable potassium and total body water the only determinants of the plasma water sodium concentration? Nephrol. Dial. Transplant. 2003;18(7):1266–1271. doi: 10.1093/ndt/gfg112. [DOI] [PubMed] [Google Scholar]
- 21.Spasovski G., Vanholder R., Allolio B., et al. Clinical practice guideline on diagnosis and treatment of hyponatremia. Nephrol. Dial. Transplant. 2014;29(2):1–39. doi: 10.1093/ndt/gfu040. [DOI] [PubMed] [Google Scholar]
- 22.Bartoli E., Bergamasco L., Castello L., Sainaghi P.P. Methods for the quantitative assessment of electrolyte disturb-ances in hyperglycaemia. Nutr. Metab. Cardiovasc. Dis. 2009;19(1):67–74. doi: 10.1016/j.numecd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Bartoli E., Castello L., Sainaghi P.P., Bergamasco L. Quantitative assessment of the abnormalities of hyperosmolar coma when glucose excess is larger than Na deficit. Exp. Clin. Endocrinol. Diabetes. 2009;117(10):587–592. doi: 10.1055/s-0029-1225354. [DOI] [PubMed] [Google Scholar]
- 24.Bergamasco L., Sainaghi P.P., Castello L., Vitale E., Casagranda I., Bartoli E. Assessing water-electrolyte changes of hyperglycaemic hyperosmolar coma. Exp. Clin. Endocrinol. Diabetes. 2012;120(5):296–302. doi: 10.1055/s-0031-1273751. [DOI] [PubMed] [Google Scholar]
- 25.Monnet X., Teboul J.L. Transpulmonary thermodilution: advantages and limits. Crit. Care. 2017;21(1):147–158. doi: 10.1186/s13054-017-1739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jozwiak M., Monnet X., Teboul J.L. Monitoring: from cardiac output monitoring to echocardiography. Curr. Opin. Crit. Care. 2015;21(5):395–401. doi: 10.1097/MCC.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 27.McGrath S.P., Ryan K.L., Wendelken S.M., Rickards C.A., Convertino V.A. Pulse oximeter plethysmographic waveform changes in awake, spontaneously breathing, hypovolemic volunteers. Anesth. Analg. 2011;112(2):368–374. doi: 10.1213/ANE.0b013e3181cb3f4a. [DOI] [PubMed] [Google Scholar]
- 28.Vetrone F., Santarelli S., Russo V., et al. Copeptin decrease from admission to discharge has favorable prognostic value for 90-day events in patients admitted with dyspnea. Clin. Chem. Lab. Med. 2014;52(10):1457–1464. doi: 10.1515/cclm-2014-0207. [DOI] [PubMed] [Google Scholar]
- 29.Bartoli E., Guidetti F., Bergamasco L. Estimating excess glucose, sodium and water deficits in non-ketotic hyperglycaemia. Nephrol. Dial. Transplant. 2007;22:3478–3486. doi: 10.1093/ndt/gfm427. [DOI] [PubMed] [Google Scholar]
- 30.Chiasson J.L., Aris-Jilwan N., Bélanger R., et al. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ. 2003;168(7):859–866. [PMC free article] [PubMed] [Google Scholar]
- 31.Modi A., Agrawal A., Morgan F. Euglycemic diabetic ketoacidosis: A review. Curr. Diabetes Rev. 2017;13(3):315–321. doi: 10.2174/1573399812666160421121307. [DOI] [PubMed] [Google Scholar]
- 32.Waldhäusl W., Kleinberger G., Korn A., Dudczak R., Bratusch-Marrain P., Nowotny P. Severe hyperglycemia: effects of rehydration on endocrine derangements and blood glucose concentration. Diabetes. 1979;28(6):577–54. doi: 10.2337/diab.28.6.577. [DOI] [PubMed] [Google Scholar]
- 33.Fayfman M., Pasquel F.J., Umpierrez G.E. Management of Hyperglycemic Crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med. Clin. North Am. 2017;101(3):587–606. doi: 10.1016/j.mcna.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lund-Andersen H. Transport of glucose from blood to brain. Physiol. Rev. 1979;59(2):305–352. doi: 10.1152/physrev.1979.59.2.305. [DOI] [PubMed] [Google Scholar]
- 35.Popli S., Leehey D.J., Daugirdas J.T., et al. Asymptomatic, nonketotic, severe hyperglycemia with hyponatremia. Arch. Intern. Med. 1990;150(9):1962–1964. [PubMed] [Google Scholar]
- 36.Lien Y.H., Shapiro J.I., Chan L. Effects of hypernatremia on organic brain osmoles. J. Clin. Invest. 1990;85(5):1427–1435. doi: 10.1172/JCI114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcin J.P., Glaser N., Barnett P., et al. American academy of pediatrics. The pediatric emergency medicine collaborative research committee. Factors associated with adverse outcomes in children with diabetic ketoacidosis-related cerebral edema. J. Pediatr. 2002;141(6):793–797. doi: 10.1067/mpd.2002.128888. [DOI] [PubMed] [Google Scholar]
- 38.Wolfsdorf J., Glaser N., Sperling M.A., American Diabetes Association Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150–1159. doi: 10.2337/diacare.2951150. [DOI] [PubMed] [Google Scholar]
- 39.Yun C., Xuefeng W. Association between seizures and diabetes mellitus: a comprehensive review of literature. Curr. Diabetes Rev. 2013;9(4):350–354. doi: 10.2174/15733998113099990060. [DOI] [PubMed] [Google Scholar]
- 40.Arieff A.I. Cerebral edema complicating nonketotic hyperosmolar coma. Miner. Electrolyte Metab. 1986;12(5-6):383–389. [PubMed] [Google Scholar]
- 41.Lawrence S.E., Cummings E.A., Gaboury I., Daneman D. Population-based study of incidence and risk factors for cerebral edema in pediatric diabetic ketoacidosis. J. Pediatr. 2005;146(5):688–692. doi: 10.1016/j.jpeds.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 42.Edge J.A., Hawkins M.M., Winter D.L., Dunger D.B. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch. Dis. Child. 2001;85(1):16–22. doi: 10.1136/adc.85.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glaser N.S., Wootton-Gorges S.L., Buonocore M.H., et al. Frequency of sub-clinical cerebral edema in children with diabetic ketoacidosis. Pediatr. Diabetes. 2006;7(2):75–80. doi: 10.1111/j.1399-543X.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 44.Glaser N.S., Marcin J.P., Wootton-Gorges S.L., et al. Correlation of clinical and biochemical findings with diabetic ketoacidosis-related cerebral edema in children using magnetic resonance diffusion-weighted imaging. J. Pediatr. 2008;153(4):541–546. doi: 10.1016/j.jpeds.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 45.Krane E.J., Rockoff M.A., Wallman J.K., Wolfsdorf J.I. Subclinical brain swelling in children during treatment of diabetic ketoacidosis. N. Engl. J. Med. 1985;312(18):1147–1151. doi: 10.1056/NEJM198505023121803. [DOI] [PubMed] [Google Scholar]
- 46.Bohn D., Daneman D. Diabetic ketoacidosis and cerebral edema. Curr. Opin. Pediatr. 2002;14(3):287–291. doi: 10.1097/00008480-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Glaser N.S., Wootton-Gorges S.L., Marcin J.P., et al. Mechanism of cerebral edema in children with diabetic ketoacidosis. J. Pediatr. 2004;145(2):164–171. doi: 10.1016/j.jpeds.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 48.Glaser N.S., Wootton-Gorges S.L., Buonocore M.H., et al. Subclinical cerebral edema in children with diabetic ketoacidosis randomized to 2 different rehydration protocols. Pediatrics. 2013;131(1):e73–e80. doi: 10.1542/peds.2012-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sterns R.H., Riggs J.E., Schochet S.S., Jr Osmotic demyelination syndrome following correction of hyponatremia. N. Engl. J. Med. 1986;314(24):1535–1542. doi: 10.1056/NEJM198606123142402. [DOI] [PubMed] [Google Scholar]
- 50.Baker E.A., Tian Y., Adler S., Verbalis J.G. Blood-brain barrier disruption and complement activation in the brain following rapid correction of chronic hyponatremia. Exp. Neurol. 2000;165(2):221–230. doi: 10.1006/exnr.2000.7474. [DOI] [PubMed] [Google Scholar]
- 51.Lien Y.H. Role of organic osmolytes in myelinolysis. A topographic study in rats after rapid correction of hyponatremia. J. Clin. Invest. 1995;95(4):1579–1586. doi: 10.1172/JCI117831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kote S.S., Khandelwal A., Pathak D.G., Nath R. An unusual case of osmotic demyelination syndrome without electrolyte changes in a patient with diabetes. J. Neuroanaesth. Crit. Care. 2016;3:145–148. [Google Scholar]
- 53.Rodríguez-Velver K.V., Soto-Garcia A.J., Zapata-Rivera M.A., Montes-Villarreal J., Villarreal-Pérez J.Z., Rodríguez-Gutiérrez R. Osmotic demyelination syndrome as the initial manifestation of a hyperosmolar hyperglycemic state. Case Rep. Neurol. Med. 2014;2014:652523. doi: 10.1155/2014/652523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hegazi M.O., Mashankar A. Central pontine myelinolysis in the hyperosmolar hyperglycaemic state. Med. Princ. Pract. 2013;22(1):96–99. doi: 10.1159/000341718. [DOI] [PMC free article] [PubMed] [Google Scholar]