Abstract
Fluorescent dye staining combined with fluorescence microscopy or flow cytometry is becoming a routine way to monitor microorganism viability that is necessary for food safety, antibiotic development, and human health. However, the conventional live/dead assay dyes suffer from high cost, inconvenient staining steps, and high cytotoxicity, which is urgently needed to overcome. Herein, cheap carbon dots, CDs-EPS605, were reported to successfully assess microbial viability in a convenient way with neglectable cytotoxicity. The fluorescent N-doped CDs-EPS605 could be facilely prepared from bacterial amino exopolysaccharide (EPS) by one-step hydrothermal carbonization, which is cost-effective and sustainable. The negatively charged CDs-EPS605 consisted of C, H, O, N, P, and S, and featured various functional groups, including -COOH, -OH, -CONH-, and -NH2. CDs-EPS605 were observed to sensitively and selectively stain dead microorganisms instead of live ones to enable discrimination of live/dead microorganisms. The labeling method with CDs-EPS605 did not require protection from light, or washing, which is convenient. Additionally, CDs-EPS605 displayed better photostability and much less cytotoxicity compared to the commercial counterpart. Altogether, CDs-EPS605 represent a simple, yet powerful staining agent for microbial viability assessment, and at the same time enrich the current applications of microbial EPS.
Keywords: carbon-based nanomaterial, polysaccharides, microbial viability assessment, biocompatibility, photostability
Introduction
Microbial viability assessment is commonly performed during pathogen detection, antibiotic development and microbial monitoring in both industrial and biomedical fields (Breeuwer and Abee, 2000). It is also a routine assay in microorganism-related study in the lab. Conventionally, microbial viability assessment was carried out by plate counting that is time-consuming and labor-intensive with low sensitivity and large deviation (Zhao et al., 2014). Other methods like atomic force microscopy (AFM) (Fantner et al., 2010), fourier transform infrared spectroscopy (FT-IR) (Notingher et al., 2003), surface-enhanced raman scattering (Zhou et al., 2015) and nucleic acid sequence-based amplification (Keer and Birch, 2003) have been under development to gain higher accuracy, but they are time-consuming and costly, requiring pretreatment of organisms. Compared to these methods, fluorescent dye staining paired with fluorescence microscopy or flow cytometry is far more used for the differentiation of live/dead microorganism due to that it is simple, fast, sensitive, and visible (Breeuwer and Abee, 2000; Zhao et al., 2014). However, the commercial dyes of this fluorescence-based method, such as propidium monoazide (PMA) and propidium iodide (PI), that selectively label dead cells, suffer from high cost, toxicity, unstability, photobleaching, and cumbersome protocol (Breeuwer and Abee, 2000; Zhao et al., 2014). Therefore, developing desirable dyes for microbial viability evaluation is of great value.
Carbon dots (CDs) are commonly carbon nanoparticles with sizes less than 10 nm that may also contain oxygen, hydrogen, and other elements. CDs are well known for their high water-dispersity, superior photostability, good biocompatibility, and low cost, making them attractive photoluminescent nanomaterials. They have found a wide range of biological applications including bioimaging, biosensing, thermometer, photothermal therapy, photodynamic therapy, and drug delivery (Reckmeier et al., 2016). For bioimaging, they have been successfully utilized to discriminate gram positive/negative bacteria (Yang et al., 2016), and live/dead microorganisms (Hua et al., 2017). The amphiphilic C-dots have been synthesized and employed as an excellent tool for labeling biomimetic and cellular membranes (Nandi et al., 2014, 2015), and imaging biofilm matrix (Ritenberg et al., 2016). CDs synthesized from Lactobacillus plantarum by one-step hydrothermal carbonization were used successfully for imaging the biofilm-encased microorganisms (Lin et al., 2017).
Exopolysaccharides (EPSs) secreted out of microorganisms are sugar biopolymers consisting of homo- or hetero-saccharides that are linked together by glycosidic bonds to generate long linear or branched chains. The naturally occurring EPSs are abundant, readily available, biodegradable, and potentially non-toxic (Moscovici, 2015; Bhatia, 2016). EPSs have found a vast range of applications in nanotechnology, like green synthesis of metal nanoparticles (Kanmani and Lim, 2013; Sathiyanarayanan et al., 2017), hydrogel (Na et al., 2000; Maiti et al., 2011; Koop et al., 2015), coating materials (Jang et al., 2013; You et al., 2014; Bondarenko et al., 2016), stabilizers (Dey et al., 2016), nanoparticles (Wang et al., 2013; Guo et al., 2014; Zhang et al., 2016), capping agents (Wu et al., 2012; Yan et al., 2015). Even more, EPS can self-assembled into different nanostructures like nanofibers (Xu et al., 2013; Chen et al., 2016) and nanoparticles (Li et al., 2017), which could be employed as promising bio-active carriers for DNA (Liu et al., 2014, 2015), protein (Takahashi et al., 2014), and drug (Takedatsu et al., 2012). They can be further modified into nanocomplexes for drug delivery applications. Though many bio-macromolecules including cellulose, chitosan, proteins, and gelatin have used as molecular precursors of CDs (Reckmeier et al., 2016), so far there are few study on bacterial EPS-based CDs.
In this study, fluorescent N-doped CDs were successfully prepared by a facile hydrothermal method using bacterial amino EPS as the precursors. The microstructure, chemical compositions, optical properties of these CDs were investigated. The as-synthesized CDs have been demonstrated to be capable of discriminating live/dead microorganisms, which was evaluated and compared to the commercial counterpart in terms of universality, photostability, and biocompatibility.
Materials and Methods
Lactobacillus plantarum LLC-605 was obtained from the traditional Chinese fermented food FuYuan Pickles (Yunnan, China) with excellent exopolysaccharide-producing ability in our lab (Li et al., 2017). E. coli, S. aureus, M. luteus, B. subtilis, and P. pastoris were ordered from China Center of Industrial Culture Collection (CICC, Beijing, China). Extracellular polysaccharide EPS-605 was purified from the fermentation broth of L. plantarum LCC-605, as described in our previous study (Li et al., 2017). Dialysis membranes (Spectra/Por6 Dialysis membranes, Regenerated Cellulose) with a molecular weight cutoff of 1 kDa were purchased from Sangon Biotech. Co., Ltd. (Shanghai, China). Lysogeny broth (LB), potato dextrose agar (PDA), and potato dextrose broth (PDB) were ordered from Beijing Land Bridge Technology (Beijing, China).
CDs Preparation
CDs were fabricated from the purified EPS-605 by one-step hydrothermal carbonization. EPS-605 was dissolved in 10 mL Milli-Q water to reach the final concentration of 2 mg/mL, transferred into a 50 mL Teflon-lined stainless-steel autoclave, and then incubated at 200°C for 24 h. The resulting dark brown solution was cooled down at room temperature and centrifuged at 12000 rpm for 10 min. The supernatant was filtered through a 0.22 μm filter membrane to further remove large particles/aggregates. At last, the CD solution was dialyzed in a 1 kDa cutoff membrane against Milli-Q water for 48 h, and stored at 4°C for further use.
Characterizations of CDs
The produced CDs from EPS-605 were characterized in terms of the size, morphology and element components. A drop of CD solution was deposited on a 400-mesh carbon-coated copper grid and examined using a transmission electron microscope (JEM-2100, JEOL Ltd., Japan). Ultraviolet-visible (UV-vis) and fluorescence spectra of CDs in pure water or phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, 1.7 mM KH2PO4, pH 7.4) were measured by a UV–vis spectrophotometer (UV-2600, Shimadzu, Japan) and a spectrofluorophotometer (RF-5301PC, Shimadzu, Japan), respectively. Fourier transform infrared (FTIR) spectroscopy of CDs was obtained on an FTIR spectrometer (Nicolet iS50, Thermo Scientific, United States). X-ray photoelectron spectroscopic (XPS) experiment was carried out with a Japan Kratos Axis Ultra HAS spectrometer. Zeta potential values of CDs were measured using a Zetasizer instrument (Malvern Instruments, Nano ZS, United Kingdom). The fluorescence QY of the CDs was calculated according to the method previously reported (Yang et al., 2015; Pramanik et al., 2016a,b). Briefly, a comparative assay was utilized using the following equation (Yang et al., 2015):
Where ∅, I, A, and η presents the fluorescence quantum yield, the integrated area in the emission spectrum, UV-vis absorbance at the fluorescent excitation wavelength, and the refractive index of the solvent, respectively. The subscripts “c” and “s” denote the CDs solution and the standard solution of quinine sulfate in 0.1 M H2SO4, respectively. Ac and As obtained in a 1 cm cuvette should be less than 0.1 to avoid the reabsorption effect. The QY of a quinine sulfate solution excited at 306 nm is reported to be 54% (Yang et al., 2015).
Differentiation of Live/Dead Microorganisms
Bacteria were grown in LB at 37°C for 14–18 h, while fungi were cultured in PDB at 32°C for 24–48 h. Dead cells were prepared by boiling at 100°C for 30 min. Both live and dead cells were incubated with 0.8 mg/mL CDs-EPS605 for 90 min without light protection, and then observed immediately on a fluorescent confocal microscope (TCS SP8, Leica, Germany) without washing. The excitation wavelengths were 405, 488, and 552 nm with the corresponding emissions detected in the range of 405–490, 500–565, and 570–630 nm, respectively.
Comparison Between CDs-EPS605 and PI on the Live/Dead Cell Assessment
The imaging performance of CDs-EPS605 and the commercial dye PI was compared. A mixture of live and dead S. aureus was incubated with the mixture of CDs-605 (800 ug/mL) and PI for 90 min in dark, and then imaged under a confocal microscope. PI has an excitation/emission maxima of 488/615 nm. The imaging conditions were finely tuned to realize that only the desired dye PI showed red fluorescence under the excitation wavelength of 488 nm, so that the effect of the spectral overlap between CDs-EPS605 and PI in the co-labeling experiments was avoided. Namely, under the tested conditions in this study, the red fluorescence intensity of CDs-EPS605 was much lower than that of PI, so we reduced the excitation power to achieve that CDs-EPS605 did not display red fluorescence that was evidenced with dead cell samples stained only with CDs-EPS605, while PI still had red fluorescence that was demonstrated with dead cell samples labeled with CDs-EPS605 alone.
Photostability Assay
For the UV irradiation experiment, the corresponding PL intensities of the CD solutions were recorded after irradiation under a UV lamp of 365 nm (20 mW) for different times as indicated in the text. The CD solutions with different pH values (3, 5, 7, 9, and 11) were made with the pH adjusted via concentrated HCl or NaOH solution, whose PL intensities were assayed at 500 nm (λex = 460 nm) on the spectrofluorophotometer. The PL intensities of the CD solutions at various temperatures (4, 25, 40, 55, 70, 85, and 100°C) and different buffer concentrations (0, 10, 100, 200, 300, 400, 500, 600, 700, 800, and 900 mM) were measured. The same experiments were performed for the small molecular dye PI as a comparison.
Effect of CDs-EPS605 on Microorganism Growth
Both E. coli and S. aureus were grown in LB overnight with 180 rpm at 37°C, while P. pastoris was grown in PDA with 180 rpm at 32°C. The cell culture was diluted 1:100 in PDA or LB with different concentrations of CDs-EPS605 (0, 0.1, 0.2, 0.4, 0.8, 1, and 3 mg/mL), of which 200 μL/well was transferred into the 96-well plates (Costar, Corning, United States). The inoculated 96-well plates were incubated for 24 h at 37°C with 180 rpm. Then, the OD of the cell cultures was recorded at 600 nm.
Colony forming unit (CFU) counting method was performed to further study the cytotoxicity of CDs-EPS605 to E. coli. Briefly, E. coli in log phase were grown in LB in the presence of 3 mg/mL CDs-EPS605 at 37°C with 180 rpm for 24 h. Each culture was diluted with LB with a dilution factor of 1 × 105. The diluted microbial culture were plated on LB agar plates in triplicate and incubated at 37°C for 24 h. Then, the cell colonies of E. coli were measured. Three biological replicates were carried out. The cytotoxicity was determined by comparing the number of CFUs in treated groups to that in the control group.
Results and Discussion
Synthesis and Characterization of Carbon Dots
We considered microbial exopolysaccharide EPS-605 as an excellent raw material for CDs synthesis, given that it contains elements C, H, O, S, P, and N (Li et al., 2017), providing both the major carbon framework and other elements for doping to produce CDs without the utilization of passive agents (Reckmeier et al., 2016). To prove this, purified EPS-605 from strain L. plantarum LLC-605 was exploited to prepare CDs by one-step hydrothermal reaction. The as-produced CDs were referred to as CDs-EPS605. The TEM image showed that CDs-EPS605 were well monodispersed and quasi-spherical, with an average diameter of 4.3 nm (Figure 1A). In the high-resolution image of CDs-EPS605 (Figure 1B), a crystalline structure was observed. CDs-EPS605 were negatively charged with a zeta potential of -24 mV (Figure 1C), owing to that the raw material EPS-605 has a highly negative charge of -32.6 mV (Li et al., 2017). CDs-EPS605 exhibited strong blue fluorescence when excited at 365 nm with an ultraviolet lamp (Figure 1D), demonstrating CDs-EPS605 were well dispersed in aqueous solution. The UV-VIS absorption spectrum of CDs-EPS605 exhibited a huge absorption peak at 320 nm (Figure 1D), which might be due to the n-π∗ transition of C = O group on the surface of CDs-EPS605 (Figures 2A,E; Song et al., 2017). Except this, no characteristic absorption peak was found as reported in other carbon nanomaterials (Zhao et al., 2011; Jiang et al., 2017; Lin et al., 2017).
FIGURE 1.
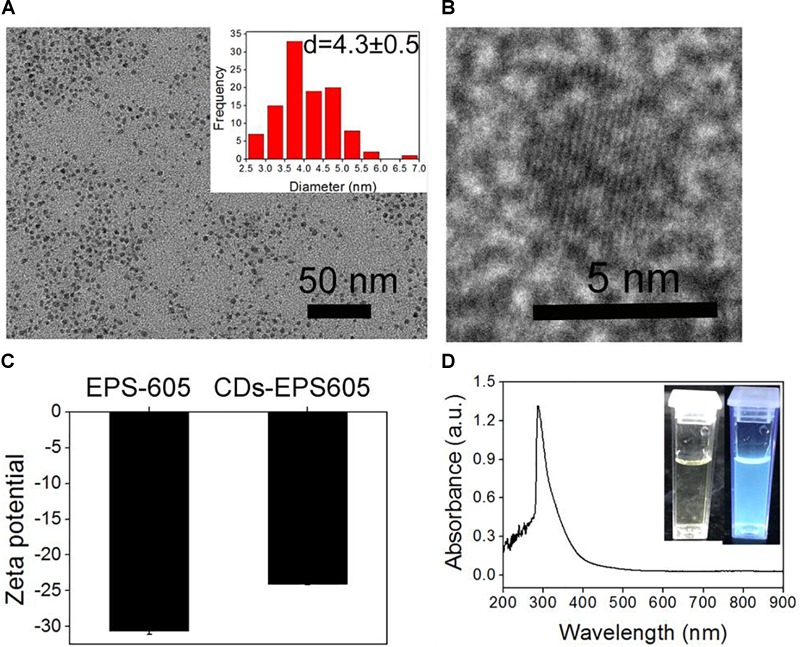
Profiling of CDs-EPS605. (A) TEM image of CDs-EPS605. Inset: the corresponding size histogram. (B) The high resolution TEM of CDs-EPS605. (C) The zeta potential of CDs-EPS605. (D) The UV-Vis absorption spectra of CDs-EPS605 in H2O. Inset: photographs of CDs-EPS605 in H2O without and with the UV irradiation at 365 nm.
FIGURE 2.
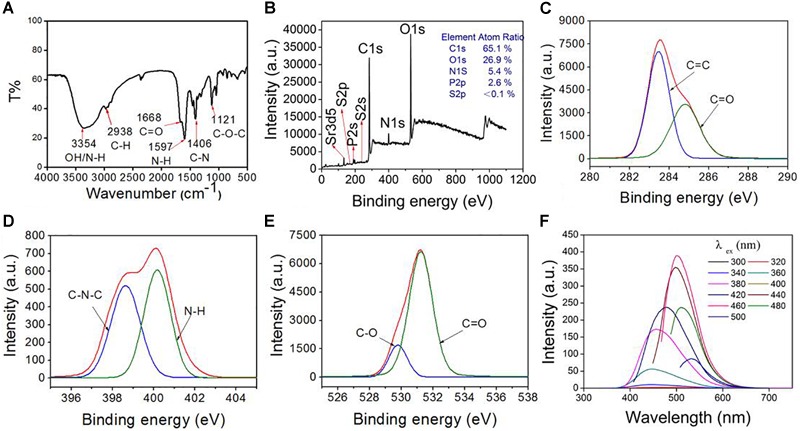
The compositions of CDs-EPS605 were determined by FTIR (A) and XPS (B). The high-resolution XPS peaks of C1s (C), N1s (D), and O1s (E). (F) Fluorescence spectra of CDs-EPS605 in water.
Compositions of CDs-EPS605
The compositions of CDs-EPS605 were detected by FTIR and XPS. The broad absorption band around 3354 cm-1 was assigned to the stretching vibrations of O-H [ν(O-H)] and/or N-H [ν(N-H)] (Figure 2A). The peak at 1668 cm-1 belonged to the C = O stretching vibration, ν(C = O), due to the -COOH group or the amide bond (-CONH-). The peak at 1597 cm-1 was attributed to the N-H bending vibration δ(N-H), due to the NH2 group or the amide bond (-CONH-). The peak at 1406 cm-1 might originated from the O-H/C-H bending vibration [δ(O-H)/δ(C-H)], and the one at 1121 cm-1 was owning to the C-O stretching vibrations [ν(C-O)].
CDs-EPS605 contains the elements of C (65.1%), O (26.9%), N (5.4%), P (2.6%), and S (<0.1%) as determined by XPS (Figure 2B). In the high-resolution C1s spectrum (Figure 2C), the two fitted peaks at 284.6 and 285.9 eV were attributed to carbon-containing groups C-C/C = C (sp2 carbons) and C-N, respectively (Bao et al., 2015). The N1s peak at 399.7 and 401.3 eV (Figure 2D) were separately ascribed to amide nitrogen C-N-C and the amino nitrogen N-H (Yang et al., 2014; Zhang and Chen, 2014). For the O1s spectrum, the two fitted peaks at 530.9 and 532.3 eV (Figure 2E) were attributed to the oxygen element in the forms of C = O and C-O, respectively (Zhang and Chen, 2014; Ding et al., 2016). Overall, the FTIR and XPS results demonstrated that the as-fabricated CDs-EPS605 contains the elements C, H, O, S, P, and N, and the functional groups -OH, -COOH, -CONH-, and -NH2. These findings show that the functional groups of the starting material EPS-605 are conferred onto CDs-EPS605 (Li et al., 2017).
Optical Properties of CDs-EPS605
When the excitation wavelength was changed from 360 to 420 nm or from 440 to 500 nm, no red-shift was observed for the emission wavelength. Clearly, the fluorescence emission spectrum of CDs-EPS605 only partly relies on the excitation wavelength. The emission intensity of CDs-EPS605 rose steadily from 300 nm to 460 nm with a peak at 460 nm, and then fell from 480 nm to 520 nm (Figure 2F). The maximum fluorescence emission intensity was found at 500 nm with an excitation wavelength of 460 nm. The QY of CDs-EPS was 3.86%. Obviously, CDs-EPS605 were N-doped and multi-colored luminescent.
The starting material for CDs-EPS605, exopolysaccharide EPS-605, can be readily obtained from L. plantarum LCC-605 that is abundant, renewable, and biocompatible. Meanwhile, the synthesis method is simple and facile. Compared to other EPSs derived from animals like chitin or from plants like cellulose and gelatin that have been employed as precursors for production of CDs (Chowdhury et al., 2012; Shchipunov et al., 2015; Shen et al., 2016), the production of microbial EPS from fermentation is timesaving and controllable with much higher production titer (Moscovici, 2015). The defined and reproducible EPS production by easily controlled fermentation parameters would ensure a high quality of CDs-EPS production. By contrast, using biomass for CD fabrication such as galic (Zhao et al., 2015), fruit juice(Sahu et al., 2012) and bacteria (Hua et al., 2017), whose components is very complicated and difficult to be profiled, suffer from uncontrolled quality from batch to batch (Reckmeier et al., 2016). Furthermore, EPS605 contains 7% element N as measured by XPS (Li et al., 2017) and thus no dopants or surface passivation agents is required for the synthesis of N-doped CDs-EPS605. When using polysaccharides without N element, dopants such as urea (Shen et al., 2016) and ethylenediamine (Chen et al., 2017) were added to introduce into CD structures N element that has a favorable effect on the CD optical properties. Other N-containing polysaccharides like chitin and chitosan have also been used as raw materials for CD production (Chowdhury et al., 2012; Shchipunov et al., 2015). Nevertheless, these two polysaccharide suffer from poor water solubility, and strong acid is used like nitric acid (Shchipunov et al., 2015) and acetic acid (Chowdhury et al., 2012) in the hydrothermal reaction. Notably, EPS605 represent an ideal precursor for the production of green N-doped CDs for high sustainability, readily availability, controlled quality, N-containing, and good water solubility.
CDs-EPS605 Can Discriminate Live/Dead S. aureus
Both live and dead S. aureus were incubated with CDs-EPS605 without light protection and then examined under confocal laser scanning microscopy (CLSM) without washing (Figure 3). According to the fluorescence spectra of CDs-EPS605 (Figure 2F), the sample was viewed in three channels including blue, green and red when excited at 405, 488, and 552 nm, respectively. No fluorescence was observed for the live and healthy S. aureus cultured at 37°C overnight, indicating they were not labeled by CDs-EPS605. In contrast, strong fluorescence was detected in all three tested channels for dead S. aureus killed by boiling for 10 min, demonstrating that the dead cells were stained by CDs-EPS605. Therefore, CDs-EPS605 can be used to discriminate between live/dead S. aureus cells. Meanwhile, the three-color imaging of dead S. aureus of CDs-EPS605 enables flexibility in terms of the laser options for excitation.
FIGURE 3.
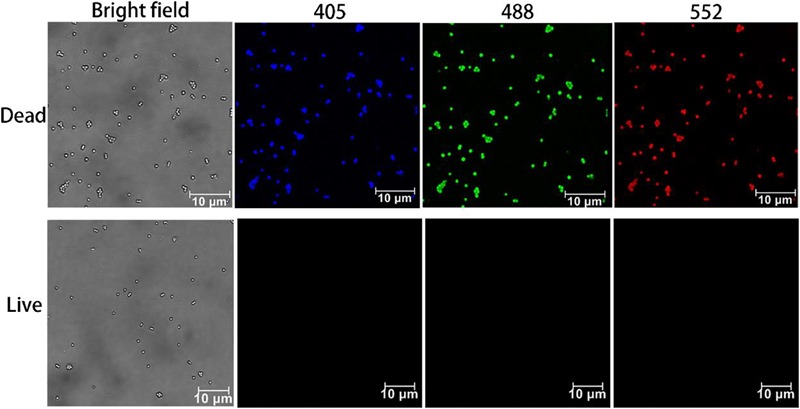
Confocal images of live and dead S. aureus labeled with CDs-EPS605. Fluorescence images were obtained under the excitation of 405, 488, and 552 nm, respectively. Bright field images were also presented for reference.
To further confirm the live/dead microorganism discrimination ability of CDs-EPS605, a mixture of live and dead S. aureus was co-stained by CDs-EPS605 and the commercial live/dead assay dye PI that is well-known for selectively labeling dead cells but not live ones to distinguish live/dead cells. The fluorescence of CDs-EPS605 co-localized well with that of PI, and live S. aureus were not labeled by either PI or CDs-EPS605 (Figure 4), confirming that CDs-EPS605 could only stain dead S. aureus.
FIGURE 4.
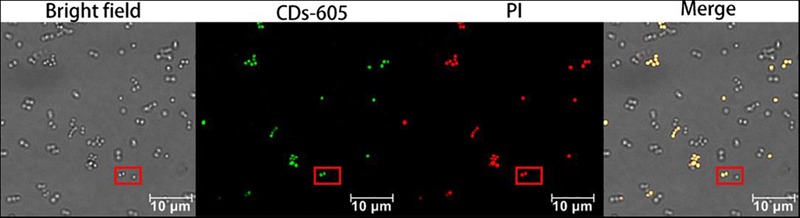
Confocal fluorescence images of live and dead S. aureus co-labeled with CDs-EPS605 and PI. The excitation wavelength was 488 and 552 nm for CDs-EPS605 and PI, respectively. In the red squares, two dead S. aureus cells were co-labeled by both CDs-EPS605 and PI, while one live S. aureus cell was not stained.
CDs-EPS605 Possess Excellent Photostability
Good photostability of dyes under various experimental conditions is important and valuable for practical applications. Hence, the effect of UV irradiation time, temperature, buffer concentration and pH on the photoluminescence (PL) property of both CDs-EPS605, and the commercial dye PI in solutions, was evaluated (Figure 5). The PL of both CDs-EPS605 and PI had a slight decrease under the continuous UV irradiation for 90 min (Figure 5A). The PL of CDs-EPS605 remained almost unchanged as the buffer concentration or the temperature was increased, which is different from that the PL of PI was reduced noticeably (Figures 5B,C). At pH 3–11, a larger extend of fickleness of PL was observed for CDs-EPS605 than PI, indicating CDs-EPS was more sensitive to pH than PI (Figure 5D). In all tested conditions, at least 80% PL of CDs-EPS605 was maintained, showing the excellent photostability of CDs-EPS605 under various environment conditions (Figure 5). Obviously, the photostability of CDs-EPS605 is better than PI, making the live/dead microorganism assessment more flexible and convenient.
FIGURE 5.
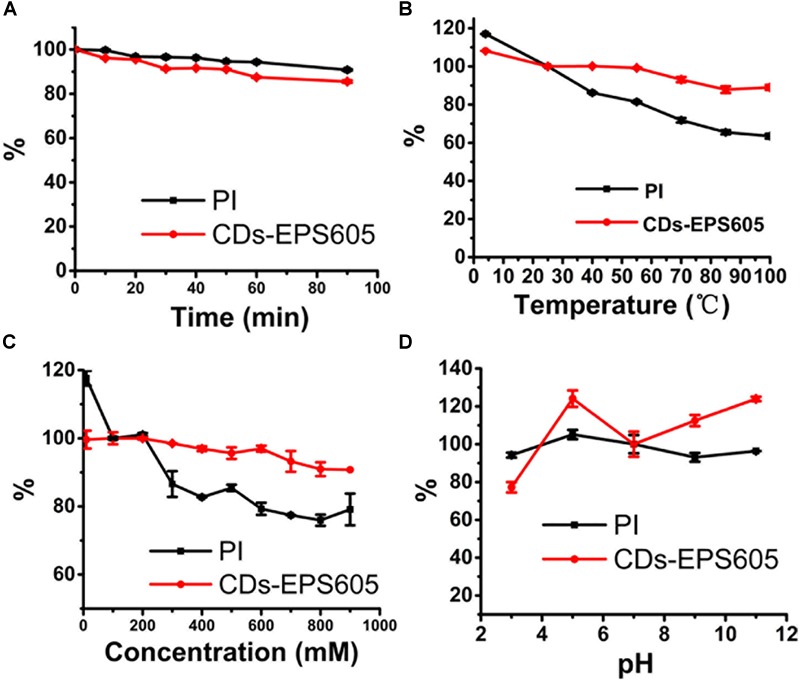
PL properties of CDs-EPS605 as a function of UV irradiation time (A), temperature (B), ionic strength (different concentrations of PBS solution, pH = 7.4) (C) and pH (D). The PL intensity was measured at 514 nm (λex = 488 nm). The PL intensity of CDs-605 in PBS (pH = 7.4) without laser irradiation was measured at 25°C and was arbitrarily assigned as 100%.
The Live/Dead Cell Distinction by CDs-EPS605 Is Microorganism-Universal
The universality of the live/dead cell differentiation with CDs-EPS605 is explored by incubating CDs-EPS605 with different bacteria, both dead and live, including M. luteus (Gram positive), S. subtilis (Gram positive), E. coli (Gram negative), and fungus P. pastoris (Figure 6 and Supplementary Figure S1). For all tested microorganisms, only dead microorganisms show strong florescence in the blue, green and red channels excited with 405, 488, and 552 nm, respectively (Figure 6), whereas no fluorescence was observed for the corresponding live ones (Supplementary Figure S1). These findings demonstrate CDs-EPS605 could selectively stain dead microorganisms regardless of their species and can be applied to discriminate between live and dead microorganisms.
FIGURE 6.
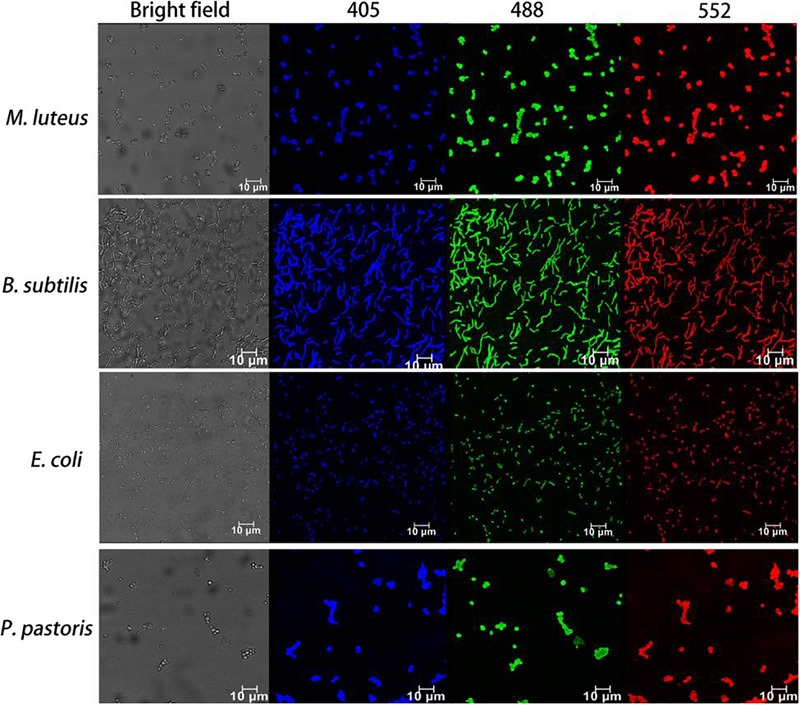
Fluorescence images of dead microorganisms covering two other Gram-positive bacteria (M. luteus and B. subtilis), one Gram-negative bacterium (E. coli) and yeast (P. pastoris) labeled with CDs-EPS605. The corresponding bright field was also presented. Fluorescence images were observed with the excitation length of 405, 488, and 552 nm, respectively.
CDs-EPS605 Are Non-toxic Toward Microorganisms
To assess the toxicity of CDs-EPS605 on microorganisms, the effect of CDs-EPS605 on microorganism growth was figured out by determining the optical density of cell culture at 600 nm (OD600). S. aureus, E. coli, and P. pastoris, each as the model for Gram-positive bacteria, Gram-negative bacteria, and fungi, respectively, were chosen for the evaluation. The OD600 of cell culture from these three microorganisms remained unaltered when grown for 24 h in the presence of CDs-EPS605 of 0, 0.1, 0.2, 0.4, 0.8, 1, and 3 mg/mL. This implied that CDs-EPS605 had no toxicity toward microorganisms. This result was further proved by the plate counting result using E. coli as an example (Supplementary Figure S2). It is reported that PI caused a significant toxicity toward both S. aureus and yeast at its working concentration of 30 μM, leading to significantly reduced cell viability of these two microorganisms treated with 30 μM PI for 24 h as compared to the untreated control (Hua et al., 2017). Hence, CDs-EPS605 are more suitable for long-time, accurate labeling of dead microbes than PI.
Currently, the expensive commercial dyes for live/dead assay require picky storage condition (-20°C), sample avoidance from light when staining, and sample washing after the staining, which was not required for CDs-EPS605. The starting material EPS-605 can be generated abundantly by fermentation of L. plantarum using MSA as the carbon source with a high yield of 940 mg/L (Li et al., 2017). MSA is sold at ∼45 $/250 g in China. Meanwhile, the hydythermal reaction is done at 200°C for 24 h, which is also a cheap process. Based on these, CD-EPS605 is considered cost-effect. Furthermore, CDs-EPS605 featured prominent photostability (Figure 5) and no cytotoxicity (Figure 7). Obviously, the discrimination of live/dead microorganisms by CDs-EPS605 is easier and safer than those commercial counterparts, while it is also cheap.
FIGURE 7.
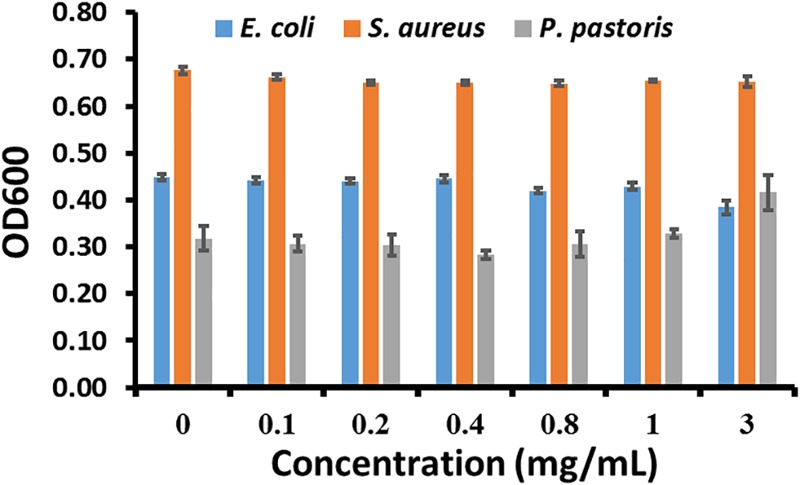
The effect of CDs-EPS605 on growth of microorganisms. The OD600 of E. coli, S. aureus, and P. pastoris for 24 h was measured, respectively in culture media containing 0–3 mg/mL CDs-EPS605 at 37°C with 180 rpm.
Through zeta potential measurement, FTIR, and XPS, it was found that the main surface group of CDs-EPS605 is the negatively charged carboxyl group, leading to a strong electrostatic repulsion interaction between CDs-EPS605 and the negatively charged microorganisms. Additionally, there are hydrophilic groups such as -COOH and -OH on CDs-EPS605, preventing the hydrophobic interaction between CDs-EPS605 and microorganisms. Such highly negative and hydrophilic surface of CDs-EPS605 impedes them from penetrating into the live microorganisms. In contrast, the ultrasmall size of CDs-EPS605 can enter the dead microorganisms with compromised cell walls and plasma membranes, due to that the integrity of these microorganisms’ walls and membranes might be damaged or lost (Strauber and Muller, 2010), achieving the selective staining of these dead ones.
Conclusion
In this study, we reported amino EPS from lactate acid bacteria (LAB) was first time explored as a single precursor to produce fluorescence N-doped CDs viz. CDs-EPS605 through simple one-step hydrothermal reaction without any dopants and passivation agents. The participating amino EPS derived from fermentation of L. plantarum is readily produced and renewable, making the synthesis route green and eco-friendly. Characterization of CDs-EPS605 showed that CDs-EPS605 had an average diameter of 3.1 nm and display good dispersibility. CDs-EPS605 consisting of the elements C, H, O, N, P, and S with the functional groups -OH, -CONH-, -NH2, and -COOH, were negatively charged and carry multicolor under excitation at different wavelengths. More important, CDs-EPS605 were found to be capable of selectively labeling dead microbial cells but not live ones, which can be applied in the microbial viability assay. Compared to the current live/dead assay dyes such as PI, CDs-EPS605 possessed fascinating merits including multicolor fluorescence emission property, negligible cytotoxicity, excellent photostability, low cost, and convenient staining process. Furthermore, the synthetic strategy of CDs-EPS605 is facile, eco-friendly and economic, enabling the large-scale production of live/dead microbial differentiation kits based on CDs-EPS605, which would broaden the researches of microorganism detection. Our study advances the applications of bacterial EPSs and offers a new, renewable, and eco-friendly dye CDs-EPS605 as an excellent alternative to current commercial live/dead microbial assay dyes.
Author Contributions
FL conceived and designed the study. FL carried out the majority of the experiments. CL conducted the preparation of EPS-605 and CDs-EPS605. CL, FL, and ZC analyzed the data and drafted the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer NKR and handling Editor declared their shared affiliation.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (31700040), the Fundamental Research Funds for the Central Universities and a project funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions. ZC acknowledges the support from University of Michigan.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02697/full#supplementary-material
References
- Bao L., Liu C., Zhang Z. L., Pang D. W. (2015). Photoluminescence-tunable carbon nanodots: surface-state energy-gap tuning. Adv. Mater. 27 1663–1667. 10.1002/adma.201405070 [DOI] [PubMed] [Google Scholar]
- Bhatia S. (2016). “Microbial polysaccharides as advance nanomaterials,” in Systems for Drug Delivery: Safety, Animal, and Microbial Polysaccharides. (Cham: Springer International Publishing; ) 29–54. 10.1007/978-3-319-41926-8_2 [DOI] [Google Scholar]
- Bondarenko O. M., Ivask A., Kahru A., Vija H., Titma T., Visnapuu M., et al. (2016). Bacterial polysaccharide levan as stabilizing, non-toxic and functional coating material for microelement-nanoparticles. Carbohydr. Polym. 136 710–720. 10.1016/j.carbpol.2015.09.093 [DOI] [PubMed] [Google Scholar]
- Breeuwer P., Abee T. (2000). Assessment of viability of microorganisms employing fluorescence techniques. Int. J. Food. Microbiol. 55 193–200. 10.1016/S0168-1605(00)00163-X [DOI] [PubMed] [Google Scholar]
- Chen C., Meng Y., Li S., Wu W., Liu C., Xu X., et al. (2016). Single chain morphology and nanofiber-like aggregates of branched beta-(1 - > 3)-d-glucan in water/dimethylsulfoxide solution. Carbohydr. Polym. 137 287–294. 10.1016/j.carbpol.2015.10.090 [DOI] [PubMed] [Google Scholar]
- Chen J., Wang Q., Zhou J., Deng W., Yu Q., Cao X., et al. (2017). Porphyra polysaccharide-derived carbon dots for non-viral co-delivery of different gene combinations and neuronal differentiation of ectodermal mesenchymal stem cells. Nanoscale 9 10820–10831. 10.1039/c7nr03327 [DOI] [PubMed] [Google Scholar]
- Chowdhury D., Gogoi N., Majumdar G. (2012). Fluorescent carbon dots obtained from chitosan gel. RSC Adv. 2 12156-12159. 10.1039/c2ra21705h 25498649 [DOI] [Google Scholar]
- Dey S., Sherly M. C. D., Rekha M. R., Sreenivasan K. (2016). Alginate stabilized gold nanoparticle as multidrug carrier: evaluation of cellular interactions and hemolytic potential. Carbohydr. Polym. 136 71–80. 10.1016/j.carbpol.2015.09.016 [DOI] [PubMed] [Google Scholar]
- Ding H., Yu S. B., Wei J. S., Xiong H. M. (2016). Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 10 484–491. 10.1021/acsnano.5b05406 [DOI] [PubMed] [Google Scholar]
- Fantner G. E., Barbero R. J., Gray D. S., Belcher A. M. (2010). Kinetics of antimicrobial peptide activity measured on individual bacterial cells using high-speed atomic force microscopy. Nat. Nanotech. 5 280-285. 10.1038/nnano.2010.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Liu Y., Wang Y., Wu J., Yang X., Li R., et al. (2014). Ph-sensitive pullulan-based nanoparticle carrier for adriamycin to overcome drug-resistance of cancer cells. Carbohydr. Polym. 111 908–917. 10.1016/j.carbpol.2014.05.057 [DOI] [PubMed] [Google Scholar]
- Hua X. W., Bao Y.-W., Wang H.-Y., Chen Z., Wu F.-G. (2017). Bacteria-derived fluorescent carbon dots for microbial live/dead differentiation. Nanoscale 9 2150–2161. 10.1039/C6NR06558A [DOI] [PubMed] [Google Scholar]
- Jang H., Ryoo S.-R., Kostarelos K., Han S. W., Min D.-H. (2013). The effective nuclear delivery of doxorubicin from dextran-coated gold nanoparticles larger than nuclear pores. Biomaterials 34 3503–3510. 10.1016/j.biomaterials.2013.01.076 [DOI] [PubMed] [Google Scholar]
- Jiang Y. W., Gao G., Zhang X., Jia H.-R., Wu F.-G. (2017). Antimicrobial carbon nanospheres. Nanoscale 9 15786–15795. 10.1039/C7NR04679K [DOI] [PubMed] [Google Scholar]
- Kanmani P., Lim S. T. (2013). Synthesis and characterization of pullulan-mediated silver nanoparticles and its antimicrobial activities. Carbohydr. Polym. 97 421–428. 10.1016/j.carbpol.2013.04.048 [DOI] [PubMed] [Google Scholar]
- Keer J. T., Birch L. (2003). Molecular methods for the assessment of bacterial viability. J. Microbiol. Methods 53 175–183. 10.1016/S0167-7012(03)00025-3 [DOI] [PubMed] [Google Scholar]
- Koop H. S., de Freitas R. A., de Souza M. M., Savi-Jr R., Silveira J. L. M. (2015). Topical curcumin-loaded hydrogels obtained using galactomannan from schizolobium parahybae and xanthan. Carbohydr. Polym. 116 229–236. 10.1016/j.carbpol.2014.07.043 [DOI] [PubMed] [Google Scholar]
- Li C., Zhou L., Yang H., Lv R., Tian P., Li X., et al. (2017). Self-assembled exopolysaccharide nanoparticles for bioremediation and green synthesis of noble metal nanoparticles. ACS Appl. Mater. Interfaces 9 22808–22818. 10.1021/acsami.7b02908 [DOI] [PubMed] [Google Scholar]
- Lin F., Li C., Dong L., Fu D., Chen Z. (2017). Imaging biofilm-encased microorganisms using carbon dots derived from l. Plantarum. Nanoscale 9 9056–9064. 10.1039/C7NR01975K [DOI] [PubMed] [Google Scholar]
- Liu Q., Wang C., Cao Y., Xu X., Zhang L. (2014). A novel gene carrier prepared from triple helical [small beta]-glucan and polydeoxyadenylic acid. J. Mater. Chem. B 2 933–944. 10.1039/C3TB21195A [DOI] [PubMed] [Google Scholar]
- Liu Q., Xu H., Cao Y., Li M., Xu X., Zhang L. (2015). Transfection efficiency and internalization of the gene carrier prepared from a triple-helical [small beta]-glucan and polydeoxyadenylic acid in macrophage raw264.7 cells. J. Mater. Chem. B 3 3789–3798. 10.1039/C4TB02127D [DOI] [PubMed] [Google Scholar]
- Maiti S., Ranjit S., Mondol R., Ray S., Sa B. (2011). Al + 3 ion cross-linked and acetalated gellan hydrogel network beads for prolonged release of glipizide. Carbohydr. Polym. 85 164–172. 10.1016/j.carbpol.2011.02.010 [DOI] [Google Scholar]
- Moscovici M. (2015). Present and future medical applications of microbial exopolysaccharides. Front. Microbiol. 6:1012. 10.3389/fmicb.2015.01012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na K., Park K.-H., Kim S. W., Bae Y. H. (2000). Self-assembled hydrogel nanoparticles from curdlan derivatives: characterization, anti-cancer drug release and interaction with a hepatoma cell line (hepg2). J. Control. Release 69 225–236. 10.1016/S0168-3659(00)00256-X [DOI] [PubMed] [Google Scholar]
- Nandi S., Malishev R., Parambath Kootery K., Mirsky Y., Kolusheva S., Jelinek R. (2014). Membrane analysis with amphiphilic carbon dots. Chem. Commun. 50 10299–10302. 10.1039/c4cc03504f [DOI] [PubMed] [Google Scholar]
- Nandi S., Ritenberg M., Jelinek R. (2015). Bacterial detection with amphiphilic carbon dots. Analyst 140 4232–4237. 10.1039/c5an00471c [DOI] [PubMed] [Google Scholar]
- Notingher I., Jones J. R., Verrier S., Bisson I., Embanga P., Edwards P., et al. (2003). Application of ftir and raman spectroscopy to characterisation of bioactive materials and living cells. Spectroscopy 17 275–288. 10.1155/2003/893584 [DOI] [Google Scholar]
- Pramanik A., Biswas S., Kole A. K., Tiwary C. S., Krishnaraj R. N., Kumbhakar P. (2016a). Template-free hydrothermal synthesis of amphibious fluorescent carbon nanorice towards anti-counterfeiting applications and unleashing its nonlinear optical properties. RSC Adv. 6 99060–99071. 10.1039/C6RA20442B [DOI] [Google Scholar]
- Pramanik A., Kole A. K., Krishnaraj R. N., Biswas S., Tiwary C. S., Varalakshmi P., et al. (2016b). A novel technique of synthesis of highly fluorescent carbon nanoparticles from broth constituent and in-vivo bioimaging of C. elegans. J. Fluoresc. 26 1541–1548. 10.1007/s10895-016-1854-8 [DOI] [PubMed] [Google Scholar]
- Reckmeier C. J., Schneider J., Susha A. S., Rogach A. L. (2016). Luminescent colloidal carbon dots: optical properties and effects of doping [invited]. Opt. Express 24 A312–A340. 10.1364/oe.24.00a312 [DOI] [PubMed] [Google Scholar]
- Ritenberg M., Nandi S., Kolusheva S., Dandela R., Meijler M. M., Jelinek R. (2016). Imaging pseudomonas aeruginosa biofilm extracellular polymer scaffolds with amphiphilic carbon dots. ACS chem. biol. 11 1265–1270. 10.1021/acschembio.5b01000 [DOI] [PubMed] [Google Scholar]
- Sahu S., Behera B., Maiti T. K., Mohapatra S. (2012). Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chem. Commun. 48 8835–8837. 10.1039/c2cc33796g [DOI] [PubMed] [Google Scholar]
- Sathiyanarayanan G., Dineshkumar K., Yang Y. H. (2017). Microbial exopolysaccharide-mediated synthesis and stabilization of metal nanoparticles. Crit. Rev. Microbiol. 43 731–752. 10.1080/1040841x.2017.1306689 [DOI] [PubMed] [Google Scholar]
- Shchipunov Y. A., Khlebnikov O. N., Silant’ev V. E. (2015). Carbon quantum dots hydrothermally synthesized from chitin. Polym. Sci. Ser. B 57 16–22. 10.1134/s1560090415010121 [DOI] [Google Scholar]
- Shen P., Gao J., Cong J., Liu Z., Li C., Yao J. (2016). Synthesis of cellulose-based carbon dots for bioimaging. ChemistrySelect 1 1314–1317. 10.1002/slct.201600216 [DOI] [Google Scholar]
- Song P., Zhang L., Long H., Meng M., Liu T., Yin Y., et al. (2017). A multianalyte fluorescent carbon dots sensing system constructed based on specific recognition of fe(iii) ions. RSC Adv. 7 28637–28646. 10.1039/C7RA04122E [DOI] [Google Scholar]
- Strauber H., Muller S. (2010). Viability states of bacteria–specific mechanisms of selected probes. Cytometry A 77 623–634. 10.1002/cyto.a.20920 [DOI] [PubMed] [Google Scholar]
- Takahashi D., Dai H., Hiromasa Y., Krishnamoorthi R., Kanost M. R. (2014). Self-association of an insect β-1,3-glucan recognition protein upon binding laminarin stimulates prophenoloxidase activation as an innate immune response. J. Bio. Chem. 289 28399–28410. 10.1074/jbc.M114.583971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takedatsu H., Mitsuyama K., Mochizuki S., Kobayashi T., Sakurai K., Takeda H., et al. (2012). A new therapeutic approach using a schizophyllan-based drug delivery system for inflammatory bowel disease. Mol. Ther. 20 1234–1241. 10.1038/mt.2012.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen H., Liu Y., Wu J., Zhou P., Wang Y., et al. (2013). Ph-sensitive pullulan-based nanoparticle carrier of methotrexate and combretastatin a4 for the combination therapy against hepatocellular carcinoma. Biomaterials 34 7181–7190. 10.1016/j.biomaterials.2013.05.081 [DOI] [PubMed] [Google Scholar]
- Wu J., Zhang F., Zhang H. (2012). Facile synthesis of carboxymethyl curdlan-capped silver nanoparticles and their application in sers. Carbohydr. Polym. 90 261–269. 10.1016/j.carbpol.2012.05.033 [DOI] [PubMed] [Google Scholar]
- Xu S., Lin Y., Huang J., Li Z., Xu X., Zhang L. (2013). Construction of high strength hollow fibers by self-assembly of a stiff polysaccharide with short branches in water. J. Mater. Chem. A 1 4198–4206. 10.1039/C3TA00050H [DOI] [Google Scholar]
- Yan J.-K., Liu J.-L., Sun Y.-J., Tang S., Mo Z.-Y., Liu Y.-S. (2015). Green synthesis of biocompatible carboxylic curdlan-capped gold nanoparticles and its interaction with protein. Carbohydr. Polym. 117 771–777. 10.1016/j.carbpol.2014.10.048 [DOI] [PubMed] [Google Scholar]
- Yang J., Zhang X., Ma Y. H., Gao G., Chen X., Jia H. R., et al. (2016). Carbon dot-based platform for simultaneous bacterial distinguishment and antibacterial applications. ACS Appl. Mater. Interfaces 8 32170–32181. 10.1021/acsami.6b10398 [DOI] [PubMed] [Google Scholar]
- Yang L., Jiang W., Qiu L., Jiang X., Zuo D., Wang D., et al. (2015). One pot synthesis of highly luminescent polyethylene glycol anchored carbon dots functionalized with a nuclear localization signal peptide for cell nucleus imaging. Nanoscale 7 6104–6113. 10.1039/c5nr01080b [DOI] [PubMed] [Google Scholar]
- Yang X., Luo Y., Zhu S., Feng Y., Zhuo Y., Dou Y. (2014). One-pot synthesis of high fluorescent carbon nanoparticles and their applications as probes for detection of tetracyclines. Biosens. Bioelectron. 56 6–11. 10.1016/j.bios.2013.12.064 [DOI] [PubMed] [Google Scholar]
- You D. G., Saravanakumar G., Son S., Han H. S., Heo R., Kim K., et al. (2014). Dextran sulfate-coated superparamagnetic iron oxide nanoparticles as a contrast agent for atherosclerosis imaging. Carbohydr. Polym. 101 1225–1233. 10.1016/j.carbpol.2013.10.068 [DOI] [PubMed] [Google Scholar]
- Zhang C., An T., Wang D., Wan G., Zhang M., Wang H., et al. (2016). Stepwise ph-responsive nanoparticles containing charge-reversible pullulan-based shells and poly(β-amino ester)/poly(lactic-co-glycolic acid) cores as carriers of anticancer drugs for combination therapy on hepatocellular carcinoma. J. Control. Release 226 193–204. 10.1016/j.jconrel.2016.02.030 [DOI] [PubMed] [Google Scholar]
- Zhang R., Chen W. (2014). Nitrogen-doped carbon quantum dots: facile synthesis and application as a “turn-off” fluorescent probe for detection of hg2 + ions. Biosens. Bioelectron. 55 83–90. 10.1016/j.bios.2013.11.074 [DOI] [PubMed] [Google Scholar]
- Zhao E., Hong Y., Chen S., Leung C. W., Chan C. Y., Kwok R. T., et al. (2014). Highly fluorescent and photostable probe for long-term bacterial viability assay based on aggregation-induced emission. Adv. Healthc. Mater. 3 88–96. 10.1002/adhm.201200475 [DOI] [PubMed] [Google Scholar]
- Zhao H. X., Liu L. Q., Liu Z. D., Wang Y., Zhao X. J., Huang C. Z. (2011). Highly selective detection of phosphate in very complicated matrixes with an off-on fluorescent probe of europium-adjusted carbon dots. Chem. Commun. 47 2604–2606. 10.1039/C0CC04399K [DOI] [PubMed] [Google Scholar]
- Zhao S., Lan M., Zhu X., Xue H., Ng T. W., Meng X., et al. (2015). Green synthesis of bifunctional fluorescent carbon dots from garlic for cellular imaging and free radical scavenging. ACS Appl. Mater. Interfaces 7 17054–17060. 10.1021/acsami.5b03228 [DOI] [PubMed] [Google Scholar]
- Zhou H., Yang D., Ivleva N. P., Mircescu N. E., Schubert S., Niessner R., et al. (2015). Label-free in situ discrimination of live and dead bacteria by surface-enhanced raman scattering. Anal. Chem. 87 6553–6561. 10.1021/acs.analchem.5b01271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


