Abstract
Objective:
Cisplatin is an anticancer drug used for treating childhood solid tumors. Symptoms related to cisplatin-induced cardiovascular adverse effects may be mild or severe. Rutin (vitamin P1) has many properties, including as antioxidant, anticancer, antidiabetic, antimicrobial, antiulcer, and tissue renewal properties. Therefore, we aimed to biochemically, histopathologically, and immunohistochemically demonstrate the effect of rutin on cisplatin-induced cardiotoxicity in rats.
Methods:
The rats included in our study were divided into four groups: Healthy group (HE), 5-mg/kg cisplatin group (CP), 50 mg/kg rutin+5-mg/kg cisplatin (CR-50), 100-mg/kg rutin+5-mg/kg cisplatin (CR-100) group.
Results:
CP group administered cisplatin had significantly increased blood, serum, and cardiac tissue malondialdehyde (MDA), interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), troponin I, creatine kinase (CK), and CK-MB levels compared to the HE group, whereas there was a significant decrease in the total glutathione (tGSH) levels. Rutin was observed to prevent the increase in MDA, IL-1β, TNF-α, troponin I, CK, and CK-MB levels as well as prevent the decrease in tGSH levels more significantly when administered at a 100-mg/kg dose than at a 50-mg/kg dose. Histopathologically, cardiac necrosis, dilated/congested blood vessels, hemorrhage, polymorphonuclear leukocyte, edema, and cells with pyknotic nuclei were observed in the CP group. Rutin was shown to prevent cisplatin-induced cardiac damage more effectively when used at a100-mg/kg dose than at a 50-mg/kg dose.
Conclusion:
These results suggest that rutin is useful for preventing cisplatin-related cardiovascular damage
Keywords: cisplatin, cardiac damage, rutin, rat
Introduction
Cisplatin [cis -diamminedichloroplatinum (II)], which is a chemotherapeutic agent, is one of the most commonly used drugs for treating cancers (1). It is an anticancer drug that contains the heavy metal, platinum, and is commonly used in the treatment of childhood solid tumors (2). Cisplatin is widely used primarily in the treatment of testicular cancer as well as in head and neck, cervical, breast, lung, ovarian, gastric, and urinary bladder cancers (3). Cisplatin acts as a heavy-metal DNA-alkylating agent. Its therapeutic effect significantly increases with the increased dose (4), but higher dosages can lead to severe adverse effects (5, 6). Although cardiotoxicity is not considered as a typical adverse effect of cisplatin, in the recent years, a spectrum of cardiotoxic findings that develop during or shortly after cisplatin infusion have been reported (7, 8). These include mild cardiovascular adverse effects as well as severe ones, such as cardiac failure, pericarditis, myocarditis, arrhythmia, hypertension, and rarely, cardiac ischemia, cardiac tamponade, and endomyocardial fibrosis (8, 9). However, the mechanisms underlying the cardiotoxic effects of cisplatin have not been fully identified (7). Primarily, increase in free radical levels and a decrease in antioxidant enzymes are held responsible for the pathogenesis of cardiotoxicity (10). Considering this hypothesis, the usefulness of antioxidants against cisplatin-induced toxicity was investigated, and some antioxidants were found to be protective (11).
Rutin (vitamin P1), whose effect on cisplatin-induced cardiotoxicity is the subject of this study, has many properties such as antioxidant, anticancer, antidiabetic, antimicrobial, antiulcer, and tissue renewal properties (12, 13). This suggests that rutin can be effective for preventing cisplatin-induced cardiotoxicity. However, no research on the prophylactic effect of rutin (P1) against cisplatin-induced cardiotoxicity has been reported so far. Therefore, the aim of this study is to biochemically, histopathologically, and immunohistochemically investigate the effect of rutin on cisplatin-induced oxidative cardiac damage.
Methods
Animals
In the study, we used a total of 24 male albino Wistar rats obtained from University Medical Experimental Practice and Research Center. The weight of the rats ranged between 260–280 grams, and they were kept and fed at normal room temperature (22°C) before the experiment. Ethics committee approval was obtained from University Animal Experiments Local Ethics Committee (24.08.2017, 7/105).
Chemical agents
Sodium thiopental was purchased from IE Ulagay (Turkey), Rutin was purchased from Solgar (USA), and cisplatin from Liba (Turkey).
Experimental groups
Rats used in the study were divided into four groups: healthy (HE), 5-mg/kg cisplatin (CP), 50-mg/kg rutin+5-mg/kg cisplatin (CR-50), and 100-mg/kg rutin+5-mg/kg cisplatin (CR-100) group.
Experimental procedure
Oral catheters were used to administer 50-mg/kg rutin to the rats in the CR-50 group (n=6) and 100-mg/kg rutin to the rats in the CR-100 group (n=6). Distilled water was administered as a solvent through the same route in the CP (n=6) and HE (n=6) groups. One hour after the administration of rutin and distilled water, 5-mg/kg cisplatin was injected intraperitoneally (i.p.) into the rats in the CR-50, CR-100, and CP groups once in every 2 days for a total of 8 days. Rutin and distilled water were administered once a day for 8 days. At the end of this period, all animals were sacrificed using a high-dose anesthetic (50-mg/kg sodium thiopental) and cardiac tissues were removed. Malondialdehyde (MDA), total glutathione (tGSH), interleukin 1 beta (IL-1β), and tumor necrosis factor alpha (TNF-α) levels in the collected cardiac tissues were measured. Troponin I (TPI), creatine kinase (CK), CK-MB levels were measured using blood samples collected before sacrifice. Moreover, cardiac tissues were examined histopathologically and immunohistochemically. Results obtained from the rutin group were compared with the results obtained from the HE and CP groups.
Biochemical analysis
Tissue malondialdehyde measurements
Malondialdehyde measurements were based on the method used by Ohkawa et al. (14), involving spectrophotometric measurement of absorbance of the pink-stained complex formed by thiobarbituric acid (TBA) and MDA. The tissue homogenate sample (0.1 mL) was added to a solution containing 0.2 ml of 80 g/L sodium dodecyl sulfate, 1.5 mL of 200 g/L acetic acid, 1.5 mL of 8 g/L 2-thiobarbiturate, and 0.3 mL distilled water. The mixture was incubated at 95°C for 1 h. Upon cooling, 5 mL of n-butanol: pyridine (15:1) was added. The mixture was vortexed for 1 min and centrifuged for 30 min at 4000 rpm. The absorbance of the supernatant was measured at 532 nm. The standard curve was obtained using 1,1,3,3-tetramethoxypropane (14).
Total glutathione measurements
According to the method defined by Sedlak and Lindsay (15) DTNB [5,5′-dithiobis (2-nitrobenzoic acid)] disulfite is chromogenic in the medium, and DTNB is reduced easily by sulfhydryl groups. The yellow stain produced during the reduction is measured by spectrophotometry at 412 nm. For measurement, a cocktail solution [5.85 mL of 100-mM Na-phosphate buffer, 2.8 mL of 1-mM DTNB 3.75 mL of 1-mM Nicotinamide adenine dinucleotide phosphate (NADPH), and 80 µL of 625-U/L glutathione reductase] was prepared. Before measurement, 0.1 mL of meta-phosphoric acid was added onto 0.1 mL of tissue homogenate and centrifuged for 2 min at 2000 rpm for deproteinization. Then, 0.15 mL of the cocktail solution was added to 50 µL of the supernatant. The standard curve was obtained using glutathione disulfide (GSSG).
Interleukin 1 beta and tumor necrosis factor alpha measurements in tissue
Interleukin 1 beta and tumor necrosis factor alpha concentrations in the tissue homogenate were measured using the following rat-specific sandwich enzyme-linked immunosorbent assay kits: Rat Interleukin 1β ELISA Kit (Cat no: YHB0616Ra, Shanghai LZ) and Rat Tumor Necrosis Factor α ELISA kits (Cat no: YHB1098Ra, Shanghai LZ). Analyses were performed according to the manufacturers’ instructions. Briefly, the wells of the microplates were coated with the monoclonal antibody specific for rat IL-1β and TNF-α. The tissue homogenate, standards, and biotinylated monoclonal antibody specific and streptavidin-HRP were pipetted onto the wells and then incubated at 37°C for 60 min. After washing, chromogen reagent A and chromogen reagent B were added, which are acted upon by the bound enzyme to produce a color. It was incubated at 37°C for 10 min. Then, the stop solution was added. The color intensity of this product is directly proportional to the concentration of rat IL-1β and TNF-α present in the original specimen. At the end of the procedure, the well plates were read at 450 nm using a microplate reader (Bio-Tek, USA). The absorbance of the samples was estimated using formulas obtained from standard charts.
Troponin I measurement
Troponin I levels in the plasma obtained from the animals were measured by enzyme-linked fluorescent assay using the VIDAS Troponin I Ultra kit. Readily available test reagents in the kit were used to automatically perform all steps of the test in the VIDAS equipment. The sample was transferred to the well containing alkaline phosphatase (conjugate)-labeled anti-cardiac troponin I antibodies. Sample–conjugate mix was placed in the solid phase receptacle to ensure the binding of the antigen to the conjugate and troponin I, which is bound to the inner wall of the solid phase receptacle. The unbound content was washed away. The conjugated enzyme catalyzes the hydrolysis of 4-methyl umbelliferyl phosphate (the substrate) to 4-methylumbelliferone, whose fluorescence is measured at 450 nm. The fluorescence intensity is directly proportional to the antigen concentration in the sample.
CK measurement
Photometric measurement of the CK in the plasma obtained from the animals was performed using Roche/Hitachi Cobas c 701 system. Readily available test reagents were used to perform all steps of the test according to the procedure. UV test is performed according to the following reactions. Equimolar NADPH and ATP are produced at the same rate. The rate of NADPH formation, which is photometrically measured at 340 nm, is directly proportional to the CK activity.
CK-MB measurement
Measurement of the CK-MB in the plasma obtained from the animals was performed using Roche/Hitachi Cobas c 701 system. Readily available test reagents were used to perform all steps of the test using immunological UV test, according to the procedure. CK-MB isoenzyme is composed of the two subunits CK-M and CK-B, both of which have an active site. With the help of CK-M-specific antibodies, catalytic activities of the CK-M subunit in the sample are 99.6% inhibited without affecting the CK-B subunit. There maining CK-B activity, which is equivalent to the half of the CK-MB activity, is measured using total CK method.
Histopathological examination
Cardiac tissues obtained from the rats were fixed in 10% formalin solution for 24h. After routine tissue processing, 4-µm-thick sections were obtained from the paraffin blocks and were stained with hematoxylin&eosin. All sections were examined under a light microscope (Olympus BX 52, Tokyo, Japan) by two pathologists who were blinded to the treatment protocol.
Immunohistochemical procedures
For immunohistochemical staining, primary antibodies of caspase-3 antibody Santa Cruz Biotechnology, Dallas, TX: 1/100 and, Cell Signaling Technology Inc., Danvers, MA were used. Sections were stained using a fully automated immunohistochemistry (IHC) device (LeicaBond-Max, LeicaBiosystems, Melbourne, Australia). After being processed in the IHC device, sections were dehydrated through a graded series of ethanol to xylene and enclosed with a mounting medium (Entellan, Merck Millipore, Darmstadt, Germany). From the rat heart samples incubated in 10% formalin solution for immunohistochemical processing, 4-µm-thick sections were made on a positively charged microscope slide. Results of the analysis under Olympus BX51 microscope were evaluated on the basis of caspase-3 staining of the heart using the grading system below. In this evaluation, diffuseness and intensity were considered separately. Diffuseness represents the areas the dye can be found and the intensity represents the color intensity. For diffuseness, grade I represents coloration in <10%, grade II represents coloration between 10%–50%, and grade III represents coloration in >50% cells. For intensity, grade I represents mild, grade II represents intermediate, and grade III represents intense coloration of the cells.
Statistical analyses
The results obtained from the experiments are depicted as “mean±standard deviation” (×±SD). The significance level of the intergroup difference was identified using one-way analysis of variance. Then, Fisher’s posthoc least significant difference was performed. All statistical analyses were performed using “IBM SPSS Statistics Version 20” program, and a p value of <0.05 was considered significant.
Results
Biochemical results
As can be seen in Figure 1a, CP group, to which cisplatin was administered, a statistically significant increase in MDA levels in the cardiac tissue was detected compared to the HE group (p<0.0001). Rutin administration decreased this increase in the CP groups. However, he difference between CR-100 group and HE group was not statistically significant (p>0.05).
Figure 1.
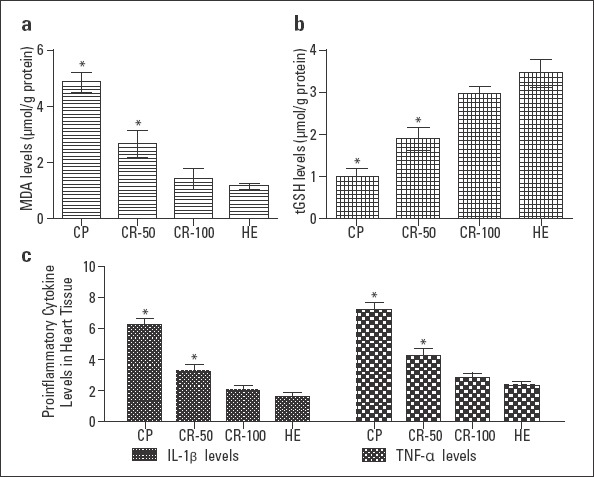
MDA (A), tGSH (B), and proinflammatory cytokine (C) levels in the heart tissues of the CP, CR-50, CR-100, and HE groups. The HE group is compared with other groups (n=6; *=P<0.0001)
Cisplatin administration significantly decreased tGSH levels in the cardiac tissue, and a significant difference was detected in the CP groups when compared to the HE group (p<0.0001; Fig. 1b). In groups administered rutin, particularly when 100 mg dosing was used, decrease in tGSH levels was prevented compared to the CP group and the values were close to those in the HE group.
IL-1β levels in the cardiac tissue were higher in the CP group than in the HE group (p<0.0001). No statistically significant difference was detected between the CR-100 group and HE group (p>0.05; Fig. 1c). TNF-α levels were found to be increased in the CP group than the HE group, and the difference was statistically significant (p<0.0001). TNF-α levels decreased significantly in the groups administered rutin, particularly in the CR-100 group, and reached to the levels similar to those in the HE group (Fig. 1c).
Troponin I levels were significantly increased in the CP groups compared to the HE group (p<0.0001). Rutin administration significantly decreased troponin I levels. The difference between the groups administered rutin and HE group was not statistically significant (p>0.05; Fig. 2a).
Figure 2.
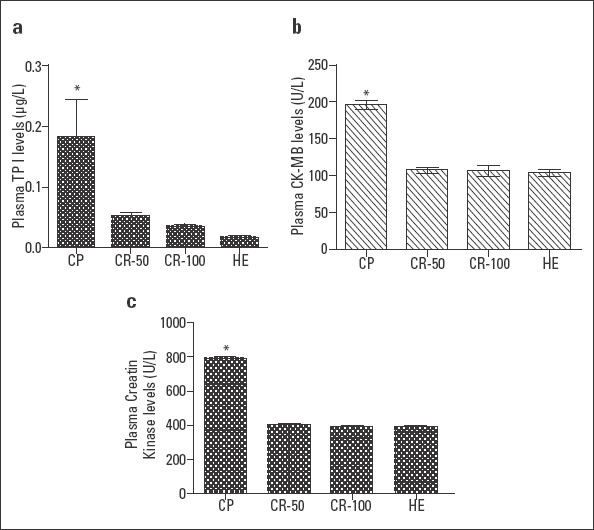
Plasma troponin I (A), CK-MB (B), and CK (C) levels of the CP, CR-50, CR-100, and HE groups. The HE group is compared with other groups (n=6; *=P<0.0001)
Plasma CK-MB levels in the CP group increased significantly compared to the HE group, and the difference between the two groups was statistically significant (p<0.0001). Rutin administration halted this cisplatin-dependent increase. The difference between CR-50, CR-100, and HE groups was not statistically significant (p>0.05; Fig. 2b).
As can be seen in Figure 2c, cisplatin administration increased plasma CK levels. Rutin administration, however, significantly decreased this increase. In particular, the difference between CR-100 and HE groups was not significant (p>0.05).
Histopathological results
In Figure 3a, the healthy cardiac muscle can be seen. In cardiac muscle administered cisplatin, sporadic cardiac necrosis (solid arrow), dilated congested blood vessel (dashed arrow) were observed (Fig. 3b). Cardiac muscle of the CP group rats showed hemorrhage (solid arrow), polymorpho nuclear leukocyte (dashed arrow), pyknotic nucleus (circle marked arrow) (Fig. 3c). In Figure 3d, dilated congested vein structure in the tissue administered cisplatin+rutin (solid arrow) and near-normal appearance was observed. In Figure 3e, an appearance similar to normal healthy tissue was observed in the cardiac muscle administered 100-mg/kg rutin.
Figure 3.
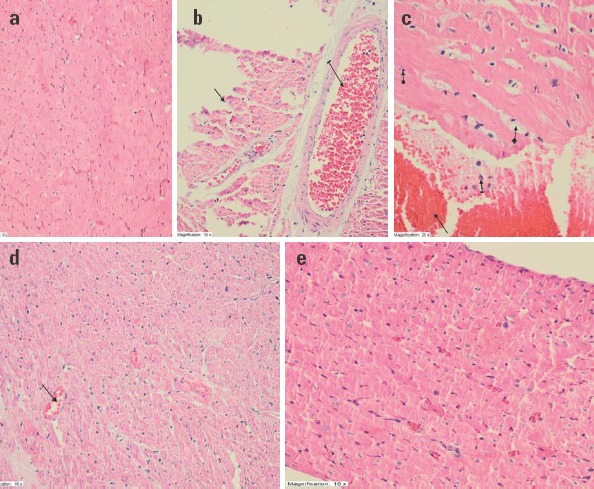
Histopathological appearance of the cardiac tissues of rats in the study groups. (a) Healthy cardiac muscle-H&E 200X. (b) Sporadic cardiac necrosis (solid arrow) and dilated congested blood vessel (dashed arrow)- H&E 200X. (c) Hemorrhage (solid arrow), polymorphonuclear leukocyte (dashed arrow), edema (square marked arrow), pyknotic nucleus (circle marked arrow) in cardiac muscle administered cisplatin-H&E 400X. (d) Near-normal appearance and continuous dilated congested vein structure (solid arrow) in tissue administered cisplatin+50-mg/kg rutin- H&E 200X. (e) Normal, healthy-like appearance in cardiac muscle administered cisplatin+100-mg/kg rutin- H&E 200X
Immunohistochemical results
The diffuseness and intensity of tissue by caspase-3 was regarded as grade I in the HE group healthy hearts (Fig. 4a) whereas diffuseness and intensity were evaluated as grade II in the CP groups (Fig. 4b). The diffuseness and intensity of tissues by caspase-3 were regarded as grade I in the CR groups (Fig. 4c and 4d, respectively).
Figure 4.
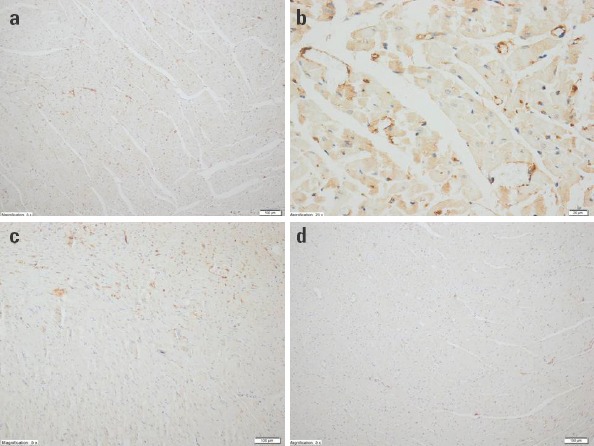
Immunohistochemical evaluation of the cardiac tissues of rats in the study groups. (a) The HE group, diffuseness and intensity of staining by caspase-3: Grade I (H&E 100X). (b) The CP group, diffuseness and intensity of staining by caspase-3: Grade II (H&E 400X). (c) The CR-50 group, diffuseness and intensity of staining by caspase-3: Grade I (H&E 100X). (d) The CR-100 group, diffuseness and intensity of staining by caspase-3: Grade I (H&E 100X)
Discussion
In this study, the effect of rutin on cisplatin-induced cardiotoxicity in rats was evaluated biochemically and histopathologically. The results of biochemical and histopathological experiments indicate that cisplatin causes oxidative stress in cardiac tissue. Cisplatin is known to cause early and late stage cardiotoxic effects in children in particular, because children are more sensitive to cardiotoxicity than adults (16, 17). Although cisplatin-induced cardiac toxicity is not fully defined, cisplatin has been shown to cause mitochondrial dysfunction, nuclear damage, activation of apoptotic pathways, and inflammation in the cardiac tissues (18, 19). Moreover, cisplatin has been shown to induce cardiac toxicity by increasing the production of free oxygen radicals (20). Demkow et al. (20), Noori et al. (21) have reported that oxidative stress develops after the cisplatin infusion. One of the most important indicators of the oxidative damage is the end product of lipid peroxidation, MDA (22). In oxidative tissue damage, an increase in the amount of MDA is observed whereas a decrease is observed in the amount of endogenous antioxidant molecule, tGSH (23, 24). In our study, a significant increase was observed in MDA levels in CP group, whereas a significant decrease was observed in tGSH levels compared to the HE group.
Moreover, in our study, it was found that the levels of proinflammatory cytokines such as IL-1β and TNF-α increase in the cardiac tissue of animals administered cisplatin. In the literature, it was reported that IL-1β and TNF-α can cause systemic tissue damage (25). IL-1β plays an important role in inflammatory cascade by causing apoptosis and leukocyte infiltration (26). TNF-α and IL-1β emerge in the early stage of inflammation, and they carry out many functions, such as oxidative explosion the neutrophils and release of reactive oxygen species, via their common signalling molecules (26, 27). The higher TNF-α and IL-1β levels in the group administered cisplatin than in the HE found in our study show that the results corroborate with the literature.
Serum levels of cardiac enzymes such as TPI, CK, and CK-MB have gained importance in the recent years in the detection of cardiac damage. Cardiac TPI is one of the highly sensitive and specific parameters of myocardial damage (28, 29). Cisplatin has the potential to disrupt the cell membranes, which enables the release of intracellular proteins such as cardiac TPI, CK, and CK-MB (28). El-Awady et al. (8) reported significant increases in the serum CK, CK-MB, and plasma cardiac TPI activity, compared with control groups, following the administration of a single cisplatin dose. In our study, significantly high cardiac TPI, CK, and CK-MB levels compared to the healthy control group were observed in rats administered cisplatin.
The results of our experiments showed that rutin significantly prevents the increase in MDA levels and the decrease in tGSH levels caused by cisplatin, depending on the dose. Rutin has an antioxidant and cardioprotective effect (12, 30). In the literature, there are no studies on the protective effect of rutin against cisplatin-induced cardiotoxicity. However, there are studies showing that rutin decreases doxorubicin-induced cardiotoxicity (31). In the study by Umarani et al. (32) it was reported that rutin decreases MDA levels and increases tGSH levels in fluoride-induced cardiotoxicity. Annapurna et al. (33) reported that rutin is effective in repairing diabetes-dependent oxidative myocardial damage in rats. In another study, it was shown that rutin increases tissue tGSH levels in cases of isoproterenol-induced cardiac damage, thus decreasing cardiac toxicity (34).
Rutin was shown to have not only an antioxidant but also anti-inflammatory properties (35, 36). Alhoshani et al. (37) showed that rutin significantly decreased TNF-α levels and was effective in repairing kidney damage in cases of cisplatin-induced kidney damage. Wu et al. (38) showed that rutin decreased the increased TNF-α levels in patients with lung cancer. In another study, rutin was shown to significantly decrease TNF-α and IL-1β levels (39). A significant, dose-dependent decrease in TNF-α and IL-1β levels after rutin administration observed in our study show that our results corroborate with the literature.
In our study, the CR group showed significant dose-dependent decreases in TPI, CK, and CK-MB levels compared to the CP group. In the literature, there are no studies on the protective effect of rutin against cisplatin-induced cardiotoxicity. However, in rats administered isoprenaline, rutin was shown to decrease cardiac TPI levels and decrease cardiac toxicity (40). In another study, it was proven that rutin significantly decreased isoproterenol-induced cardiac TPI and CK levels (41). In carfilzomib-induced cardiac toxicity, rutin was shown to decrease CK and CK-MB levels, and was effective in repairing cardiac damage (42).
Histopathologically, polymorphonuclear leukocyte infiltration, dilated congested blood vessels, hemorrhage in cardiac muscle, edema, necrosis, and pyknotic nucleus were observed in the cardiac tissue of the CP group. In our study, continuous dilated congested vessel structure was found in the cardiac tissue of rats in the CR-50 group, near-normal appearance was observed in the cardiac tissue of rats in the CR-100 group. In the literature, it was shown that PNL cell infiltration produces free radicals, and these free radicals lead to lipid peroxidation, followed by events that lead to cell necrosis (43). Tousson et al. (44) reported that oxidative stress causes PNL infiltration and edema in the cardiac tissue. It is well known that edema, and PNL infiltration are the symptoms of inflammatory reaction with cell death (45). In this study, the results of our biochemical and histopathological experiments are also supported by immunohistochemical examination results. Significant apoptosis was observed in the CP group, whereas rutin showed a dose-dependent antiapoptotic effect. Apoptosis is the final form of cell damage. Its fundamental mechanism i.e., physiologically and genetically regulated apoptosis is considered as a form of programmed cell death (46). Abnormal apoptosis was shown to prevent the disease severity and progression in several diseases (47).
Study limitations
The small sample size of our study is a limitation. This study should be interpreted as a preliminary study and its findings should be interpreted with caution. Further studies are required to elucidate the precise mechanisms underlying cisplatin-induced cardiac toxicity and the effects of rutin on preventing them.
Conclusion
It was biochemically, histopathologically, and immunohistochemically shown that cisplatin increases MDA, TNF-α and IL-1β levels, and decreases tGSH levels in the cardiac tissue, leading to oxidative damage. Rutin at doses of 50 and 100 mg/kg prevented the cardiac tissue from cisplatin-induced oxidative cardiac toxicity. However, rutin was more effective in 100-mg/kg dose. These results suggest that rutin can be useful for preventing cisplatin-related cardiac toxicity.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – İ.T., A.Ö.B., D.A.; Design – İ.T., D.A.; Supervision – İ.T., A.Ö.B., F.K.Ç., N.K., Z.S., Y.B., A.Ö., D.A.; Fundings – None; Materials – İ.T., F.K.Ç., N.K.; Data collection &/or processing – İ.T., A.Ö.B., F.K.Ç., N.K., Z.S., Y.B., A.Ö., D.A.; Analysis &/or interpretation – İ.T., A.Ö.B., F.K.Ç., N.K., D.A.; Literature search – İ.T., A.Ö.B., D.A.; Writing – İ.T., D.A.; Critical review – İ.T., A.Ö.B., F.K.Ç., N.K., Z.S., Y.B., A.Ö., D.A.
References
- 1.Wheate NJ, Walker S, Craig GE, Oun R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010;39:8113–27. doi: 10.1039/c0dt00292e. [DOI] [PubMed] [Google Scholar]
- 2.Sheikh-Hamad D, Cacini W, Buckley AR, Isaac J, Truong LD, Tsao CC, et al. Cellular and molecular studies on cisplatin-induced apoptotic cell death in rat kidney. Arch Toxicol. 2004;78:147–55. doi: 10.1007/s00204-003-0521-4. [DOI] [PubMed] [Google Scholar]
- 3.Dasari S, Tchounwou PB. Cisplatin in cancer therapy:molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabuncuoğlu S, Özgüneş H. Cisplatin toxicity:Importance of oxidative stress and effect of antioxidants. J Ist Faculty Med. 2011;74:18–25. [Google Scholar]
- 5.Sakamoto M, Kaga K, Kamio T. Extended high-frequency ototoxicity induced by the first administration of cisplatin. Otolaryngol Head Neck Surg. 2000;122:828–33. doi: 10.1016/S0194-59980070009-X. [DOI] [PubMed] [Google Scholar]
- 6.Bodiga VL, Bodiga S, Surampudi S, Boindala S, Putcha U, Nagalla B, et al. Effect of vitamin supplementation on cisplatin-induced intestinal epithelial cell apoptosis in Wistar/NIN rats. Nutrition. 2012;28:572–80. doi: 10.1016/j.nut.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Patanè S. Cardiotoxicity:cisplatin and long-term cancer survivors. Int J Cardiol. 2014;175:201–2. doi: 10.1016/j.ijcard.2014.04.238. [DOI] [PubMed] [Google Scholar]
- 8.El-Awady E-SE, Moustafa YM, Abo-Elmatty DM, Radwan A. Cisplatin-induced cardiotoxicity:Mechanisms and cardioprotective strategies. Eur J Pharmacol. 2011;650:335–41. doi: 10.1016/j.ejphar.2010.09.085. [DOI] [PubMed] [Google Scholar]
- 9.Aktürk E, Kurtoğlu E, Harputluoğlu H. Cardiovascular Side Effects of Cancer Drugs:How to Identify, Treat, and Follow up These Side Effects?İnönüÜniversitesi Tıp Fakültesi Dergisi. 2011;18:137–42. [Google Scholar]
- 10.Demir F, Narin F, Akgün H, Üzüm K, Saraymen R, Baykan A, et al. Effects of melatonin on experimental cardiotoxicity induced by doxorubicin. Çocuk SağHast Derg. 2004;47:260–8. [Google Scholar]
- 11.Francescato HD, Coimbra TM, Costa RS, Bianchi Mde L. Protective effect of quercetin on the evolution of cisplatin-induced acute tubular necrosis. Kidney Blood Press Res. 2004;27:148–58. doi: 10.1159/000078309. [DOI] [PubMed] [Google Scholar]
- 12.Richetti SK, Blank M, Capiotti KM, Piato AL, Bogo MR, Vianna MR, et al. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav Brain Res. 2011;217:10–5. doi: 10.1016/j.bbr.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Nassiri-Asl M, Mortazavi SR, Samiee-Rad F, Zangivand AA, Safdari F, Saroukhani S, et al. The effects of rutin on the development of pentylenetetrazole kindling and memory retrieval in rats. Epilepsy Behav. 2010;18:50–3. doi: 10.1016/j.yebeh.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 16.Lipshultz SE, Rifai N, Sallan SE, Lipsitz SR, Dalton V, Sacks DB, et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation. 1997;96:2641–8. doi: 10.1161/01.cir.96.8.2641. [DOI] [PubMed] [Google Scholar]
- 17.Bano N, Najam R, Qazi F. Adverse cardiac manifestations of cisplatin-a review. Int J Pharm Sci Rev Res. 2013;18:80–5. [Google Scholar]
- 18.Ma H, Jones KR, Guo R, Xu P, Shen Y, Ren J. Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure:role of endoplasmic reticulum stress. Clin Exp Pharmacol Physiol. 2010;37:460–5. doi: 10.1111/j.1440-1681.2009.05323.x. [DOI] [PubMed] [Google Scholar]
- 19.Zsengellér ZK, Ellezian L, Brown D, Horváth B, Mukhopadhyay P, Kalyanaraman B, et al. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J Histochem Cytochem. 2012;60:521–9. doi: 10.1369/0022155412446227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demkow U, Biatas-Chromiec B, Stelmaszczyk-Emmel A, Radzikowska E, Wiatr E, Radwan-Rohrenschef P, et al. The cardiac markers and oxidative stress parameters in advanced non-small cell lung cancer patients receiving cisplatin-based chemotherapy. EJIFCC. 2011;22:6–15. [PMC free article] [PubMed] [Google Scholar]
- 21.Noori S, Mahboob T. Antioxidant effect of carnosine pretreatment on cisplatin-induced renal oxidative stress in rats. Indian J Clin Biochem. 2010;25:86–91. doi: 10.1007/s12291-010-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hideg K, Kálai T. Novel antioxidants in anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7:160–4. doi: 10.1007/s12012-007-0019-z. [DOI] [PubMed] [Google Scholar]
- 23.Nazıroğlu M, Karaoğlu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004;195:221–30. doi: 10.1016/j.tox.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Kisaoglu A, Borekci B, Yapca OE, Bilen H, Suleyman H. Tissue damage and oxidant/antioxidant balance. Eurasian J Med. 2013;45:47–9. doi: 10.5152/eajm.2013.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grace PA. Ischaemia-reperfusion injury. Br J Surg. 1994;81:637–47. doi: 10.1002/bjs.1800810504. [DOI] [PubMed] [Google Scholar]
- 26.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–8. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 27.Semenzato G. Tumour necrosis factor:a cytokine with multiple biological activities. Br J Cancer. 1990;61:354–61. doi: 10.1038/bjc.1990.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertinchant JP, Polge A, Juan JM, Oliva-Lauraire MC, Giuliani I, Marty-Double C, et al. Evaluation of cardiac troponin I and T levels as markers of myocardial damage in doxorubicin-induced cardiomyopathy rats, and their relationship with echocardiographic and histological findings. Clin Chim Acta. 2003;329:39–51. doi: 10.1016/s0009-8981(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 29.Kismet E, Varan A, Ayabakan C, Alehan D, Portakal O, Büyükpamukçu M. Serum troponin T levels and echocardiographic evaluation in children treated with doxorubicin. Pediatr Blood Cancer. 2004;42:220–4. doi: 10.1002/pbc.10368. [DOI] [PubMed] [Google Scholar]
- 30.Javed H, Khan M, Ahmad A, Vaibhav K, Ahmad M, Khan A, et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience. 2012;210:340–52. doi: 10.1016/j.neuroscience.2012.02.046. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y, Yang L, Ma J, Lu L, Wang X, Ren J, et al. Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochim Biophys Acta. 2017;1863:1904–11. doi: 10.1016/j.bbadis.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Umarani V, Muvvala S, Ramesh A, Lakshmi B, Sravanthi N. Rutin potentially attenuates fluoride-induced oxidative stress-mediated cardiotoxicity, blood toxicity and dyslipidemia in rats. Toxicol Mech Methods. 2015;25:143–9. doi: 10.3109/15376516.2014.1003359. [DOI] [PubMed] [Google Scholar]
- 33.Annapurna A, Reddy CS, Akondi RB, Rao SR. Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type I diabetic rats. J Pharm Pharmacol. 2009;61:1365–74. doi: 10.1211/jpp/61.10.0014. [DOI] [PubMed] [Google Scholar]
- 34.Punithavathi VR, Shanmugapriya K, Prince PS. Protective effects of rutin on mitochondrial damage in isoproterenol-induced cardiotoxic rats:an in vivo and in vitro study. Cardiovasc Toxicol. 2010;10:181–9. doi: 10.1007/s12012-010-9077-8. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Lai YC, Wang HT, Wang RK, Meng Q, Zheng WH. Protective effect of rutin against oxidative injury in neuronal cells. Zhong Yao Cai. 2014;37:640–4. [PubMed] [Google Scholar]
- 36.Choi KS, Kundu JK, Chun KS, Na HK, Surh YJ. Rutin inhibits UVB radiation-induced expression of COX-2 and iNOS in hairless mouse skin:p38 MAP kinase and JNK as potential targets. Arch Biochem Biophys. 2014;559:38–45. doi: 10.1016/j.abb.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Alhoshani AR, Hafez MM, Husain S, Al-sheikh AM, Alotaibi MR, Al Rejaie SS, et al. Protective effect of rutin supplementation against cisplatin-induced Nephrotoxicity in rats. BMC Nephrol. 2017;18:194. doi: 10.1186/s12882-017-0601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu F, Chen J, Fan LM, Liu K, Zhang N, Li SW, et al. Analysis of the effect of rutin on GSK-3βand TNF-αexpression in lung cancer. Exp Ther Med. 2017;14:127–30. doi: 10.3892/etm.2017.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bispo da Silva A, Cerqueira Coelho PL, Alves Oliveira Amparo J, Alves de Almeida Carneiro MM, Pereira Borges JM, Dos Santos Souza C, et al. The flavonoid rutin modulates microglial/macrophage activation to a CD150/CD206 M2 phenotype. Chem Biol Interact. 2017;274:89–99. doi: 10.1016/j.cbi.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Filipsky T, Ríha M, Hasková P, Pilarová V, Nováková L, Semecky V, et al. Intravenous rutin in rat exacerbates isoprenaline-induced cardiotoxicity likely due to intracellular oxidative stress. Redox Report. 2017;22:78–90. doi: 10.1080/13510002.2016.1159817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanely Mainzen Prince P, Priya S. Preventive effects of rutin on lysosomal enzymes in isoproterenol induced cardio toxic rats:Biochemical, histological and in vitro evidences. Eur J Pharmacol. 2010;649:229–35. doi: 10.1016/j.ejphar.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 42.Imam F, Al-Harbi NO, Al-Harbia MM, Korashy HM, Ansari MA, Sayed-Ahmed MM, et al. Rutin attenuates carfilzomib-induced cardiotoxicity through inhibition of NF-κB, hypertrophic gene expression and oxidative stress. Cardiovasc Toxicol. 2017;17:58–66. doi: 10.1007/s12012-015-9356-5. [DOI] [PubMed] [Google Scholar]
- 43.Sahna E, Deniz E, Aksulu HE. Myocardial ischemia-reperfusion injury and melatonin. Anadolu Kardiyol Derg. 2006;6:163–8. [PubMed] [Google Scholar]
- 44.Tousson E, Hafez E, Zaki S, Gad A. The cardioprotective effects of L-carnitine on rat cardiac injury, apoptosis, and oxidative stress caused by amethopterin. Environ Sci Pollut Res Int. 2016;23:20600–8. doi: 10.1007/s11356-016-7220-1. [DOI] [PubMed] [Google Scholar]
- 45.Crawford J, Cotran R, Kumar V, Collins T. Robbins Pathologic Basis of Disease. 6th ed. Philadelphia: W.B. Saunders; 1999. [Google Scholar]
- 46.Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–6. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 47.Carson DA, Ribeiro JM. Apoptosis and disease. Lancet. 1993;341:1251–4. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]


