Abstract
Objective:
The objective of this study was to highlight the impact of increased cardiac output (CO) and/or pulmonary vascular resistance (PVR) on the occurrence and evolution of pulmonary hypertension (PH) in hyperthyroidism and to follow their evolution in patients under therapy.
Methods:
Our study group consisted of 142 women with hyperthyroidism of different severities and etiologies. We divided our patients into three groups: groups A (overt hyperthyroidism), B (recurrent disease), and C (subclinical forms). We performed echocardiography to determine echocardiographically estimated systolic pulmonary arterial pressure (eePAP), CO, and PVR before and at 3, 6, and 12 months after treatment with thyroid suppression therapy and beta-blockers.
Results:
In our study group we documented PH of various severities in 73 patients (51.4%). Increased CO, induced mostly by hyperthyroidism-specific tachycardia, was frequently detected in overt hyperthyroidism and also augmented PVR, as documented in 43.66% of patients with severe and recurrent forms. For all patients with PH, we emphasized a strong correlation between eePAP and PVR level (r=0.854, p<0.0001) and a moderate one with CO (r=0.437, p<0.0001) and with hyperthyroidism duration (r=0.545, p<0.0001). Under therapy, CO rapidly normalized and PVR significantly decreased in groups A and C. In group B, the reduction was modest and statistically significant.
Conclusion:
The pathophysiological mechanisms responsible for the occurrence of PH are elevated CO and PVR. While increased CO is rapidly alleviated under therapy, elevated eePAP and PVR persist in recurrent cases and are responsible for the perpetuation of PH.
Keywords: pulmonary hypertension, hyperthyroidism, cardiac output, pulmonary vascular resistance
Introduction
One of the most serious cardiovascular complications in hyperthyroidism is pulmonary hypertension (PH) (1, 2). This type of PH is assigned to group 5 with unclear and/or multifactorial mechanisms (3, 4). Its severity ranges from mild forms, with prompt resolution, to clinically significant ones that persist or even worsen over time, leading to right heart failure. Current data regarding the prevalence of PH among hyperthyroid patients are conflicting, with 24% (5), 36% (6), and 65% (7) being reported.
Although the pathogenesis of type 5 PH and/or right heart failure in thyrotoxicosis is uncertain, several theories have been proposed. There are basically two assumptions: 1) hyperthyroidism leads to a hyperkinetic cardiovascular status often associated with high cardiac output (CO) and elevated circulatory volume (8), which determines an increased and rapid venous return to the right ventricle (RV). These factors lead to pressure overload in RV followed by dilatation, tricuspid regurgitation (TR), and augmentation of pulmonary arterial pressure (PAP). 2) More recent data suggest a direct influence of thyroid hormones on the pulmonary circulation (1). Possible explanations are represented by a) enhanced sensitivity to catecholamines of the pulmonary vasculature, followed by reduced compliance and vasoconstriction with augmentation of pulmonary vascular resistance (PVR); b) altered metabolism of vasoconstrictor mediators such as serotonin, endothelin 1, and thromboxane; and c) enhanced metabolism of intrinsic pulmonary vasodilators such as nitric oxide and prostacyclin. In some pathologies (Graves’ and Hashimoto disease), there are autoimmune processes involving thyroid stimulating hormone (TSH) receptor antibodies (TRAbs) that could mediate endothelial cell injury (9, 10). This results in apoptosis-resistant endothelial cells, with vascular remodeling leading to PH aggravation (1, 11, 12). Evidencing these pathophysiological processes is challenging and assessing hemodynamic parameters involves right heart catheterization, but echocardiography offers the possibility of accurately estimating them without the risks of invasive procedures (13, 14).
The main aim of this study was to highlight, by means of echocardiography, the prevailing etiopathogenic mechanism responsible for the augmentation of PAP-increased CO and/or alterations of PVR-and to document the relationship between them in hyperthyroid patients. The second aim was to demonstrate the evolution of CO and/or PVR under proper therapy of hyperthyroidism with prognostic impact.
Methods
Study population
Our study included 142 female patients aged 26–55 years (mean age=42.21±6.54 years) admitted to the Endocrinology Clinic of our hospital in a period of 4 years (2013-2017).
Inclusion criteria:
Hyperthyroidism of various etiologies, severities, and durations.
Exclusion criteria:
Patients diagnosed with arterial PH-idiopathic or heritable forms, those with chronic connective tissue diseases or congenital heart diseases, or those exposed to medication and/or toxins that could induce PH of group 1 and 1’.
Patients with significant left heart pathology that could lead to PH of group 2.
Patients with chronic respiratory diseases that could cause PH of group 3.
Patients with chronic thromboembolic PH or other pulmonary artery obstructions that could cause PH of group 4.
Patients with hematological, systemic, metabolic (except hyperthyroidism), or other diseases that could induce PH of group 5 to prevent the influence of these factors on the apparition of group 5 PH.
Patients exposed to medications for PH (e.g., calcium channel blocker, prostacyclin analogs or endothelin receptor antagonists, phosphodiesterase type 5 inhibitors, and guanylate cyclase stimulators) to avoid the influence of vasodilator drugs on PH.
Postmenopausal women and male patients to limit the impact of estrogen status on the pulmonary vasculature (15).
Study groups: depending on the severity and duration of hyperthyroidism and thyroid hormones levels, the patients were assigned into three groups:
- Group A (80 untreated hyperthyroid women with recent onset of thyroid disease, whose severity ranged from mild to severe forms with thyrotoxicosis)
- Group B (33 women with recurrent hyperthyroidism, diagnosed for over 2 years, with several relapses, with a new episode of thyrotoxicosis at the time of enrolment in the study)
-Group C (29 patients with subclinical hyperthyroidism, suppressed TSH levels, and normal thyroid hormone levels).
Endocrinological evaluation
Diagnosis of hyperthyroidism was based on clinical picture and confirmed by suppressed TSH levels (<0.01 mIU/L) and increased free thyroxine (fT4) and/or free triiodothyronine (fT3) levels. Subclinical hyperthyroidism was established based on the suppressed TSH and normal fT4 and fT3 levels. Graves’ disease was confirmed by increased titres of TRAbs.
Serum TSH, fT4, and fT3 levels were determined using chemiluminescent microparticle immunoassay, with the normal range considered being as follows: TSH, 0.465-4.560 mIU/L, fT4, 0.71–1.85 ng/mL (9.13–23.81 pmol/L), and fT3, 1.71–3.71 pg/mL (2.65–5.69 pmol/L). TRAbs were determined by enzyme-linked immunosorbent assay, with positive titres being considered if levels were >1.75 IU/mL.
Thyroid ultrasonography (2D mode and color Doppler) was performed using the Siemens ultrasound system with a linear transducer (5.0–14 MHz). In patients with suppressed TSH levels associated with thyroid nodule(s), the diagnosis of toxic adenoma and toxic multinodular goiter was confirmed by 99mTc thyroid scintigraphy.
Cardiological evaluation
All echocardiographic examinations were performed with an Acuson Sequoia C 512 ultrasound machine and by the same echocardiographist to avoid interobserver differences. On echocardiography, after a regular examination of cardiac morphology and function, we pursued the evolution of the following parameters:
-
For the left ventricle (LV)
- Ejection fraction determined by the Simpson method.
- CO calculated on the basis of stroke volume (SV) and heart rate (HR).
-
B. For the RV:
- Right atrial (RA) diameter and aria.
- RV diameter.
- TR velocity.
- Tricuspid annular plane systolic excursion (TAPSE) values of <16 mm were considered suggestive of RV dysfunction.
-
Echocardiographic estimated PVR has been shown to provide a reliable non-invasive estimation of PVR (13). In this study, we considered PVR of >2 WU as elevated and PVR of >3 WU as significant (3, 13, 18).These measurements were repeated after 3, 6, and 12 months of therapy when patients achieved euthyroid state.
Statistical analysis
Data analysis was performed using SPSS v.24.0 (Statistical Package for the Social Sciences, Chicago, IL, USA). Continuous variables were presented as mean and standard deviation (SD) or median and interquartile range, and categorical variables were presented as frequency and percentages. Bias-corrected and accelerated bootstrap interval (1000 bootstrap samples) was used to calculate the 95% confidence interval. We performed descriptive and inferential statistics analysis to summarize the characteristics of the study population. To evaluate the proportion of PH in the groups, we applied the chi-squared test (χ2). The results of the Shapiro-Wilk normality test showed a non-Gaussian distribution; therefore, we continued to use nonparametric tests. To highlight the prevailing etiopathogeny of PH, we analyzed the strength of a linear relationship between eePAP and CO, SV, and PVR using Spearman’s rank-order correlation. To compare patients with/without PH, characteristics in Table 1 and specific group-pairs with each other, we used the Mann-Whitney U test. For comparing the three groups (A, B, C), we used the Kruskal-Wallis H test, followed by a post-hoc analysis with Dunn’s pairwise test, and Bonferroni correction was applied for the three pairs of groups. Friedman’s test followed by a post-hoc analysis with Wilcoxon signed-rank test were conducted with a Bonferroni correction to evaluate the evolution of eePAP, CO, and PVR in our three groups over a follow-up period of 12 months. A p value of <0.05 was considered to indicate a statistically significant difference.
Table 1.
Characteristics and results of echocardiography in the study group
| Clinical, laboratory, and echocardiography results | Group A | Group B | Group C | pf | ||
|---|---|---|---|---|---|---|
| 80 patients | 33 patients | 29 patients | ||||
| A-B | A-C | B-C | ||||
| TSH, 0.46-4.68 µUI/mLa | 0.01 (0.005-0.052) | 0.004 (0.0009-0.008) | 0.3 (0.26-0.36) | 0.001 | <0.001 | <0.001 |
| Confidence intervalb | [0.0085; 0.015] | [0.003; 0.004] | [0.3; 0.34] | |||
| Age, yearsc | 38.25±5.93 | 39.93±6.62 | 42.92±6.52 | 0.188 | <0.006 | 0.079 |
| fT4, 10-28.2 pmol/L | 34.4 (28.22-45) | 59 (40.64-62.14) | 13.2 (12-17.04) | <0.001 | <0.001 | <0.001 |
| fT3, 4.28-8.1 pmol/L | 17 (10.5-26.4) | 12.5 (6.97-22.67) | 5.13 (4.32-5.98) | 0.047 | <0.001 | <0.001 |
| TRAb | 10.3 (5-40.1) | 40.6 (33.15-51.75) | 1 (0.9-1.25) | 0.019 | <0.001 | <0.001 |
| Onset of hT, (months) | 4.6 (3.1-5.7) | 25 (19.5-27) | 4 (3.4-4.5) | <0.001 | 0.117 | <0.001 |
| Heart rate (b/min) | 100 (82-107.7) | 99 (88-100) | 78(70-87.5) | 0.212 | <0.001 | <0.001 |
| Paroxysmal AFd, e | 4 (5%) | 5 (15.15%) | 0 | 0.070 | 0.220 | 0.029 |
| Parameters of LV | ||||||
| Ejection fraction (%) | 68 (63.22-70) | 67 (67-70) | 76 (74-77) | 0.176 | <0.001 | <0.001 |
| Stroke volume (mL) | 90 (83.25-98.75) | 85 (76.5-96) | 80 (79-81.5) | 0.002 | <0.001 | 0.026 |
| Cardiac output (L/min) | 8.3 (7.9-9.27) | 7.8 (7-8.85) | 6.24 (5.68-6.87) | 0.06 | <0.001 | <0.001 |
| Parameters of RV | ||||||
| RA aria (cm2) | 18.4 (13.73-24.6) | 23 (18.2-28.67) | 11.4 (7.3-14.1) | <0.001 | <0.001 | <0.001 |
| Tricuspid ring diameter | 3.7 (3.3-4) | 3.3 (3-3.8) | 2.2 (2-2.5) | <0.001 | 0.001 | <0.001 |
| RV basal diameter (cm) | 2.8 (2.51-3) | 3 (3-3.25) | 2.4 (2.3-2.6) | <0.001 | <0.001 | <0.001 |
| TAPSE | 23 (20-27) | 17 (16-18) | 27 (25.5-28) | <0.001 | 0.007 | <0.001 |
| TR: mild | 28 (35%) | 10 (30.3%) | 13 (44.8%) | 0.631 | 0.349 | 0.237 |
| moderate | 21 (26.25%) | 13 (39.39%) | 0 | 0.166 | 0.002 | <0.001 |
| severe | 8 (10%) | 8 (24.24%) | 0 | 0.027 | 0.100 | 0.004 |
| eePAP (<35 mm Hg) | 40 (25-48) | 50 (34-59.5) | 5 (5-5) | 0.017 | <0.001 | <0.001 |
| PVR estimated (<2 WU) | 2 (1.2-3) | 3.8 (1.8-5.7) | 0.16 (0.16-0.16) | 0.003 | <0.001 | <0.001 |
Median and associated quartiles (Q1–Q3);
Bias-corrected and accelerated bootstrap interval (1000 bootstrap samples);
Mean and standard deviation (SD);
Counts (percentages);
Chi-squared test;
Mann-Whitney U test
Group A: newly diagnosed overt hyperthyroidism; Group B: recurrent hyperthyroidism; Group C: subclinical hyperthyroidism
TSH - thyroid stimulating hormone; fT4 - free thyroxin; fT3 - free triiodothyronine; TRAb - TSH receptor antibodies; hT - hyperthyroidism; AF - atrial fibrillation; LV - left ventricle; RV - right ventricle; RA - right atria; TAPSE - tricuspid annular plane systolic excursion; TRV - tricuspid regurgitation velocity; eePAPs - echocardiographically estimated systolic pressure in the pulmonary artery; PVR - pulmonary vascular resistance; WU - Wood Units
The study was approved by the Ethics Committee of our hospital and all patients signed a written consent.
Results
All female patients included in our study had hyperthyroidism of various etiologies: Graves’ disease (119%-83.8%), multinodular goiter (12%-8.45%), and toxic adenoma (11%-7.74%). From our group, 113 patients (79.57%) had overt hyperthyroidism, and 33 women had been diagnosed more than 2 years ago, with several relapses, treated with antithyroid drugs. These women, for one reason or another, have refused/delayed surgery or radioactive iodine therapy and were treated only with oral antithyroid drugs. Due to poor compliance to therapy, they remained insufficiently controlled, with at least two relapses of thyrotoxicosis occurring during their evolution. From this subgroup, 27 patients (81.81%) had Graves’ disease and 6 (18.18%) had multinodular goiter.
Thyroid dysfunctions were treated according to their etiologies and severities-either surgery or therapy with radioactive iodine or oral antithyroid drugs-so that patients could achieve euthyroid state as soon as possible. Most of them also received beta-blockers to treat associated cardiovascular symptoms-tachycardia, arrhythmias, or elevated blood pressure.
After a careful evaluation, any other etiology that could have determined PH was ruled out. Although some patients had systemic hypertension grade 1 or mild mitral or aortic lesions, no one had significant cardiovascular pathology.
The results of clinical, laboratory, and echocardiographic examinations are presented in Table 1, and the distribution of eePAP, PVR, CO, and SV in patients with and without PH are presented in Figure 1.
Figure 1.
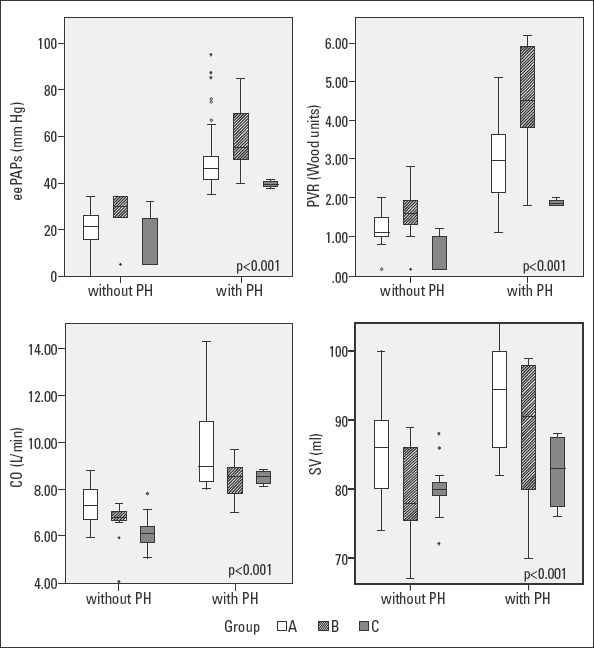
Distributions of eePAP, PVR, CO, and SV levels in patients with and without PH
Group A - newly diagnosed overt hyperthyroidism; Group B - recurrent hyperthyroidism; Group C - subclinical hyperthyroidism; PH - pulmonary hypertension; PVR - pulmonary vascular resistance; eePAPs - echocardiographically estimated pulmonary artery pressure; CO - cardiac output; SV - stroke volume; Box plots of PAPs; PVR; CO, SV.Kruskal-Wallis H test (p<0.001)
With regard to the 80 patients with newly diagnosed overt hyperthyroidism, 33 (41.25%) had no PH, but 47 patients (58.75%) had PH with the median of eePAP being 46 (43–52) mm Hg. These patients had various severities-5 (10.63%) severe, 17 (36.17%) moderate, and 25 (53.19%) mild. All 47 patients with PH had elevated CO due to increased HR. All patients had normal SV. Referring to PVR, 39 patients with PH (81.35%) had elevated values [median=3 (2.2–3.7) WU], and 24 had values >3 WU; all of them also had elevated CO, but normal SV. We found that eePAP had a strong positive, linear correlation with CO (r=0.803, p<0.001, CI=0.709–0.869), but a moderate one with SV (r=0.571, p<0.001, CI=0.402–0.702) and with HR (r=0.660, p<0.001, CI=0.515–0.768). We determined a strong positive linear correlation between mean eePAP and PVR level (r=0.917, p<0.001, CI=0.874–0.946) and with the duration of the disease (r=0.76, p<0.001, CI=0.649–0.839) (Fig. 2).
Figure 2.
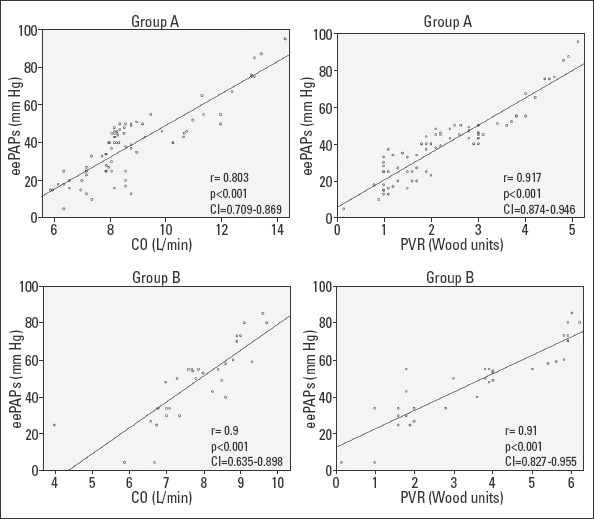
Correlations between eePAP and CO and between eePAP and PVR in groups A and B
Group A - newly diagnosed overt hyperthyroidism; Group B - recurrent hyperthyroidism; Group C - subclinical hyperthyroidism; PH - pulmonary hypertension; PVR - pulmonary vascular resistance; eePAPs - echocardiographically estimated pulmonary artery pressure; CO - cardiac output; the analyse of the strength of linear relationship between levels of PAPs and CO respective PVR was performed with Spearman’s rank-order correlation
Referring to group B (33 patients with recurrent hyperthyroidism) with relapse and thyrotoxicosis at the time of the study, 22 (66.66%) patients were detected with increased eePAP value with a median of 55 (50–70.7) mm Hg. There were 8 patients (36.36%) with severe PH, 10 (45.45%) with moderate, and 5 (22.72%) with mild PH. We documented elevated CO values in 13 patients (59.09%), with a median of 8.55 (7.78–8.92) L and a median HR of 94.5 (90–100) b/min, but neither of them had increased SV, median=90.5 (79.5–98) mL. Elevated PVR was determined in 20 patients with PH (90.9%), all with levels >3 WU and a median of 5.2 (3.9–5.9) WU. In patients with recurrent hyperthyroidism, we found a positive strong relationship between eePAP and PVR (r=0.91, p<0.001, CI=0.827–0.955) and CO (r=0.9, p<0.001, CI=0.635–0.898), but a moderate one between eePAP and SV (r=0.56, p=0.001, CI=0.278–0.761) and HR (r=0.539, p<0.001, CI=0.241–0.744). The correlation between eePAP and the duration of the disease was strong (r=0.944, p<0.001, CI=0.889–0.972) (Fig. 2).
From the group of 29 patients with subclinical hyperthyroidism, 25 patients (86.2%) had no PH and only 4 (13.79%) had mild PH [median of eePAP=39.5 (38.25–40.75) mm Hg]. Of these 4 women, 3 (75%) had mildly increased CO [median=8.52 (8.16–8.77) L] and 2 (50%) had slightly increased PVR [median=1.85 (1.80–1.97) WU], neither of them with values of >3 WU. We determined a moderate, positive, linear correlation between eePAP and CO (r=0.514, p=0.004, CI=0.182–0.74) and HR (0.587, p=0.005, CI=0.281–0.784), but not between eePAP and SV (r=0.128, p=0.504, CI=−0.472 to −0.25). eePAP was strongly correlated with PVR (r=0.978, p<0.001, CI=0.954–0.989) and moderately with the disease duration (r=0.656, p<0.001, CI=0.382–0.824).
Of the total 142 patients, 73 (51.4%) had PH of various severity with a median PAP of 49 (43–56.5) mmHg. Regarding the left and right cardiac performance, estimated by FE and TAPSE, all patients had values within the normal ranges. In 64 patients (45.07%) from our study group, mainly in those with new-onset overt hyperthyroidism, we documented increased CO [median=8.8 (8.36–10.29) L], but this elevation was mostly determined by hyperthyroidism-specific tachycardia taking into account that median HR was 100 (90.75–110) b/min. On the other hand, in 62 patients (43.66%) with overt hyperthyroidism, especially in severe and recurrent cases, we documented augmented PVR (>2 WU) [median=3 (2.2–4.1) WU], with 44 of them having values of >3 WU.
For patients with PH from all three subgroups, we found that eePAP had a strong positive correlation with PVR (r=0.854, p<0.001, CI=0.242–0.664), a moderate one with CO (r=0.437, p<0.001, CI=0.414-0.792) and with the duration of the thyroid disease (r=0.545, p<0.001, CI=0.18–0.614), and a weak one with SV (r=0.313, p=0.007, CI=0.242–0.664).
Referring to the etiology of hyperthyroidism in patients with PH, 44 women (93.61%) from group A and 20 (90.9%) from group B had Graves’ disease. This pathology was absent in patients with subclinical hyperthyroidism and PH. Using Spearman’s rank-order correlation test, we documented moderate positive correlations between eePAP and PVR and between eePAP and TRAb (r=0.512, p<0.001, CI=0.302–0.687 and r=0.601, p<0.001, CI=0.318–0.826, respectively). The correlation between CO and TRAb was weaker (r=0.371, p<0.006, CI=0.131–0.591) in patients with Graves’ disease and PH.
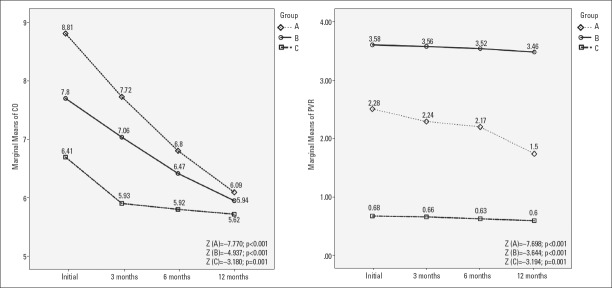
We followed the evolution of eePAP, CO, and PVR at 3, 6, and 12 months. Friedman’s test demonstrated a statistically significant difference for all three groups (p<0.001). Post-hoc analysis with Wilcoxon signed-rank tests was conducted and a Bonferroni correction was applied, resulting in a significance level at p<0.008. Referring to initial levels of eePAP, we noticed a significant reduction every 3 months in patients with newly diagnosed hyperthyroidism (group A), leading to a decrease of 22.9% at 12 months (Z=−7.706, p<0.001). In group B, eePAP registered a significant reduction only after the first 6 months, which reached 3.56% at 12 months (Z=−3.601, p<0.001). In group C, the reduction of eePAP at 12 months was 7.35% (Z=−3.077, p=0.002) (Fig. 3).
Figure 3.
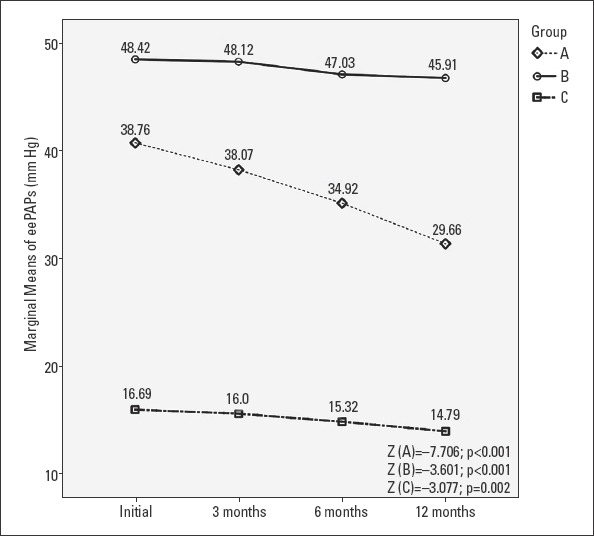
Evolution of eePAP values at 3, 6, and 12 months in patients with PH
Group A - newly diagnosed overt hyperthyroidism; Group B - recurrent hyperthyroidism; Group C - subclinical hyperthyroidism; eePAPs - echocardiographically estimated pulmonary artery pressure; the Friedman test followed by a post-hoc analysis with Wilcoxon signed-rank test was conducted with a Bonferroni correction applied resulting in a significance level set at p<0.008
Referring to CO, although at the beginning of the study, we registered the most elevated median of CO in patients from group A (with newly diagnosed overt hyperthyroidism). In the evolution, especially in the first 6 months, after restoring the euthyroid state, under specific treatment of hyperthyroidism and beta-blockers, we documented an extremely significant regression of CO for groups A and B, but not for group C. At 12 months, we noticed a significant regression of CO, with 26.56% in group A (Z=−7.770, p<0.001), with 23.76% in group B (Z=−4.937, p<0.001), and with 9.93% in group C (Z=−3.180, p=0.001) (Fig. 4).
Figure 4.
Evolution of CO and PVR values at 3, 6, and 12 months in patients with PH
Group A - newly diagnosed overt hyperthyroidism; Group B - recurrent hyperthyroidism; Group C - subclinical hyperthyroidism; PVR - pulmonary vascular resistance; CO - cardiac output; the Friedman test followed by a post-hoc analysis with Wilcoxon signed-rank test was conducted with a Bonferroni correction applied resulting in a significance level set at p<0.008
Discussing the evolution of PVR, we initially documented increased medians in group A and especially in group B, but not in group C. These values decreased significantly, but slowly, over time in group A, with a total reduction of 32.81% at 12 months (Z=−7.638, p<0.001). In groups B and C, the reduction was more modest, thus statistically significant [with 8.763% at 12 months (Z=−3.644, p<0.001) in group B and 11.84% at 12 months in group C (Z=−3.194, p=0.001)] (Fig. 4).
We used the Pearson chi-squared test (χ2) to determine the risk ratio for developing PH in groups A and B using group C as reference and we obtained 5.15 for group B and 4.61 for group A.
Discussion
In this study, we documented PH of various severities in 73 female patients (51.4%). The incidence of PH in hyperthyroidism is a controversial topic in the literature, with data ranging from 24% (5) to 65% (7), depending on the selection criteria of the study group and assessment methods. The most common etiology of hyperthyroidism in our patients was Graves’ disease (119 patients, 83.8%), followed by multinodular goiter (12 patients, 8.45%) and toxic adenoma (11 patients, 7.74%), which is characteristic for our geographic region. Patients with Graves’ disease had a higher incidence of PH (54.62%), supporting the hypothesis that autoimmune processes, associated with endothelial damage, may play a major role in the pathogenesis of PH (1, 11). For patients with overt Graves’ disease and PH, we documented the presence of a significant moderate positive correlation between eePAP and PVR and between eePAP and TRAb. PH prevailed in patients with recurrent hyperthyroidism (66.66%), followed by those with overt forms (58.75%), but was also detected in subclinical disease (13.79%).
To preclude other etiologies of PH, we used very strict inclusion/exclusion criteria in our study, neither of our patients having pathological conditions that could induce PH by themselves. Our study included only hyperthyroid women. Generally, hyperthyroidism is more frequent in women, and Graves’ disease is known to have an incidence ten times higher in female patients than in male patients. Smoking is also more common in men and is responsible for an increased incidence of chronic obstructive pulmonary disease and therefore of group 3 PH. Therefore, only 11 men met the inclusion criteria for our study and for enhanced homogeneity of the study group, without the influence of sex, we excluded them from our study. Similarly, we have excluded postmenopausal women. In the literature, there are debates on sex differences and the influence of estrogen on the pathogeny of PH (15).
In our study, we used echocardiography for the assessment of eePAP, CO, SV, and PVR. However, some authors (19) have presented conflicting data regarding the correlation between eePAP, assessed by Doppler echocardiography, and mean PAP measured via right heart catheterization (20). Despite its diagnostic accuracy, this last method has limitations: it is invasive, has potentially serious risks, and is not ethically justified only for screening purpose. On the other hand, echocardiography offers a reliable estimation of eePAP and PVR. In their studies, Abbas et al. (13) demonstrated a strong correlation between eePAP and PVR, determined both by Doppler echocardiography and right heart catheterization, being a reliable method to identify patients with elevated PVR and providing an improved non-invasive method, aspect debated also in other articles (16-18).
In terms of the pathophysiological mechanisms involved in the occurrence of PH in hyperthyroidism, there are few inconsistent data in the literature (10, 21). We detected all patients with new-onset overt hyperthyroidism, elevated CO, related mostly to hyperthyroidism-specific tachycardia, taking into account that few of them (12.67%) had increased SV. This group was heterogeneous regarding the severity of hyperthyroidism, which included mild, moderate, and severe forms as well as thyrotoxicosis, which explains the wide variation of CO and eePAP. Regarding patients with recurrent hyperthyroidism, 59.09% of patients with PH had elevated CO, but neither increased SV, similar to the results reported by Teasdale (21). The correlation between eePAP and CO was very strong only in overt disease. In all patients with new-onset hyperthyroidism, CO was rapidly alleviated under therapy with beta-blockers and antithyroid drugs, aspect debated in other studies (21).
Analyzing the impact of PVR’s elevation on the pathogenesis of PH, we documented PVR of >3 WU in 90.9% of patients with recurrent hyperthyroidism and PH. By comparison, only 43.66% of patients with overt disease presented abnormal PVR sustaining the hypothesis that this dysfunction gradually develops, as a consequence of pulmonary vascular remodeling triggered by autoimmune and humoral mechanisms.
The statistical analysis revealed that in all patients from our study, the strongest correlation was that between eePAP and PVR.
For patients with PH from all three subgroups, we found that eePAP has a very strong positive correlation with PVR, a moderate one with CO and SV, and a strong one with the thyroid disease duration.
We followed up the evolution of PH over a period of 1 year. In patients with newly diagnosed overt hyperthyroidism, we observed a gradual regression of eePAP, CO, and PVR, parallel with the restoration of euthyroidism, with an extremely significant difference between the initial and final levels (p<0.001); this was also described in other studies (22, 23). The fastest regression was observed for CO levels and HR. In patients with recurrent forms, the reduction of PVR was modest, thus statistically significant. It is possible that the duration of follow-up for these patients was too small to draw any significant conclusions.
In our study, the risk ratio analysis for developing PH in overt hyperthyroidism evidenced an increased risk, especially in recurrent cases in which it was five times higher than that in subclinical ones.
As our knowledge of RV physiology and biology progresses, it is obvious that a comprehensive approach to pulmonary circulation and RV’s physiology and their interactions will be beneficial in both clinical management of PH patients and scientific research (13, 14, 24). The evolution of RV pathology from normal to a compensated (hypertrophied) and then decompensated state parallels the evolution of pulmonary vascular physiology from a vasodilated, high capacitance state to vasoconstricted arteries and early loss of endothelial cells/capillaries to an end-stage proliferative and obliterative vascular remodeling. Therefore, it is important to study the physiology of the RV and PH comprehensively and simultaneously as a unit (24).
Study limitations
In this study we relied only on the echocardiographic assessment of eePAP, CO, SV, and PVR and have not validated our data with invasive methods. Another aspect was that in this study, we have not debated the impact of the severity of hyperthyroidism, expressed by the levels of serum fT4, fT3, and TSH, on the gravity of PH. Group A was very heterogeneous, including patients with overt hyperthyroidism of various severity, ranging from mild forms to moderate and severe ones with thyrotoxicosis, and therefore, eePAP, CO, and PVR values varied widely. To diminish the statistical significance of the results, we decided not to divide group A into subgroups, according to the severity of hyperthyroidism.
Conclusion
The pathophysiological mechanisms responsible for the occurrence of PH in our patients were the elevation of CO, mainly as a consequence of hyperthyroidism-specific tachycardia, and the augmentation of PVR. In hyperthyroid patients, CO gradually alleviated under therapy with antithyroid drugs and beta-blockers, reaching normal limits. The fastest regression of CO was observed in newly diagnosed cases, who also had a reduction by more than a third of the increased PVR in a year. In recurrent cases, the reduction of eePAP and PVR at a year was modest, thus statistically significant, but remained in the pathological domain being responsible for the perpetuation of PH.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – C.T., M.T.; Design – C.T., M.T.; Supervision – C.T., M.T.; Fundings – C.T., M.T.; Materials – M.V., M.B., F.P.; Data collection &/or processing – C.T., M.T., M.V., M.B., G.N.P.; Analysis &/or interpretation – C.T., M.T., G.N.P.; Literature search – C.T., M.T., M.B., F.P.; Writing – C.T., M.T., G.N.P.; Critical review – C.T., M.T., M.V., F.P.
References
- 1.Biondi B, Kahaly GJ. Cardiovascular involvement in patients with different causes of hyperthyroidism. Nat Rev Endocrinol. 2010;6:431–43. doi: 10.1038/nrendo.2010.105. [DOI] [PubMed] [Google Scholar]
- 2.Marvisi M, Balzarini L, Mancini C, Mouzakiti P. Thyroid gland and pulmonary hypertension. What's the link? Panminerva Med. 2013;55:93–7. [PubMed] [Google Scholar]
- 3.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension:The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS):Endorsed by:Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Li JH, Safford RE, Aduen JF, Heckman MG, Crook JE, Burger CD. Pulmonary hypertension and thyroid disease. Chest. 2007;132:793–7. doi: 10.1378/chest.07-0366. [DOI] [PubMed] [Google Scholar]
- 6.Sugiura T, Yamanaka S, Takeuchi H, Morimoto N, Kamioka M, Matsumura Y. Autoimmunity and pulmonary hypertension in patients with Graves'disease. Heart Vessels. 2015;30:642–6. doi: 10.1007/s00380-014-0518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armigliato M, Paolini R, Aggio S, Zamboni S, Galasso MP, Zonzin P, et al. Hyperthyroidism as a cause of pulmonary arterial hypertension:a prospective study. Angiology. 2006;57:600–6. doi: 10.1177/0003319706293131. [DOI] [PubMed] [Google Scholar]
- 8.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–35. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 9.Zuhur SS, Baykiz D, Kara SP, Sahin E, Kuzu I, Elbuken G. Relationship Among Pulmonary Hypertension, Autoimmunity, Thyroid Hormones and Dyspnea in Patients with Hyperthyroidism. Am J Med Sci. 2017;353:374–80. doi: 10.1016/j.amjms.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Scicchitano P, Dentamaro I, Tunzi F, Ricci G, Carbonara S, Devito F, et al. Pulmonary hypertension in thyroid diseases. Endocrine. 2016;54:578–87. doi: 10.1007/s12020-016-0923-8. [DOI] [PubMed] [Google Scholar]
- 11.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension:a perspective. Eur Respir J. 2005;26:1110–8. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- 12.Jabbar A, Pingitore A, Pearce SHS, Zaman A, Iervasi G, Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol. 2017;14:39–55. doi: 10.1038/nrcardio.2016.174. [DOI] [PubMed] [Google Scholar]
- 13.Abbas AE, Franey LM, Marwick T, Maeder MT, Kaye DM, Vlahos AP, et al. Noninvasive assessment of pulmonary vascular resistance by Doppler echocardiography. J Am Soc Echocardiogr. 2013;26:1170–7. doi: 10.1016/j.echo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Naing P, Kuppusamy H, Scalia G, Hillis GS, Playford D. Non-Invasive Assessment of Pulmonary Vascular Resistance in Pulmonary Hypertension:Current Knowledge and Future Direction. Heart Lung Circ. 2017;26:323–30. doi: 10.1016/j.hlc.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Dempsie Y, MacLean MR. The influence of gender on the development of pulmonary arterial hypertension. Exp Physiol. 2013;98:1257–61. doi: 10.1113/expphysiol.2012.069120. [DOI] [PubMed] [Google Scholar]
- 16.Nickson C Critical Care Compendium. [Updated May 6 2018]. Available from:URL: https://lifeinthefastlane.com/ccc/
- 17.Badesch DB, Champion HC, Sanchez MAG, Hoeper MM, Loyd JE, Manes A, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S55–66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Lindqvist P, Söderberg S, Gonzalez MC, Tossavainen E, Henein MY. Echocardiography based estimation of pulmonary vascular resistance in patients with pulmonary hypertension:a simultaneous Doppler echocardiography and cardiac catheterization study. Eur J Echocardiogr. 2011;12:961–6. doi: 10.1093/ejechocard/jer222. [DOI] [PubMed] [Google Scholar]
- 19.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–21. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaymaz C, Mutlu B, Küçükoğlu MS, Kaya B, Akdeniz B, Kılıçkıran Avcı B, et al. Preliminary results from a nationwide adult cardiology perspective for pulmonary hypertension:RegiStry on clInical outcoMe and sUrvival in pulmonaRy hypertension Groups (SIMURG) Anatol J Cardiol. 2017;18:242–50. doi: 10.14744/AnatolJCardiol.2017.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teasdale SL, Warrick J, Stowasser M, Stanton T. Hyperdynamic Right Heart Function in Graves'Hyperthyroidism Measured by Echocardiography Normalises on Restoration of Euthyroidism. Heart Lung Circ. 2017;26:580–5. doi: 10.1016/j.hlc.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Marmouch H, Boubaker F, Arfa S, Slim T, Sayadi H, Jmal M, et al. Transient pulmonary hypertension in patients with Graves'disease. Endocrine Abstracts 2015;In 17 th European Congress of Endocrinology;2015 May 16-20, Dublin, Ireland. Endocrine Abstracts. 2015;37:EP936. [Google Scholar]
- 23.Marvisi M, Zambrelli P, Brianti M, Civardi G, Lampugnani R, Delsignore R. Pulmonary hypertension is frequent in hyperthyroidism and normalizes after therapy. Eur J Intern Med. 2006;17:267–71. doi: 10.1016/j.ejim.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Badano LP, Ginghina C, Easaw J, Muraru D, Grillo MT, Lancellotti P, et al. Right ventricle in pulmonary arterial hypertension:haemodynamics, structural changes, imaging, and proposal of a study protocol aimed to assess remodelling and treatment effects. Eur J Echocardiogr. 2010;11:27–37. doi: 10.1093/ejechocard/jep152. [DOI] [PubMed] [Google Scholar]