Abstract
Objective:
Type A aortic dissection (AD) and ascending thoracic aortic aneurysm (AA) are thoracic vascular diseases with similar initial pathology but inequable clinical features and outcomes, where local and systemic inflammation play an important part. We aimed to observe and analyze the differences and correlation between inflammation and pathological changes in the aorta and biomechanical strength between AD and AA.
Methods:
From August 2011 to February 2013, 20 patients with AD (AD group) and 13 patients with AA (AA group) who underwent aorta surgery were included. Serum concentrations of total cholesterol (TC), triglycerides (TG), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) levels were measured just before surgical anesthesia. The longitudinal vessel samples of the affected ascending aorta were harvested during surgery and prepared for subsequent pathological observation and uniaxial tension test to measure the longitudinal tensile strength (TS). Samples were also prepared for further measurement of tissue homogenized TNF-α and IL-6 concentrations.
Results:
No significant difference was seen between the two groups with respect to baseline data, and the serum concentrations of TC and TG of both the groups were within the normal range (p>0.05). Blood and tissue homogenized levels of IL-6 and TNF-α were significantly higher in the AD group than in the AA group (p<0.001). Pathological observation of the aortic tissue showed more inflammatory cells infiltration and elastic fiber destruction in the AD group than in the AA group, indicating significant aortic medial degeneration. Uniaxial tensile tests showed that the longitudinal TS was significant lower in the AD group than in the AA group (p<0.001). The longitudinal TS showed negative correlations with serum and tissue homogenized concentrations of IL-6 and TNF-α in the AD group (p<0.05), whereas no such significant correlation was seen in the AA group.
Conclusion:
Patients with AD had acute systemic inflammation, along with acute inflammation and declined biomechanical strength of the affected aorta. The serum and tissue homogenized concentrations of IL-6 and TNF-α showed a significant correlation with the biomechanical strength of affected aorta in AD.
Keywords: aortic dissection, inflammation, tensile strength, interleukin-6, tumor necrosis factor-α
Introduction
Aortic dissection (AD) is a critical illness that progresses rapidly and thus can be termed as a medical emergency condition. Cardiovascular surgery needs to be performed immediately as this condition has an extremely high fatality rate. The fatality rate in patients with AD increases by about 1%–2% every hour since its onset, so the fatality rate can rise up to 50% within 48 h (1). In patients with AD, aortic rupture and multiple organ dysfunction are the leading causes of death (2, 3). Surgical and interventional treatments are the conventional therapeutic approaches used in the treatment of patients with acute AD; surgical treatments are particularly recommended for Stanford A AD cases involving the aorta. However, due to acute inflammation of the local blood vessels, it is difficult to treat AD with surgical and interventional treatments: these approaches are characterized with high risk factors. Inflammation contributes to the occurrence and development of AD: local chronic inflammations causing aortic medial degeneration are involved in the pathophysiology of AD (4-7). During this process, inflammatory mediators such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) play a major role. After the occurrence of AD, there is a massive and rapid development of local chronic inflammations. Consequently, the condition of patients worsens as they rapidly develop signs and symptoms of acute systemic inflammation. Finally, such patients develop systemic inflammatory response syndrome (SIRS) or even multiple organ dysfunction syndrome (MODS). In clinical practice, we have observed that acute inflammations of the aortic wall severely affect the structural strength of the aorta, such as significant regional inflammation and edema which lead to brittle and vulnerable aorta tissue. These factors cause anastomotic hemorrhage and complications while performing surgery. While performing interventional treatments, changes in the aortic walls can significantly affect the stabilities of the implanted covered stents and increase the possibility of endoleaks. Therefore, acute inflammations of the aortic walls cause complications while performing cardiovascular surgery and interventional treatments in patients with acute AD and even subacute AD, in whom the disease course ranges between 2 weeks and 2 months.
Aortic aneurysm (AA) is another macrovascular disease that is clinically different from AD. Although the two diseases have the same pathophysiology, aortic medial degeneration, the degrees of their systemic and local inflammatory responses are different. Multiple organ dysfunction caused by systemic inflammatory responses is a rare phenomenon in patients with AA. Moreover, while performing surgery, it was discovered that the vascular lesions in patients with AA were significantly milder than those in patients with AD, thereby significantly reducing the possibility of developing complications, such as vascular anastomosis and anastomotic hemorrhage. Currently, no research studies have compared the differences in the biomechanical strength of aortic vessels of patients with AD or AA; therefore, no data regarding the correlations between these differences and degrees of inflammatory responses are available.
In this study, we aim to preliminarily explore the degree of systemic inflammations and local vessel inflammation in AD, to detect their influence on vessel tensile properties, and to compare them with those in AA. We hypothesized that there is a correlation between inflammation and decrease in vessel strength in AD.
Methods
Subjects and grouping
From August 2011 to February 2013, 20 patients with Stanford A AD and 13 with AA who underwent surgical treatments were enrolled in the AD and AA groups respectively, at the Department of Cardiovascular Surgery of West China Hospital, Sichuan University. Before enrolling, informed consents were obtained from all the patients. All of them voluntarily participated in the entire study.
This study was approved by the Ethics Committee of West China Hospital.
Inclusion and exclusion criteria
We included patients with Stanford A AD who underwent graft replacement of the ascending aorta (graft replacement of the aortic arch was performed in some patients with aortic arch lesions) and patients with ascending thoracic AA who underwent graft replacement or arterioplasty of the ascending aorta at our hospital. The affected aortic walls were either partially or completely removed during the surgical process.
We excluded patients with a history of chronic diseases, such as chronic obstructive pulmonary disease, diabetes, kidney disease, and liver cirrhosis that resulted in organ dysfunctions. We also excluded patients whose condition was complicated by cancers, autoimmune diseases, or other inflammatory diseases.
Methods
Test items
On the planned operation day, serum samples were collected from every patient before administering anesthesia; these samples were used to detect the serum concentrations of total cholesterol (TC), triglycerides (TG), IL-6, and TNF-α.
The longitudinal specimens of the lesioned aorta were collected for tests below: (1) uniaxial tensile test; (2) pathomorphological observation under the hematoxylin–eosin (HE) and elastic fiber (EF) staining; (3) preparation of tissue homogenates to test IL-6 and TNF-α concentration.
Detection of serum concentrations of IL-6 and TNF-α
An IMMULITE 1000 Immunoassay System (Siemens, Munich, Germany) equipped with human TC, TG, IL-6, and TNF-α detection kits (Siemens, Munich, Germany) was used.
Collection and preservation of the vessel specimen
Surgeons cut out longitudinal section of the aortic tissue on the basis of the following specific conditions for the different groups of patients: aortic vessel specimens were obtained from the normal-looking nondissected aorta tissues near the dissected aortic lesions in the AD group; whereas in the AA group, aortic vessel specimens were obtained from the aortic aneurysm walls. The specimens with regular edges had to be ≥30.0 mm in length and ≥5.0 mm in width. These aortic vessel specimens were divided into three categories. The first category of aortic vessel specimens were immediately embedded in paraffin for HE and EF staining and the resultant pathomorphological changes of the vascular tissues were observed. The second category of aortic vessel specimens were homogenized (final tissue concentration of 10%) for detecting IL-6 and TNF-α concentrations. The third category of aortic vessel specimens was first washed with normal saline and then wrapped in saline-soaked gauzes. Thereafter, these wrapped specimens were stored in a refrigerator at −20°C. All the specimens were postoperatively sent for tensile testing within 1 month.
Determination of IL-6 and TNF-α concentrations in the aortic vessel specimens
The 10% tissue homogenates were prepared for the double antibody sandwich ELISA test for determining of IL-6 and TNF-α concentrations using the IL-6/TNF-α ELISA kits (NeoBioscience, Shenzhen, China).
Tensile tests of the aortic vessel specimens
Instron 8744 Servohydraulic Test Systems (Instron, Grove City, US) was used to perform the uniaxial tension test, and the longitudinal TS (as the maximum external force the specimen can tolerate before fracture when being stretched) of the aortic vessel specimens was measured. The test process is shown in Figure 1.
Figure 1.
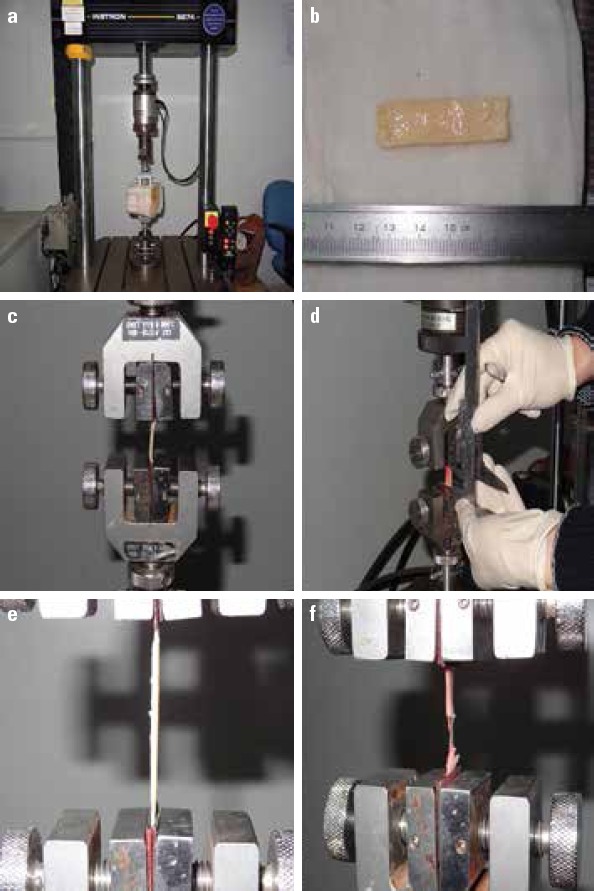
Tensile test of the aortic vessel specimens
(a) Instron 8744 servohydraulic test systems (Instron, Grove City, US)
(b) Prepared aortic vessel specimen with the required shape
(c) Specimen longitudinally loaded onto the test system
(d) Measure of the initial specimen lengths using vernier caliper
(e) Specimen stretched on the system with a certain load range and pulling speed
(f) Data of tensile strength that can indicate the mechanical strength of the specimen was collected at the time when the specimen was stretched to fracture
After thawing, each specimen was cut into three rectangular pieces having a minimal size of 30.0 mm×5.0 mm and regular edges. Each specimen was loaded onto the system longitudinally, and the testing specimen’s initial length, width, and thickness measured by a vernier caliper were input using the testing software affiliated to the mechanical testing system. A 100-N force sensor was used to conduct the experiment on the aortic vessel specimens at a load range of 20 N and a pulling speed of 10 mm/min; the test process was terminated when the specimen was just completely stretched to fracture by the pulling process. During the tensile process, the testing software was used to automatically record loads, tensile elongations, stresses, and strains of the specimens at each time point. The longitudinal TS of the aortic vessel specimen was defined as the maximal stress the sample can tolerate just before being completely stretched to fracture; for each patient, the mean value of TS obtained from the three tissue pieces was used for statistical analysis. Measure values and definitions of the tensile test are shown in Table 1.
Table 1.
Measure values and definitions of the tensile test
| Name | SI unit | Definition | Formula |
|---|---|---|---|
| Load, F | N | Force applied uniformly in a direction of a sample | |
| Area, S | M2 | The cross-sectional area of the sample perpendicular to the load direction | |
| Stress, σ | Pa | Load on the sample per unit of area | σ=F/S |
| Tensile Strength, TS | the maximum stress the sample can tolerate before fracture when being stretched |
Statistical analysis
SPSS 19.0 software (SPSS Inc., Chicago, USA) was used for data input and statistical analysis. GraphPAD Prism 5 software (GraphPad Software Inc., La Jolla, USA) was utilized to create the graphical charts. Measurement data were expressed as means±standard deviations, enumeration data were analyzed using the chi-squared test. Student t test was applied to analyze the mean differences of normally distributed continuous variables between the two groups, and Pearson linear correlation analysis was used to assess the correlations between the corresponding measurement data of the two groups.
Results
Baseline data of the enrolled patients
The baseline data of the patients is shown in Table 2. Age and sex data of both the groups were collected, and no significant difference was seen between the two groups (age: p=0.331, gender: p=0.250). Typical clinical manifestations of the AD group were recorded: severe viscera and limb ischemia was not observed, four patients in the AD group presented symptoms of respiratory failure and noninvasive mechanical ventilation was performed. No significant difference was seen in the serum concentrations of TC and TG (TC: p=0.192, TG: p=0.515), and all values were within the normal range.
Table 2.
Baseline data of the enrolled patients in aortic dissection and aortic aneurysm groups
| AD group (n=20) | AA group (n=13) | P value | |
|---|---|---|---|
| Male | 14 (70%) | 10 (77%) | 0.331 |
| Age (years) | 42.8±10.6 | 45.3±18.6 | 0.250 |
| Onset time till surgery (h) | 502.9±280.1 | ||
| Pain in chest and back | 18 (90%) | ||
| Renal failure | 0 | ||
| Gastrointestinal ischemia | 0 | ||
| Limb ischemia | 0 | ||
| Respiratory failure | 4 (20%) | ||
| Serum total | 4.25±1.65 | 3.94±2.08 | 0.192 |
| cholesterol (mmol/L) | |||
| Serum triglycerides | |||
| (mmol/L) | 1.02±0.23 | 1.21±0.45 | 0.515 |
Data are presented as mean±SD or as numbers and percentages. P<0.05 was considered statistically significant.
AA - aortic aneurysm; AD - aortic dissection
Pathomorphological observation of the aortic vessel specimens under HE and EF staining
We performed pathomorphological observations under HE and EF staining. HE staining revealed abundant inflammatory cell infiltrations in the aortic vessel specimens of the AD group, whereas few inflammatory cells were observed in those of the AA group. Moreover, the consecutive structures of EF in the medial wall were clear in the aortic vessel specimens of the AA group. In contrast, these clear structures were rarely observed in the specimens of the AD group. EF staining revealed different distributions of the elastic fiber in the aortic vessel specimens of AD and AA groups: structures of the elastic fiber in the medial wall of the AD group were disordered and inconsecutive, with frequent fiber breakages; whereas the elastic fibers of the AA group were integrated in the form of continuous and parallel bundles with few breakages (see Fig. 2).
Figure 2.
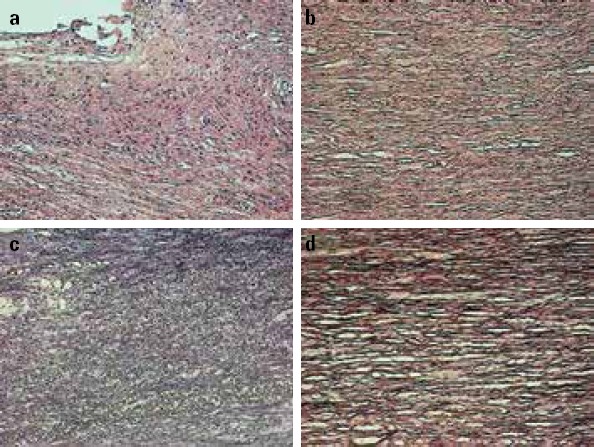
Pathomorphological observations of the aortic vessel specimens of the aortic dissection (AD) and aortic aneurysm (AA) groups using hematoxylin–eosin (HE) and elastic fiber (EF) staining technique under 200× magnification
(a) HE staining of the specimen in the AD group
(b) HE staining of the specimen in the AA group
(c) EF staining of the specimen in the AD group
(d) EF staining of the specimen in the AA group
Comparisons of IL-6 and TNF-α concentrations in the serum and vessel tissue homogenates
In the AD group, the serum concentrations of IL-6 and TNF-α were significantly higher than those in the AA group (IL-6: 72.15±38.68 pg/mL versus 5.20±3.51 pg/mL, p<0.001; TNF-α: 57.83±23.63 pg/mL versus 10.29±5.05 pg/ml, p<0.001). The serum concentrations of IL-6 and TNF-α in the AA group were within the normal range (normal range of serum IL-6 and TNF-α concentrations: 0-7.00 pg/mL and 0-20.00 pg/mL). Similar results were obtained in the comparison of levels of IL-6 and TNF-α in vessel tissue homogenates of AD and AA groups (IL-6: 59.89±21.88 pg/mL versus 28.50±19.09 pg/mL, p<0.01; TNF-α: 595.83±232.56 pg/mL versus 46.73±28.52 pg/mL, p<0.001). The results are shown in Figure 3.
Figure 3.
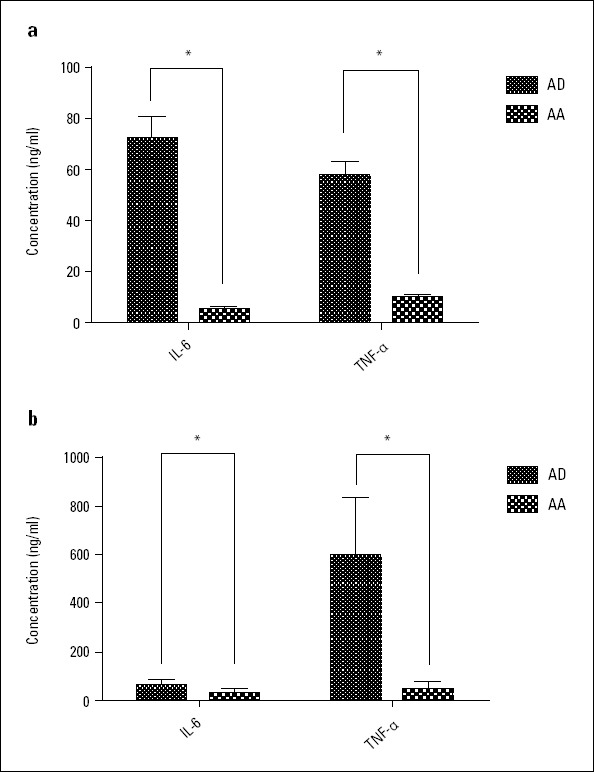
IL-6 and TNF-α concentrations in the serum and vessel tissue homogenates of the AD and AA groups
(a) Blood IL-6 and TNF-α concentrations
(b) Vessel tissue homogenate IL-6 and TNF-α concentrations
*Significant difference, P<0.05
Comparison of longitudinal TS in the aortic vessel specimens
The longitudinal TS of the aortic vessel specimens was 0.57±0.19 MPa in the AD group and 1.05±0.22 MPa in the AA group. As shown in Figure 4, the longitudinal TS of the aortic vessel specimen in the AD group was significantly lower than that in the AA group (p<0.001).
Figure 4.
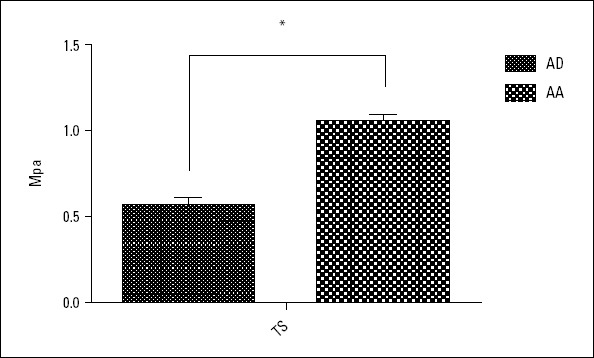
Longitudinal TS in the aortic vessel specimens of the AD and AA groups
*Significant difference, P<0.05
Correlation analyses of the longitudinal TS of the aortic vessel specimens and IL-6 and TNF-α concentrations in the serum and tissue homogenates
In the AD group, the longitudinal TS of the aortic vessel specimen negatively correlated with IL-6 and TNF-α concentrations in the serum and tissue homogenates (blood IL-6: r=−0.50, p=0.026, blood TNF-α: r=−0.52, p=0.018; tissue homogenate IL-6: r=−0.49, p=0.028; tissue homogenate TNF-α: r=−0.50, p=0.025, see Fig. 5), and no significant correlations were found between the longitudinal TS of the aortic vessel specimens and the IL-6 and TNF-α concentrations in serum and tissue homogenates in the AA group (blood IL-6: p=0.761, blood TNF-α: p=0.980; tissue homogenate IL-6: p=0.217; tissue homogenate TNF-α: p=0.878, see Fig. 6). It revealed that decrease in tissue strength was associated with systemic (serum) and local (vessel tissue) inflammation response of AD group.
Figure 5.
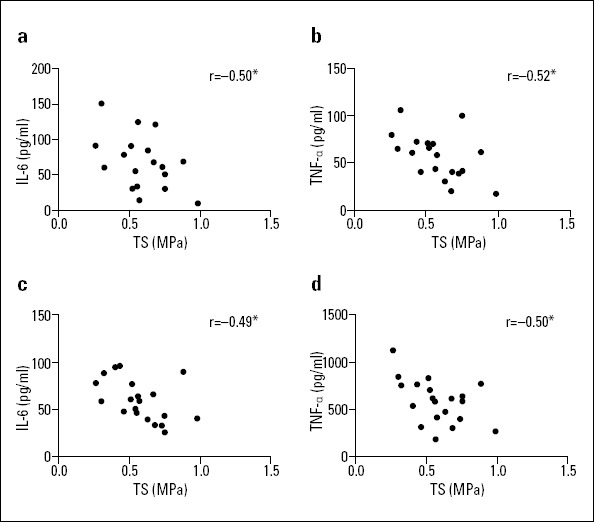
Correlation of the longitudinal TS of the aortic vessel specimens and IL-6 and TNF-α concentrations in the serum and tissue homogenates of the AD group
(a) Serum level IL-6/TS correlation
(b) Serum level TNF-α/TS correlation
(c) Tissue level IL-6/TS correlation
(d) Tissue level TNF-α/TS correlation
*Significant difference, P<0.05
Figure 6.
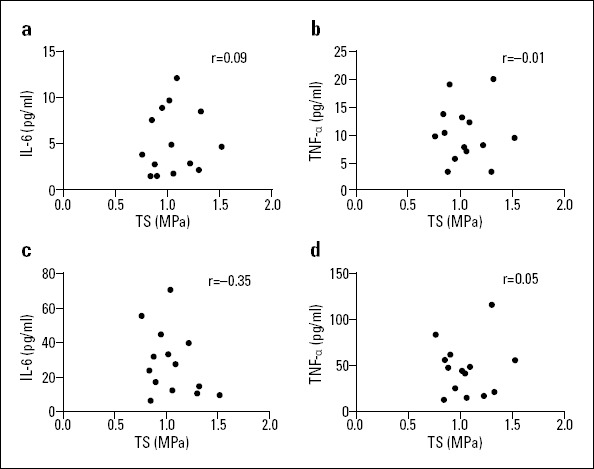
Correlation of the longitudinal TS of the aortic vessel specimens and IL-6 and TNF-α concentrations in the blood and tissue homogenates of the AA group
(a) Blood level IL-6/TS correlation
(b) Blood level TNF-α/TS correlation
(c) Tissue level IL-6/TS correlation
(d) Tissue level TNF-α/TS correlation
All P>0.05
Discussion
AD is a critical cardiovascular illness that has a sudden onset, a rapid disease progression, and an extremely high fatality rate. Recent studies have confirmed the direct involvement of inflammatory responses in the occurrences and progressions of aortic diseases such as AD and AA, which are mainly characterized by aortic medial degeneration. Prior to the onset of AA and AD in patients, local infiltration of T-lymphocytes and macrophages is found in the aortic medial wall. These two cells would activate the Fas system, promote the apoptosis of smooth muscle cells, and release inflammatory mediators such as IL-6 and TNF-α (4). These two mediators will upregulate the expression of matrix metalloproteinases (MMPs) through NF-κB, ERK1/2, and JNK pathways, resulting in the degeneration of EFs and medial degeneration (8, 9). The degradation products of MMPs introduce strong leukocyte chemotaxis, which can promote local inflammation cell infiltration in feedback and release IL-6 and TNF-α (10, 11). This self-promoted aortic medial degeneration surges continuously and leads to AA if the arterial wall is not separated. Conversely, it leads to AD if the arterial wall is separated. After the onset of AD, the contact between blood and the dissected aortic wall further instigates a traumatic inflammation by inducing a hemodynamic change that triggers the release of massive inflammatory mediators such as IL-6 and TNF-α (12, 13). As a consequence, there is an outbreak of an acute inflammation in the large blood vessels, causing further damages to the medial walls that extend the dissection. When the above inflammatory mediators enter the peripheral blood flow, their blood concentrations significantly rises in patients with AAD (6). Systemic distribution of these massive inflammatory mediators in the circulating blood induces and aggravates systemic inflammatory response, resulting in the development of SIRS and even MODS. The two important inflammatory cytokines, IL-6 and TNF-α directly participate in the local and systemic inflammatory responses before and after the onsets of AA and AD in patients. The pathological detections of IL-6 and TNF-α have directly confirmed the differences in the local and systemic inflammatory responses of patients with AA and AD. Due to the difference in clinical features and outcome in AA and AD, the differences in inflammation response in both groups and their contribution toward clinical significance need to be confirmed.
The course of AA and AD can damage the involved aorta and lead to decrease in vessel strength, as the AD involved vessels are more brittle and vulnerable than AA involved ones. The difference in vessel strength is a major problem, which presents a great challenge for AD surgical treatments. Previous basic biomechanical studies of aorta wall have proved that the mechanical properties of the aortic vessel’s wall depend on the structure of the medial wall, which in turn depends on the nature, content, and spatial configuration of its collagen fibers, EFs, and smooth muscles. Among all the arteries, aorta has the thickest medial wall owing to a high content ratio of EF to collagen fiber and gets the lowest content of smooth muscle, so its biomechanical properties are mainly dependent on the EFs of the medial wall (14). Therefore, aortic medial degeneration and EF damages caused by the inflammatory mediators in AA and AD have a great impact on the biomechanical properties of the affected tissues in the aortic vessel. As the mechanical strength of the aortic vessel wall gets impaired, vessel strength, i.e., TS may get significantly reduced, thereby causing great complications during surgical procedure. So, we assumed that the degree of inflammation response might directly affect tissue strength. Therefore, we used the uniaxial tensile test to measure the longitudinal TS of the aortic vessels, comparing the difference in its biomechanical strength between AA and AD groups.
Aortic specimens were collected from the AD and AA group. We harvested the specimen in the AD group from the normal-looking nondissected aorta tissues near the dissected aortic lesions, since we assumed the inflammation caused by dissection might even affect the tissue strength of nondissected aorta with complete three layers of intima, media and adventitia. For biomechanical tests, the aorta vessel specimens were frozen stored for centralized tests, and it was confirmed that frozen storage of vessel specimen at −20°C did not induce weakening of biomechanical properties (15-17). Since no significant difference was seen between the serum levels of TC and TG in the two groups, the possibility of hyperlipemia-induced aorta lesions was denied. Our results indicated that the serum and tissue homogenized IL-6 and TNF-α concentrations in the AD group were significantly different from that in the AA group. The pathomorphological results revealed that inflammatory cell infiltrations in the medial wall and EF damages of the aortic vessel were more severe in the AD group than in the AA group. Compared with the AA group, the AD group had more significant systemic and local inflammatory responses, the latter of which was directly reflected in the different biomechanical strengths determined through sequential mechanical tensile testing of the aortic vessel specimens: biomechanical strength, the longitudinal TS of the aortic vessels in the AD group was significantly lower than that in the AA group, indicating that impaired vessels of the AD group had a significantly reduced vessel strength and a higher possibility of being damaged when exposed to a foreign force. These differences in the strength of the aortic vessel in the AD and AA groups coincided with the clinical practice.
Due to ethical issues, we could not obtain aorta specimens from healthy subjects for further study. However, previous studies proved that the biomechanical strength of the AA vessels were significantly poorer than those of the normal vessels. Vorp et al. (18) compared the biomechanical properties between AA vessel specimens obtained during surgical processes and normal ascending aorta wall specimens obtained from autopsy of dead subjects. They found that compared with the normal ascending aorta wall specimens, the longitudinal and latitudinal TS of the ATAA vessel specimens were decreased by 34% and 29%, respectively (18). Iliopoulos et al. (19) also proved that TS in AA vessels was significantly decreased compared with that in the normal vessels. Moreover, they clearly pointed out that this decrease was directly correlated with the EF damages of the medial wall in patients with AA (19). In this study, we did not get the biomechanical data of normal aorta specimens, but it had been reported that the longitudinal TS of the ascending aorta specimens of normal subjects was of 1.71±0.14 MPa (20). Compared with the longitudinal TS of ascending aorta wall specimens in normal subjects, the longitudinal TS of the impaired ascending aorta wall specimens of the AD and AA groups in our study was decreased by 67% and 38%, respectively. Therefore, the difference and gradient of longitudinal TS among AD involved aorta, AA involved aorta and normal aorta was obvious. This observation agreed well with that observed in clinical practice.
The correlation analysis performed between IL-6 and TNF-α concentrations in both blood and tissue homogenates and longitudinal TS of vessel specimens in the AD group showed a significant negative correlation with the corresponding IL-6 and TNF-α concentrations in both serum and tissue homogenates, but no significant correlation was found in the AA group, neither in the serum nor in the tissue homogenates. These findings further proved the existence of acute inflammatory responses in the impaired aortic vessels of patients with AD and the significantly negative impact of acute inflammatory responses on the strengths of aortic vessel: severe inflammatory responses can cause sharp decline in the strength of aortic vessel. The massive releases of IL-6 and TNF-α in the lesioned aortic vessels of patients with AD could be attributed to their direct participation in the local inflammatory responses. Thus, this phenomenon would negatively affect the strength of aortic vessel. Furthermore, these inflammatory mediators may also be involved in systemic inflammatory responses. Therefore, further studies must be conducted to decipher the clinical application of IL-6 and TNF-α concentrations in the serum. The findings can be used to predict the strength of the impaired aortic vessels in patients with acute AD. Thus, we can optimize the surgical timing and minimize complications encountered during surgery. In other words, we can sharply reduce both intraoperative and postoperative complications.
Study limitations
Our research had several limitations. As mentioned earlier, we faced constraints of ethical issues as we could not measure the biomechanical properties of the ascending aorta in normal people. Therefore, we could not compare the differences of longitudinal TS of the aortic vessel between patients with AD and AA and normal people, but we compared them with previously recorded data. We had also planned to measure the aorta in normal autopsy series, which had passed ethic reviews, but we were unable to get enough autopsy samples in time. Maybe, in the future, we can get enough controlled samples for statistical comparison. Moreover, the impaired aortic vessel should be maximally reserved for arterioplasty or facilitating the wrapping of the graft in the surgical graft replacement for hemostasis. Therefore, we could obtain only limited numbers of aortic vessel specimens and it was difficult to get latitudinal specimen for testing latitudinal TS along with longitudinal TS. In addition, to evaluate the degrees of inflammatory responses in tissue vessels, we preliminarily adopted the ELISA assays and detected TNF-α and IL-6 concentrations in tissue homogenates. This was a simple method to yield quantitative data, which would facilitate sequential correlation analysis. Due to the limitations of miscellaneous factors involved in the preparation of tissue homogenate and ELISA assay, a further qualitative analysis must be performed for measuring tissue concentration of TNF-α and IL-6 using western blot and immunohistochemistry techniques.
Conclusion
More significant inflammatory responses developed in patients with AD than in those with AA, such as acute inflammatory responses and declining mechanical properties of the aortic vessel wall, further leading to systemic inflammation. IL-6 and TNF-α are a part of and play important roles in both systemic and local vessel inflammatory responses; these responses adversely affected the biomechanical properties of the involved aortic vessel. The serum concentrations of IL-6 and TNF-α, which were clinically easy to measure, might somehow be used to indicate the decrease in biomechanical strength of the affected aorta in AD.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – Z.B., W.M.; Design – Z.B., J.G.; Supervision – W.M.; Fundings – Y.S.; Materials – W.M.; Data collection &/or processing – Z.B.; Analysis &/or interpretation – Z.B.; Literature search – J.G.; Writing – Z.B.; Critical review – W.M.
References
- 1.Franco KL, Verrier ED. Advanced therapy in cardiac surgery. Hamilton, Ont.; Saint Louis: B.C. Decker; 1999. [Google Scholar]
- 2.Aalberts JJ, Boonstra PW, van den Berg MP, Waterbolk TW. In-hospital mortality and three-year survival after repaired acute type A aortic dissection. Neth Heart J. 2009;17:226–31. doi: 10.1007/BF03086252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JB, Chung CH, Moon DH, Ha GJ, Lee TY, Jung SH, et al. Total arch repair versus hemiarch repair in the management of acute DeBakey type I aortic dissection. Eur J Cardiothorac Surg. 2011;40:881–7. doi: 10.1016/j.ejcts.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 4.He R, Guo DC, Estrera AL, Safi HJ, Huynh TT, Yin Z, et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg. 2006;131:671–8. doi: 10.1016/j.jtcvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Sbarouni E, Georgiadou P, Marathias A, Geroulanos S, Kremastinos DT. D-dimer and BNP levels in acute aortic dissection. Int J Cardiol. 2007;122:170–2. doi: 10.1016/j.ijcard.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 6.del Porto F, Proietta M, Tritapepe L, Miraldi F, Koverech A, Cardelli P, et al. Inflammation and immune response in acute aortic dissection. Ann Med. 2010;42:622–9. doi: 10.3109/07853890.2010.518156. [DOI] [PubMed] [Google Scholar]
- 7.Li JJ, Zhu CG, Yu B, Liu YX, Yu MY. The role of inflammation in coronary artery calcification. Ageing Res Rev. 2007;6:263–70. doi: 10.1016/j.arr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Samuvel DJ, Sundararaj KP, Lopes-Virella MF, Huang Y. IL-6 and high glucose synergistically upregulate MMP-1 expression by U937 mononuclear phagocytes via ERK1/2 and JNK pathways and c-Jun. J Cell Biochem. 2010;110:248–59. doi: 10.1002/jcb.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skjøt-Arkil H, Barascuk N, Larsen L, Dziegiel M, Henriksen K, Karsdal MA. Tumor necrosis factor-αand receptor activator of nuclear factor-κB ligand augment human macrophage foam-cell destruction of extracellular matrix through protease-mediated processes. Assay Drug Dev Technol. 2012;10:69–77. doi: 10.1089/adt.2010.0366. [DOI] [PubMed] [Google Scholar]
- 10.Cheuk BL, Cheng SW. Differential expression of elastin assembly genes in patients with Stanford Type A aortic dissection using microarray analysis. J Vasc Surg. 2011;53:1071–8. e2. doi: 10.1016/j.jvs.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 11.LeMaire SA, Wang X, Wilks JA, Carter SA, Wen S, Won T, et al. Matrix metalloproteinases in ascending aortic aneurysms: bicuspid versus trileaflet aortic valves. J Surg Res. 2005;123:40–8. doi: 10.1016/j.jss.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Cuhlmann S, Van der Heiden K, Saliba D, Tremoleda JL, Khalil M, Zakkar M, et al. Disturbed blood flow induces RelA expression via c-Jun N-terminal kinase 1: a novel mode of NF-kappaB regulation that promotes arterial inflammation. Circ Res. 2011;108:950–9. doi: 10.1161/CIRCRESAHA.110.233841. [DOI] [PubMed] [Google Scholar]
- 13.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, et al. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–51. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harkness ML, Harkness RD, McDonald DA. The collagen and elastin content of the arterial wall in the dog. Proc R Soc Lond B Biol Sci. 1957;146:541–51. doi: 10.1098/rspb.1957.0029. [DOI] [PubMed] [Google Scholar]
- 15.Adham M, Gournier JP, Favre JP, De La Roche E, Ducerf C, Baulieux J, et al. Mechanical characteristics of fresh and frozen human descending thoracic aorta. J Surg Res. 1996;64:32–4. doi: 10.1006/jsre.1996.0302. [DOI] [PubMed] [Google Scholar]
- 16.Chow MJ, Zhang Y. Changes in the mechanical and biochemical properties of aortic tissue due to cold storage. J Surg Res. 2011;171:434–42. doi: 10.1016/j.jss.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Delgadillo JOV, Delorme S, El-Ayoubi R, DiRaddo R, Hatzikiriakos S. Effect of freezing on the passive mechanical properties of arterial samples. J Biomedical Science and Engineering. 2010;3:645–52. [Google Scholar]
- 18.Vorp DA, Schiro BJ, Ehrlich MP, Juvonen TS, Ergin MA, Griffith BP. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Ann Thorac Surg. 2003;75:1210–4. doi: 10.1016/s0003-4975(02)04711-2. [DOI] [PubMed] [Google Scholar]
- 19.Iliopoulos DC, Kritharis EP, Giagini AT, Papadodima SA, Sokolis DP. Ascending thoracic aortic aneurysms are associated with compositional remodeling and vessel stiffening but not weakening in age-matched subjects. J Thorac Cardiovasc Surg. 2009;137:101–9. doi: 10.1016/j.jtcvs.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Vorp DA, Raghavan ML, Muluk SC, Makaroun MS, Steed DL, Shapiro R, et al. Wall strength and stiffness of aneurysmal and nonaneurysmal abdominal aorta. Ann N Y Acad Sci. 1996;800:274–6. doi: 10.1111/j.1749-6632.1996.tb33330.x. [DOI] [PubMed] [Google Scholar]


