Abstract
The neuroplastic mechanisms in the fish brain that underlie sex reversal remain unknown. Gonadotropin-releasing hormone 3 (GnRH3) neurons control male reproductive behaviours in Mozambique tilapia and show sexual dimorphism, with males having a greater number of GnRH3 neurons. Treatment with androgens such as 11-ketotestosterone (KT), but not 17β-estradiol, increases the number of GnRH3 neurons in mature females to a level similar to that observed in mature males. Compared with oestrogen, the effect of androgen on neurogenesis remains less clear. The present study examined the effects of 11-KT, a non-aromatizable androgen, on cellular proliferation, neurogenesis, generation of GnRH3 neurons and expression of cell cycle-related genes in mature females. The number of proliferating cell nuclear antigen-positive cells was increased by 11-KT. Simultaneous injection of bromodeoxyuridine and 11-KT significantly increased the number of newly-generated (newly-proliferated) neurons, but did not affect radial glial cells, and also resulted in newly-generated GnRH3 neurons. Transcriptome analysis showed that 11-KT modulates the expression of genes related to the cell cycle process. These findings suggest that tilapia could serve as a good animal model to elucidate the effects of androgen on adult neurogenesis and the mechanisms for sex reversal in the fish brain.
Introduction
Sex reversal is a well-known phenomenon in fishes, with some naturally changing their phenotype during their lifetime and others switching sexes in response to environmental factors or hormone treatment. Fish which have undergone sex reversal have both gonads, but the reproductive behaviour of the sex they have reversed to1. Kobayashi et al. have reported that hormonal treatments induce heterotypical sexual behaviours in goldfish and carp2; however, they also retain and exhibit sexual behaviours of their original sex in response to key stimulus3. These findings indicate that fish tend to have some plasticity in their neural circuits.
Nile and Mozambique tilapias, commercially important fish species in aquaculture, are often subjected to artificial sex reversal during their early life stages because male fish grow faster than females. As such, androgen administration has been adopted for generating monosex (all male) tilapia4,5. Thorough investigation of the mechanisms underlying gonadal sex differentiation and reversal in tilapias have revealed that these processes are driven by the suppression of genes responsible for the production of one sex hormone and the activation of genes responsible for the production of the opposite sex hormone in the gonads6–9. However, it remains unclear how sex reversal takes place in the brain, though transcriptome analysis was recently applied to elucidate the mechanisms for the sex reversal in the brain10,11. The difficulty in elucidating this issue is partly because the specific neurons critically related to reproductive behaviours are not identified in sex reversal fish.
Gonadotropin-releasing hormones (GnRHs) are neuropeptides responsible for reproduction and other biological events in both vertebrates and non-vertebrates (for review, see12–14). Most teleost fishes (including tilapias) have three GnRH subtypes (GnRH1, GnRH2, and GnRH3). Using Nile tilapia, Ogawa et al.15 established that GnRH3 is a potent neuromodulator for male sexual behaviours such as nesting and aggression15. They found that injection of anti-GnRH antisera into the third ventricle of the male fish brain (intraventricular GnRH-immunoneutralization) resulted in significantly decreased nest-building ability, nest size, and aggressive behaviour15. Our previous study16 that focused on GnRH3 neurons in the tilapia brain, revealed that GnRH3 neurons are localized to the terminal nerve (TN, terminal ganglion) and that mature males have significantly more GnRH3 neurons than mature females. Additionally, androgens such as 11-ketotestosterone (11-KT, fish specific androgen) and methyltestosterone (potent synthetic fish androgen), but not oestrogen, could annul the difference in the number of GnRH3 neurons between the sexes16. In other words, the number of GnRH3 neurons in androgen-treated females were increased to a level similar to mature males, revealing that the brains of androgen-treated females were sexually reversed in terms of GnRH3 neurons. Furthermore, androgen injection produced male-specific nest-building behaviour in about 70% of mature females, within 2 weeks after the 11-KT injection16. Taken together, these studies indicate that GnRH3 neurons are important in regulating reproductive behaviours of males and are also involved in sex reversal of the brain in females. However, it is still uncertain how 11-KT increases GnRH3 neurons in the female brain. Hence, it is especially important to answer whether or not 11-KT increases GnRH3 neurons via adult neurogenesis.
Numerous studies using various vertebrate species have shown that oestrogen can induce neuronal proliferation in adults17 (and see18–20 for reviews on the positive effects of oestrogen on adult neurogenesis in rodents). In contrast, some studies have reported inhibitory effects of androgens on adult neurogenesis in the mouse brain and rhesus macaque hippocampus21,22, whereas other studies on song birds have shown positive effects of androgen on adult neurogenesis23–26. However, since testosterone is converted to estradiol by aromatase in the brain, it is difficult to determine whether testosterone itself has effects on adult neurogenesis. Therefore, it is of great importance to know whether androgens themselves can induce adult neurogenesis and generate new neurons with identified functions like GnRH neurons. A non-aromatizable androgen, 11-KT is the most potent natural androgen in teleosts27,28 and is a non-aromatizable androgen. This property of 11-KT makes it easier to address the question whether the androgen itself, but not oestrogen converted from androgen, is responsible for stimulating adult neurogenesis in androgen-treated fish.
The present study thus aimed to investigate two questions by using mature female Mozambique tilapia. The first question is whether 11-KT can cause cellular proliferation, adult neurogenesis, and altered gene expressions related to cell cycle in the brain and the second question is whether 11-KT can increase GnRH3 neurons, via adult neurogenesis, during masculinization in the females.
Results
11-KT-induces increase in proliferating cells (PCNA-positive) in the periventricular regions of mature females
Our previous study demonstrated that GnRH3 neurons were sexually dimorphic in the Mozambique tilapia; males have a greater number of GnRH3 neurons in the TN and treatment with androgens, such as 11-KT and methyltestosterone, increases the number of GnRH3 neurons in mature females to a level similar to mature males16. In order to examine whether 11-KT affects cellular proliferation in this brain region, we compared the number of proliferating cell nuclear antigen (PCNA)-positive cells between control and 11-KT-injected females.
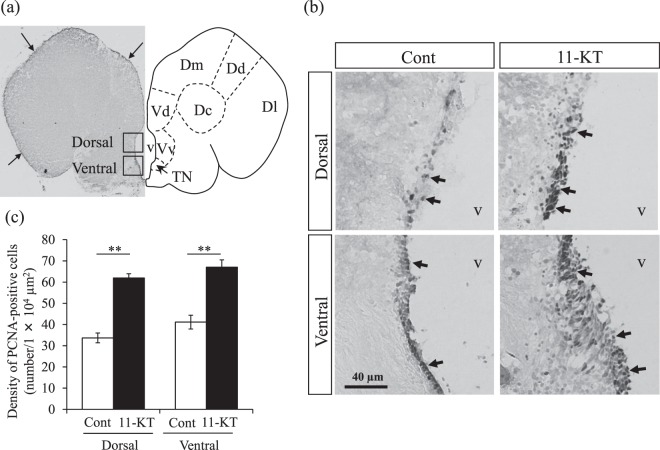
Immunohistochemistry using anti-PCNA antibody revealed that PCNA-positive nuclei were present along the outer surface of the brain (arrows in Fig. 1a) and around the ventricle (periventricular regions, arrows in Fig. 1b). Since the TN is located near the ventricle16,29, we evaluated the effect of 11-KT on cellular proliferation around the ventricle. As shown in Fig. 1b, PCNA-positive cells were observed in the dorsal and ventral periventricular regions of both control and 11-KT-injected females; interestingly, PCNA-positive cells were much more densely detected in 11-KT-injected females than in controls.
Figure 1.
PCNA immunostaining in the female tilapia brain. (a) Left: Representative frontal section of the female tilapia brain crossing the terminal nerve (TN) immunostained with PCNA antibody. The two inserted squares correspond to regions in the dorsal and ventral areas used for counting cells displaying a PCNA-positive nucleus (each area, 140 µm × 140 µm) around the ventricle. Arrows indicate PCNA-positive cells. Right: Atlas of the brain corresponding to the left image. Dm = medial zone of dorsal telencephalic area; Dd = dorsal zone of dorsal telencephalic area; Dl = lateral zone of dorsal telencephalic area; Vd = dorsal nucleus of ventral telencephalic area; Vv, = ventral nucleus of ventral telencephalic area; v = ventricle. (b) Representative image of PCNA-positive cells in the dorsal and ventral areas around the ventricle at the level of the TN in 11-KT-treated and control (Cont) female tilapia. Arrows indicate PCNA-positive nuclei. (c) Density of PCNA-positive cells in control and 11-KT-treated females in the dorsal and ventral areas around the ventricle. **Indicates p < 0. 01 (unpaired Student’s t-test). Data are expressed as mean ± SEM.
Bilateral counts of PCNA-positive cells in the dorsal and ventral periventricular regions (indicated in black squares in Fig. 1a), measured in the frontal section cutting through the TN, revealed that the mean density of PCNA-positive cells was significantly higher in 11-KT injected females than in controls (p < 0.01, Student t-test, Fig. 1c). Thus, these studies demonstrated that administration of 11-KT significantly increased cellular proliferation along the ventricle.
Androgen increased generation of neurons, but not radial glial cells around the ventricle
In order to determine the phenotype of the newly proliferated cells in the brain area around the TN, we combined BrdU-tracing with immunostaining against a neuron marker, Hu, or a radial glial marker, glial fibrillary acidic protein (GFAP). BrdU-tracing experiments were performed by simultaneous injections of BrdU either with 11-KT or with the vehicle (oil).
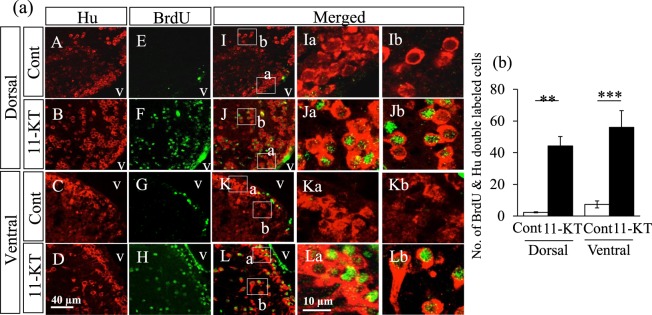
Female fish simultaneously injected with BrdU and 11-KT (or vehicle) had Hu-positive neurons in the dorsal and ventral parts of the brain (red cells in Fig. 2a-A–D and I–L). Only a few BrdU-positive nuclei were detected in controls (green nuclei in Fig. 2a-E and G); however, numerous BrdU-positive nuclei were observed along the ventricle and in substantial areas in the dorsal and ventral parts of the brain in 11-KT-injected females (green nuclei in Fig. 2a-F and H). Similarly, 11-KT-treated female fish displayed many Hu-positive cells with a BrdU-labelled nucleus (newly-generated/proliferated neurons) along the ventricle and in substantial areas of the dorsal and ventral parts of the brain (red cells with green nuclei in Fig. 2a-J and L). In contrast, the double-labelled cells were scarcely detected in control females (Fig. 2a-I and K). Higher magnification images further confirmed our observation that double-labelled cells (BrdU- and Hu-positive cells) were scarcely observed in control females along the ventricle (Fig. 2a-Ia and Ka) or substantial areas of the brain in the controls (Fig. 2a-Ib and Kb), while 11-KT-treated females displayed multiple double-labelled cells in these areas (Fig. 2a-Ja, Jb, La, and Lb).
Figure 2.
Representative images obtained from double immunohistochemistry with BrdU and Hu antibodies. (a) Hu-positive cells (red) observed in dorsal and ventral areas around the ventricle in control (cont) and 11-KT-treated (11-KT) females (A,B,C,D). Although few BrdU-labelled nuclei (green) were detected in control animals (E,G), BrdU-labelled nuclei were frequently observed in 11-KT-treated females (F,H). I,J,K and L show merged images. Ia,b,Ja,b,Ka,b and La,b show enlarged images of the areas inset in I,J,K, and L, respectively; v = ventricle. (b) The number of Hu-positive cells with a BrdU-labelled nucleus. White and black bars indicate the numbers of double-labelled cells in control (Cont) and 11-KT-treated (11-KT) females. **Indicates p < 0. 01; ***Indicates p < 0. 001 (unpaired Student’s t-test). Data are expressed as mean ± SEM.
As shown in Fig. 2b, the number of newly-generated neurons (Hu-positive cells with a BrdU-labelled nucleus) in the dorsal and ventral periventricular regions, in a frontal section cutting through the TN, were markedly increased in 11-KT-injected females, compared with controls (p < 0.01 or p < 0.001; controls vs 11-KT-treated females, unpaired Student’s t-test). These results demonstrate that 11-KT increased adult neurogenesis in the dorsal and ventral regions along the ventricle in females.
GFAP-immunoreactive ventricular radial glial cells in teleost fish are considered as astrocytic subpopulations and neuronal progenitors in mammals30. We examined whether 11-KT could affect the proliferation of radial glial cells around the ventricle, in the frontal section cutting through the TN, in female tilapia. Figure 3 shows representative images of immunostaining with antibodies against GFAP and BrdU. We found many GFAP-positive cells in the dorsal and ventral areas around the ventricle in both control and 11-KT-treated females (red cells in Fig. 3); however, no GFAP-positive cells with a BrdU-labelled nucleus were observed (Fig. 3A’–D’). These findings indicated that 11-KT did not increase the proliferation of ventricular radial glial cells around the TN in the female brain.
Figure 3.
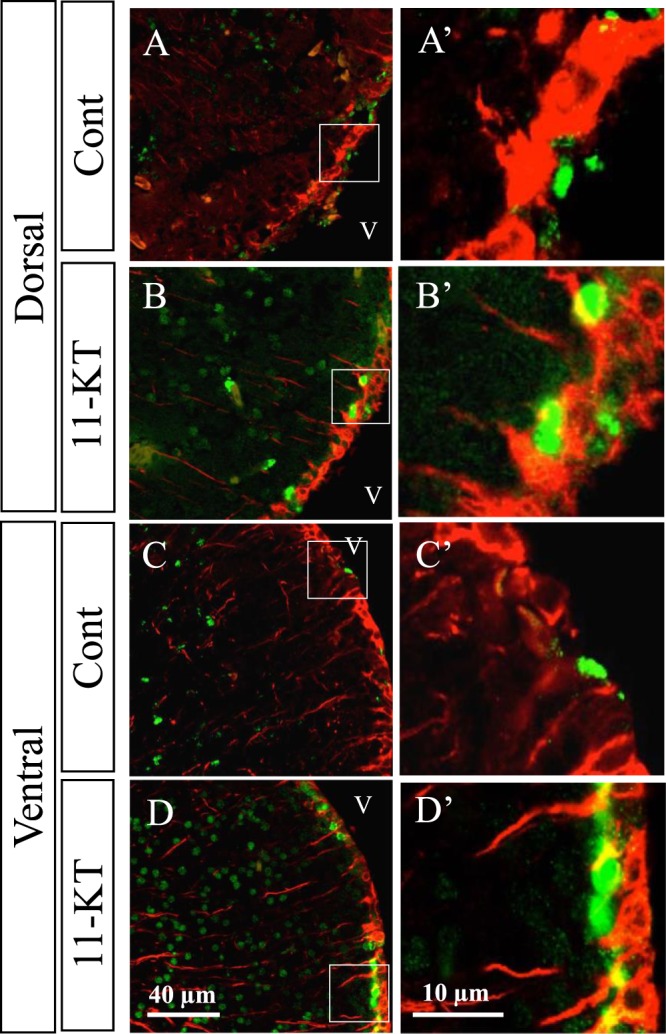
Representative merged images of sections double-stained with BrdU and GFAP antibodies. GFAP-positive cells (red) visualized on the inner surface of the ventricle, with long processes in the dorsal and ventral areas around the ventricle in control (Cont) and 11-KT-treated (11-KT) females. (A,B) dorsal and ventral areas, respectively, of control animals. (C,D) dorsal and ventral areas, respectively, of 11-KT-treated females. A’,B’,C’ and D’ show enlarged views of inset squares in A,B,C, and D, respectively; v = ventricle.
11-KT-generated GnRH3 neurons in female tilapia
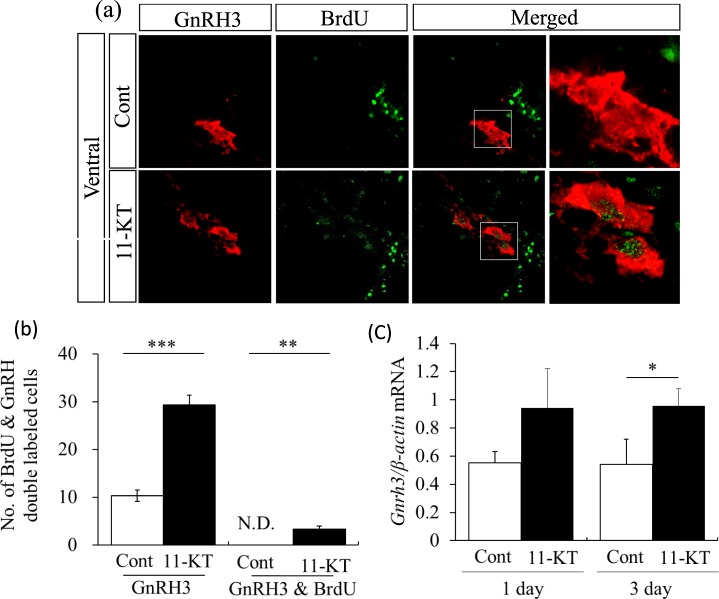
Further examination of the effect of 11-KT on GnRH3 neurons revealed that no control females exhibited GnRH3-positive neurons (red) with a BrdU-labelled nucleus (Fig. 4a, “Cont”). In contrast, GnRH3 neurons (red) with a BrdU-positive nucleus (green) were found in female tilapia injected with 11-KT (Fig. 4a). These findings indicated that 11-KT treatment could induce newly-generated GnRH3 neurons in the female tilapia brain.
Figure 4.
Representative images of sections double-stained with BrdU and GnRH3 antibodies. (a) GnRH3 neurons (red), BrdU-labelling nuclei (green) and merged images in control (Cont) and 11-KT treated (11-KT) females. GnRH3 neurons with BrdU-labelling in the nucleus were observed in 11-KT treated females, but not in controls. The rightmost column shows enlarged views of inserted squares in the second from the right column. (b) Number of GnRH3 neurons in control (Cont, white column) and 11-KT-treated (11-KT, black column) females (left) three days after 11-KT injection. Number of double labelled cells (GnRH & BrdU) in control (Cont, white column) and 11-KT treated (11-KT, black column) animals (right); N. D. = not detected. (c) Relative expression levels of GnRH3 mRNA in control (Cont, white column) and 11-KT treated (11-KT, black column) females one day (left) and three days (right) after 11-KT injection. **Indicates p < 0.01; ***Indicates p < 0.001 (unpaired Student’s t-test). Data are expressed as mean ± SEM.
As shown in Fig. 4b, the mean number of GnRH3-positive neurons was significantly increased in 11-KT-treated fish, compared with control females at three days after 11-KT injection (p < 0.001, Cont vs 11-KT in the left side columns in Fig. 4b). GnRH3 neurons with a BrdU-labelled nucleus were detected in 11-KT-treated females (Fig. 4b), but not in controls.
Our transcriptome analysis showed that the expression level of GnRH3 mRNA was increased by 11-KT one day after treatment (fold change = 1.135, Supplementary Table 3). As shown in Fig. 4c, quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) analysis showed that the expression of GnRH3 mRNA was increased significantly at 3 days after 11-KT injection (p < 0.05, student t-test), but not significantly at day 1.
11-KT-induced changes in cell cycle-related gene expressions
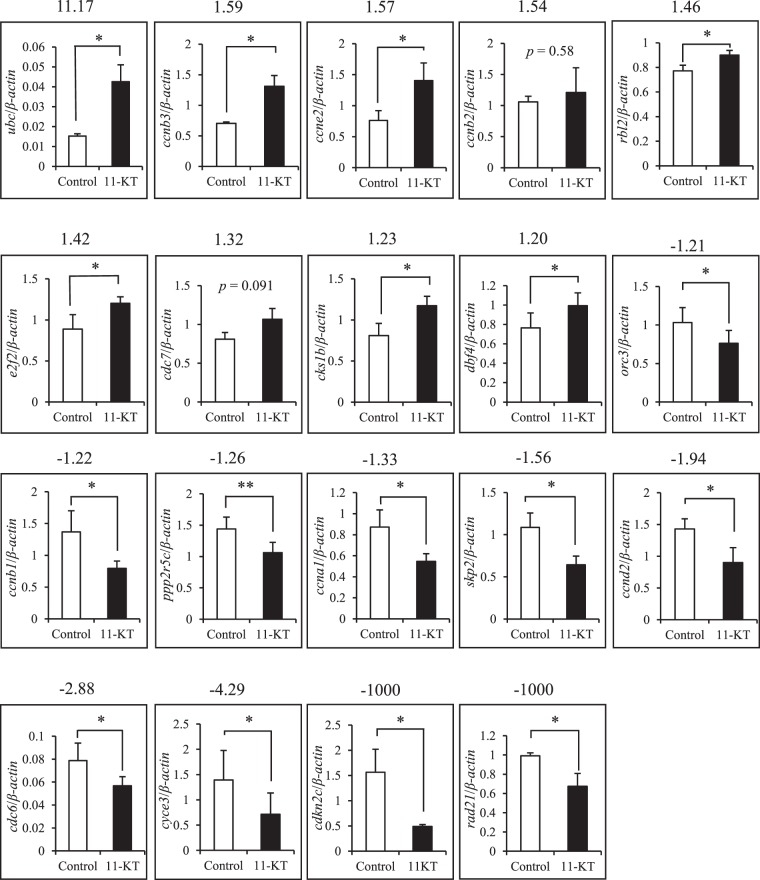
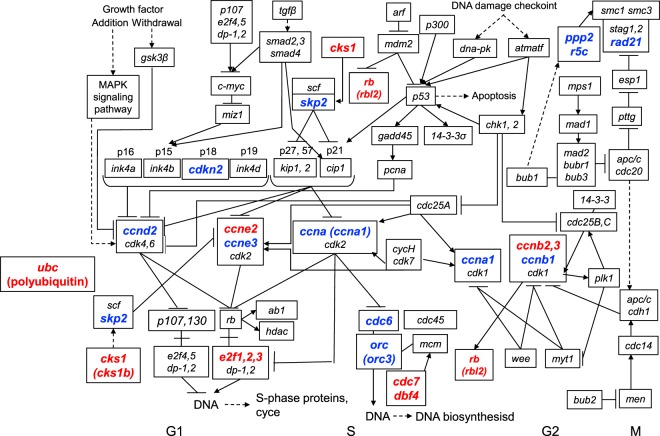
Transcriptome analysis was conducted to compare changes in gene expression in the brain region around the TN between control and 11-KT-treated female groups. Transcriptome profiling revealed that 1122 genes (among a total number of 6851 assembled unigenes) were identified as having a 1.2-fold change due to treatment with 11-KT; 641 genes were upregulated by 11-KT treatment, while 481 genes were downregulated. To evaluate the androgen effects on cellular proliferation, we focused on transcripts that were clustered within “Cyclins and Cell Cycle Regulation” pathway in PathCards and “Cell Cycle” pathway according to the Kyoto Encyclopedia of Genes and Genomes (KEGG). It was found that 103 genes were identified based on the transcriptome profiling. As shown in Table 1, using a plus 1.2-fold change as the threshold, we found that 9 transcripts were up-regulated by 11-KT, namely, polyubiquitin C (ubc), cyclin B3 (ccnb3), cyclin E2 (ccne2), cyclin B2 (ccnb2), retinoblastoma-like protein 2 (rbl2), E2F transcription factor 2 (e2f2), cell division control protein 7 (cdc7), cyclin-dependent kinases regulatory subunit 1 (cks1b), and activator of S phase kinase (ask or dbf4). Similarly, using a minus 1.2-fold change as the threshold, the following 10 genes were down-regulated by 11-KT, namely, origin recognition complex subunit 3 (orc3), cyclin B1 (ccnb1), protein phosphatase 2 regulatory subunit B’gamma (ppp2r5c), cyclin A1 (ccna1), S-phase kinase-associated protein 2 (skp2), cyclin D2 (ccnd2), cell division control protein 7 (cdc6), G1/S-specific cyclin E3 (cyce3), cyclin-dependent kinase N2C (cdkn2c), and cohesion complex subunit SCC1 (rad21). Our qRT-PCR analysis that examined expression of these 19 genes (Supplementary Table S2) revealed that levels, except ccnb2 and cdc7, were significantly affected by 11-KT in the female brain (Fig. 5). In addition, the tendency of 11-KT-induced effects on ccnb2 and cdc7, as shown by qRT-PCR assay, was consistent with the results obtained from our transcriptome analysis. As shown in Fig. 6 and Table 1, these 19 genes are inferred to play key roles in the cell cycle regulation as positive or negative regulators, mostly in G1 and S phases.
Table 1.
Cell cycle related gene expressions which were affected by 11-KT.
| Gene Symbol | Gene Name | Accession No. | Fold Change (11-KT/Cont) |
Function | |
|---|---|---|---|---|---|
| ubc | polyubiquitin | XM_019365938 | 11.17 | ubiquitin gene, Ubiquitination has been associated with protein degradation, DNA repair, cell cycle regulation, kinase modification, endocytosis, and regulation of other cell signaling pathways. | |
| ccnb3 | cyclin B3 |
XM_005458602 XM_003453924 |
1.586 | Regulator of cyclin dependent kinase | |
| ccne2 | cyclin E2 | XM_005472790 | 1.567 | Regulator of cyclin dependent kinase | |
| ccnb2 | cyclin B2 |
XM_005457408 XM_003452594 |
1.541 | Regulator of cyclin dependent kinase.The B-type cyclins, B1 and B2, associate with p34cdc2 and are essential components of the cell cycle regulatory machinery | |
| rbl2 | retinoblastoma-like protein 2 |
XM_019360388 XM_019360390 XM_019360401 XM_005447651 XM_019360394 |
1.464 | Key regulator of entry into cell division | |
| e2f2 | transcription factor E2F2 |
XM_003453243 XM_005477160 |
1.422 | The E2F family plays a crucial role in the control of cell cycle and action of tumor suppressor proteins and is also a target of the transforming proteins of small DNA tumor viruses. | |
| cdc7 | cell division control protein 7 |
XM_005476033 XM_005476034 |
1.321 | ATP binding, kinase activity, metal ion binding, protein binding, protein kinase activity, protein serine/threonine kinase activity | |
| cks1b | CDC28 protein kinase regulatory subunit 1B | XM_003452448 | 1.234 | cyclin-dependent protein serine/threonine kinase activator activity, histone binding, protein binding, protein kinase binding, ubiquitin binding | |
| dbf4 | ask | activator of S phase kinase |
XM_003450951 XM_005472703 XM_019364488 |
1.204 | Regulatory subunit for CDC7 which activates its kinase activity thereby playing a central role in DNA replication and cell proliferation. |
| orc3 | origin recognition complex subunit 3 | XM_003453228 | −1.211 | DNA replication origin binding, protein binding | |
| ccnb1 | cyclin B1 | XM_003440084 | −1.223 | Regulator of cyclin dependent kinase.The B-type cyclins, B1 and B2, associate with p34cdc2 and are essential components of the cell cycle regulatory machinery | |
| ppp2r5c | protein phosphatase 2 regulatory subunit B’gamma |
XM_003440783 XM_005449702 XM_005449704 |
−1.257 | Phosphatase 2 A regulatory subunit B family. It is implicated in the negative control of cell growth and division. | |
| ccna1 | cyclin A1 | XM_003458268 | −1.335 | Regulator of cyclin dependent kinase | |
| skp2 | fbxl1 | F-box and leucine-rich repeat protein 1 (S-phase kinase-associated protein 2) | XM_005449432 | −1.557 | SKP2 has been implicated in double negative feedback loops with both p21 and p27, that control cell cycle entry and G1/S transition |
| ccnd2 | cyclin D2 | XM_003442521 | −1.941 | Regulator of cyclin dependent kinase | |
| cdc6 | cell division control protein 6 | XM_003454088 | −2.878 | ATP binding, kinase binding, nucleotide binding, protein binding | |
| cyce3 | cyclin-E3 |
XM_019351010 XM_019351007 XM_019351009 XM_005452832 |
−4.291 | Regulator of cyclin dependent kinase | |
| cdkn2c | cyclin-dependent kinase inhibitor 2 C |
XM_019351452 XM_019351453 XM_019351454 |
−1000 | cyclin-dependent protein serine/threonine kinase inhibitor activity | |
| rad21 | scc1,mcd1 | cohesin complex componet | XM_003447160 | −1000 | The RAD21 gene provides instructions for making a protein that is involved in regulating the structure and organization of chromosomes during cell division. |
The table provides a description that includes the gene symbol, name and function for each gene affected by 11-KT treatment. It also gives the fold change (11-KT/control) for each gene that was affected by 11-KT treatment.
Figure 5.
Changes in expressions of genes related to cell cycle after 11-KT treatment. Expressions of 19 genes listed in Table 1 were examined by real-time RT-qPCR analysis and compared between control (white column) and 11-KT treated (11-KT, black column) females. The numeric values on the top of each graph indicates the fold change as revealed by the transcriptome analysis. *Indicates p < 0. 05, **Indicates p < 0.01 (unpaired Student’s t-test). Data are expressed as mean ± SEM.
Figure 6.
Cell cycle regulation pathway and the genes whose expressions were altered by 11-KT. The figure was slightly modified from ‘Cell cycle’ pathway (map04110, KEGG). Genes whose expressions were increased (red, fold change >1. 2) or decreased (blue, fold change < -1. 2) by 11-KT treatment were marked in the figure. Ubc coding polyubiquitin-C precursor regulates each phase of the cell cycle through the degradation of cyclins.
Discussion
This study revealed the promotive activity of androgens on cellular proliferation, adult neurogenesis, and the production of GnRH3 neurons, partly via adult neurogenesis, in the female tilapia brain. Since testosterone, the major androgen in mammals and birds31, can be converted to estradiol by aromatase, it is difficult to discern the effects of testosterone from that of estradiol in these animals. Importantly, 11-KT is a non-aromatizable androgen and the most potent natural androgen in teleosts27,28 and helped identify effects of androgen on mature female tilapia in this study. A previous study by our group has shown that 11-KT and methyltestosterone, but not estradiol, increased the number of GnRH3 neurons in female tilapia, suggesting the promotive role of androgen in the proliferation and/or differentiation of GnRH3 neurons16.
Immunostaining with PCNA antibody revealed that 11-KT treatment had a positive effect on cellular proliferation in the brain. BrdU-tracing combined with Hu-immunostaining suggested that 11-KT induced adult neurogenesis in mature females. On the other hand, BrdU-tracing combined with GFAP-immunostaining indicated that 11-KT treatment did not stimulate the production of newly-generated ventricular radial glial cells, which are regarded as progenitor cells in the adult fish brain30. Although they are mostly transient in developing mammals, radial glial cells are maintained in the adult central nervous system of fish, and serve as guides for migrating neurons as well as the major source of neural and glial precursor cells [for review, see32]. Perez et al.33 have reported that PCNA-positive cells correspond to GFAP-positive radial glial cells in fish33. These radial glial cells in fish are reported to express steroid receptors including androgen receptors, and are directly targeted by steroids34,35. Thus, combined with our present results, it is indicated that 11-KT may have induced mitosis of radial glia cells (PCNA-positive cells) which produced newly-generated neurons (Hu- and BrdU-double labelled cells) but not newly-generated radial glial cells (GFAP- and BrdU-double labelled cells).
In addition, based on BrdU-tracing combined with GnRH3-immunostaining, 11-KT treatment produced newly-proliferated GnRH3 neurons. These results revealed that some of the 11-KT increased GnRH3 neurons in the female brain16 are probably generated via adult neurogenesis. GnRH3 neurons can be generated through one of three possible mechanisms: (1) proliferation from progenitor cells and differentiation after cell division, (2) differentiation from pre-existing cells without proliferation, or (3) the combination of both. Our data support the idea that, at least, some of the new GnRH3 neurons could have resulted from adult neurogenesis. However, since the GnRH3- and BrdU-double labelled cells were only a small part of GnRH3 neurons (Fig. 4b), it is a possible scenario that differentiation into GnRH3 neurons from pre-existing cells may be a major contributor. As a next step, we will be examining the precise pathways possibly involved in the neurogenesis and differentiation of GnRH3 neurons induced by 11-KT in mature females. Because it is difficult to investigate the direct role of androgen and pathways in vivo, a pharmacological approach using brain slices including the terminal ganglion area will be applied.
The generation of GnRH neurons during development has been well studied in vertebrates, GnRH (or luteinizing hormone-releasing hormone) neurons originate in the medial olfactory placode of the developing rodent nose and migrate into the septal-preoptic area and hypothalamus36–38. In the sockeye salmon, Parhar et al.29,39 established that GnRH3 neurons originated in the medial olfactory placode and migrated into the basal forebrain during development. Using the gnrh3-GFP transgenic medaka, Okubo et al.40. visualized the migration pathway of GnRH neurons including GnRH3 neurons during development. On the contrary, the generation of GnRH neurons, including GnRH3 neurons, in adult animals remains unclear. In the Tg (GnRH3:EGFP) zebrafish line, after specific ablation of GnRH3 neurons in the olfactory region, GnRH3 neurons were regenerated only when the ablation was conducted 2 days after fertilization41. Interestingly, bilateral GnRH3 soma ablation at 4 or 6 days after fertilization resulted in lack of GnRH3 neurons and arrested oocytes in adults. Additionally, in female zebrafish without hormonal treatments, the only source of GnRH3 neurons in the olfactory region in embryos41. On the other hand, new-born GnRH1 neurons, with GnRH1-immunoreactivity and BrdU labelling in the nuclei, were detected in the adult ring dove forebrain after brain lesion and the recruitment of new GnRH1-immunoreactive neurons is sensitive to the reproductive stage of the birds42. Cortés-Campos et al.43 demonstrated that adult zebrafish-derived hypothalamic neurospheres generate GnRH neurons, including GnRH3 ones. They also demonstrated that the number of GnRH neurons increased following exposure to testosterone or GnRH3. In accordance with the latter two studies, the present result supports the idea that GnRH neurons in some vertebrates can be generated in certain conditions, like lesions or hormonal treatments such as androgen exposure.
Tilapia have distinct sexual dimorphism in the number of GnRH3 neurons; mature males have more GnRH3 neurons than mature females16. Multiple studies have reported sex differences in GnRH3 neurons or gnrh3 expression. For example, female half spotted goby had more and larger GnRH cells in the TN44. Similarly, in medaka, the expression of gnrh3 was slightly higher in females than males, although no sex differences in the total area of gnrh3 expression were detected45. In tilapia, other than courtship behaviours, sexual reproductive behaviours were clearly different between the sexes; males exhibited nest building and aggressive behaviours, whereas in females maternal oral egg incubation was observed. It is reported that GnRH3 is a potent neuromodulator of male reproductive behaviours in tilapia15. Thus, the strong neuromodulatory role of GnRH3 neurons on the male-specific reproductive behaviours is related to the large population of GnRH3 neurons in male tilapia and the promotive effect of 11-KT on them. In addition, we confirmed that the expression level of GnRH3 mRNA was also increased by 11-KT treatment.
Among the gonadal hormones, estradiol has been recognized as a major modulator of adult neurogenesis due to its capacity to facilitate both survivability and proliferation of neurons in mammals and birds17–20. In addition, aromatase is localized in radial glial cells in the adult teleost30,46, [see47 for review] and oestrogens have been suggested to play significant roles in the brains of these fishes. However, actions of oestrogen in fish still remains controversial. Diotel et al.48 established the inhibitory action of estradiol on cell neurogenesis, and migration at the olfactory bulbs/telencephalon junction and in the mediobasal hypothalamus of the adult zebrafish brain. Blocking the aromatase activity with 1,4, 6-androstatriene-3,17-dione or oestrogen receptors with ICI 182,780 increased the number of proliferative cells, suggesting an inhibitory role for estrogen in the proliferation in the fish brain. On the contrary, Lin et al.49 showed that estradiol treatment in the early brain development enhanced cellular proliferation and gene expressions of aromatase and radial glial cell marker (Blbp) in black porgy indicating a stimulatory effect of estradiol on the proliferation and development of radial glial cells in the fish brain49. Research studies reporting the androgen-induced neurogenesis in vertebrates are much less compared to those that reporting neurogenesis induced by oestrogen. Most of these studies focused on the song control centre (HVC) of the brain in song birds and reported testosterone-induced enlargement of the HVC due to neurogenesis, angiogenesis and neuronal survivability increased by the hormone23–26,50–53. In most of these studies, however, the effect of oestrogen aromatized from testosterone cannot be excluded. In mammals, there is a non-aromatizable androgen, 5α-dihydrotestosterone (5α-DHT). In addition, androgenic mechanisms of sexual differentiation of the central nervous system and behavior have been well documented in mammals during development, especially by using genetically modified mice or mutants [see for reviews54,55]. In contrast with mammals, sex reversal can occur in many species of mature fishes, whose brain holds plenty of neural progenitor cells during adulthood. However, little information is available on whether androgen may induce sex reversal of the brain, or whether it may induce adult neurogenesis in the fish brain. We provide the first evidence that androgen can positively affect adult neurogenesis in the fish brain. This may partly constitute the mechanisms by which the sex reversal of the brain occurs under androgen exposure.
The mechanistic machinery for 11-KT to induce neurogenesis in the mature female tilapia brain remains unsolved. Bases on our transcriptome data, we suggest that among the Arα, Arβ, Erα, and Erβ genes, Arβ gene expression is indeed upregulated by 11-KT in females (Supplementary Table S3). Furthermore, present transcriptome profiling with qRT-PCR analysis revealed that 11-KT affected the expressions of genes related to the cell-cycle pathway in the region around the TN (Figs 5 and 6). We found that among the members of the cyclin family proteins which control progression of the cell cycle, transcript expressions of ccnb3, ccnb2 and ccne2 were increased, while ccnb1, ccna1, ccnd2, and cyce3 were decreased, by 11-KT treatment. Importantly, each cyclin homolog is differentially expressed at various cell cycle phases. For example, it has been reported that cyclin B1 functions during the M phase, while cyclin B3 is a mitotic cyclin that promotes the metaphase-anaphase transition56. E-type cyclins function in driving cell cycle progression57, whereas cyclin A1 functions mainly in the G1/S cell cycle progression58. In addition to cyclins, expression of other genes known as cell cycle regulators, was positively or negatively affected by 11-KT. For example, gene expressions of rbl2 and e2f2, crucial regulators of cell division59, were stimulated by 11-KT treatment. In contrast, the expressions of skp2 and cdkn2c, which are reported to control CDK activity60,61 were depressed. The large increment of ubc mRNA induced by 11-KT (Fig. 6) supports the importance of the ubiquitin-proteasome degradation system’s involvement in cell cycle regulation, similar to as seen in rodents62. Since most proteins translated from these genes positively or negatively regulate each other through phosphorylation or degradation during the cell cycle, it is difficult to decide from these data whether 11-KT has a positive or negative effect on cell cycle. In conclusion, these results obtained from the transcriptome analysis and real-time qRT-PCR analysis support the idea that 11-KT can influence cellular proliferation and fit the results from PCNA-staining and BrdU-tracing experiments in the female tilapia brain.
Methods
Animals
Experiments were conducted according to the principles and procedures approved by the Institutional Animal Care and Use Committees of Toyo University and Academia Sinica. Mozambique tilapia (Oreochromis mossambicus) were maintained at 25 °C. The total number of tilapia used was 115.
Comparison of the number of proliferating (PCNA-positive) cells around the TN between control and 11-KT-treated females
11-KT injection
Sexually mature female Mozambique tilapia of almost equal size (n = 6; approximately 30 g in body weight (BW) and 15 cm in length) were used. 11-KT (Cosmo Bio, Japan) was dissolved in sesame oil at a concentration of 1.0 mg/ml. Three mature females (11-KT-treatment) were intraperitoneally injected with 11-KT at 5 µg/g BW, and three mature females (controls) were injected with sesame oil alone. After the injections, fish were housed individually in tanks (50 L glass tanks) for 7 days.
Brain fixation
Seven days after the injection, animals were anesthetized with 0.2% 3-aminobenzoic acid ethyl ester (MS222, Sigma, St. Louis, MO) and transcardially perfused with saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.3). Brains were then removed and post-fixed in the same fixative overnight.
Brain sectioning
Fixed brains were washed in 0.1 M phosphate-buffered saline (PBS), and then immersed in 0.1 M PB containing 20% sucrose overnight. Serial frontal sections (20 μm thickness) were cut on a cryostat (CM-3050-S, Leica Microsystems, Wetzlar, Germany), thaw-mounted onto MAS-coated glass slides (Matsunami Glass, Osaka, Japan) in three parallel series, and stored at −20 °C until further use.
Immunohistochemistry for localization of GnRH3 neurons in the TN
Two of the three series of brain sections from each fish were used for PCNA immunohistochemistry, while the other series was used for GnRH3 labelling to confirm the location of the TN. Immunohistochemistry with an anti-GnRH3 antibody was performed using a custom-made primary anti-GnRH3 antibody (produced by Protein Purify, Isezaki, Japan) and Alexa 555-labelled goat anti-rabbit IgG (ThermoFisher Scientific LTD)16. GnRH3-positive neurons were observed under a fluorescence microscope (Axio Imager A1, Zeiss, Jena, Germany) and sections with the highest number of GnRH3-positive cells were identified.
Proliferating cell nuclear antigen (PCNA) immunohistochemistry and cell counts
For PCNA immunohistochemistry, we chose the sections immediately adjacent to the one identified as having the highest number of GnRH3 neurons. These sections were incubated in methanol containing 0.3% hydrogen peroxide to block endogenous peroxidase activity. Next, sections were immunohistochemically stained with mouse monoclonal anti-PCNA antibody (1:3000, P8825, Sigma-Aldrich) diluted in 0.1 M PBS containing 0.03% Triton X-100 and 10% Block Ace and with biotinylated goat anti-mouse IgG (1:500, Vector Laboratories, Burlingame, CA, USA). After incubation with an avidin-biotin-peroxidase-complex (ABC Method kit, Vector Laboratories), the sections were incubated with 0.02% solution of 3, 3′-diaminobenzidine (Wako Pure Chemical Industries) in 0.175 M sodium acetate buffer containing 0.125% nickel chloride (Wako Pure Chemical Industries, Osaka, Japan) and 0.0026% hydrogen peroxide (Wako Pure Chemical Industries).
In every PCNA-stained sections obtained per animal, the number of cells with PCNA-positive nuclei (PCNA-positive cells) were counted in the dorsal and ventral areas (each area, 140 × 140 µm) around the ventricle (Fig. 1a). Average dorsal and ventral densities of PCNA-positive cells per animal were calculated, and mean densities and standard errors of PCNA-positive cells were then determined for control (n = 3) and 11-KT injected (n = 3) animal groups.
Identification of newly-generated cells via in vivo BrdU tracing
Experimental design for BrdU injections and fixation
Sexually mature female tilapia of almost equal size (n = 6; approximately 30 g in BW and 15 cm length) were used for this study. Three females were simultaneously injected with BrdU (10 mM BrdU (Sigma) at 10 μl/g BW, i.p.) and 11-KT (5 µg/g BW, i.p.), whereas another three were injected with BrdU and the vehicle (sesame oil) for controls. After the injection, the fishes were individually kept in tanks for 7 days. Next, they were anesthetized and transcardially perfused with the fixative as described above. Serial frontal sections were cut at 16 μm thickness on a cryostat. Sections were then thaw-mounted onto MAS-coated glass slides in five parallel series, and stored at −20 °C until further use.
Double immunohistochemistry with anti-BrdU and either Hu, GFAP or GnRH3 antibodies
Brain sections obtained as described above were subjected to double immunohistochemistry with anti-BrdU and an antibody against either Hu, GFAP or GnRH3. One series was first stained with anti-GnRH3 to determine the region of the TN where GnRH3-positive neurons were localized. Among the remaining four series of sections, we selected three series of sections, adjacent to the section with the highest number of GnRH3 neurons, to examine the occurrence of newly-generated neurons, GFAP-positive radial glial cells, and GnRH3 neurons. Sections were incubated with anti-Hu C/D (1:100, mouse monoclonal antibody, Molecular Proves, USA), anti-GFAP (1:1000, mouse monoclonal antibody, G3893, Sigma-Aldrich), or anti-GnRH3 antibody. In the next step, sections were incubated in secondary antibodies conjugated with Alexa Flour 555 as follows: goat anti-mouse IgG (1:500, Life Technologies) for Hu C/D (Hu) and GFAP detection, and goat anti-rabbit IgG (1:500, Life Technologies) for GnRH3 detection. For BrdU detection, sections were again fixed in 4% paraformaldehyde and treated with 2N HCl. Immunostaining against BrdU was performed with anti-BrdU antibody (1:400, rat monoclonal antibody, OBT0030, Bio-Rad) and the secondary antibody (Alexa Fluor 488 anti-rat IgG, 1:500, Life Technologies). Stained sections were observed under a confocal laser scanning microscope (LSM5 Pascal, Zeiss). To examine the specificity of the immunoreaction, sections were either processed without the primary antibody, or were incubated with normal rabbit IgG in place of the specific antibody.
Transcriptome analysis of cellular proliferation-related genes
TN sample preparation
Sexually mature female fish of similar size (n = 103, average BW = 28. 6 g and length = 9. 4 cm) were used for this study. Under anesthesia with MS222, 11-KT dissolved in sesame oil was injected intraperitoneally at 5 µg/g BW to nearly half of the fish (n = 53), while the rest (n = 50) were injected with sesame oil. At 24 h after injection, fish were anesthetized and ovarian maturation was confirmed for each fish. From only the matured females (control, n = 40; 11-KT, n = 35), brain regions around the TN were bilaterally removed, weighed (approximately 15 mg/fish), frozen in liquid nitrogen, and stored at −80 °C until use. Next, specimens from three animals were mixed into one sample and RNA extraction was performed with the QIAGEN RNeasy Plus Universal Mini Kit (QIAGEN, Hikden) according to the manufacturer’s protocols. It was found that RNA concentration of each sample, total amount of RNA, RNA integrity number, and 28 S/18 S ratio, were higher than 1 µg/µl, 20 µg, 8.0, and 1.5, respectively. Four control and four 11-KT-treated counterparts were then processed by Genomic BioSci & Tech (New Taipei City, Taiwan) for transcriptome analysis using next generation sequencing (NGS). The rest samples were used for real-time quantitative PCR analysis.
Library preparation, sequencing and primary analysis
Sampled total RNA were subjected to mRNA isolation and fragmentation, cDNA synthesis, adapter ligation and enrichment amplification. cDNA library was constructed and sequenced using Illumina HiSeq 2000 sequencer (San Diego, CA, USA). CLC genomics workbench (v. 8.0, QIAGEN) was used to conduct various bioinformatics analyses. Readings from Illumina HiSeq sequencing were first trimmed; next, “map read to reference” and “RNA-seq analysis” options were both utilized to map trimmed reads onto Oreochromis niloticus genome.
Differential gene expression cluster analysis
We used gitools v. 2.3 to cluster differentially expressed genes from different treatments. Expression level (RPKM) was calculated using total exon reads/mapped reads (millions) x exon length (kb)). Next, we calculated RPKM Z-score for each gene using Z = (x − μ)/σ, where μ and σ represent the mean and standard deviation, respectively, and used it for cluster analyses with parameters including cluster = 50 and interaction = 300. Subsequently, differentially expressed genes with fold change >1.2, by comparison of RPKM value, were further selected.
Genes regulating cellular proliferation
Based on the results of our transcriptome analysis, we selected the genes included in the “Cyclins and Cell Cycle Regulation” pathway in PathCards (https://pathcards.genecards.org/Card/cyclins_and_cell_cycle_regulation?queryString=cell%20cycle%20) and “ Cell Cycle” map (map04110) in Kyoto Encyclopedia Genes and Genomes (KEGG) pathway (http://www.genome.jp/dbgetbin/www_bget?pathway:map04110). Identified genes were listed in terms of differential expression between control and 11-KT-treated animals. We used NIH Gene (http://www. ncbi. nlm. nih. gov/gene) to identify the specific function of the genes in the pathways. For the genes whose expression ratios (11-KT treated/control) were greater than 1.2 or less than 1/1.2, we further examined their expression levels by using quantitative real-time RT-PCR analysis.
Real-time qRT-PCR
The remaining of RNA samples collected for the transcriptome analysis were used. In addition, RNA samples were obtained from the brain regions around the TN of sexually mature female fish at three days after 11-KT injection. Reverse transcription and real-time qRT-PCR were performed as previously reported63. The pairs of primers used are listed in Supplementary Table S2.
Statistical analysis
The numbers of PCNA-, BrdU-, and GnRH3-positive cells, as well as the double-labelled cells (BrdU/Hu, BrdU/GFAP, BrdU/GnRH3) were compared between 11-KT- and control fish using unpaired Student’s t-test. Gene expression levels were also compared between these groups using unpaired Student’s t-test. p < 0.05 was considered statistically significant.
Electronic supplementary material
Supplementary Dataset (Table S1, S2, S3)
Acknowledgements
This work was partly supported by The INOUE ENRYO Memorial Grant (TOYO University).
Author Contributions
R.O.K. designed this research and interpreted data. Ya.N. and A.T. performed PCNA experiments and analysed the data. Ya.N. and T.K. performed BrdU study and analysed the data. Yu.N., R.D.C., K.H. and R.O.K. collected samples for transcriptome analysis and RT-PCR analysis. Y.C.T., K.J.R., P.P.H., M.A. and R.O.K. analysed the data. M.A. and K.S. performed real-time RT-PCR analysis. R.O.K. wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35303-9.
References
- 1.Munakata A, Kobayashi M. Endocrine control of sexual behavior in teleost fish. Gen Comp Endocrinol. 2010;165:456–468. doi: 10.1016/j.ygcen.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi M, Nakanishi T. 11-ketotestosterone induces male-type sexual behavior and gonadotropin secretion in gynogenetic crucian carp, Carassius auratus langsdorfii. Gen Comp Endocrinol. 1999;115:178–187. doi: 10.1006/gcen.1999.7314. [DOI] [PubMed] [Google Scholar]
- 3.Saoshiro S, Kawaguchi Y, Hayakawa Y, Kobayashi M. Sexual bipotentiality of behavior in male and female goldfish. Gen Comp Endocrinol. 2013;181:265–270. doi: 10.1016/j.ygcen.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Pandian TJ, Sheela SG. Hormonal induction of sex reversal in fish. Aquaculture. 1995;138:1–22. doi: 10.1016/0044-8486(95)01075-0. [DOI] [Google Scholar]
- 5.Phelps, R. P. & Popma, T. J. Sex reversal of tilapia. Tilapia aquaculture in the Americas, Vol. 2. The World Aquaculture Society, 34–59 (2000).
- 6.Kobayashi T, Kajiura-Kobayashi H, Nagahama Y. Induction of XY sex reversal by estrogen involves altered gene expression in a teleost, tilapia. Cytogenet Genome Res. 2003;101:289–294. doi: 10.1159/000074351. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T, Kajiura-Kobayashi H, Guan G, Nagahama Y. Sexual dimorphic expression of DMRT1 and Sox9a during gonadal differentiation and hormone-induced sex reversal in the teleost fish Nile tilapia (Oreochromis niloticus) Dev Dyn. 2008;237:297–306. doi: 10.1002/dvdy.21409. [DOI] [PubMed] [Google Scholar]
- 8.Sun LN, et al. Transdifferentiation of differentiated ovary into functional testis by long-term treatment of aromatase inhibitor in Nile tilapia. Endocrinology. 2014;155:1476–1488. doi: 10.1210/en.2013-1959. [DOI] [PubMed] [Google Scholar]
- 9.Wang DS, et al. Doublesex- and Mab-3-related transcription factor-1 repression of aromatase transcription, a possible mechanism favoring the male pathway in tilapia. Endocrinology. 2010;151:1331–1340. doi: 10.1210/en.2009-0999. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso SD, Gonçalves D, Goesmann A, Canário AVM, Oliveira RF. Temporal variation in brain transcriptome is associated with the expression of female mimicry as a sequential male alternative reproductive tactic in fish. Mol Ecol. 2018;27:789–803. doi: 10.1111/mec.14408. [DOI] [PubMed] [Google Scholar]
- 11.Beal AP, Martin FD, Hale MC. Using RNA-seq to determine patterns of sex-bias in gene expression in the brain of the sex-role reversed Gulf Pipefish (Syngnathus scovelli) Mar Genomics. 2018;37:120–127. doi: 10.1016/j.margen.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Gorbman A, Sower SA. Evolution of the role of GnRH in animal (Metazoan) biology. Gen Comp Endocrinol. 2003;134:207–213. doi: 10.1016/j.ygcen.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Morgan K, Millar RP. Evolution of GnRH ligand precursors and GnRH receptors in protochordate and vertebrate species. Gen Comp Endocrinol. 2004;139:191–197. doi: 10.1016/j.ygcen.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Tsai PS. Gonadotropin-releasing hormone in invertebrates: structure, function, and evolution. Gen Comp Endocrinol. 2006;148:48–53. doi: 10.1016/j.ygcen.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa S, et al. Immunoneutralization of gonadotropin-releasing hormone type-III suppresses male reproductive behavior of cichlids. Neurosci Lett. 2006;403:201–205. doi: 10.1016/j.neulet.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 16.Kuramochi A, Tsutiya A, Kaneko T, Ohtani-Kaneko R. Sexual dimorphism of gonadotropin-releasing hormone type-III (GnRH3) neurons and hormonal sex reversal of male reproductive behavior in Mozambique tilapia. Zoolog Sci. 2011;28:733–739. doi: 10.2108/zsj.28.733. [DOI] [PubMed] [Google Scholar]
- 17.Kaba H, Rosser AE, Keverne EB. Hormonal enhancement of neurogenesis and its relationship to the duration of olfactory memory. Neuroscience. 1988;24:93–98. doi: 10.1016/0306-4522(88)90314-4. [DOI] [PubMed] [Google Scholar]
- 18.Fowler CD, Liu Y, Wang Z. Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res Rev. 2008;57:342–351. doi: 10.1016/j.brainresrev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galea LA. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Galea LA, et al. Sex hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013;25:1039–1061. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- 21.Allen KM, Fung SJ, Rothmond DA, Noble PL, Weickert CS. Gonadectomy increases neurogenesis in the male adolescent rhesus macaque hippocampus. Hippocampus. 2014;24:225–238. doi: 10.1002/hipo.22217. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, et al. Effects of androgens on early post-ischemic neurogenesis in mice. Transl Stroke Res. 2014;5:301–311. doi: 10.1007/s12975-013-0298-6. [DOI] [PubMed] [Google Scholar]
- 23.Absil P, Pinxten R, Balthazart J, Eens M. Effect of age and testosterone on autumnal neurogenesis in male European starlings (Sturnus vulgaris) Behav Brain Res. 2003;143:15–30. doi: 10.1016/S0166-4328(03)00006-8. [DOI] [PubMed] [Google Scholar]
- 24.Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamura T, Barker JM, Balthazart J, Ball GF. Androgens and estrogens synergistically regulate the expression of doublecortin and enhance neuronal recruitment in the song system of adult female canaries. J Neurosci. 2011;31:9649–9657. doi: 10.1523/JNEUROSCI.0088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Ye R, Goldman SA. Testosterone modulation of angiogenesis and neurogenesis in the adult songbird brain. Neuroscience. 2013;239:139–148. doi: 10.1016/j.neuroscience.2012.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borg B. Androgens in teleost fishes. Comp Biochem Physiol. 1994;109C:219–245. [Google Scholar]
- 28.Lokman PM, et al. 11-Oxygenated androgens in female teleosts: prevalence, abundance, and life history implications. Gen Comp Endocrinol. 2002;129:1–12. doi: 10.1016/S0016-6480(02)00562-2. [DOI] [PubMed] [Google Scholar]
- 29.Parhar IS, et al. Neurons synthesizing gonadotropin-releasing hormone mRNA subtypes have multiple developmental origins in the medaka. J Comp Neurol. 1998;401:217–226. doi: 10.1002/(SICI)1096-9861(19981116)401:2<217::AID-CNE5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 30.Pellegrini E, et al. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J Comp Neurol. 2007;501:150–167. doi: 10.1016/j.jsbmb.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Hirschenhauser K, Oliveira R. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim Behav. 2006;71:265–277. doi: 10.1016/j.anbehav.2005.04.014. [DOI] [Google Scholar]
- 32.Pinto L, Götz M. Radial glial cell heterogeneity–the source of diverse progeny in the CNS. Prog Neurobiol. 2007;83:2–23. doi: 10.1016/j.pneurobio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Pérez MR, et al. Relationships between radial glial progenitors and 5-HT neurons in the paraventricular organ of adult zebrafish - potential effects of serotonin on adult neurogenesis. Eur J Neurosci. 2013;38:3292–301. doi: 10.1111/ejn.12348. [DOI] [PubMed] [Google Scholar]
- 34.Diotel N, et al. The brain of teleost fish, a source, and a target of sexual steroids. Front Neurosci. 2011;5:137. doi: 10.3389/fnins.2011.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pellegrini E, et al. Steroid modulation of neurogenesis: Focus on radial glial cells in zebrafish. J Steroid Biochem Mol Biol. 2016;160:27–36. doi: 10.1016/j.jsbmb.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- 37.Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA. 1989;86:8132–8136. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daikoku-Ishido H, Okamura Y, Yanaihara N, Daikoku S. Development of the hypothalamic luteinizing hormone-releasing hormone-containing neuron system in the rat: in vivo and in transplantation studies. Dev Biol. 1990;140:374–387. doi: 10.1016/0012-1606(90)90087-Y. [DOI] [PubMed] [Google Scholar]
- 39.Parhar IS, Iwata M, Pfaff DW, Schwanzel-Fukuda M. Embryonic development of gonadotropin-releasing hormone neurons in the sockeye salmon. J Comp Neurol. 1995;362:256–270. doi: 10.1002/cne.903620208. [DOI] [PubMed] [Google Scholar]
- 40.Okubo K, et al. Forebrain gonadotropin-releasing hormone neuronal development: insights from transgenic medaka and the relevance to X-linked Kallmann syndrome. Endocrinology. 2006;147:1076–1084. doi: 10.1210/en.2005-0468. [DOI] [PubMed] [Google Scholar]
- 41.Abraham E, Palevitch O, Gothilf Y, Zohar Y. Targeted gonadotropin-releasing hormone-3 neuron ablation in zebrafish: effects on neurogenesis, neuronal migration, and reproduction. Endocrinology. 2010;151:332–340. doi: 10.1210/en.2009-0548. [DOI] [PubMed] [Google Scholar]
- 42.Cheng MF, Alexander K, Zhou S, Bonder E, Chuang LS. Newborn GnRH neurons in the adult forebrain of the ring dove. Horm Behav. 2011;60:94–104. doi: 10.1016/j.yhbeh.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Cortés-Campos C, Letelier J, Ceriani R, Whitlock KE. Zebrafish adult-derived hypothalamic neurospheres generate gonadotropin-releasing hormone (GnRH) neurons. Biol Open. 2015;4:1077–1086. doi: 10.1242/bio.010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maruska KP, Mizobe MH, Tricas TC. Sex and seasonal co-variation of arginine vasotocin (AVT) and gonadotropin-releasing hormone (GnRH) neurons in the brain of the halfspotted goby. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:129–144. doi: 10.1016/j.cbpa.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Kawabata Y, Hiraki T, Takeuchi A, Okubo K. Sex differences in the expression of vasotocin/isotocin, gonadotropin-releasing hormone, and tyrosine and tryptophan hydroxylase family genes in the medaka brain. Neuroscience. 2012;218:65–77. doi: 10.1016/j.neuroscience.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 46.Forlano PM, et al. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xing L, Goswami M, Trudeau VL. Radial glial cell: critical functions and new perspective as a steroid synthetic cell. Gen Comp Endocrinol. 2014;201:181–185. doi: 10.1016/j.ygcen.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Diotel N, et al. Effects of estradiol in adult neurogenesis and brain repair in zebrafish. Horm Behav. 2013;63:193–207. doi: 10.1016/j.yhbeh.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Lin CJ, Fan-Chiang YC, Dufour S, Chang CF. Activation of brain steroidogenesis and neurogenesis during the gonadal differentiation in protandrous black porgy, Acanthopagrus schlegelii. Dev Neurobiol. 2016;76:121–136. doi: 10.1002/dneu.22303. [DOI] [PubMed] [Google Scholar]
- 50.Kim DH, et al. Testosterone-induced matrix metalloproteinase activation is a checkpoint for neuronal addition to the adult songbird brain. J Neurosci. 2008;28:208–216. doi: 10.1523/JNEUROSCI.3674-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Louissaint A, Jr., Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/S0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 52.Shevchouk OT, Ghorbanpoor S, Ball GF, Cornil CA, Balthazart J. Testosterone-induced neuroendocrine changes in the medial preoptic area precede song activation and plasticity in song control nuclei of female canaries. Eur J Neurosci. 2017;45:886–900. doi: 10.1111/ejn.13530. [DOI] [PubMed] [Google Scholar]
- 53.Shevchouk OT, Ball GF, Cornil CA, Balthazart J. Studies of HVC Plasticity in Adult Canaries Reveal Social Effects and Sex Differences as Well as Limitations of Multiple Markers Available to Assess Adult Neurogenesis. PLoS One. 2017;12:e0170938. doi: 10.1371/journal.pone.0170938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu MV1, Shah NM. Control of masculinization of the brain and behavior. Curr Opin Neurobiol. 2010;21:116–23. doi: 10.1016/j.conb.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swift-Gallant A, Monks DA. Androgenic mechanisms of sexual differentiation of the nervous system and behavior. Front Neuroendocrinol. 2017;46:32–45. doi: 10.1016/j.yfrne.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Wolgemuth DJ, Manterola M, Vasileva A. Role of cyclins in controlling progression of mammalian spermatogenesis. Int J Dev Biol. 2013;57:159–168. doi: 10.1387/ijdb.130047av. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caldon CE, Musgrove EA. Distinct and redundant functions of cyclin E1 and cyclin E2 in development and cancer. Cell Div. 2010;5:2. doi: 10.1186/1747-1028-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji P, et al. Cyclin A1, the alternative A-type cyclin, contributes to G1/S cell cycle progression in somatic cells. Oncogene. 2005;24:2739–2744. doi: 10.1038/sj.onc.1208356. [DOI] [PubMed] [Google Scholar]
- 59.Stevaux O, Dyson NJ. A revised picture of the E2F transcriptional network and RB function. Curr Opin Cell Biol. 2002;14:684–691. doi: 10.1016/S0955-0674(02)00388-5. [DOI] [PubMed] [Google Scholar]
- 60.Bretones G, Delgado MD, León J. Myc and cell cycle control. Biochim Biophys Acta. 2015;1849:506–516. doi: 10.1016/j.bbagrm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 61.James MK, Ray A, Leznova D, Blain SW. Differential modification of p27Kip1 controls its cyclin D-cdk4 inhibitory activity. Mol Cell Biol. 2008;28:498–510. doi: 10.1128/MCB.02171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryu KY, et al. The mouse polyubiquitin gene UbC is essential for fetal liver development, cell-cycle progression and stress tolerance. EMBO J. 2007;26:2693–2706. doi: 10.1038/sj.emboj.7601722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsutiya A, Nishihara M, Goshima Y, Ohtani-Kaneko R. Mouse pups lacking collapsin response mediator protein 4 manifest impaired olfactory function and hyperactivity in the olfactory bulb. Eur J Neurosci. 2015;42:2335–2345. doi: 10.1111/ejn.12999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Dataset (Table S1, S2, S3)