Abstract
This study aimed to investigate the use of golden thread extract (GTE), clove extract (CE), and commercially available nitrite for retarding lipid and protein oxidation and for maintaining color stability and sensory attributes in beef patties stored at 4℃. GTE, CE, and nitrite treatment samples were found to be efficient in retarding lipid oxidation as all three treatments resulted in low thiobarbituric acid reactive substance (TBARS) content (p<0.05). By using GTE, CE, and nitrite into beef patties, protein oxidation was not developed. Incorporation of GTE and CE into beef patties maintained color stability by protecting against the decrease of L*, a*, b*, chroma, and hue angle values and exhibited significant influence on sensory characteristics, including color and odor of beef patties (p<0.05). Compared to commercially available nitrite, GTE and CE were more effective as antioxidants for inhibiting lipid oxidation, and preserving color stability of fresh beef patties. The study indicated that GTE and CE could be utilized efficiently to extend the shelf life of beef patties.
Keywords: beef patties, natural antioxidants, oxidation stability, color stability, sensory attributes
Introduction
The beef patty is a popular, widely consumed meat product for fast meals due to changing life styles (Mohamed and Mansour, 2012). Factors affecting consumption such as flavor, appearance, freshness, and nutritional value were applied for the evaluation of beef meat quality by consumers (Cho et al., 2010). Consumers demand high quality and convenient meat products with natural flavor and the desirable appearance of beef is greatly appreciated (Hugas et al., 2002). However, minced meat and meat products undergo oxidative changes and become rancid earlier than intact muscle, as grinding exposes the muscle surface to air and the lipid membranes to metal oxidation catalysts (Devatkal et al., 2010). The oxidative process in meat induces the modification of lipids and proteins which, in turn, results in the deterioration of flavor, texture, and color of the meat, as well as the development of toxic compounds, poor shelf life, and nutrient loss (Falowo et al., 2014). Discoloration of meat occurs during the oxidation process because the aftereffect of lipid oxidation is the formation of pro-oxidants capable of reacting with oxymyoglobin, which leads to the formation of metmyoglobin (Frankel, 1998). Furthermore, antioxidants are compounds that can be added to fresh and processed meats to delay, retard, or prevent oxidative reaction, hinder the development of off-flavor, and increase color stability (Lorenzo et al., 2014).
Nitrites are well-known for their antioxidant effect in meat products (Honikel, 2004). Generally, nitrites provide the typical cured red color, inhibition of microbial contamination, antioxidant effects and expansion of shelf-life in meat products (Honikel, 2014). However, nitrites are identified as potentially toxic in cured meat with chemical toxicity and carcinogenic potential (reactions with some biogenic amines and formation of N-nitrosamines) in meat products (Pegg and Shahidi, 1997). In addition, reports of various adverse health effects of synthetic antioxidants (BHT, BHA, and others) have led to increasing interest in the use of natural sources of antioxidants in meat products because of their safety, purchaser acceptability, and greater application in expanding the shelf life of foods (Mokhtar et al., 2014).
Golden thread (Coptis chinensis Franch) is an important traditional medicinal herb. The principal active constituents of golden thread are protoberberine alkaloids (PBAs), consisting of berberine, coptisine, palmatine, epiberberine, and jatrorrhizine (Fan et al., 2008). One of the major components of alkaloidal content in golden thread extract (GTE) is berberine, which has many beneficial effects (Chung et al., 1999; Yu et al., 2005). Berberine, the most important extract of C. chinensis, exhibits antimicrobial, anti-diarrhoea, antineoplastic, anti-inflammatory, anti-cancer activities, and inhibitory effects on the growth of M. aeruginosa (Chung et al., 1999; Zhang et al., 2010).
The clove is the dried, unexpanded flower bud of Syzygium aromaticum L. trees belonging to the Myrtaceae family. Commercially available cloves are used as a condiment or spice to preserve food against spoilage (El-Maatia et al., 2016). Clove extracts have been widely reported as having especially potent antioxidant properties (Shi et al., 2014). The main bioactive compounds of clove extract (CE) are eugenol and eugenyl acetate, which show high potential antioxidant properties (El-Maatia et al., 2016; Lee and Shibamoto, 2001).
In recent years, the application of natural antioxidants has increased for enhancing the oxidative stability of meat products (Armenteros et al., 2016). For instance, CE has been recently reported to be a potential antioxidant to improve the oxidative stability of meat products (Zhang et al., 2016). However, to the best of our knowledge, there has been no investigation of the antioxidant effects on beef patties of using GTE and CE as natural antioxidant ingredients.
Therefore, the objectives of this study were to investigate the antioxidant effect of GTE and CE on the lipid and protein stability against oxidation, color stability, and sensory evaluation of beef patties.
Materials and Methods
Natural antioxidants preparation and chemicals
Golden thread and clove as natural antioxidants were purchased from a local market in Jinju, Korea. The status of natural antioxidants were dry matter. The extracts of golden thread and clove powder were obtained by applying the reflux extraction process. This method was applied separately for each sample extract. The powders and distilled water were mixed at the ratio of 1:5 (w/v) and displaced for extraction at 90℃ for 6 h (fraction 1). The extractions of residues were performed using distilled water (1:5) at 90℃ for 12 h (fraction 2). These two fractions were pooled after cooling at room temperature. The aqueous solutions were filtered with Whatman No. 4 filter paper and then concentrated using a vacuum rotary evaporator on 250 rpm at 90℃. The concentrated GTE and CE were freeze dried and stored at –70℃ until use. Sodium nitrite and all other chemicals were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All chemicals used in this study were of high purity analytical grade.
Beef patties preparation
Three beef rounds (Semimembranosus muscle, SM) from different steers (n=4) and beef back fat were purchased from a local packing plant at 3 d post mortem. Lean beef round was trimmed of visible fat and connective tissues, and their fat contents were determined. The lean beef round and beef back fat were ground separately using a meat grinder (GG-22, German Knife, Germany) through a plate with an 8-mm steel plate of twice. After mixing, the ground beef round and beef fat were divided to 3 batches (approximately 8 kg/batch) for study.
The beef patties were prepared in the meat processing laboratory, and the same formulation was used for all beef patties. The raw materials were thoroughly mixed at the appropriate ratio using a mixer (5K45SSWH, Whirlpool, USA). The basic recipe consisted of 79.0 mg/kg lean beef meat, 10.0 mg/kg beef back fat, 10.0 mg/kg iced water, and 1.0 mg/kg salt (sodium chloride). Portions of equal parts of the meat mixture were mixed with sodium nitrite and two antioxidants of GTE and CE. Four different types of beef patties were prepared according to the following formulations: without sodium nitrite, GTE, or CE (control); with 0.007% sodium nitrite (T1); with 0.05% GTE (T2); with 0.05% CE (T3). Beef patties (approximately 80 g) were prepared using a hand-held patty maker and placed on plastic trays. These were packaged individually in oxygen-permeable bags (polyethylene, 15×20 cm). The beef patties were then brought to the Animal Foods Processing laboratory or analytical laboratory at Gyeongsang National University to determine the thiobarbituric acid reactive substance (TBARS) value, carbonyl content, MetMb content, pH, cooking loss, color, and sensory attributes after1, 3, 5, and 7 days of cold storage at 4℃.
Ultra-performance liquid chromatography-quadrupole-time-of-flight (UPLC-Q-TOF) MS analysis
Metabolites extracted from GTE and CE using methanol and distilled water (6:4, v/v) were analyzed with a UPLC system (Waters, Milford, MA, USA). The samples were infused into an Acquity UPLC BEH C18 column (2.1 mm×100 mm×1.7 μm; Waters) balanced by water with 0.1% formic acid added and eluted in a gradient of acetonitrile with 0.1% formic acid with a flow rate of 0.35 mL/min for 10 min. Three replicates were used for metabolic analysis of the extracts. Metabolites were analyzed by aquadrupole-time-of-flight mass spectrometer (waters) in electro-spray ionization (Q-TOF MS in ESI)-positive mode. The sampling cones and voltages of the capillary were fixed at 40 V and 3 kV, respectively. The temperatures of the ion source and desolvation were calibrated at 100℃ and 400℃, respectively, and the desolvation gas flow rate was 900 L/h. The data of TOF MS were collected in the m/z 50–1,500 range with a scan time of 0.2 s. The MS/MS spectra of the metabolites were accumulated in the m/z 50–1,500 range by using a collision energy ramp from 20 to 40 eV.
Physicochemical properties
pH determination
The pH was measured using a digital pH meter (MP230, Mettler Toledo, Greifensee, Switzerland). A beef patty (3 g) was dispersed in 27 mL of distilled water and then homogenized with a polytron homogenizer (T25 basic, IKA, Selangor, Malaysia). The pH meter was calibrated using the standard buffers of pH 4.0 and 7.0 at 25℃ before measuring.
Cooking loss
The loss of beef patty due to cooking was determined gravimetrically following a modified method of Boles and Swan (1996). Twenty five grams of sample was transferred into a 50 mL centrifuge tube and then heated in a water bath at 70℃ for 30 min followed by centrifugation at 850 g for 10 min. The total amount of fluid released was expressed as a percentage of the same weight.
Where, W1 is the weight of uncooked patty and W2 is the weight of the cooked patty.
Metmyoglobin (MetMb) content
The content of MetMb was determined as described by Krzywicki (1982). The absorbance of the extract was measured at 572, 565, 545, and 525 nm using a spectrophotometer (Agilent Co., CA. USA). In order to remove the influence of the additive dye, the same amount of additive was used for the correction. The content of MetMb was calculated for beef patties muscles using the following equation:
Where, R1, R2, and R3 are absorbance ratios of A572/A525, A565/A525, and A545/A525, respectively.
Preparation of myofibrillar protein (MP) and measurement of protein oxidation
MP was prepared as described by Liu and Xiong (2015). Approximately 2 g of each dissected sample was minced and homogenized in 10 volumes of rigor buffer (0.1 M KCl, 2 mM MgCl2, 1 mM EGTA, and 10 mM K2PO4, pH 7.0) at 15,000 rpm for 15s with a homogenizer (T25, IKA, Staufen, Germany). The homogenates were centrifuged at 5,000 g for 15 min at 4℃ (1736R, Labogene, Seoul, Korea). MP pellets were kept on ice and used within 12 h after measuring the protein concentration by the biuret method.
The extent of protein oxidation was determined by observing carbonyl content using the spectrophotometric assay including the sample derivation method as described by Castegna et al. (2003). Soglia et al. (2016) stated that the carbonyl content is determined by reading the absorbance at the optimum wavelength (λ=280 and 370 nm) of each sample against its appropriate blank using the following equation:
Lipid oxidation measurement
The TBARS content in beef patty samples was determined using the TBA distillation procedure of Buege and Aust (1978). The sample was analyzed at 531 nm against a blank containing 2 mL of deionized distilled water and 4 mL of TBA/TCA solution. The amounts of TBA were expressed as mg of malondialdehyde (MDA) per kg of sample.
Color measurement
The measurement of color (International Commission on Illumination L*, a*, b*) was performed at the surface of the beef patties using a Minolta Chromameter (Minolta CR 301, Tokyo, Japan) standardized with a white calibration plate (Y=93.5; x=0.3132; y=0.3198). The chroma and hue angle were calculated using the equation:
Sensory evaluation of cooked beef patties
Samples of beef patties from each treatment were evaluated by a 10-member trained expert descriptive attribute sensory panel at Gyeongsang National University. The panelists were selected and trained according to the method of Meilgaard et al. (1999). Panelists were served samples representing anchor points for each attribute and training sessions using patties with GTE and CE in the Animal Foods processing lab. The panelists were trained using a 5-point scale (“5 extremely intense” and “1 slightly intense”) for patty color, flavor, and texture attributes (hardness and juiciness). Final anchor point ratings were decided upon by the training panel after a preparatory session of evaluation and discussion. For the samples, panelists determined the samples for color, flavor, odor, and flavor of extract (FOE) applying a 9-point hedonic scale, where 1 was “very inappropriate” and 9 was “very appropriate” as described by Meilgaard et al. (1999). Each panelist received at least 2 cubes per sample and evaluated 8 randomly ordered samples (with/without GTE and CE) per day.
Statistical analysis
The experiment design of this study was completely randomized design with three independent batches. All data were analyzed by using mixed procedure of SAS 9.3 (2014). A fixed effect of model was treatment or storage, and random effect of model was batch (physicochemical and color data) or panelist (sensory evaluation data). Least square means test was used to determine the level of differences among treatments or storage. Least squares means for all traits were separated (F-test, p<0.05) by using least significant differences generated by the PDIFF option in SAS.
Results and Discussion
Extracted metabolites content of clove extract and golden thread extract
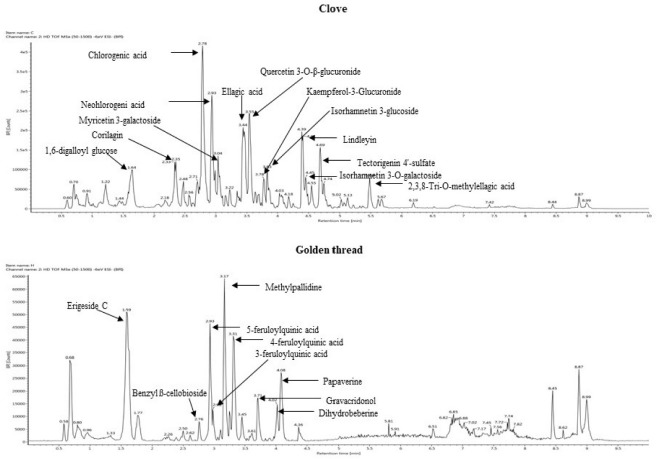
A typical ultra-performance liquid chromatogram of the metabolites extract isolated from CE and GTE is presented in Fig. 1. The extracted metabolites in CE included chlorogenic acid, neochlorogenic acid, quercetin-3-O-β-glucuronide, ellagic acid, lindleyin, tectorigenin-4'-sulfate, myricetin 3-galactoside, corilagin, 1,6-digalloyl glucose, isorhamnetin-3-glucoside, isorhamnetin-3-O-galactoside, kaempferol-3-glucuronide, and 2,3,8-tri-o-methylellagic acid, all of which are phenolic compounds. The metabolites extracted from GTE included methylpallidine, erigeside C, 5-feruloylquinic acid, 4-feruloylquinic acid, papaverine, gravacridonol, dihydroberberine, 3-feruloylquinic, and benzyl-beta-cellobioside. Among these metabolites extracted from GTE, the major metabolites were phenolic compounds and the others were alkaloids. It can be stated that CE and GTE containing phenolic compounds exhibited antioxidant activities, in accordance with Huang and Frankel (1997), who reported that phenolic groups showed a significant role in antioxidative activity. Lee and Shibamoto (2001) found that the main chemicals extracted as aroma compounds from clove buds were eugenol, eugenyl acetate, benzyl alcohol, and 1-actyloxy-2-propanol, which had antioxidant properties. However, Fan et al. (2008) revealed that the major active compounds of golden thread (C. chinensis) were PBAs, including berberine, coptisine, palmatine, epiberberine, and jatrorrhizine, which showed inhibitory effects.
Fig. 1. Presentative UPLC-Q-TOF MS profiles of clove extract and golden thread extract.
Physicochemical properties
The pH values
Beef patties with added nitrite, GTE, and CE had higher pH values than that of the control, but there were no significant differences (p>0.05) in pH values among control patties and patties treated with nitrite (0.007%), GTE, and CE during 1, 3, 5, and 7 days of cold storage at 4℃ (Table 1). So, no significant effects of nitrite, GTE and CE on pH values were observed at all days of the storage period. Mokhtar et al. (2012) indicated that the pH value increase might be due to the liberation of ammonia compounds as a result of proteolytic microbial flora present in the raw meat. Furthermore, Georgantelis et al. (2007a) observed that fresh pork sausages containing chitosan, rosemary and chitosan, as well as α-tocopherol and chitosan exhibited homogeneous (p>0.05) pH values over the entire storage time.
Table 1. Effect of natural antioxidants on physicochemical properties of beef patty.
| Storage
period (day) |
Treatment1) | pH | Cooking
loss (%) |
MetMB
content (%) |
Carbonyl
content (nmol/mg protein) |
|---|---|---|---|---|---|
| 1 | C | 5.53 | 61.29 | 4.91d | 0.04bc |
| T1 | 5.55 | 64.73 | 25.61ab | 0.05bc | |
| T2 | 5.56 | 63.35 | 24.79ab | 0.04c | |
| T3 | 5.55 | 63.68 | 14.57c | 0.08bc | |
| 3 | C | 5.52 | 57.98 | 7.07d | 0.10bc |
| T1 | 5.55 | 59.30 | 24.32b | 0.12b | |
| T2 | 5.58 | 60.62 | 24.34bc | 0.10bc | |
| T3 | 5.57 | 60.92 | 10.56bc | 0.11bc | |
| 5 | C | 5.49 | 66.23 | 12.52cd | 0.21a |
| T1 | 5.52 | 66.39 | 27.18bc | 0.10bc | |
| T2 | 5.53 | 65.49 | 24.18bc | 0.11bc | |
| T3 | 5.50 | 66.41 | 25.73bc | 0.07bc | |
| 7 | C | 5.54 | 68.43 | 20.58b | 0.28a |
| T1 | 5.58 | 65.32 | 24.23bc | 0.07bc | |
| T2 | 5.58 | 65.33 | 24.91b | 0.12b | |
| T3 | 5.56 | 66.01 | 19.93bc | 0.12bc | |
| SEM | 0.04 | 3.30 | 2.07 | 0.04 | |
a–d Means with different letters are significantly different in column (p<0.05).
1) C, control; T1, added 0.007% nitrite; T2, added 0.05% golden thread extract; T3, added 0.05% clove extract.
Cooking loss
Beef patties made with the addition of nitrite, GTE, and CE showed higher cooking loss than that of the control; however, no significant differences (p>0.05) in cooking loss were occurred among nitrite, GTE, and CE treated patties and control patties during 1, 3, 5, and 7 days of cold storage at 4℃ (Table 1). The results have been previously obtained, where the addition of plant extracts such as Ulam Raja and green tea into beef patties increased the cooking loss compared to that in the control (Reihani et al., 2014).
Metmyoglobin (MetMb) content
Both the natural antioxidants treatment and storage time influenced the MetMb content. Beef patties with added nitrite, GTE, and CE had significantly higher MetMb content compared to control patties during 1 and 3 days of cold storage at 4℃ (p<0.05). Nitrite and GTE-treated patties showed significantly higher MetMb content among all types of patties at 1 and 3 days of storage time (p<0.05). The data indicate the effectiveness of the used antioxidants in hindering myoglobin and/or oxymyoglobin oxidation as follows: CE>GTE>nitrite (Table 1). Again, MetMb content for nitrite, GTE, and CE treated beef patties exhibited more than that of control patties at the period of 5 and 7 days cold (4℃) storage, but significant differences in MetMb content were not shown between control patties and all treated patties (p>0.05). These results matched with the observations of Bekhit et al. (2003), who showed that beef patties treated with the natural antioxidant carnosine increased accumulation of MetMb and had 50% more MetMb than the control after 6 days of storage in which this could be owing to several concentration of carnosine as natural antioxidant applied in this study. However, Liu et al. (2015) found that, compared to the control group, the treatment of beef patties with several natural antioxidants resulted in a higher MetMb% after 6 days of storage but a lower MetMb% after 8 days of storage.
Protein oxidation
The carbonyl content of different formulas of beef patties is shown in Table 1. Treatment of beef patties with nitrite and natural antioxidants of GTE and CE resulted in lower carbonyl content than the control patties during 5 and 7 days of cold storage at 4℃. However, no significant differences in carbonyl content were found between all treated patties and the control patties over the cold storage time of 1, 3, 5, and 7 days (p>0.05). The carbonyl content in all treated beef patties increased gradually with the storage time from 1 to 3, and 5 to 7 days. Cooked pork meat patties containing several plant materials showed decreased carbonyl content compared to that in the control patties (Salminen et al., 2006). Mercier et al. (2004) showed that the carbonyl content increased during the first 30 min of incubation but slightly decreased until 5 h in vitamin E-containing mixed diet-treated beef homogenates. Furthermore, Batifoulier et al. (2002) observed that the carbonyl content decreased after a long incubation period in microsomal membranes from turkey muscle supplemented with vitamin E.
Lipid oxidation
Fig. 2 shows the effect of GTE and CE on lipid oxidation in beef patties after 7 days of cold storage at 4℃. TBARS formation increased rapidly in the control patties over cold storage time from 1 to 7 days at 4℃. Beef patties with a dose of either nitrite or GTE and CE showed lower TBARS values compared to the control (p<0.05), which indicates that nitrite, GTE, and CE were effective in protecting beef patties from lipid oxidation. There was no significant difference between patties with added nitrite, GTE, and CE. However, patties containing GTE and CE showed the lowest TBARS values. These results are in agreement with Shi et al. (2014) who observed lower TBARS values for silver carp fillets treated with clove buds extract because of its antioxidant activity compared with those in the control and other extracts. Janero (1990) mentioned that TBARS values could be inhibited by the reaction of malonaldehyde with sugars, amino acids, and nitrite complex formulations. In addition, nitrite has shown antioxidant activity against lipid oxidation. Nitrite has the antioxidant property that it can form myoglobin-stable substances for making iron (as the oxidation catalyst) inaccessible in cured meat (Honikel, 2008). A study conducted by Zhang et al.(2016) showed that the addition of CE alone and in combination with rosemary extract could be used as a natural antioxidant in raw chicken meat, resulting in significantly lower levels of TBARS values during storage at 4℃ (p<0.05). Beef patties treated with rosemary extract alone and in combination with carnosine and chitosan had lower TBARS values during storage compared to the control (Mokhtar et al., 2014). Therefore, GTE and CE are efficient natural antioxidants for use in controlling lipid oxidation in beef patties.
Fig. 2. Effects of natural antioxidants extract on TBARS of beef patty according to storage periods.
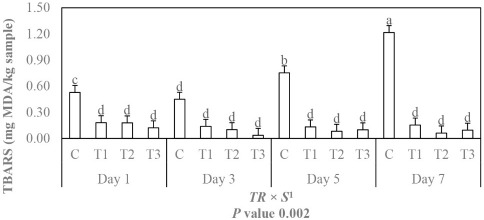
C, control; T1, added 0.007% nitrite; T2, added 0.1% golden thread extract; T3, added 0.1% clove extract; TBARS, thiobarbituric acid reactive substance. 1 Interaction of treatment and storage. a–d Means with different letters are significantly different in interaction of storage and treatment.
Color evaluation
The color attributes resulting from different treatments of fresh beef patties are presented in Table 2. For lightness (L*) value of fresh beef patties, there were no significant differences in L* values among the control patties, and patties with added nitrite, GTE, and CE at the cold (4℃) storage period of 1, 3, 5, and 7 days (p>0.05). In regard to the redness (a*) value, no significant differences in a* value were observed among the control patties and patties with added GTE and CE during cold storage of 1, 3, 5, and 7 days (p>0.05). However, nitrite-treated patties had a significantly lower a* value than those of the control patties and patties treated with GTE and CE over all days of storage (p<0.05). Again, the yellowness (b*) value of fresh beef patties treated with GTE was significantly (p<0.05) higher than those of the control patties and patties with added nitrite and CE at the storage time of 1, 3, 5, and 7 days, which may have occurred because of the presence of slightly colored substances in GTE. However, significant differences in b* values were not recorded among patties with added nitrite and CE, and control patties during the cold storage period of 3, 5, and 7 days (p>0.05). Regarding the chroma value, fresh beef patties added with GTE showed significantly the highest chroma value among the control and all treated patties at the storage period of 3 days (p<0.05). However, no significant differences in chroma value were observed between CE-added patties and control patties over all days of storage at 4℃ (p>0.05). Again, nitrite-treated patties showed significantly the lowest chroma value among all types of beef patties during entire storage days (p<0.05). For hue angle value, fresh beef patties containing nitrite and GTE had significantly higher hue angle values than those of the control and CE-treated patties at the cold storage period of 1 and 3 days (p<0.05), and no significant difference in hue angle value was shown for patties with added nitrite and GTE (p>0.05). However, no significant differences in hue angle values were obtained between the control and CE-added patties during storage period of 1, 3 and 5 days at 4℃ (p>0.05).
Table 2. Effect of natural antioxidants on color of fresh beef patty.
| Storage
period (day) |
Treatment1) | Lightness (L*) |
Redness (a*) |
Yellowness (b*) |
Chroma | Hue angle |
|---|---|---|---|---|---|---|
| 1 | C | 42.25 | 18.95a | 9.77c | 21.35ab | 27.04d |
| T1 | 41.94 | 7.49d | 7.93d | 10.92e | 46.45b | |
| T2 | 42.49 | 17.15ab | 13.95a | 22.17a | 38.90bc | |
| T3 | 42.03 | 17.01ab | 9.13cd | 19.35bc | 28.25d | |
| 3 | C | 40.51 | 14.96bc | 8.90cd | 17.44c | 31.00cd |
| T1 | 42.00 | 7.72d | 8.83cd | 11.74e | 48.75ab | |
| T2 | 42.20 | 14.22bc | 13.48a | 19.64b | 43.45b | |
| T3 | 41.37 | 15.13b | 8.47d | 17.36c | 29.18d | |
| 5 | C | 42.80 | 10.93c | 9.25cd | 14.44d | 40.55bc |
| T1 | 44.50 | 7.47d | 9.01cd | 11.75e | 50.30ab | |
| T2 | 43.51 | 12.68c | 13.65a | 18.69bc | 47.01ab | |
| T3 | 41.99 | 13.37bc | 8.36d | 15.79cd | 31.91cd | |
| 7 | C | 42.67 | 10.46c | 10.20c | 14.83d | 44.97b |
| T1 | 45.09 | 7.26d | 9.26cd | 11.86e | 51.34a | |
| T2 | 40.65 | 11.17c | 12.34b | 16.75cd | 47.62ab | |
| T3 | 42.57 | 12.73c | 9.05cd | 15.65cd | 34.95c | |
| SEM | 1.66 | 0.86 | 0.53 | 0.71 | 2.59 | |
a–e Means with different letters are significantly different in column (p<0.05).
1) C, control; T1, added 0.007% nitrite; T2, added 0.05% golden thread extract; T3, added 0.05% clove extract.
Table 3 shows the effect of natural antioxidants on the color attributes of cooked beef patties. For the L* value, no significant differences in L* values were reported among cooked patties with added nitrite, GTE and CE, and control patties during storage period of 1, 3, 5, and 7 days at 4℃ (p>0.05). Regarding the a* value, nitrite-treated patties had higher a* value than the control patties and patties treated with GTE and CE, but no significant differences in a* values were observed among control patties and patties with added nitrite, GTE, and CE over all storage days at 4℃ (p>0.05). For the b* value of cooked beef patties, significant differences in b* value were not recorded among patties with added nitrite, GTE and CE, and control patties at the cold storage period of 1, 3, 5, and 7 days (p>0.05). In regard to the chroma value, cooked patties with added nitrite, GTE and CE exhibited no significant differences in chroma value compared with the control patties during storage period of 1, 3, 5, and 7 days at 4℃ (p>0.05). For hue angle value, The hue angle values of GTE-treated patties were higher than those of the control patties and, nitrite and CE treated patties; however, no significant differences in hue angle values were obtained among the control patties and patties with added nitrite, GTE, and CE over total cold storage days (p>0.05).
Table 3. Effect of natural antioxidants on color of cooked beef patty.
| Storage
period (day) |
Treatment1) | Lightness (L*) |
Redness (a*) |
Yellowness (b*) |
Chroma | Hue angle |
|---|---|---|---|---|---|---|
| 1 | C | 51.84 | 6.38 | 7.11 | 9.81 | 49.16 |
| T1 | 52.41 | 8.06 | 7.08 | 11.02 | 43.29 | |
| T2 | 51.24 | 4.23 | 8.66 | 9.66 | 64.29 | |
| T3 | 50.74 | 3.77 | 6.33 | 7.41 | 58.41 | |
| 3 | C | 50.63 | 4.32 | 7.17 | 8.40 | 58.70 |
| T1 | 51.58 | 10.24 | 6.48 | 12.14 | 32.19 | |
| T2 | 50.83 | 3.95 | 9.42 | 10.24 | 67.40 | |
| T3 | 48.02 | 5.29 | 8.43 | 9.98 | 58.09 | |
| 5 | C | 50.81 | 6.97 | 8.52 | 11.41 | 52.75 |
| T1 | 52.20 | 8.41 | 8.14 | 12.04 | 45.05 | |
| T2 | 51.12 | 4.20 | 9.56 | 10.60 | 66.35 | |
| T3 | 49.53 | 4.39 | 7.64 | 8.87 | 59.51 | |
| 7 | C | 53.26 | 5.56 | 6.68 | 8.97 | 51.85 |
| T1 | 52.34 | 8.01 | 7.31 | 11.10 | 44.62 | |
| T2 | 49.89 | 4.24 | 9.10 | 10.13 | 64.44 | |
| T3 | 49.22 | 4.89 | 6.44 | 8.09 | 52.69 | |
| SEM | 1.30 | 1.28 | 0.60 | 0.77 | 6.42 | |
1) C, control; T1, added 0.007% nitrite; T2, added 0.05% golden thread extract; T3, added 0.05% clove extract.
Our results agreed with the findings of Reihani et al. (2014) who observed that Ulam Raja and green tea extracts-treated beef patties exhibited no significant differences in lightness value with the control beef patties. Zhang et al. (2016) found that chicken meat treated with clove and rosemary extract exhibited an increased lightness value compared to the control over the total storage time. The findings of this study are related to the results of Zhang et al. (2016) who showed that the a* value was higher in chicken meat with added clove and rosemary extract compared to the control. Again, Sánchez-Escalante et al. (2001) found that beef patties treated with rosemary and carnosine showed significantly increased redness values compared to the control patties after the same storage period (p<0.05). The raw chicken meat treated with clove and rosemary extracts had higher b* values compared to the evaluated value of the control over the full length of storage (Zhang et al., 2016). Gibis and Weiss (2012) also mentioned that discoloration of fried beef patties occurred because of the addition of grape seed extract as a natural antioxidant. Bekhit et al. (2003) reported that carnosine, quercetin, resveratrol, and rutin-treated beef patties exhibited significantly increased chroma values compared to the control during 9 days of storage. Bekhit et al. (2003) revealed that beef patties with added carnosine, quercetin, resveratrol, and rutin demonstrated significantly lower hue angle values than the control patties during the storage period between 1 and 9 days.
Sensory evaluation
The color scores of cooked beef patties treated with CE were significantly higher than those of the control patties and patties with added nitrite and GTE during storage period of 3, 5, and 7 days at 4℃ (p<0.05) (Table 4). Cooked patties treated with GTE had significantly higher color score than control patties and nitrite-treated patties at 3 and 5 days storage time (p<0.05), but no significant differences were observed among GTE and nitrite-containing patties, and control patties at 7 days storage period (p>0.05). In regard to flavor, GTE and CE-containing patties exhibited lower flavor scores than the control patties; however, no significant differences in flavor scores were shown among control patties and patties treated with nitrite, GTE, and CE over entire storage days at 4℃ (p>0.05). Again, beef patties formulated by nitrite, GTE, and CE were not recognized significant differences in odor scores with the control patties during storage period of 1, 3, 5, and 7 days at 4℃ (p>0.05). Regarding the FOE score, no significant differences in FOE scores were observed among control patties, and patties treated with nitrite, GTE, and CE at all storage days (p>0.05). Georgantelis et al. (2007b) found that the chitosan-treated beef burger contained a more intense red color compared to the rosemary extract and α-tocopherol-treated burgers and the controls. All beef patties with added all-natural antioxidants showed significantly increased odor and color scores compared to the measured score of the control during the storage period (p<0.05) (Mokhtar et al., 2014). Zhang et al. (2016) reported that clove and rosemary extract-added chicken meat received a higher odor score than the control during the entire length of storage (p<0.05). Mohamed and Mansour (2012) observed that beef patties formulated without the addition of marjoram and rosemary as antioxidants exhibited significantly lower flavor scores than those of other formulas (p<0.05). Thus, the addition of GTE and CE did not influence the sensory attributes of flavor in cooked beef patties, but GTE and CE influenced the color as a sensory characteristic in cooked beef patties. Thus, GTE and CE addition were more effective for acquiring greater intensity of color in cooked beef patties.
Table 4. Effect of natural antioxidants on sensory characteristics of cooked beef patty.
| Storage period (day) | Treatment1) | Color | Flavor | Odor | FOE |
|---|---|---|---|---|---|
| 1 | C | 6.33ab | 4.83 | 2.33 | 1.50 |
| T1 | 2.00d | 4.00 | 1.17 | 1.83 | |
| T2 | 5.33b | 4.00 | 1.17 | 1.83 | |
| T3 | 6.67a | 4.00 | 2.33 | 2.00 | |
| 3 | C | 3.60c | 5.05 | 3.65 | 2.05 |
| T1 | 2.60cd | 4.88 | 2.20 | 2.45 | |
| T2 | 5.00b | 3.69 | 1.99 | 2.45 | |
| T3 | 6.60a | 3.67 | 1.84 | 1.85 | |
| 5 | C | 3.50c | 5.67 | 3.67 | 2.00 |
| T1 | 2.67cd | 5.17 | 3.50 | 2.33 | |
| T2 | 5.00b | 4.33 | 3.33 | 2.67 | |
| T3 | 6.50a | 3.83 | 2.50 | 2.33 | |
| 7 | C | 2.60cd | 5.85 | 2.45 | 2.25 |
| T1 | 2.40cd | 4.48 | 2.00 | 2.45 | |
| T2 | 4.60bc | 3.89 | 1.39 | 2.65 | |
| T3 | 7.00a | 3.87 | 2.44 | 2.05 | |
| SEM | 0.42 | 0.70 | 0.68 | 0.60 | |
a–d Means with different letters are significantly different in column (p<0.05).
1 C, control; T1, added 0.007% nitrite; T2, added 0.05% golden thread extract; T3, added 0.05% clove extract.
FOE, flavor of extract.
Conclusion
The results demonstrate the efficiency of GTE and CE (as natural antioxidants) and of commercially available nitrite in retarding the increases in TBA resulting in the inhibition of lipid oxidation of beef patties during cold storage at 4℃. Protein oxidation was not developed by using GTE, CE, and nitrite into beef patties. Incorporation of GTE and CE stabilized the color of beef patties and had a significant impact on sensory characteristics, including color and odor of the beef patties. Furthermore, the antioxidant effects of GTE, CE, and nitrite on lipid oxidation were more prominent than those on protein oxidation. Among the three treatments, GTE and CE had more potential for antioxidant activity in beef patties compared with that of the commercially available nitrite. This could be due to the high content of phenolic bioactive components in CE and GTE. Therefore, GTE and CE can be applied as natural antioxidants to prevent lipid and protein oxidation and extend the shelf-life of meat products to achieve highly desirable and valuable healthy meat products.
Acknowledgements
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food Agriculture, Forestry and Fisheries, Ministry of Agriculture, Food and Rural Affairs (Project No. 316064-02-2-HD030).
References
- Armenteros M, Morcuende D, Ventanas J, Estévez M. The application of natural antioxidants via brine injection protects Iberian cooked hams against lipid and protein oxidation. Meat Sci. 2016;116:253–259. doi: 10.1016/j.meatsci.2016.02.027. [DOI] [PubMed] [Google Scholar]
- Batifoulier F, Mercier Y, Gatellier P, Renerre M. Influence of vitamin E on lipid and protein oxidation induced by H2O2-activated MetMb in microsomal membranes from turkey muscle. Meat Sci. 2002;61:389–395. doi: 10.1016/s0309-1740(01)00209-1. [DOI] [PubMed] [Google Scholar]
- Bekhit AED, Geesink GH, Ilian MA, Morton JD, Bickerstaffe R. The effects of natural antioxidants on oxidative processes and metmyoglobin reducing activity in beef patties. Food Chem. 2003;81:175–187. doi: 10.1016/S0308-8146(02)00410-7. [DOI] [Google Scholar]
- Boles JA, Swan JE. Effect of post-slaughter processing and freezing on the functionality of hot-boned meat from young bull. Meat Sci. 1996;44:11–18. doi: 10.1016/s0309-1740(96)00076-9. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Method Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Castegna A, Drake J, Pocernich C, Butterfield DA. Methods in biological oxidative stress. Humana Press Inc.; Totowa NJ: 2003. Protein carbonyl levels-an assessment of protein oxidation; pp. 161–168. [Google Scholar]
- Cho SH, Kim J, Park BY, Seong PN, Kang GH, Kim JH, Jung SG, Im SK, Kim DH. Assessment of meat quality properties and development of a palatability prediction model for Korean Hanwoo steer beef. Meat Sci. 2010;86:236–242. doi: 10.1016/j.meatsci.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Chung JG, Wu LT, Chu CB, Jan JY, Ho CC, Tsou MF, Lu HF, Chen GW, Lin JG, Wang TF. Effects of berberine on arylamine N-acetyltransferase activity in human bladder tumour cells. Food Chem Toxicol. 1999;37:319–326. doi: 10.1016/s0278-6915(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Devatkal SK, Narsaiah K, Borah A. Antioxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci. 2010;85:155–159. doi: 10.1016/j.meatsci.2009.12.019. [DOI] [PubMed] [Google Scholar]
- El-Maatia MFA, Mahgoubb SA, Labiba SM, Al-Gabya AMA, Ramadan MF. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. Eur J Integr Med. 2016;8:494–504. doi: 10.1016/j.eujim.2016.02.006. [DOI] [Google Scholar]
- Falowo AB, Fayemi PO, Muchenje V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res Int. 2014;64:171–181. doi: 10.1016/j.foodres.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Fan DL, Xiao XH, Ma XJ. Calorimetric study of the effect of protoberberine alkaloids in Coptis chinensis Franch on Staphylococcus aureus growth. Thermochim Act. 2008;480:49–52. doi: 10.1016/j.tca.2008.09.008. [DOI] [Google Scholar]
- Frankel EN. Lipid oxidation. The Oily Press Ltd.; Dundee Scotland: 1998. pp. 55–77. [Google Scholar]
- Georgantelis D, Ambrosiadis I, Katikou P, Blekas G, Georgakis SA. Effect of rosemary extract, chitosan and α-tocopherol on microbiological parameters and lipid oxidation of fresh pork sausages stored at 4℃. Meat Sci. 2007a;76:172–181. doi: 10.1016/j.meatsci.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Georgantelis D, Blekas G, Katikou P, Ambrosiadis I, Fletouris DJ. Effect of rosemary extract, chitosan and a-tocopherol on lipid oxidation and color stability during frozen storage of beef burgers. Meat Sci. 2007b;75:256–264. doi: 10.1016/j.meatsci.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Gibis M, Weiss J. Antioxidant capacity and inhibitory effect of grape seed and rosemary extract in marinades on the formation of heterocyclic amines in fried beef patties. Food Chem. 2012;134:766–774. doi: 10.1016/j.foodchem.2012.02.179. [DOI] [PubMed] [Google Scholar]
- Honikel KO. Curing agents. In: Devine C, Dikeman M, Jensen WK, editors. Encyclopedia of meat sciences. Elsevier; Oxford, UK: 2004. pp. 195–201. [Google Scholar]
- Honikel KO. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008;78:68–76. doi: 10.1016/j.meatsci.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Honikel KO. Chemical analysis for specific compounds/curing agents. In: Devine G, Dikeman M, editors. Encyclopedia of meat sciences. 2nd ed. Academic Press; Oxford, UK: 2014. pp. 200–205. [Google Scholar]
- Huang SW, Frankel EN. Antioxidant activity of tea catechins in different lipid systems. J Agri Food Chem. 1997;45:3033–3038. doi: 10.1021/jf9609744. [DOI] [Google Scholar]
- Hugas M, Garriga M, Monfort JM. New mild technologies in meat processing: High pressure as a model technology. Meat Sci. 2002;62:359–371. doi: 10.1016/s0309-1740(02)00122-5. [DOI] [PubMed] [Google Scholar]
- Janero DR. Malonaldehyde and thiobarbituric acid-reactivity as diagnostics indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Krzywicki K. The determination of haem pigments in meat. Meat Sci. 1982;7:29–36. doi: 10.1016/0309-1740(82)90095-X. [DOI] [PubMed] [Google Scholar]
- Lee KG, Shibamoto T. Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum (L.) Merr. et Perry] Food Chem. 2001;74:443–448. doi: 10.1016/S0308-8146(01)00161-3. [DOI] [Google Scholar]
- Liu C, Xiong YL. Oxidation-initiated myosin subfragment cross-linking and structural instability differences between white and red muscle fiber types. J Food Sci. 2015;80:288–297. doi: 10.1111/1750-3841.12749. [DOI] [PubMed] [Google Scholar]
- Liu F, Xu Q, Dai R, Ni Y. Effects of natural antioxidants on colour stability, lipid oxidation and metmyoglobin reducing activityin raw beef patties. Technol Aliment. 2015;14:37–44. doi: 10.17306/J.AFS.2015.1.4. [DOI] [PubMed] [Google Scholar]
- Lorenzo JM, Sineiro J, Amado IR, Franco D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014;96:526–534. doi: 10.1016/j.meatsci.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Meilgaard MC, Carr BT, Civille GV. Sensory evaluation techniques. 3rd ed. CRC Press; Boca Raton, FL: 1999. p. 354. [Google Scholar]
- Mercier Y, Gatellier P, Renerre M. Lipid and protein oxidation in vitro, and antioxidant potential in meat from Charolais cows finished on pasture or mixed diet. Meat Sci. 2004;66:467–473. doi: 10.1016/S0309-1740(03)00135-9. [DOI] [PubMed] [Google Scholar]
- Mohamed HMH, Mansour HA. Incorporating essential oils of marjoram and rosemary in the formulation of beef patties manufactured with mechanically deboned poultry meat to improve the lipid stability and sensory attributes. LWT- Food Sci Technol. 2012;45:79–87. doi: 10.1016/j.lwt.2011.07.031. [DOI] [Google Scholar]
- Mokhtar S, Mostafa G, Taha R, Eldeep GSS. Effect of different starter cultures on the biogenic amines production as a critical control point in fresh fermented sausages. Eur Food Res Technol. 2012;235:527–535. doi: 10.1007/s00217-012-1777-9. [DOI] [Google Scholar]
- Mokhtar SM, Youssef KM, Morsy NE. The effects of natural antioxidants on colour, lipid stabilityand sensory evaluation of fresh beef patties stored at 4℃. J Agroaliment Proc Technol. 2014;20:282–292. [Google Scholar]
- Pegg RB, Shahidi F, Fox JB., Jr Unraveling the chemical identity of meat pigments. Crit Rev Food Sci Nutr. 1997;37:561–589. doi: 10.1080/10408399709527789. [DOI] [PubMed] [Google Scholar]
- Reihani SFS, Tan TC Huda N, Easa AM. Frozen storage stability of beef patties incorporated with extracts from Ulam raja leaves (Cosmos caudatus) Food Chem. 2014;155:17–23. doi: 10.1016/j.foodchem.2014.01.027. [DOI] [PubMed] [Google Scholar]
- Salminen H, Estévez M, Kivikari R, Heinonen M. Inhibition of protein and lipid oxidation by rapessed, camelina and soy meal in cooked pork meat patties. Eur Food Res Technol. 2006;223:461–468. doi: 10.1007/s00217-005-0225-5. [DOI] [Google Scholar]
- Sánchez-Escalante A, Djenane D, Torrescano G, Beltrán JA, Roncalés P. The effects of ascorbic acid, taurine, carnosine and rosemary powder on colour and lipid stability of beef patties packaged in modified atmosphere. Meat Sci. 2001;58:421–429. doi: 10.1016/s0309-1740(01)00045-6. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS user’s guide. Version 9.3. SAS Institute Inc.; Cary NC, USA: 2014. [Google Scholar]
- Shi C, Cui J, Yin X, Luo Y, Zhou Z. Grape seed and clove bud extracts as natural antioxidants in silver carp (Hypophthalmichthys molitrix) fillets during chilled storage: Effect on lipid and protein oxidation. Food Cont. 2014;40:134–139. doi: 10.1016/j.foodcont.2013.12.001. [DOI] [Google Scholar]
- Soglia F, Petracci M, Ertbjerg P. Novel DNPH-based method for determination of protein carbonylation in muscle and meat. Food Chem. 2016;197:670–675. doi: 10.1016/j.foodchem.2015.11.038. [DOI] [PubMed] [Google Scholar]
- Yu HH, Kim KJ, Cha JD, Kim HK, Lee YE, Choi NY, You YO. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005;8:454–46. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- Zhang H, Wu J, Guo X. Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality. Food Sci Hum Wellness. 2016;5:39–48. doi: 10.1016/j.fshw.2015.11.003. [DOI] [Google Scholar]
- Zhang S, Zhang B, Xing K, Zhang X, Tian X, Dai W. Inhibitory effects of golden thread (Coptis chinensis) and berberine on microcystis aeruginosa. Water Sci Technol. 2010;61:763–769. doi: 10.2166/wst.2010.857. [DOI] [PubMed] [Google Scholar]


