Abstract
The aim of this study was to determine meat quality characteristics and fatty acid composition of Korean native black goats (KNBG) finished on intensive feeding of alfalfa (ALF) and conventional feeding of commercial concentrate pellets (CCP) with low-energy common grasses. Ten KNBG (12 months old) were divided into two groups and subjected to either ALF or CCP treatments. The goats were slaughtered after 6 months of feeding with experimental diets to investigate meat quality characteristics and fatty acid compositions of longissimus lumborum muscle. There were no significant differences in proximate chemical composition, collagen, or myoglobin content between ALF and CCP groups of goats. Meat color, water-holding capacity, or tenderness was not significantly different between the two groups either. However, proportions of monounsaturated fatty acids and polyunsaturated fatty acids were significantly (p<0.05) different between the two groups. The proportion of oleic acid was significantly (p<0.05) higher in ALF goat whereas proportion of linoleic and arachidonic acids were significantly (p<0.05) higher in CCP goat. Results suggest that KNBG finished with intensive feeding of alfalfa could produce goat meat with desirable fatty acids for human diets.
Keywords: Korean native black goat, goat meat quality, fatty acid profile, alfalfa, goat feed
Introduction
Goats are appropriate under semi-arid and punitive environmental conditions because of their browsing behaviors and adaptation to severe climates. For these reasons, in many Asian countries, goat meat is the main source of animal protein. Korean native black goat (KNBG; Capra hircuscoreanae) has been domesticated about 2000 years back in Korea. Consumption of KNBG has been improved significantly with increasing consumption of meat products in last decades. KNBG has conventionally been known as healthy food, with nutritional features superior in other meat sources generally consumed. According to Kim et al. (1993), KNBG has lower cholesterol and saturated fat contents compared to beef and pork. In general, as compared to other ruminants, goat meat has desirable fatty acids with moderately higher proportion of polyunsaturated fatty acids (Mushi et al., 2009).
It is well known that diet leads to alterations in meat quality traits and nutrients, because biological characteristics of muscular tissue can be affected by diet changes (Renand et al., 2001). It has been shown that diet is one of the most important factor influencing carcass yield, cutability and meat qualities of many species (Wood et al., 2008), especially goats (Warmington and Kirton, 1990). Alfalfa (Medicago sativa L.) is valuable grass due to its uniform distribution and high growth rate during the growing season. Alfalfa also has an outstanding nutritive value, leading to tremendous outcomes for feeding lambs (Fraser et al., 2004). Alfalfa is the main pasture legume for numerous species of livestock (Van Keuren and Matches, 1988) and alfalfa hay is often used to supply crude protein in finishing diets for ruminants. It is cultivated in more than 80 countries in an area exceeding 35 million ha (Radović et al., 2009). World production of alfalfa was around 436 million tons in 2006 (FAO, 2006). The cost of high quality of alfalfa bales is US $80–100/Metric Tons.
In general, various kinds of forages are available for small ruminants in relation to finishing system such as energy and protein. Because growing animals require more nutrients than mature animals, the selection of forage species is much important. However, the effect of intensive alfalfa feeding on meat quality and fatty acid profile of KNBG is currently unclear. Therefore, the objective of this study was to assess meat quality characteristics and fatty acid compositions of KNBG finished on intensively-managed alfalfa or conventional feeding of commercial concentrate pellets (CCP) with ad libitum low-energy common grasses in Korea.
Material and Methods
Animals and sampling
Ten KNBG (approximately 12 month of age) were allocated into two groups and subjected to either intensive feeding of alfalfa grasses (ALF: crude protein, 15.3%; fiber, 28.6%) or conventional feeding of CCP (crude protein, 15.15%; crude fat, 3.88%; crude ash, 6.75%; moisture 11.5%; TDN, 68%) with ad libitum low-energy common grasses including rice straw, Italian ryegrass, and Timothy grass. Because the most abundant roughage for ruminants is rice straw in Korea, it was used as major source of low-energy common grass in this experiment. Animals were stratified by two dietary treatments and assigned into five pens for each dietary group. They were slaughtered at 6 months after feeding experiment diets. Feed were provided ad libitum twice daily. Animals were allowed free access to freshwater placed in water buckets.
Goats were slaughtered according to standard protocol at an average live body weight of 48.3±3.8 kg. They were subjected to electrical stunning (about 220 voltages) followed by exsanguination, skinning, evisceration, and washing procedures. The chilling process of carcasses were carried out in chilling room at 2°C for 24 h. Left and right longissimus lumborum (LL) muscles were cut to measure meat quality traits.
Meat quality traits
Meat color was determined by measuring L* (lightness), a*(redness/greenness) and b* (yellowness/blueness) values using a Minolta Chromameter (Minolta CR-300, Japan) that was calibrated with a white plate (Y=93.5, X=0.3132, y=0.3198). An average value of three different locations on the meat surface was used for statistical analysis.
Water-holding capacity (WHC) was evaluated by three methods including the released water (RW) %, drip loss (DL) %, and cooking loss (CL) %, DL % and CL % were determined as described previously (Hwang et al., 2010). RW % was determined by the method of Joo (2018). A meat sample (3.0±0.05 g) was placed on a previously dried and weighed filter paper (Whatman No. 1 of 11 cm of diameter) with two thin plastic films. After weighing them with electrical balance, the filter-paper and plastic film with meat sample were positioned between Plexiglas plates. A load of 2.5 kg and free mechanical force was applied for 5 min. The wet filter paper and plastic films were quickly weighed after precisely removing the compressed meat. The percentage of RW was calculated as follows: RW %=[(Damp filter-paper and plastic films weight)–(filter-paper and plastic films weight)/Meat sample weight]×100.
Warner-Bratzler shear force (WBSF) was carried out for the cooked sample based on AMSA (1995) guideline. Toward parallel into myofiber direction sample core (1.0 cm-diameter) were inserted. The cores were sheared perpendicular to myofiber’s direction using an Instron tensile testing machine (Model 4443, Instron Corp., USA) with a V-shaped shear blade. Ultimate force was obtained using 100 N load cell tension applied at a crosshead speed of 250 mm/min. The full-scale load was 50 kg. Maximum peak force recorded during the test was stated as shear force. The average of five readings was recorded for each sample group.
Chemical analysis
Moisture content and crude ash content were determined by applying the oven-drying method (AOAC, 1995). Crude protein was determined by the Kjeldahl method (AOAC, 1995). Fat content was determined using the Folch method (Folch et al., 1957) as described previously (Hwang et al., 2010).
Collagen content was measured by the method of AOAC (1995). Briefly, a 4 g of meat sample was taken and 30 mL of H2SO4 was added to the sample for hydrolysis at 105°C for 16 h. Water was pour into funnel to reach up to total volume of 500 mL after hydrolysis. A filtered solution of 5 mL was diluted to 100 mL. For measurement of hydroxyproline content, 2 mL diluted solution was taken after adding 1 mL of oxidation solution (50 mM of chloramine-T hydrate, 156 mM citric acid, 375 mM NaOH, 661 mM sodium acetate trihydrate, 29% v/v 1-ptopanol, pH 6.0). Vortexing of samples were carried out and then samples were kept at room temperature for 20 min by adding 1 mL of color reagent. The vortexed samples were covered with aluminum foil and placed in water bath at 60°C for 15 min. Before measuring the absorbance level, the samples were cooled down by holding it to running water for 3 min. The absorbance level was carried out by using UV spectrometer at wavelength of 558 nm. From standard curve (1.2, 2.4, 3.6, and 4.8 μg hydroxyproline per mL of H2O), Hydroxyproline content was calculated. Results were computed by following formula: Total collagen=hydroxyproline×8.
Myoglobin (Mb) concentration was determined by the method of Warriss (1979). Fatty acids composition was determined using a gas chromatograph (HP6890N, Hewlett-Packard, USA) equipped with a HP7683 (Hewlett-Packard) automatic sampler as described previously (Hwang et al., 2010). Atherogenic index was calculated as content ratio of saturated fatty acid (SFA)/unsaturated fatty acid (UFA) using the following formula suggested by Ulbricht and Southgate (1991): AI (atherogenic index)=[C12:0z+4(C 14:0)+C16:0]/[MUFA+PUFA].
Statistical Analysis
Statistical analysis was performed with one-way analysis of variance (one-way ANOVA) by applying SPSS (SPSS 16.0, Chicago, IL, USA). Differences between mean values of two dietary treatments were obtained by Duncan’s multiple range test. Significance was defined at p<0.05. Results were expressed as the least square mean values of three independent replications except that WBSF was recorded as the average of five measurements.
Results and Discussion
Changes in meat quality traits
Meat color measurements of LL muscles from KNBG finished on ALF and CCP are shown in Table 1. There were no significant differences in lightness (L*), redness (a*), or yellowness (b*) between ALF and CCP goats. These results support previous reports that dietary treatments have no effect on meat color (Dransfield et al., 1990). Instead, meat color is more affected by breed (Kadim et al., 2003), slaughter weight (Beriain et al., 2000), and aging (Kadim et al. 2003; Kannan et al., 2006). According to Priolo et al. (2002), feeding of low-energy diets to ruminants causes darker color (lower L* values) of Longissimus muscle as compared to finishing them with high energy diets. Finishing lambs with grass had darker color meat as compared to concentrate fed lambs (Priolo et al., 2002). Realini et al. (2004) have also reported that pasture-fed steers had darker color on the surface of Longissimus muscle in contrast to concentrate-fed steers. However, steers that fed on several combinations of forage-concentrate diets for a short period had similar instrumental color values of Longissimus muscle (French et al., 2001). It is recommended that feeding period should be extended to improve the meat color by diet. In current study, there was no significant difference in myoglobin content in loins between the two groups of goats either. This implies that intensive feeding of alfalfa does not influence myoglobin concentration or meat color of KNBG.
Table 1. Color measurements and myoglobin % of longissimus lumborum muscle from goat finished on intensive feeding of alfalfa (ALF) or conventional feeding of commercial concentrate pellets (CCP) with low-energy common grasses.
| Color traits | ALF | CCP |
|---|---|---|
| Lightness (L*) | 33.37±0.58 | 33.11±0.54 |
| Redness (a*) | 17.41±0.72 | 17.50±0.86 |
| Yellowness (b*) | 1.87±0.23 | 1.77±0.19 |
| Myoglobin (mg/g) | 7.34 ±0.25 | 6.93±0.59 |
Data are presented as least square mean±SE.
As shown in Fig. 1, there was no significant difference in WHC of goat meats between the two groups (ALF and CCP). There were no significant differences in RW % or DL % between the two groups either. Beriain et al. (2000) have reported a higher range of WHC in longissimus dorsi (LD) muscles of lambs by slaughter weight. Kadim et al. (2003) have also reported differences in WHC of different muscles among goat breeds (Batina, Dhofari, and Jabal Akdhar) in Oman. Although several studies reported that WHC of goat meat was influenced by breeds, slaughter weight, animal age, and muscle differences, our data clearly showed that the WHC of KNBG loin was not significantly affected by intensive feeding (alfalfa or CCP for goat).
Fig. 1. Water holding capacity (A: released water %, B: drip loss %) of longissimus lumborum from goat finished on intensive feeding of alfalfa (ALF) or conventional feeding of commercial concentrate pellets (CCP) with low-energy common grasses.
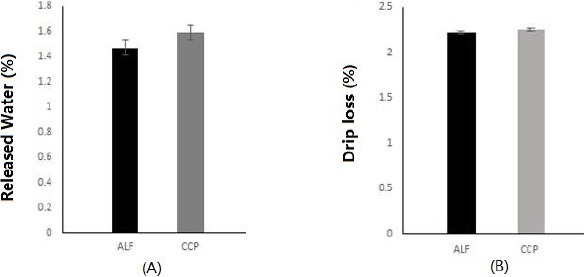
Data are presented as least square mean±SE.
Results of WBSF and collagen content of ALF and CCP goats are presented in Table 2. There were no significant differences in WBSF or collagen content between ALF and CCP goats. This might be due to the lack of significant difference in meat color and WHC parameters between the two treatments. Safari et al. (2009) have reported no changes in CL or shear force among goat fed on different nutritional regimes. Similar results have been reported by Kannan et al. (2006) and Smith et al. (1979). Connective tissue is a crucial element of organisms. It applies structural function as an aggregator to support cells. Meat tenderness is strongly related to collagen content and solubility. Our results clearly showed no difference in WBSF due to no difference in collagen content between ALF and CCP goats. This implies that tenderness or collagen content is not influenced by diet of KNBG. This is similar to results of Van Niekerk and Casey (1988) on Boer goats. Our results showed that meat quality traits including color, WHC, and tenderness were not changed by intensive feeding of alfalfa compared to conventional feeding CCP for KNBG.
Table 2. Shear force and collagen content of longissimus lumborum muscle from goat finished on intensive feeding of alfalfa (ALF) or conventional feeding of commercial concentrate pellets (CCP) with low-energy common grasses.
| Parameters | ALF | CCP |
|---|---|---|
| WBSF(kg/cm2) | 3.92±0.14 | 4.05±0.16 |
| Collagen (%) | 0.67±0.18 | 0.90±0.16 |
Data are presented as least square mean±SE.
Proximate chemical and fatty acid composition
Effects of intensive feeding of alfalfa on proximate chemical composition of KNBG are shown in Table 3. Goat meat did not significantly differ in moisture, crude protein, fat, or ash content between ALF and CCP groups. Generally, the important factors which effect the chemical composition of meat are breed, age, sex, anatomical location of muscle and nutrition (Lawrie, 1998). However, in the current study, the chemical composition of KNBG meat was not influenced by different feeding of diet for 6 months before slaughter. It is normally recognized that alfalfa contains higher crude protein than common grasses (Turner et al., 2014). This designates that intensive feeding of alfalfa could substitute conventional feeding of CCP for KNBG because of its higher protein content. Schmidt et al. (2013) have stated that total ash, protein, or lipid concentration in longissimus muscle does not vary when beef steers are finished on monocultures of alfalfa. Results of proximate chemical analysis of KNBG were similar to those reported by Hopkins-Shoemaker (2006). Animals fed on higher concentrate diet had higher fat content in muscle (French et al., 2001; Geay et al., 2001). Marino et al. (2006) have also reported that the fat content in LD muscle of concentrate fed steers were two times higher compared to pasture-fed steers.
Table 3. Proximate chemical analysis of longissimus lumborum muscle from goat finished on intensive feeding of alfalfa (ALF) or conventional feeding of commercial concentrate pellets (CCP) with low-energy common grasses.
| Parameters | ALF | CCP |
|---|---|---|
| Moisture | 75.82±0.09 | 76.00±0.15 |
| Protein | 21.14±0.07 | 21.06±0.17 |
| Fat | 1.91±0.06 | 1.64±0.08 |
| Ash | 1.13±0.04 | 1.30±0.07 |
Data are presented as least square mean±SE.
Fatty acid compositions of KNBG meat finished on ALF and CCP are summarized in Table 4. Proportions of monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) were significantly (p<0.05) different between ALF and CCP goats. However, proportions of SFA were not significantly different between ALF (43.17%) and CCP (42.92%) in goats. ALF goat had significantly (p<0.05) higher proportion of MUFA than CCP goat mainly due to higher proportion of oleic acid (C18:1). The significantly lower proportion of PUFA in ALF goat was due to lower proportions of linoleic acid (C18:2) and arachidonic acid (C20:4). However, proportion of palmitic acid (C16:0) or stearic acid (C18:0) in ALF goat was not significantly different from that in CCP goat, resulting in no difference in SFA between ALF and CCP goats. All these results suggest that fatty acid composition of KNBG meat could be strongly influenced by intensive feeding of alfalfa.
Table 4. Fatty acid profiles of longissimus lumborum muscle from goat finished on intensive feeding of alfalfa (ALF) or conventional feeding of commercial concentrate pellets (CCP) with low-energy common grasses.
| Fatty acids | ALF | CCP | SEM | p-level |
|---|---|---|---|---|
| C12:0 | 0.08 | 0.08 | 0.006 | 0.3466 |
| C14:0 | 2.00A | 1.78B | 0.09 | 0.0061 |
| C14:1 | 0.13 | 0.10 | 0.02 | 0.1281 |
| C16:0 | 23.89 | 22.83 | 2.07 | 0.4398 |
| C16:1 | 3.26A | 2.89B | 0.192 | 0.01 |
| C18:0 | 17.12 | 18.16 | 1.59 | 0.3773 |
| C18:1 n9 | 44.58A | 41.00B | 1.240 | 0.001 |
| C18:2 n6 | 4.56B | 6.00A | 0.360 | 0.002 |
| C18:3 n3 | 1.71 | 2.05 | 0.51 | 0.3227 |
| C20:0 | 0.08 | 0.08 | 0.01 | 0.7422 |
| C20:4 n6 | 1.61B | 3.35A | 0.522 | 0.0008 |
| C20:5 n3 | 0.73B | 1.47A | 0.371 | 0.0137 |
| C22:6 n3 | 0.10B | 0.21A | 0.061 | 0.0186 |
| SFA | 43.17 | 42.92 | 1.129 | 0.7314 |
| MUFA | 47.97A | 44.00B | 1.30 | 0.0014 |
| PUFA | 8.86B | 13.08A | 1.346 | 0.001 |
| PUFA/SFA | 0.21B | 0.30A | 0.035 | 0.002 |
| n6 | 6.17B | 8.12A | 1.780 | 0.1222 |
| n3 | 2.53B | 3.73A | 0.805 | 0.0463 |
| AI | 0.57 | 0.53 | 0.092 | 0.142 |
A,B Treatment means within a row with different letters are different at the p level indicated. SFA, saturated fatty acids, MUFA, monounsaturated fatty acids, MUFA, polyunsaturated fatty, AI, atherogenic index.
According to Webb et al. (2005) and Marino et al. (2006), the meat obtained from pasture-fed animals consist equivalent quantity of SFA, a lower amount of MUFA and higher content of PUFA in comparison to concentrate-fed animals. Fraser et al. (2004) reported the similar amount of fatty acid in comparison to present study, showing that C18:1, C16:0, and C18:0 were the majority of fatty acids in longissimus muscle from lambs finished on various kinds of grasses including alfalfa. Solaiman et al. (2011) have also reported that C18:1, C16:0, and C18:0 are the major fatty acids in longissimus muscle of Boer goat kids fed on annual ryegrass. In the present study, proportions of C18:1, C16:0, and C18:0 in LL muscles from ALF goat were 44.58%, 23.89%, and 17.12%, respectively. These results suggest that prominent fatty acids in KNBG meat might not change with intensive feeding of alfalfa. However, our data showed that intensive feeding of alfalfa might improve the palatability of KNBG because oleic acid (C18:1 cis-9) and stearic acid (C18:0) were the major constituents of intramuscular fat in goats (Rhee et al., 2000).
Proportion of most PUFA in ALF goat were significantly (p<0.05) lower than those in CCP goat. Particularly, proportions of linoleic acid (C18:2) and arachidonic acid (C20:4) as prominent n-6 PUFA were significantly (p<0.05) lower in ALF goat. However, there was no significant (p>0.05) difference in proportion of linolenic acid (C18:3), the prominent n-3 PUFA, between ALF and CCP goats. These differences in fatty acid compositions might be due to different crude protein and TDN contents in goat diet. Alteration in fatty acid compositions could be attributed to changes in microbial distribution in the rumen due to increased crude protein and TDN level (Majdoub-Mathlouthi et al., 2013). Our data suggest that desirable composition of fatty acids for human health could be obtained by intensive feeding with alfalfa to decrease n-3 and n-6 PUFA simultaneously. In addition, our results clearly showed that AI was not significantly changed by intensive feeding of alfalfa.
Conclusions
KNBG finished on ALF and CCP had similar meat quality traits including color measurements, myoglobin content, WHC measurements, WBSF, and collagen content. Proximate chemical compositions of ALF goat were not significantly different from that those of CCP goat either. However, proportions of MUFA and PUFA were significantly different between ALF and CCP goats. Especially, the proportion of oleic acid was higher in ALF goat whereas proportions of linoleic and arachidonic acids were higher in CCP goat. These results suggest that KNBG finished with intensive feeding of alfalfa could produce goat meat with desirable fatty acids for human diets.
Acknowledgements
This research was supported by Cooperative Research Program for Agriculture Science and Technology Development (Project PJ01375601) from the Korean Rural Development Administration.
References
- AOAC. Official methods of analysis of AOAC. Association of Official Analytical Chemists. 1995 [Google Scholar]
- American Meat Science Association [AMSA] Research guidelines for cookery, sensory evaluation and instrumental tenderness measurements of fresh meat. American Meat Science Association in Cooperation with National Live Stock and Meat Board; Chicago, IL, USA: 1995. p. 8. [Google Scholar]
- Beriain MJ, Horcada A, Purroy A, Lizaso G, Chasco J, Mendizabal JA. Characteristics of Lacha and Rasa Aragonesa lambs slaughtered at three live weights. J Anim Sci. 2000;78:3070–3077. doi: 10.2527/2000.78123070x. [DOI] [PubMed] [Google Scholar]
- Dransfield E, Nute GR, Hogg BW, Walters BR. Carcass and eating quality of ram castrated ram and ewe lambs. Anim Sci. 1990;50:291–299. doi: 10.1017/S0003356100004748. [DOI] [Google Scholar]
- FAO. FAOSTAT. Food and Agriculture Organization of the United Nations. 2006 [Google Scholar]
- Fraser MD, Speijers MH M, Theobald VJ, Fychan R, Jones R. Production performance and meat quality of grazing lambs finished on red clover, lucerne, or perennial ryegrass swards. Grass Forage Sci. 2004;59:345–356. doi: 10.1111/j.1365-2494.2004.00436.x. [DOI] [Google Scholar]
- French P, O’Riordan EG, Monahan FJ, Caffrey PJ, Mooney MT, Troy DJ. The eating quality of meat of steers fed grass and or concentrates. Meat Sci. 2001;57:379–386. doi: 10.1016/S0309-1740(00)00115-7. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Geay Y, Bauchart D, Hocquette JF, Culioli J. Effect of nutritional factors on biochemical, structural and metabolic characteristics of muscles in ruminants, consequences on dietetic value and sensorial qualities of meat. Reprod Nutr Dev. 2001;41:1–26. doi: 10.1051/rnd:2001108. [DOI] [PubMed] [Google Scholar]
- Hopkins-Shoemaker CE. Evaluation of sustainable forage systems for meat goat production in the Southern United States. Ph.D. dissertation, Auburn Univ.; Auburn, AL, USA: 2006. [Google Scholar]
- Hwang YH, Kim GD, Jeong JY, Hur SJ, Joo ST. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 2010;86:456–461. doi: 10.1016/j.meatsci.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Joo ST. Determination of water-holding capacity of porcine musculature based on released water method using optimal load. Korean J Food Sci An. 2018;38:823–828. doi: 10.5851/kosfa.2018.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadim IT, Mahgoub O, Al-Ajmi DS, Al-Maqbaly RS, Al-Saqri NM, Ritchie A. An evaluation of the growth, carcass and meat quality characteristics of Omani goat breeds. Meat Sci. 2003;66:203–210. doi: 10.1016/S0309-1740(03)00092-5. [DOI] [PubMed] [Google Scholar]
- Kannan G, Gadiyaram KM, Galipalli S, Carmichael A, Kouakou B, Pringle TD, McMillin KW, Gelaye S. Meat quality in goats as influenced by dietary protein and energy levels, and postmortem aging. Small Rumin Res. 2006;61:45–52. doi: 10.1016/j.smallrumres.2005.01.006. [DOI] [Google Scholar]
- Kim JO, Kim MN, Ha YL. Processing of Korean black goat meat to remove goaty flavor. Food Sci Biotechnol. 1993;2:26–29. [Google Scholar]
- Lawrie RA. Meat science. 6th ed. CRC Press; Cambridge, England: 1998. [Google Scholar]
- Majdoub-Mathlouthi L, Saïd B, Say A, Kraiem K. Effect of concentrate level and slaughter body weighton growth performances, carcass traits and meat quality of Barbarine lambs fed oat hay based diet. Meat Sci. 2013;93:557–563. doi: 10.1016/j.meatsci.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Marino R, Albenzio M, Girolami A, Muscio A, Sevi A, Braghieri A. Effect of forage to concentrate ratio on growth performance, and on carcass and meat quality of Podolian young bulls. Meat Sci. 2006;7:415–424. doi: 10.1016/j.meatsci.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Mushi DE, Safari J, Mtenga LA, Kifaro GC, Eik LO. Effects of concentrate levels on fattening performance, carcass and meat quality attributes of Small East African×Norwegian crossbred goats fed low quality grass hay. Livest Sci. 2009;124:148–155. doi: 10.1016/j.livsci.2009.01.012. [DOI] [Google Scholar]
- Priolo A, Micol D, Agabriel J, Prache S, Dransfield E. Effect of grass or concentrate feeding systems on lamb carcass and meat quality. Meat Sci. 2002;62:179–185. doi: 10.1016/S0309-1740(01)00244-3. [DOI] [PubMed] [Google Scholar]
- Radović J, Sokolović D, Marković J. Alfalfa-most important perennial forage legume in animal husbandry. Biotec Anim Husban. 2009;25:465–475. doi: 10.2298/BAH0906465R. [DOI] [Google Scholar]
- Realini CE, Duckett SK, Brito GW, Dalla Rizza M, De Mattos D. Effect of pasture vs. concentrate feeding with or without antioxidants on carcass characteristics, fatty acid composition, and quality of Uruguayan beef. Meat Sci. 2004;66:567–577. doi: 10.1016/S0309-1740(03)00160-8. [DOI] [PubMed] [Google Scholar]
- Renand G, Picard B, Touraille C, Berge P, Lepetit J. Relationship between muscle characteristics and meat quality traits of young Charolais bulls. Meat Sci. 2001;59:49–60. doi: 10.1016/S0309-1740(01)00051-1. [DOI] [PubMed] [Google Scholar]
- Rhee KS, Waldron DF, Ziprin YA, Rhee KC. Fatty acid composition of goat diets vs intramuscular fat. Meat Sci. 2000;54:313–318. doi: 10.1016/S0309-1740(99)00094-7. [DOI] [PubMed] [Google Scholar]
- Safari J, Mushi DE, Mtenga LA, Kifaro GC, Eik LO. Effects of concentrate supplementation on carcass and meat quality attributes of feedlot finished small east Africa goats. Livest Sci. 2009;125:266–274. doi: 10.1016/j.livsci.2009.05.007. [DOI] [Google Scholar]
- Schmidt JR, Miller MC, Andrae JG, Ellis SE, Duckett SK. Effect of summer forage species grazed during finishing on animal performance, carcass quality and meat quality. J Anim Sci. 2013;91:4451–4461. doi: 10.2527/jas.2012-5405. [DOI] [PubMed] [Google Scholar]
- Smith ME, Kastner CL, Hunt MC, Kropf DH, Allen DM. Elevated conditioning temperature effects on beef carcasses from four nutritional regimes. J Food Sci. 1979;44:158–163. doi: 10.1111/j.1365-2621.1979.tb10031.x. [DOI] [Google Scholar]
- Solaiman SG, Kerth CR, Willian K, Min BR, Shoemaker C, Jones W, Bransby D. Growth performance, carcass characteristics and meat quality of boer-cross wether and buck goats grazing marshall ryegrass. Aisan-Australas J Anim Sci. 2011;24:351–357. doi: 10.5713/ajas.2011.10081. [DOI] [Google Scholar]
- Turner KE, Belesky DP, Cassida KA, Zerby HN. Carcass merit and meat quality in Suffolk lambs, Katahdin lambs, and meat-goat kids finished on agrass-legume pasture with and without supplementation. Meat Sci. 2014;98:211–219. doi: 10.1016/j.meatsci.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Ulbricht TLV, Southgate DAT. Coronary heart diseases: Seven dietary factors. Lancet. 1991;338:985–992. doi: 10.1016/0140-6736(91)91846-M. [DOI] [PubMed] [Google Scholar]
- Van Keuren RW, Matches GC. Pasture production and utilization. Alfalfa Alfalfa Improv. 1988;29:515–538. [Google Scholar]
- Van Niekerk WA, Casey NH. The Boer goat. II. Growth, nutrient requirements, carcass and meat quality. Small Rumin Res. 1988;1:355–368. doi: 10.1016/0921-4488(88)90061-2. [DOI] [Google Scholar]
- Warmington BG, Kirton AH. Genetic and non-genetic influence on growth and carcass traits of goats. Small Rumin Res. 1990;3:147–165. [Google Scholar]
- Warriss PD. The extraction of haem pigments from fresh meat. Int J Food Sci Technol. 1979;14:75–80. doi: 10.1111/j.1365-2621.1979.tb00849.x. [DOI] [Google Scholar]
- Webb EC, Casey NH, Simela L. Goat meat quality. Small Rumin Res. 2005;60:153–166. doi: 10.1016/j.smallrumres.2005.06.009. [DOI] [Google Scholar]
- Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, Hughes SI, Whittington FM. Fat deposition, fatty acid composition and meat quality. A review. Meat Sci. 2008;78:343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]


