Genetic tools are needed to examine gene expression in the pathogen Leptospira interrogans. We developed a reporter plasmid that replicates in L. interrogans with green fluorescent protein (GFP) as the readout of promoter activity. We demonstrated an application of the new reporter plasmid by identifying an upstream element responsible for the poor basal expression of the sph2 sphingomyelinase gene in an L. interrogans serovar Lai strain. This new tool is useful for the discovery of the molecular determinants of L. interrogans gene expression.
KEYWORDS: Leptospira, gene expression, leptospirosis, reporter gene, sphingomyelinase, transcription
ABSTRACT
Many strains of the spirochete Leptospira interrogans serovar Pomona express the osmotically inducible sphingomyelinase gene sph2 at much higher levels than strains from other serovars. We developed a new green fluorescent protein (GFP) reporter plasmid to examine sph2 gene expression determinants. The vector enables the fusion of the test promoter to the ribosome-binding site and coding region of gfp. We fused the sph2 promoters from the L. interrogans serovar Lai strain 56601 and from the L. interrogans serovar Pomona strain LC82-25 to gfp to examine the molecular determinants of differential sph2 expression between the two strains. Similar to what was observed with the native sph2 genes, the introduction of the plasmids into the Lai 56601 strain resulted in near background levels of gfp expression from the Lai sph2 promoter, while the expression from the Pomona sph2 promoter was high. The expression of both fusions increased at physiologic levels of osmolarity achieved by adding sodium chloride to the culture medium. We examined the role of a 17-bp upstream element found in all L. interrogans strains expressing low basal levels of sph2 and missing from Pomona strains that express sph2 at high levels. When the 17-bp sequence present upstream of the Lai sph2 promoter was deleted or scrambled, the fusion expression increased substantially. Conversely, the insertion of the 17-bp sequence upstream of the Pomona sph2 promoter diminished fusion expression. In contrast, the removal of an insertion sequence-like element that is found only in the Pomona sph2 upstream sequence had no effect on the expression from the Pomona sph2 fusion in the Lai strain. These findings demonstrate the utility of the gfp reporter plasmid in analyzing gene expression in L. interrogans.
IMPORTANCE Genetic tools are needed to examine gene expression in the pathogen Leptospira interrogans. We developed a reporter plasmid that replicates in L. interrogans with green fluorescent protein (GFP) as the readout of promoter activity. We demonstrated an application of the new reporter plasmid by identifying an upstream element responsible for the poor basal expression of the sph2 sphingomyelinase gene in an L. interrogans serovar Lai strain. This new tool is useful for the discovery of the molecular determinants of L. interrogans gene expression.
INTRODUCTION
Most pathogenic members of the genus Leptospira have a bimodal lifestyle. In one phase, leptospires live in moist soil and freshwater bodies located throughout the world (1). At other times, they invade vertebrate hosts to permanently colonize the renal tubules, resulting in long-term shedding into the environment by these carriers via their urine (2). The adaptation to these diverse conditions requires the regulation of large numbers of specialized genes, consistent with the results from whole-genome transcriptional array and sequencing studies of L. interrogans incubated under conditions simulating those encountered during its life cycle (3–8). Additionally, different serovars of Leptospira are generally associated with specific hosts (9), which may reflect adaptation by differential expression of the same genes in different serovars of Leptospira.
One example of a gene expressed at dissimilar levels in different serovars is sph2, which encodes an enzyme responsible for most, if not all, of the sphingomyelinase activity of L. interrogans (10). L. interrogans expresses sph2 in dialysis membrane chambers implanted into the peritoneal cavities of rats. Additionally, antisera raised against Sph2 detect the antigen in kidney sections from acutely infected hamsters (11) and in urine obtained from leptospirosis patients (12). Sph2 may contribute to the pathogenesis of leptospirosis by adhering to fibronectin, inducing apoptosis of host cells, and triggering the production of proinflammatory cytokines (13–16). Most members of L. interrogans serovar Pomona examined to date produce large amounts of sph2 transcript and Sph2 protein, whereas a strain of L. interrogans serovar Lai produces smaller amounts of transcript and undetectable levels of Sph2 during growth in conventional culture medium (10, 11). Strains of L. interrogans serovars Copenhageni and Manilae also produce negligible amounts of Sph2 (10).
The genetic basis for the increased expression of sph2 in Pomona strains is unknown. A molecular epidemiology study with PCR primers encompassing an insertion sequence (IS)-like element located upstream of sph2 found that the element is widespread among Pomona subtype kennewicki strains (17). The IS-like element is missing from the sph2 upstream sequences of the four L. interrogans strains shown to produce low basal levels of Sph2 (10). For these reasons, we hypothesized that this element is responsible for the high levels of Sph2 produced in the Pomona strains (10). However, it should be noted that this hypothesis was based on observations of a single Pomona strain (10), and Sph2 was not detected in another Pomona strain that has the IS-like element upstream of the coding region (13). Whether other Pomona strains that produce high levels of Sph2 harbor the IS-like element is unknown (11).
Several reporter constructs have been developed for studying the expression of L. interrogans genes such as sph2. Osmotic induction of the ligA-ligB and sph2 L. interrogans promoters was demonstrated by genetic fusions to gfp in the nonpathogen Leptospira biflexa (18). The kdp promoter was fused to the 5′ end of the L. biflexa β-galactosidase gene bgaL and integrated by homologous recombination into the endogenous bgaL gene to create a model system for demonstrating positive regulation by KdpE of L. interrogans (19). The β-galactosidase gene from Geobacillus stearothermophilus, bgaB, was fused downstream of the lig promoter and 5′ untranslated region (UTR) to examine expression in Escherichia coli. A mutational analysis of the 5′ UTR showed that the secondary structure sequesters the ribosome-binding site (20). In each of these cases, the regulation of pathogenic gene expression was examined in a surrogate host, because a plasmid able to replicate in L. interrogans was not available. Promoter fusions to gfp or luciferase genes have been introduced into L. interrogans by transposition (21–23). However, a mutational analysis of promoters fused to the reporter is not possible with the transposon-based system; the random insertion of the transposon carrying the fusion complicates the interpretation of expression levels, because the location of the transposon in the chromosome may influence fusion expression. To overcome this limitation, we took advantage of the fact that several replicative plasmids for L. interrogans have recently been developed (24, 25). For example, pMaORI was constructed by cloning the rep and partition loci from phage-like sequences integrated in the Leptospira mayottensis genome (24). pMaORI has been shown to be stable in the absence of antibiotic selection in seven strains of L. interrogans, the nonpathogen L. biflexa, and the intermediate species Leptospira fainei and Leptospira licerasiae. Here, we adapted the pMaORI plasmid to create a new gfp reporter vector that can be used to examine gene expression in L. interrogans. We used the gfp reporter construct to identify a sequence responsible for the low basal levels of sph2 expression in a Lai strain of L. interrogans compared to that in a Pomona strain that produces large amounts of Sph2.
RESULTS
Previously, strain LC82-25 was the only L. interrogans serovar Pomona strain with an IS-like element upstream of sph2 shown to produce high levels of Sph2 (10). For this reason, we tested two additional Pomona strains with IS-like elements for Sph2 production by Western blotting (Fig. 1). Both the RM211 and P10637-46 strains were isolated from pigs. The immunoblot shows that both strains, like the LC82-25 strain, produced high levels of Sph2 when incubated in EMJH medium. The levels of Sph2 were much higher than that produced by the L. interrogans serovar Manilae strain L495 grown in EMJH medium with sodium chloride added to achieve a physiologic osmolarity.
FIG 1.
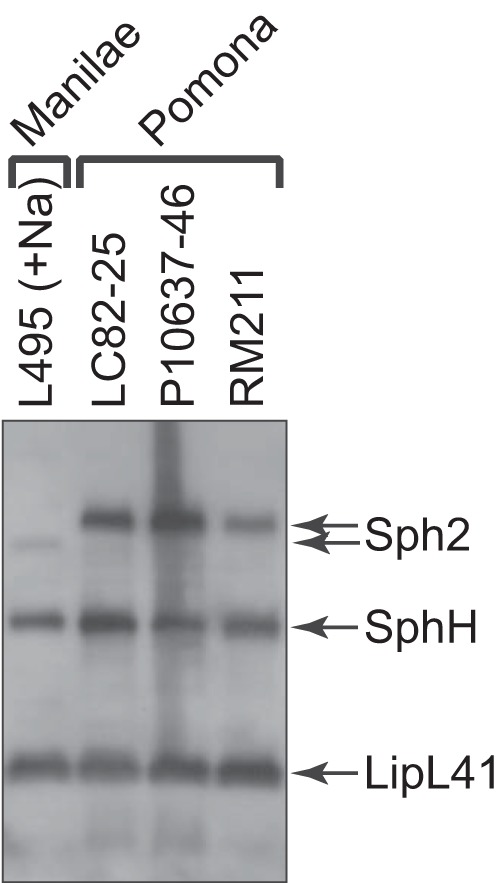
Western blot analysis of Sph2 production from three strains of L. interrogans serovar Pomona. Western blots from three L. interrogans Pomona strains grown in EMJH medium were probed with Sph2 and LipL41 antisera. The Sph2 antiserum cross-reacts with SphH. L. interrogans serovar Manilae strain L495 was grown in EMJH medium supplemented with 120 mM sodium chloride, and its lysate was included for the comparison of Sph2 levels. Sph2 produced by the L495 strain has a slightly lower molecular weight than Sph2 produced by Pomona strains.
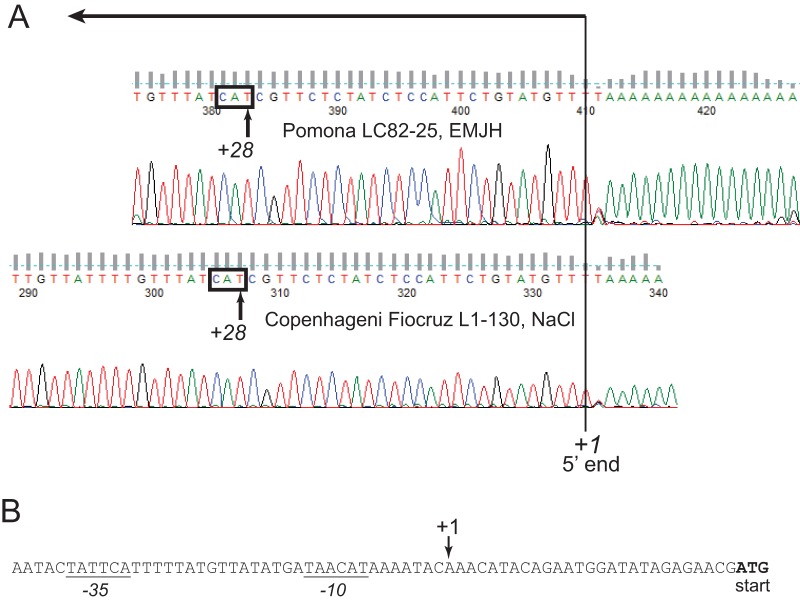
To determine whether transcription from within or adjacent to the IS-like element accounted for the high basal expression of sph2 in the Pomona LC82-25 strain, we mapped the major 5′ end of the sph2 transcript by 5′ rapid amplification of cDNA ends (RACE) (Fig. 2A). The trace shows a single 5′ end located 28 nucleotides from the sph2 start codon in both L. interrogans strain LC82-25 grown in EMJH medium and strain Fiocruz L1-130 grown at physiologic osmolarity (Fig. 2A). The mixture of the four nucleotides positioned next to the 5′ end likely stems from the terminal transferase activity of some reverse transcriptases (26). The 5′ end of the sph2 transcript was found to be located downstream of putative −10 and −35 E. coli-like promoter sequences (Fig. 2B).
FIG 2.
Mapping the major 5′ end of sph2 by 5′ RACE. (A) The 5′ ends of the sph2 transcripts from L. interrogans LC82-25 grown in EMJH medium (top) and L. interrogans Fiocruz L1-130 grown in EMJH medium with 80 mM sodium chloride (bottom) were mapped by 5′ RACE. Electropherograms from sequencing reactions of the amplicons are shown. The peaks representing the major 5′ ends of the sph2 transcripts are bisected by a vertical line. The sequence complementary to the start codon is boxed. The direction of transcription is indicated by the long arrow. (B) The nucleotide sequence around the major 5′ end of sph2 is shown. The start codon is shown in bold, and the predicted −10 and −35 promoter elements are underlined.
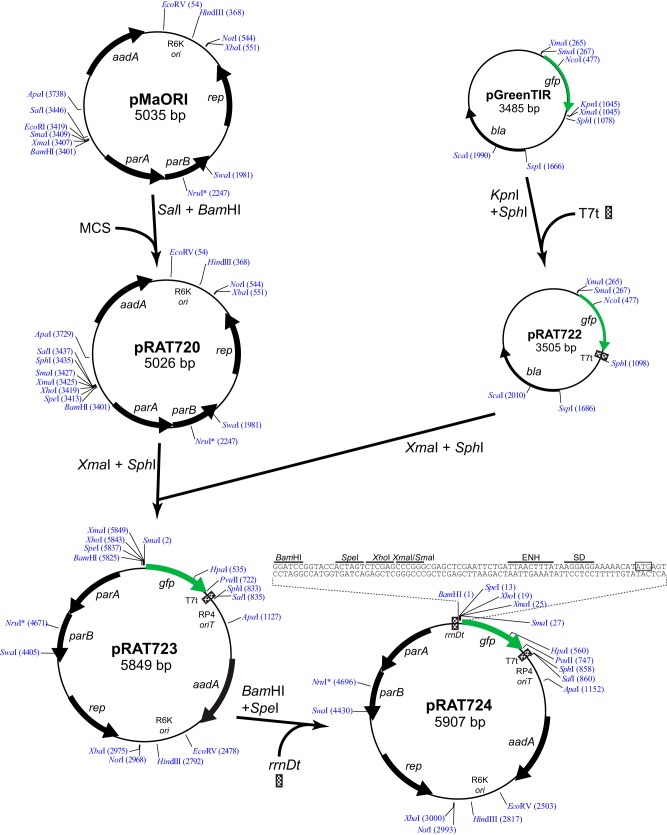
We next constructed a gfp reporter plasmid to examine sph2 gene expression in L. interrogans. We selected the gfp allele demonstrated previously to produce functional green fluorescent protein (GFP) in L. biflexa when fused to leptospiral promoters (18, 21). The gfp allele includes the S65T “red-shift” mutation and the F64L mutation that increases protein solubility (27), as well as a Shine-Dalgarno sequence complementary to the 3′ end of the L. interrogans 16S rRNA. To construct the reporter plasmid, we cloned the gfp gene, along with its translation initiation region, into the plasmid pMaORI, an RP4-based mobilizable plasmid that replicates in L. interrogans (24). Four unique restriction sites were available for cloning promoters upstream of gfp (Fig. 3). A T7 transcription terminator was inserted downstream of gfp to create pRAT723 (Fig. 3).
FIG 3.
Construction of L. interrogans gfp reporter plasmid. The thick arrows denote important open reading frames. The restriction sites shown are unique except for the XmaI sites of pGreenTIR. The numbers next to restriction enzyme names refer to the nucleotide positions after the cleavage site. The transcription terminators and a multicloning site were inserted as double-stranded oligonucleotides with overhangs compatible with the ends of the restriction-digested parent plasmid DNA. aadA, spectinomycin resistance; parA and parB, partition loci; rep, replication initiator; ENH, T7 gene 10 translational enhancer; SD, Shine-Dalgarno sequence; T7t, early T7 transcription terminator; rrnDt, E. coli rrnD transcription terminator; RP4 oriT, RP4 origin of transfer; R6K ori, R6K origin of replication; MCS, multicloning site; *, restriction site subject to dam methylation.
To minimize the transcription of gfp initiating from cryptic vector promoters upstream of gfp, an rrnD transcription terminator was placed upstream of the multicloning site (Fig. 3). E. coli transformed with pRAT723, which lacked the transcription terminator, or pRAT724, which possessed the terminator, was collected by centrifugation of liquid cultures to examine the color of the cell pellet. To quantify the terminator's activity in L. interrogans, pRAT723 and pRAT724 were transformed into an E. coli strain harboring the RP4 conjugation machinery and transferred by conjugation into the L. interrogans serovar Lai 56601 strain. Cultures were grown from four colonies of each transconjugant and were processed for fluorescence measurements. The rrnD terminator reduced GFP production by ∼50% in both L. interrogans strains (Fig. 4). The GFP levels in the absence of the rrnD terminator were similar to the levels produced from the L. interrogans lipL41 promoter (Fig. 4).
FIG 4.
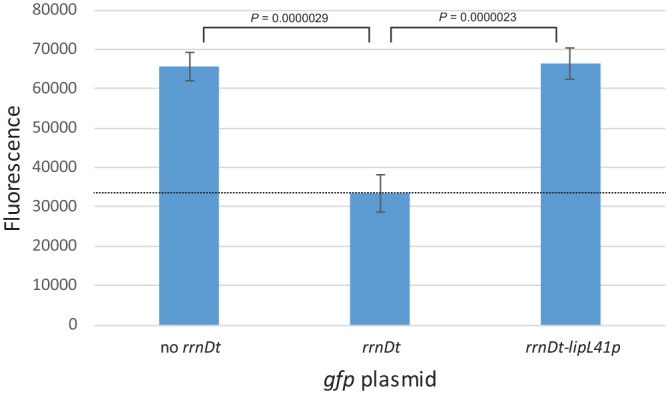
Effect of a transcriptional terminator on background gfp expression. Fluorescence measurements (arbitrary units) of L. interrogans containing plasmids with and without the E. coli rrnD transcription terminator upstream of gfp are shown. The level of GFP produced from a lipL41p-gfp fusion with an upstream rrnD transcriptional terminator is shown for comparison. The dashed line indicates the mean background reading obtained with L. interrogans carrying the empty gfp fusion vector pRAT724. Means ± standard deviations are plotted (n = 4). P values were calculated by one-way analysis of variance (ANOVA) with Tukey's honestly significant difference (HSD) post hoc test.
The sph2 transcript synthesized by the Lai 56601 strain is likely to have the same 5′ end as the sph2 transcript produced by the Fiocruz L1-130 strain, as the sequences upstream of the coding regions in the two strains are nearly identical. On the basis of the 5′ RACE result, we cloned the promoter regions of sph2 from the Lai 56601 and Pomona LC82-25 strains upstream of gfp in pRAT724. The sph2 sequence from the Lai strain (sph2pLai) comprised nucleotides −298 through +3 relative to the major 5′ end of the transcript. The sph2 promoter fragment cloned from the Pomona strain (sph2pPom) had 5′ and 3′ ends identical to those of the Lai fragment and included the IS-like element. The lipL41 promoter was also cloned into pRAT724. The resulting plasmids carrying the sph2p-gfp and lipL41p-gfp fusions, along with pRAT724, were introduced into the Lai and Pomona strains by conjugation. The four plasmids were also introduced into the nonpathogen L. biflexa, which lacks the sph2 gene.
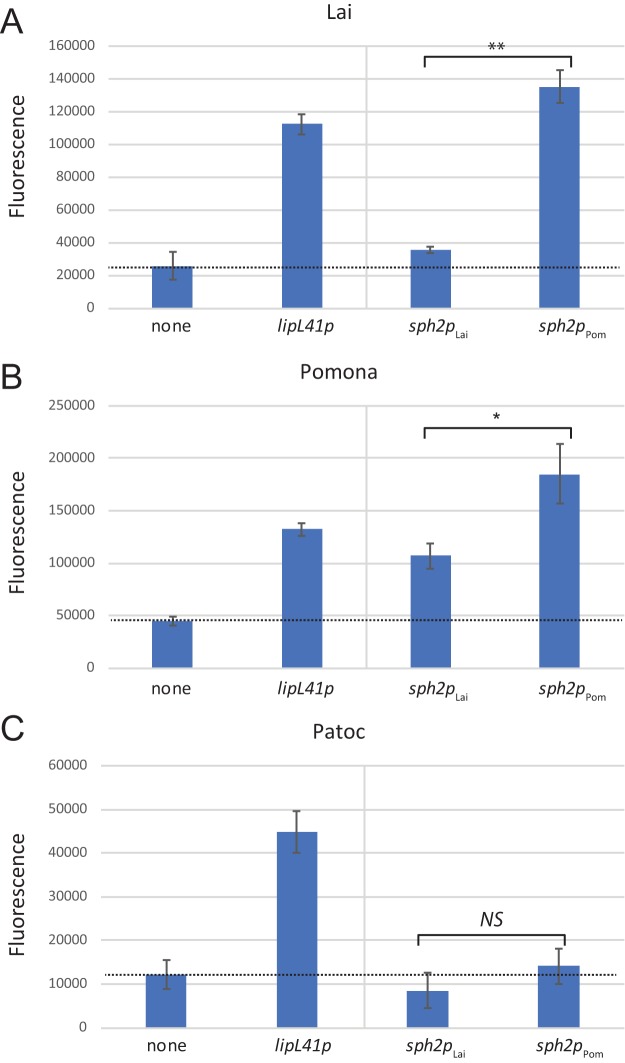
As shown in Fig. 5A, the gfp expression from the fusions in the Lai strain reflected the expression observed with native sph2 in the Lai and Pomona strains in a previous study (10). The GFP levels from the sph2pLai-gfp fusion in the Lai strain incubated in EMJH medium were near background levels. The expression from the sph2pPom-gfp fusion was much higher than from the sph2pLai-gfp fusion (Fig. 5A). The same two fusions were also examined in the Pomona strain. In contrast to its activity in the Lai strain, the sph2pLai-gfp fusion produced large amounts of GFP in the Pomona strain, although not as much as the sph2pPom-gfp fusion (Fig. 5B). These observations suggest that the activity of the (Lai) sph2 promoter was being suppressed in the Lai strain. Neither of the sph2 promoter fusions produced GFP above background levels in L. biflexa (Fig. 5C). GFP fluorescence was detected with the control lipL41p-gfp fusion in all three strains (Fig. 5).
FIG 5.
GFP production (arbitrary units) from sph2p-gfp fusions in L. interrogans and L. biflexa. Fusion plasmids were introduced into L. interrogans Lai strain 56601 (A), L. interrogans Pomona strain LC82-25 (B), and L. biflexa strain Patoc I (C) and incubated in EMJH medium with spectinomycin. The dashed lines indicate the mean background readings obtained with transconjugants carrying the promoterless gfp fusion vector pRAT724 (none). *, P < 0.05; **, P < 0.01; NS, not significant by Welch's t tests.
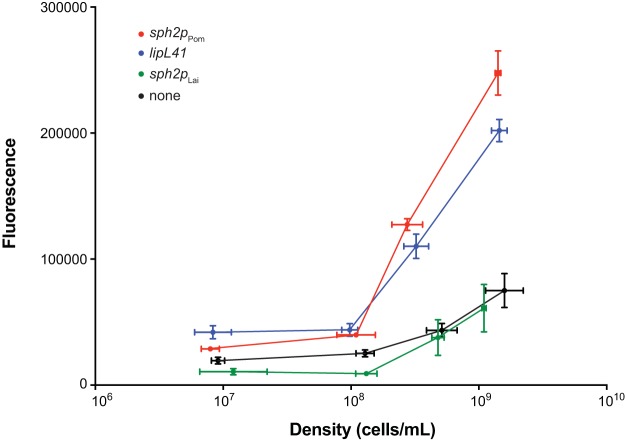
We further examined the activities of the promoters in the Lai strain under different conditions of growth. To examine fusion expression at different cell densities, cultures of the four Lai fusion strains were started at a low cell density. Once the density reached 1 × 107 cells/ml, the GFP production from the fusion strains was examined daily until early stationary phase (2 × 109 cells/ml) was reached (Fig. 6). Prior to fluorescence measurement, the densities of all cultures were adjusted to a uniform optical density. The GFP readings for all fusions, including the promoterless control, rose as the cultures reached stationary phase, although the rise was slightly greater for the sph2 promoter from the Pomona strain and the lipL41 promoter (Fig. 6). Microscopic observations revealed that cells taken at early stationary phase were noticeably shorter than cells sampled at earlier time points. Notably, the GFP level from the Lai sph2 promoter fusion never rose above background levels (Fig. 6).
FIG 6.
GFP production (arbitrary units) from sph2p-gfp fusions in L. interrogans at different cell densities. Three cultures of each of the transconjugants harboring the fusion plasmids were sampled daily starting from a cell density of 1 × 107 cells/ml. The cells were collected by centrifugation and resuspended in PBS to an OD420 of 0.4 prior to fluorescence measurement. Means ± standard deviations (n = 3) of cell counts (horizontal) and fluorescence readings (vertical) are shown.
We next determined whether the increase in native Sph2 levels observed when L. interrogans is shifted from EMJH medium to EMJH medium with sodium chloride added to achieve physiologic osmolarity (10) is also observed with the sph2 fusions. The fusion expression was higher when the transconjugants harboring the sph2p-gfp constructs were grown in EMJH medium with sodium chloride added to achieve physiologic osmolarity (Fig. 7). The sph2pPom-gfp fusion generated high levels of GFP in the Lai strain, and the expression was even higher at physiologic osmolarity (Fig. 7). As expected, the expression from the control lipL41p-gfp fusion was not affected by the sodium chloride supplement (Fig. 7).
FIG 7.
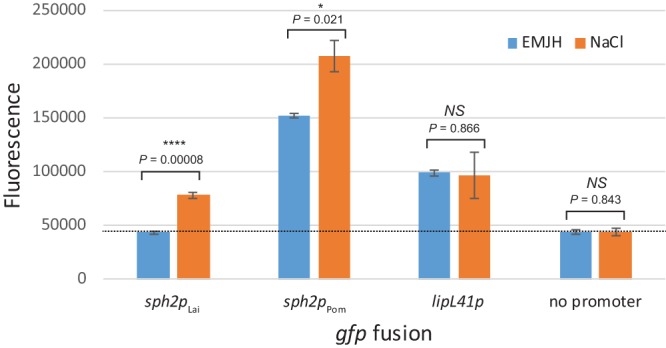
Regulation of sph2p-gfp fusion expression by sodium chloride. Fusion plasmids were introduced into L. interrogans Lai strain 56601. Fluorescence (arbitrary units) was measured from transconjugants maintained in EMJH medium (EMJH) or EMJH medium with 120 mM sodium chloride (NaCl) for 4 h. Means ± standard deviations are plotted (n = 3). *, P < 0.05; ****, P < 0.0001; NS, not significant by Welch's t tests.
To determine whether the IS-like element in the promoter region of sph2 of the Pomona strain is responsible for its high basal level of expression, the IS-like element (Fig. 8A) was precisely deleted from the sph2 promoter region in the Pomona strain, and the promoter variant was fused to gfp. Surprisingly, the GFP assay showed that the removal of the IS-like element had no significant effect on the expression of the sph2 promoter fusion when examined in the Lai strain incubated in EMJH medium and in EMJH supplemented with sodium chloride (Fig. 8B).
FIG 8.
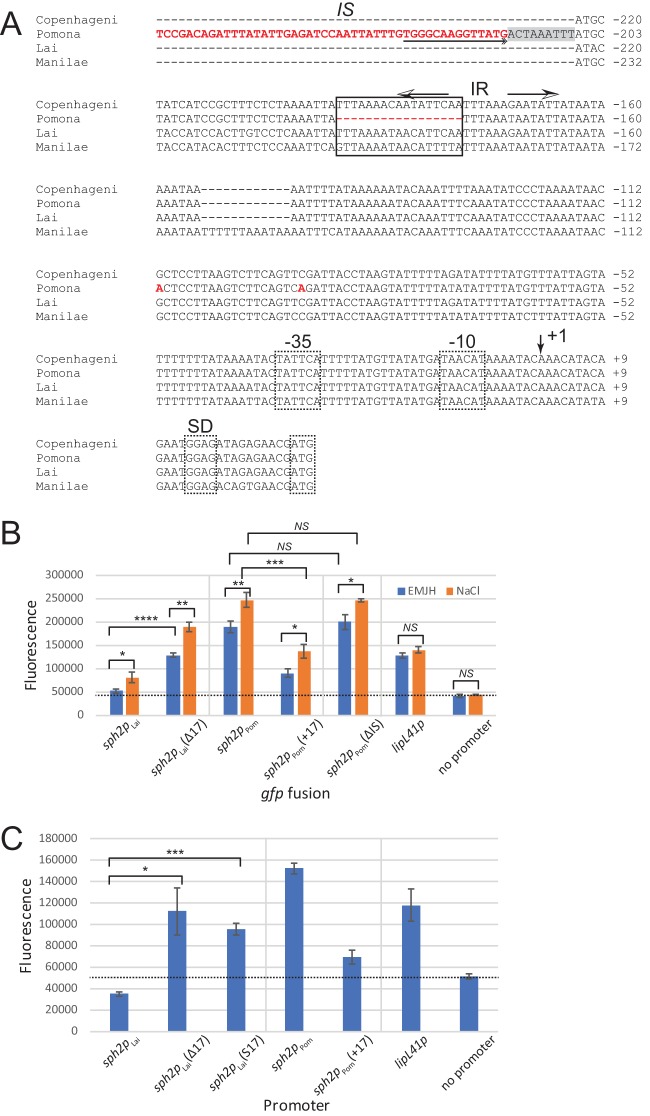
Effect of mutations introduced upstream of the sph2 promoter. (A) Multisequence alignment. Sequences upstream of sph2 from L. interrogans strains Fiocruz L1-130 (Copenhageni), LC82-25 (Pomona), 56601 (Lai), and L495 (Manilae) were aligned with Kalign. The aligned sequences extend from the 5′ ends of the promoter fragments cloned into pRAT724 to the start codon of sph2. Most of the IS-like sequences and sequences further upstream were omitted from the figure for brevity. The sequence and nucleotides unique to the Pomona LC82-25 are shown in red. An outside end of the IS-like element containing an inverted repeat is underlined with a double arrowhead at the end. The duplicated host sequence adjacent to the insertion site of the IS-like element is shaded gray. The key 17-nucleotide sequence is boxed, and the imperfect inverted repeats (IR) overlapping with the key sequence are marked with arrows. The proposed transcription start site is indicated by an arrow at position +1. The E. coli-like −10 and −35 promoter sequences, Shine-Dalgarno sequence (SD), and start codons are demarked with dashed boxes. Fusion plasmids were introduced into L. interrogans serovar Lai strain 56601. Transconjugants were incubated in EMJH medium (EMJH) or EMJH with 120 mM sodium chloride (NaCl) for 4 h (B) or in EMJH medium only (C). Fluorescence is shown in arbitrary units. The dashed lines indicate the mean background readings obtained with L. interrogans carrying the empty gfp fusion vector pRAT724. Means ± standard deviations are plotted (n = 3). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.000; NS, not significant by Welch's t test. S17, 17-bp sequence scrambled.
To search for sequences that might account for the differences of basal sph2 expression levels between the Pomona LC82-25 strain and non-Pomona strains, we aligned the sequences upstream of the sph2 coding regions from the Pomona LC82-25, Lai 56601, Copenhageni Fiocruz L1-130, and Manilae L495 strains. The 5′ ends of the aligned sequences correspond to the 5′ ends of the sph2 promoter fragments cloned into pRAT724. As expected, the multisequence alignment revealed a 321-bp IS-like sequence in the Pomona strain, located from position −216 through −536 relative to the major 5′ end of the sph2 transcript of the Pomona strain (Fig. 8A) (13). Sequence elements characteristic of transposable elements were present: a 17-bp inverted repeat at the ends of the element and a 9-bp direct repeat of host sequences immediately adjacent to the inverted repeat (Fig. 8A).
An additional unique feature of the sequence in the Pomona strain is its lack of a 17-bp segment between positions −178 and −179 relative to the major 5′ end of its sph2 transcript (Fig. 8A). To determine whether this 17-bp sequence was responsible for the low basal sph2 expression in the Lai strain, the 17-bp sequence was removed from the upstream region of the Lai sph2 promoter in the gfp fusion. The deletion mutation restored 58% (Fig. 8B), 63%, and 81% of the expression observed with the Pomona sph2p-gfp fusion over three experiments. The converse experiment was also performed by inserting the 17-bp segment upstream of the Pomona sph2 promoter in the fusion. The insertion reduced fusion expression by 68% (Fig. 8B), 70%, and 72%. The expression from all sph2 fusions was elevated at physiologic osmolarity, indicating that the 17-bp sequence is not a major contributor to osmotic induction of sph2 expression.
To rule out the possibility that removing the 17-bp sequence altered the spacing between transcriptional control elements, an additional sph2 promoter variant was constructed by scrambling the 17-bp sequence. The scrambled sequence restored 44% of the expression observed with the Pomona fusion (Fig. 8C), indicating that at least a portion of the 17-bp sequence plays a direct role in limiting the transcription from the sph2 promoter.
DISCUSSION
Differential expression of the sph2 sphingomyelinase gene from a Pomona and a non-Pomona strain of L. interrogans was examined with a new gfp fusion plasmid. This gfp fusion plasmid enabled the identification of a segment of the sph2 promoter in the Lai strain that is primarily responsible for its low levels of sph2 expression. The Copenhageni and Manilae strains, which also express low basal levels of sph2, also possess the 17-bp sequence. The three Pomona strains, which express high levels of Sph2, lack the 17-bp segment. Interestingly, the 17-bp sequence contains one arm of an inverted repeat (Fig. 8A), which may be a binding site for a trans-acting factor responsible for the low basal expression of sph2. In contrast, an upstream IS-like sequence hypothesized to increase sph2 expression did not contribute to basal expression from the Pomona sph2 promoter when examined in the Lai strain.
It is anticipated that this gfp reporter strategy can be used to study gene expression in other strains and species of Leptospira. The parent of pRAT724, pMaORI, stably replicates in at least five other strains of L. interrogans and in L. mayottensis, L. licerasiae, L. fainei, and L. biflexa (24). pMaORI carrying the fliM gene also restored the virulence of a spontaneous motility mutant of L. interrogans, indicating that pMaORI is stable in vivo in the absence of antibiotic selection (28). Nevertheless, future efforts to examine the expression of promoter fusions to gfp in vivo will require additional experiments to verify the stability of the fusion plasmid in the absence of antibiotics.
One limitation of the new fusion plasmid is that variability and irreproducibility in the GFP levels were observed at high levels of expression of the sph2 promoter fusions in the Pomona strain. For this reason, although the IS-like element did not contribute to sph2 expression in the Lai strain, we cannot firmly exclude the possibility that the IS-like element contributes to basal sph2 expression in the Pomona strain. Additionally, we observed increases in GFP from all fusions in cells entering stationary phase (Fig. 6). Because cell densities across cultures were normalized on the basis of optical density rather than cell number prior to fluorescent measurement, the cell shrinkage we observed in cultures entering stationary phase may have contributed to the increased GFP readings. Further studies are needed to determine whether an increase in plasmid copy number per cell during entry into stationary phase also contributed to the increase. Nevertheless, the development of this gfp fusion plasmid will facilitate the identification of cis-acting sequences critical for the expression of L. interrogans genes. The recent publication of the genome-wide transcription start sites for L. interrogans will aid the selection of the correct junction for promoter fusions to gfp (29). A transcription start site was not detected for sph2 in the study, consistent with the limited expression of sph2 in non-Pomona strains under standard culture conditions.
The elucidation of the mechanism for differential sph2 gene expression among L. interrogans strains raises the interesting question of whether the 17-bp promoter deletion provides a selective benefit to these strains. Increased sph2 expression appears to be unique among Pomona strains (10, 11). In contrast, sph2 is expressed poorly in strains from serovars Copenhageni, Lai, and Manilae, whose reservoir hosts are small rodents (30–32). The association of L. interrogans serovar Pomona infection with sheep and pigs (9) suggests that high levels of sph2 expression confer an adaptive benefit to the Pomona serovar for the infection of specific maintenance hosts. Additional studies comparing infection in different hosts with leptospiral strains with and without the 17-bp deletion would be helpful in understanding the role of Sph2, assessing whether there is a benefit of increased sph2 expression in some hosts, and determining whether that benefit is host specific.
The sph2 fusion constructs described here may be helpful in identifying the cis-acting site(s) involved in the osmotic regulation of sph2. In a previous study, the addition of 120 mM sodium chloride to cultures increased sph2 transcript levels by over 100-fold in both L. interrogans serovar Manilae and L. interrogans serovar Pomona (10). The fact that these increases were similar in both low and high Sph2 producers suggests that the 17-bp cis-acting element is not involved in the osmotic regulation of transcription. A previous study with the 600 bp immediately upstream of the sph2 coding region from the Fiocruz L1-130 strain of L. interrogans fused to gfp demonstrated the osmotic induction of the fusion in L. biflexa (18). We observed osmotic induction with a fragment extending from 24 to 325 nucleotides upstream of the sph2 coding region fused to gfp. Therefore, the 302-bp region serves as a starting point to locate the regulatory target. The observation that osmotic induction occurs in the nonpathogen L. biflexa suggests that a trans-acting determinant of sph2 osmotic regulation is conserved between at least some pathogenic and nonpathogenic Leptospira species.
Little is known about how cis- and trans-acting factors establish the level of gene expression in Leptospira. Several cis-acting sequences affecting the expression of Leptospira genes involving adaptation to different environmental stresses have been identified. The L. interrogans genome encodes two lexA genes (33). DNase I footprinting and gel shift assays with the recA upstream sequence revealed an SOS box bound by LexA1 (34). Additional gel shift assays demonstrated that similar sequences upstream of other SOS-induced L. interrogans genes bound to LexA1 (33). LexA2 bound only to its own promoter (33). In another study, a high-affinity binding site for the phosphorylated form of the L. biflexa HemR response regulator, which regulates heme metabolism, was enriched from a highly randomized oligonucleotide library by several rounds of affinity selection with the phosphorylated protein (35). In a recent study, the L. interrogans enhancer-binding protein EBP and the alternative sigma factor RpoN were shown to bind to various promoters by gel shift analysis (36). For L. interrogans genes such as sph2 for which the trans-acting regulatory factor remains to be identified, the pRAT724 gfp reporter plasmid will facilitate the identification of additional cis-acting sequences affecting gene expression.
MATERIALS AND METHODS
Bacterial strains.
Leptospira interrogans serovar Lai strain 56601 (37), L. interrogans serovar Copenhageni strain Fiocruz L1-130 (38), and L. interrogans serovar Pomona subtype kennewicki strains LC82-25, RM211, and P10637-46 (National Animal Disease Center, Ames, IA) were grown in EMJH medium (Probumin vaccine-grade solution, catalog number 840665, lot 103; Millipore) at 30°C with 40 μg/ml spectinomycin for plasmid selection. E. coli strains DH5α, π1, and β2163 (39) were incubated in LB with the appropriate antibiotic added to select for plasmids. The LB medium for E. coli π1 and β2163 was supplemented with 0.3 mM thymidine and 0.3 mM diaminopimelic acid, respectively (39).
Western blots.
Immunoblot analyses of L. interrogans lysates were performed as described previously (20). The blots were probed with 1:1,000 dilution of Sph2 antiserum and 1:5,000 dilution of LipL41 antiserum (40, 41).
Plasmid construction.
The plasmids used in the study are listed in Table 1. Plasmid DNA was extracted from 4 ml of overnight E. coli cultures with the Qiagen QIAprep Spin Miniprep kit (Valencia, CA) and eluted with 50 μl of 10 mM Tris-HCl, 0.1 mM EDTA, pH 8.5. Genomic DNA was purified from 5 ml of saturated L. interrogans cultures with the Promega Wizard genomic DNA purification kit and resuspended in 50 μl of 1 mM Tris-HCl, 0.1 mM EDTA, pH 7.4. Restriction enzymes and Quick ligase were supplied by New England BioLabs (Ipswich, MA). Synthetic oligonucleotides were obtained from Invitrogen, and the nucleotide sequences are provided in Table 2. PCRs were performed with Pfu DNA polymerase according to the manufacturer's instructions (Thermo Fisher Scientific).
TABLE 1.
Plasmids used in this study
| Plasmid | Description | Source or reference |
|---|---|---|
| pMaORI | Replicative plasmid for L. interrogans | 24 |
| pGreenTIR | gfp vector | 27 |
| pBluescript SK− | Cloning vector | Stratagene |
| pRAT720 | pMaORI with multicloning site | This study |
| pRAT722 | pGreenTIR with T7 early transcription terminator downstream of gfp | This study |
| pRAT723 | gfp vector in pMaORI, no rrnD transcription terminator | This study |
| pRAT724 | gfp vector in pMaORI | This study |
| pRAT725 | lipL41p-gfp in pRAT724 | This study |
| pRAT727 | sph2pLai-gfp in pRAT724 | This study |
| pRAT728 | sph2pPom-gfp in pRAT724 | This study |
| pRAT729 | sph2pPomΔIS-gfp in pRAT724 | This study |
| pRAT735 | sph2pLai in pBluescript SK− | This study |
| pRAT736 | sph2pPom in pBluescript SK− | This study |
| pRAT739 | sph2pPom(+17)-gfp in pRAT724 | This study |
| pRAT740 | sph2pLai(Δ17)-gfp in pRAT724 | This study |
TABLE 2.
Synthetic oligonucleotides used for plasmid constructions
| Oligonucleotide | Sequence (5′→3′)a | Description |
|---|---|---|
| T7Te(Kp)-5F | TAATCACACTGGCTCACCTTCGGGTGGGCCTTTCTGCGTTTATAAGGAGCATG | T7 early transcription terminator |
| T7Te(Sp)-5R | CTCCTTATAAACGCAGAAAGGCCCACCCGAAGGTGAGCCAGTGTGATTAGTAC | |
| rrnD(Bm)-2F | GATCTCAAATAAAACAAAAGGCTCAGTCGGAAGACTGGGCCTTTTGTTTTATCTGTTGGATCCGGTACCA | E. coli rrnD transcription terminator |
| rrnD(Se)-2R | CTAGTGGTACCGGATCCAACAGATAAAACAAAAGGCCCAGTCTTCCGACTGAGCCTTTTGTTTTATTTGA | |
| lipL41p(Bm)-3F | GTTCCAGGATCCTTGTAATTCAGTATCTTGTATGAGAAGT | lipL41 promoter |
| lipL41p(Xh)-4R | CACCAACTCGAGTTGATTTTGGGGAATAAGG | |
| la1029(Bm)-2F | GACCATGGATCCAGCGAGACGTTGAGTCTGA | sph2Lai promoter |
| lic12631(Bm)-32F | GACCATGGATCCAGCGAGACGCTGAGTCTGA | sph2Pom promoter |
| lic12631p(Xh)-2R | TCCAATCTCGAGGTTTGTATTTTATGTTATCATATAACATAAAAATG | sph2 promoter |
Overhangs are underlined; restriction sites are in bold font.
The gfp reporter plasmid was generated as follows (Fig. 1). A double-stranded oligonucleotide harboring the T7 early transcription terminator with 5′ KpnI and 3′ SphI single-stranded overhangs (Table 2) was inserted downstream of gfp in pGreenTIR (27) to create pRAT722. Additionally, a multicloning site was introduced into the plasmid pMaORI (24) by replacing its small BamHI-SalI fragment with the double-stranded DNA 5′-GATCCGGTACCACTAGTCTCGAGCCCGGGGCATGCG-3′/5′-TCGACGCATGCCCCGGGCTCGAGACTAGTGGTACCG-3′ (BamHI and SalI overhangs underlined), resulting in pRAT720. The gfp ribosome-binding site and coding region along with the 3′ T7 early terminator were excised from pRAT722 and inserted into XmaI-SphI-cut pRAT720 to generate pRAT723. Finally, a double-stranded oligonucleotide carrying the E. coli rrnD transcription terminator with 5′ BamHI and 3′ SpeI cohesive ends and BamHI and KpnI sites 3′ of the terminator was inserted upstream of gfp sequences in pRAT723 to generate pRAT724.
To clone the lipL41 and sph2 promoters, PCR primers were designed with BamHI and XhoI restriction sites near the 5′ ends of the upstream and downstream primers, respectively (Table 2). The lipL41 promoter was PCR amplified using L. interrogans strain Fiocruz L1-130 genomic DNA as the template. The sph2 promoters were amplified using L. interrogans serovar Lai strain 56601 and L. interrogans serovar Pomona strain LC82-25 genomic DNA as the templates. The PCR products were digested with BamHI and XhoI and inserted into the corresponding site in pRAT724 to create the promoter fusions to gfp.
Mutagenesis.
The template for oligonucleotide-directed mutagenesis of the sequence upstream of the sph2 promoter was generated by transferring the BamHI-XhoI fragment containing the promoter sequence from the wild-type sph2-gfp fusion plasmids into pBluescript SK− (Stratagene) to generate pRAT735 and pRAT736 (Table 1), which harbor the sph2 promoters from the Lai 56601 and Pomona LC82-25 strains, respectively. The desired mutations upstream of the sph2 promoter were generated as described previously (20, 42). In brief, overlapping oligonucleotides with the desired changes directed toward each strand (Table 3) were used to PCR amplify the plasmid with Pfu DNA polymerase. The template DNA was depleted by digestion with DpnI, and the amplicon was transformed into E. coli DH5α. The transformation culture was plated on LB plates containing 100 μg/ml ampicillin. Plasmid DNA was purified from overnight cultures of colonies, and the presence of the desired mutations was confirmed by Sanger sequencing with the T7 and T3 sequencing primers (Laragen, Culver City, CA).
TABLE 3.
Oligonucleotides used for site-directed mutagenesis
| Oligonucleotide | Sequence (5′→3′)a |
|---|---|
| lip0980p(−206i)-1F | TATTTAAAATAACATTCAATTTAAATAATATTATAATAAAATAAAATTTTATAAAAAATACA |
| lip0980p(−206i)-1R | AATTGAATGTTATTTTAAATAATTTTAGAGAAAGCGGATGATAG |
| la1029p(−222/−206d)-1F | CCTCAAATTA|TTTAAAGAATATTATAATAAAATAAAATTTTATAAAAAATACA |
| la1029p(−222/−206d)-1R | ATTCTTTAAA|TAATTTGAGGACAAGTGGATG |
| la1029p(−222/−206s)-2F | CCTCAAATTATGATTTTTTAGAATAATTTTAAAGAATATTATAATAAAATAAAATTTTATAAAAAATACA |
| la1029p(−222/−206s)-2R | ATTCTTTAAAATTATTCTAAAAAATCATAATTTGAGGACAAGTGGATG |
Inserted nucleotides are underlined; deletions introduced at vertical lines; scrambled sequences are in bold font.
Conjugation.
The gfp fusion plasmids were transferred into L. interrogans by conjugation from a donor E. coli strain as described (43). Briefly, E. coli β2163 was transformed with the gfp fusion plasmids and grown overnight in LB with 0.3 mM diaminopimelic acid (DAP) and 40 μg/ml spectinomycin at 37°C. The next day, 40 μl of the overnight culture was transferred to 4 ml of LB with DAP and grown to late exponential phase. L. interrogans was grown to a density of ≈108 cells/ml. Five milliliters of L. interrogans and 0.5 ml E. coli were mixed and filtered with a 0.1-μm filter (Fisher). The filter was placed face up on an EMJH plate containing 0.3 mM DAP and incubated overnight at 30°C. The bacteria were washed off the filter with 1 ml of EMJH medium and plated on three EMJH plates containing 40 μg/ml spectinomycin.
5′ end mapping of RNA.
A culture of L. interrogans Fiocruz L1-130 was started at 2 × 107 cells/ml in 25 ml EMJH medium supplemented with 80 mM NaCl and incubated for 5 days to a density of 6 × 108 cells/ml. Additionally, a culture of L. interrogans LC82-25 was initiated in 25 ml EMJH at 1 × 107 cells/ml and incubated for 3 days to a density of 5 × 108 cells/ml. RNA was extracted from L. interrogans with TRIzol (Invitrogen), and DNA was removed with Turbo DNase (Ambion). The 5′ end of the sph2 transcript was determined with the Roche 5′/3′ RACE kit, 2nd generation (Roche), using oligonucleotide sph2-8R (5′-CGTTTTGCTCTTTCATCGTGTC-3′) to prime the reverse transcription reaction and sph2-9R (5′-GCGGAGCTGCCATTTTCTG-3′) to PCR amplify the deoxyribosyladenine (dA)-tailed sph2 cDNA. The amplicon was sequenced with the sph2-9R primer to map the 5′ end.
GFP assay.
L. interrogans transconjugants carrying gfp fusion plasmids were grown in EMJH medium with 40 μg/ml spectinomycin to a density of ≈1 × 108 to 5 × 108 cells/ml, and the optical density at 420 nm (OD420) of the cultures was measured with an Ultrospec 2000 spectrophotometer (Pharmacia Biotech). Eight hundred microliters of each culture was collected by centrifugation for 5 min at 9,000 × g in an Eppendorf 5424 microcentrifuge. The cells were resuspended in a volume of phosphate-buffered saline (PBS) to obtain an OD420 reading of 0.4 (4 ml). One hundred microliters of each cell suspension was transferred to a dark-walled clear-bottom 96-well Costar plate (Thermo Fisher Scientific), and the fluorescence emitted at 528 nm (excitation 485 nm) was measured with a Synergy2 Multi-Mode microplate reader (BioTek, Winooski, VT, USA). Because the fluorescence readings of wild-type L. interrogans were similar to those of PBS, PBS was used as a blank for background subtraction. Fluorescence values from PBS were subtracted from the readings of L. interrogans carrying the gfp fusion plasmids.
Multisequence alignment.
The sph2 promoter sequences from L. interrogans strains Fiocruz L1-130, L495, 56601, and LC82-25 were aligned with Kalign (44).
Statistics.
Experiments were conducted with cultures initiated from three colonies, unless stated otherwise. Statistical analysis was conducted with R, version 3.4.2, “Short Summer” (45).
ACKNOWLEDGMENTS
We thank William G. Miller for providing pGreenTIR and Mathieu Picardeau for contributing pMaORI. We also thank Rich Zuerner for supplying the L. interrogans serovar Pomona strains. We thank Suneel Narayanavari for technical assistance and David Beenhouwer for use of the fluorescence microplate reader.
The study was funded by Veterans Affairs merit awards (to J.M. and D.A.H.) and a Public Health Service grant R21 AI128560-01A1 from the National Institute of Allergy and Infectious Diseases (to D.A.H.).
J.M. conceived the study, contributed materials, designed and performed the experiments, analyzed the data, and wrote the paper. D.A.H. helped analyze the data and write the paper.
REFERENCES
- 1.Guerra MA. 2009. Leptospirosis. J Am Vet Med Assoc 234:472–478. doi: 10.2460/javma.234.4.472. [DOI] [PubMed] [Google Scholar]
- 2.Babudieri B. 1958. Animal reservoirs of leptospires. Ann N Y Acad Sci 70:393–413. doi: 10.1111/j.1749-6632.1958.tb35398.x. [DOI] [PubMed] [Google Scholar]
- 3.Caimano MJ, Sivasankaran SK, Allard A, Hurley D, Hokamp K, Grassmann AA, Hinton JC, Nally JE. 2014. A model system for studying the transcriptomic and physiological changes associated with mammalian host-adaptation by Leptospira interrogans serovar Copenhageni. PLoS Pathog 10:e1004004. doi: 10.1371/journal.ppat.1004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iraola G, Spangenberg L, Lopes Bastos B, Grana M, Vasconcelos L, Almeida A, Greif G, Robello C, Ristow P, Naya H. 2016. Transcriptome sequencing reveals wide expression reprogramming of basal and unknown genes in Leptospira biflexa biofilms. mSphere 1:e00042-16. doi: 10.1128/mSphere.00042-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo M, Bulach DM, Powell DR, Haake DA, Matsunaga J, Paustian ML, Zuerner RL, Adler B. 2006. Effects of temperature on gene expression patterns in Leptospira interrogans serovar Lai as assessed by whole-genome microarrays. Infect Immun 74:5848–5859. doi: 10.1128/IAI.00755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo M, Murray GL, Khoo CA, Haake DA, Zuerner RL, Adler B. 2010. Transcriptional response of Leptospira interrogans to iron limitation and characterization of a PerR homolog. Infect Immun 78:4850–4859. doi: 10.1128/IAI.00435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsunaga J, Lo M, Bulach DM, Zuerner RL, Adler B, Haake DA. 2007. Response of Leptospira interrogans to physiologic osmolarity: relevance in signaling the environment-to-host transition. Infect Immun 75:2864–2874. doi: 10.1128/IAI.01619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patarakul K, Lo M, Adler B. 2010. Global transcriptomic response of Leptospira interrogans serovar Copenhageni upon exposure to serum. BMC Microbiol 10:31. doi: 10.1186/1471-2180-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis 3:757–771. doi: 10.1016/S1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 10.Narayanavari SA, Lourdault K, Sritharan M, Haake DA, Matsunaga J. 2015. Role of sph2 gene regulation in hemolytic and sphingomyelinase activities produced by Leptospira interrogans. PLoS Negl Trop Dis 9:e0003952. doi: 10.1371/journal.pntd.0003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho E, Barbosa AS, Gomez RM, Oliveira ML, Romero EC, Goncales AP, Morais ZM, Vasconcellos SA, Ho PL. 2010. Evaluation of the expression and protective potential of leptospiral sphingomyelinases. Curr Microbiol 60:134–142. doi: 10.1007/s00284-009-9519-3. [DOI] [PubMed] [Google Scholar]
- 12.Chaurasia R, Thresiamma KC, Eapen CK, Zachariah BJ, Paul R, Sritharan M. 2018. Pathogen-specific leptospiral proteins in urine of patients with febrile illness aids in differential diagnosis of leptospirosis from dengue. Eur J Clin Microbiol Infect Dis 37:423–433. doi: 10.1007/s10096-018-3187-9. [DOI] [PubMed] [Google Scholar]
- 13.Artiushin S, Timoney JF, Nally J, Verma A. 2004. Host-inducible immunogenic sphingomyelinase-like protein, Lk73.5, of Leptospira interrogans. Infect Immun 72:742–749. doi: 10.1128/IAI.72.2.742-749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinne M, Matsunaga J, Haake DA. 2012. Leptospiral outer membrane protein microarray, a novel approach to identification of host ligand-binding proteins. J Bacteriol 194:6074–6087. doi: 10.1128/JB.01119-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Wu Y, Ojcius DM, Yang XF, Zhang C, Ding S, Lin X, Yan J. 2012. Leptospiral hemolysins induce proinflammatory cytokines through Toll-like receptor 2-and 4-mediated JNK and NF-kB signaling pathways. PLoS One 7:e42266. doi: 10.1371/journal.pone.0042266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang YX, Geng Y, Yang JW, Guo XK, Zhao GP. 2008. Cytotoxic activity and probable apoptotic effect of Sph2, a sphingomyelinase hemolysin from Leptospira interrogans strain Lai. BMB Rep 41:119–125. doi: 10.5483/BMBRep.2008.41.2.119. [DOI] [PubMed] [Google Scholar]
- 17.Timoney JF, Kalimuthusamy N, Velineni S, Donahue JM, Artiushin SC, Fettinger M. 2011. A unique genotype of Leptospira interrogans serovar Pomona type kennewicki is associated with equine abortion. Vet Microbiol 150:349–353. doi: 10.1016/j.vetmic.2011.02.049. [DOI] [PubMed] [Google Scholar]
- 18.Cerqueira GM, Souza NM, Araujo ER, Barros AT, Morais ZM, Vasconcellos SA, Nascimento AL. 2011. Development of transcriptional fusions to assess Leptospira interrogans promoter activity. PLoS One 6:e17409. doi: 10.1371/journal.pone.0017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsunaga J, Coutinho ML. 2012. Positive regulation of Leptospira interrogans kdp expression by KdpE as demonstrated with a novel β-galactosidase reporter in Leptospira biflexa. Appl Environ Microbiol 78:5699–5707. doi: 10.1128/AEM.00713-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsunaga J, Schlax PJ, Haake DA. 2013. Role for cis-acting RNA sequences in the temperature-dependent expression of the multiadhesive Lig proteins in Leptospira interrogans. J Bacteriol 195:5092–5101. doi: 10.1128/JB.00663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aviat F, Slamti L, Cerqueira GM, Lourdault K, Picardeau M. 2010. Expanding the genetic toolbox for Leptospira species by generation of fluorescent bacteria. Appl Environ Microbiol 76:8135–8142. doi: 10.1128/AEM.02199-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozuru R, Saito M, Kanemaru T, Miyahara S, Villanueva SY, Murray GL, Adler B, Fujii J, Yoshida SI. 2017. Adipose tissue is the first colonization site of Leptospira interrogans in subcutaneously infected hamsters. PLoS One 12:e0172973. doi: 10.1371/journal.pone.0172973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratet G, Veyrier FJ, Fanton d'Andon M, Kammerscheit X, Nicola MA, Picardeau M, Boneca IG, Werts C. 2014. Live imaging of bioluminescent Leptospira interrogans in mice reveals renal colonization as a stealth escape from the blood defenses and antibiotics. PLoS Negl Trop Dis 8:e3359. doi: 10.1371/journal.pntd.0003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pappas CJ, Benaroudj N, Picardeau M. 2015. A replicative plasmid vector allows efficient complementation of pathogenic Leptospira strains. Appl Environ Microbiol 81:3176–3181. doi: 10.1128/AEM.00173-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu W, Wang J, Zhu Y, Tang B, Zhang Y, He P, Zhang Y, Liu B, Guo X, Zhao G, Qin J. 2015. Identification of three extra-chromosomal replicons in Leptospira pathogenic strain and development of new shuttle vectors. BMC Genomics 16:90. doi: 10.1186/s12864-015-1321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen D, Patton JT. 2001. Reverse transcriptase adds nontemplated nucleotides to cDNAs during 5′-RACE and primer extension. Biotechniques 30:574–582. doi: 10.2144/01303rr02. [DOI] [PubMed] [Google Scholar]
- 27.Miller WG, Lindow SE. 1997. An improved GFP cloning cassette designed for prokaryotic transcriptional fusions. Gene 191:149–153. doi: 10.1016/S0378-1119(97)00051-6. [DOI] [PubMed] [Google Scholar]
- 28.Fontana C, Lambert A, Benaroudj N, Gasparini D, Gorgette O, Cachet N, Bomchil N, Picardeau M. 2016. Analysis of a spontaneous non-motile and avirulent mutant shows that FliM is required for full endoflagella assembly in Leptospira interrogans. PLoS One 11:e0152916. doi: 10.1371/journal.pone.0152916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhukova A, Fernandes LG, Hugon P, Pappas CJ, Sismeiro O, Coppee JY, Becavin C, Malabat C, Eshghi A, Zhang JJ, Yang FX, Picardeau M. 2017. Genome-wide transcriptional start site mapping and sRNA identification in the pathogen Leptospira interrogans. Front Cell Infect Microbiol 7:10. doi: 10.3389/fcimb.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Faria MT, Calderwood MS, Athanazio DA, McBride AJ, Hartskeerl RA, Pereira MM, Ko AI, Reis MG. 2008. Carriage of Leptospira interrogans among domestic rats from an urban setting highly endemic for leptospirosis in Brazil. Acta Trop 108:1–5. doi: 10.1016/j.actatropica.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villanueva SY, Ezoe H, Baterna RA, Yanagihara Y, Muto M, Koizumi N, Fukui T, Okamoto Y, Masuzawa T, Cavinta LL, Gloriani NG, Yoshida S. 2010. Serologic and molecular studies of Leptospira and leptospirosis among rats in the Philippines. Am J Trop Med Hyg 82:889–898. doi: 10.4269/ajtmh.2010.09-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu W, Lin X, Yan J. 2014. Leptospira and leptospirosis in China. Curr Opin Infect Dis 27:432–436. doi: 10.1097/QCO.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 33.Fonseca LS, da Silva JB, Milanez JS, Monteiro-Vitorello CB, Momo L, de Morais ZM, Vasconcellos SA, Marques MV, Ho PL, da Costa RM. 2013. Leptospira interrogans serovar Copenhageni harbors two lexA genes involved in SOS response. PLoS One 8:e76419. doi: 10.1371/journal.pone.0076419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuñé J, Cullen P, Mazon G, Campoy S, Adler B, Barbe J. 2005. The Leptospira interrogans lexA gene is not autoregulated. J Bacteriol 187:5841–5845. doi: 10.1128/JB.187.16.5841-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morero NR, Botti H, Nitta KR, Carrion F, Obal G, Picardeau M, Buschiazzo A. 2014. HemR is an OmpR/PhoB-like response regulator from Leptospira, which simultaneously effects transcriptional activation and repression of key haem metabolism genes. Mol Microbiol 94:340–352. doi: 10.1111/mmi.12763. [DOI] [PubMed] [Google Scholar]
- 36.Hu WL, Pappas CJ, Zhang JJ, Yang YY, Yan J, Picardeau M, Yang XF. 2017. The EbpA-RpoN regulatory pathway of the pathogen Leptospira interrogans is essential for survival in the environment. Appl Environ Microbiol 83:e02377-16. doi: 10.1128/AEM.02377-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren SX, Fu G, Jiang XG, Zeng R, Miao YG, Xu H, Zhang YX, Xiong H, Lu G, Lu LF, Jiang HQ, Jia J, Tu YF, Jiang JX, Gu WY, Zhang YQ, Cai Z, Sheng HH, Yin HF, Zhang Y, Zhu GF, Wan M, Huang HL, Qian Z, Wang SY, Ma W, Yao ZJ, Shen Y, Qiang BQ, Xia QC, Guo XK, Danchin A, Saint Girons I, Somerville RL, Wen YM, Shi MH, Chen Z, Xu JG, Zhao GP. 2003. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 422:888–893. doi: 10.1038/nature01597. [DOI] [PubMed] [Google Scholar]
- 38.Ko AI, Galvao Reis M, Ribeiro Dourado CM, Johnson WD Jr, Riley LW, Salvador Leptospirosis Study Group. 1999. Urban epidemic of severe leptospirosis in Brazil. Lancet 354:820–825. doi: 10.1016/S0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 39.Demarre G, Guerout AM, Matsumoto-Mashimo C, Rowe-Magnus DA, Marliere P, Mazel D. 2005. A new family of mobilizable suicide plasmids based on broad host range R388 plasmid (IncW) and RP4 plasmid (IncPα) conjugative machineries and their cognate Escherichia coli host strains. Res Microbiol 156:245–255. doi: 10.1016/j.resmic.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Matsunaga J, Medeiros MA, Sanchez Y, Werneid KF, Ko AI. 2007. Osmotic regulation of expression of two extracellular matrix-binding proteins and a haemolysin of Leptospira interrogans: differential effects on LigA and Sph2 extracellular release. Microbiology 153:3390–3398. doi: 10.1099/mic.0.2007/007948-0. [DOI] [PubMed] [Google Scholar]
- 41.Shang ES, Summers TA, Haake DA. 1996. Molecular cloning and sequence analysis of the gene encoding LipL41, a surface-exposed lipoprotein of pathogenic Leptospira species. Infect Immun 64:2322–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng L, Baumann U, Reymond JL. 2004. An efficient one-step site-directed and site-saturation mutagenesis protocol. Nucleic Acids Res 32:e115. doi: 10.1093/nar/gnh110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slamti L, Picardeau M. 2012. Construction of a library of random mutants in the spirochete Leptospira biflexa using a mariner transposon. Methods Mol Biol 859:169–176. doi: 10.1007/978-1-61779-603-6_9. [DOI] [PubMed] [Google Scholar]
- 44.Lassmann T, Sonnhammer EL. 2005. Kalign—an accurate and fast multiple sequence alignment algorithm. BMC Bioinformatics 6:298. doi: 10.1186/1471-2105-6-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.R Core Team. 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]