Hydroxycinnamates (HCAs) are essential components of lignin and are involved in various plant functions, including defense. In nature, microbial degradation of HCAs is influential to global carbon cycling. HCA degradation pathways are also of industrial relevance, as microbial transformation of the HCA, ferulate, can generate vanillin, a valuable flavoring compound. Yet, surprisingly little is known of the genetics underlying bacterial HCA degradation. Here, we make comparisons to previously characterized bacterial HCA degraders and use a genetic approach to characterize genes involved in catabolism and uptake of HCAs in the environmentally relevant marine bacterium Sagittula stellata. We provide evidence of overlapping substrate specificity between HCA degradation pathways and uptake proteins. We conclude that S. stellata is uniquely poised to utilize HCAs found in the complex mixtures of plant-derived compounds in nature. This strategy may be common among marine bacteria residing in lignin-rich coastal waters and has potential relevance to biotechnology sectors.
KEYWORDS: bacterial catabolism, ferulate, hydroxycinnamates, p-coumarate
ABSTRACT
The hydroxycinnamates (HCAs) ferulate and p-coumarate are among the most abundant constituents of lignin, and their degradation by bacteria is an essential step in the remineralization of vascular plant material. Here, we investigate the catabolism of these two HCAs by the marine bacterium Sagittula stellata E-37, a member of the roseobacter lineage with lignolytic potential. Bacterial degradation of HCAs is often initiated by the activity of a hydroxycinnamoyl-coenzyme A (hydroxycinnamoyl-CoA) synthase. Genome analysis of S. stellata revealed the presence of two feruloyl-CoA (fcs) synthase homologs, an unusual occurrence among characterized HCA degraders. In order to elucidate the role of these homologs in HCA catabolism, fcs-1 and fcs-2 were disrupted using insertional mutagenesis, yielding both single and double fcs mutants. Growth on p-coumarate was abolished in the fcs double mutant, whereas maximum cell yield on ferulate was only 2% of that of the wild type. Interestingly, the single mutants demonstrated opposing phenotypes, where the fcs-1 mutant showed impaired growth (extended lag and ∼60% of wild-type rate) on p-coumarate, and the fcs-2 mutant showed impaired growth (extended lag and ∼20% of wild-type rate) on ferulate, pointing to distinct but overlapping roles of the encoded fcs homologs, with fcs-1 primarily dedicated to p-coumarate utilization and fcs-2 playing a dominant role in ferulate utilization. Finally, a tripartite ATP-independent periplasmic (TRAP) family transporter was found to be required for growth on both HCAs. These findings provide evidence for functional redundancy in the degradation of HCAs in S. stellata E-37 and offer important insight into the genetic complexity of aromatic compound degradation in bacteria.
IMPORTANCE Hydroxycinnamates (HCAs) are essential components of lignin and are involved in various plant functions, including defense. In nature, microbial degradation of HCAs is influential to global carbon cycling. HCA degradation pathways are also of industrial relevance, as microbial transformation of the HCA, ferulate, can generate vanillin, a valuable flavoring compound. Yet, surprisingly little is known of the genetics underlying bacterial HCA degradation. Here, we make comparisons to previously characterized bacterial HCA degraders and use a genetic approach to characterize genes involved in catabolism and uptake of HCAs in the environmentally relevant marine bacterium Sagittula stellata. We provide evidence of overlapping substrate specificity between HCA degradation pathways and uptake proteins. We conclude that S. stellata is uniquely poised to utilize HCAs found in the complex mixtures of plant-derived compounds in nature. This strategy may be common among marine bacteria residing in lignin-rich coastal waters and has potential relevance to biotechnology sectors.
INTRODUCTION
Constituting up to 30% of the cell wall of vascular plants, lignin represents the most abundant aromatic polymer on Earth and offers potential as a highly valuable source of renewable carbon for numerous applications, including hydrocarbon fuel (1) and synthetic chemical production (2). Lignin is generated from the radical polymerization of three hydroxycinnamyl alcohols (p-coumaryl [H], coniferyl [G], and sinapyl [S] alcohols), resulting in a structurally heterogeneous polymer harboring a variety of aromatic substituents linked through stable carbon-carbon and ether bonds (3). Despite this chemical complexity, many fungi and a limited number of bacteria have been demonstrated to degrade lignin in nature (4). Primary depolymerization of lignin is typically accomplished by fungi through nonspecific oxidation driven by extracellular oxidoreductases (5–7). This initial enzymatic step liberates a pool of lower-molecular-weight aromatics that can serve as carbon and energy sources for microbes with appropriate ring fission pathways (8–10).
Among the most abundant and valuable aromatic compounds associated with lignin are the hydroxycinnamates (HCAs) p-coumarate (H unit derivative) and ferulate (G unit derivative) (11, 12). Both HCAs are essential to plant integrity and offer additional potential for industrial and pharmaceutical applications (13). Ferulate and p-coumarate are common in crop plants, such as fruits, vegetables, and coffee (14, 15), but are most notably abundant in grasses (16). While p-coumarate is mostly esterified to other phenylpropanoid units of lignin (17), ferulate cross-links lignin to cell wall polysaccharides through ether and ester bonds (18). These ferulate cross-links have been identified as a primary source of recalcitrance in grass lignocellulose (19). Aside from providing a refractory architecture, HCAs belong to a family of phenylpropanoid defense molecules released in response to microbial invasion (12, 20). Not surprisingly, microbes with the ability to degrade HCAs are able to deflect these phenylpropanoid-derived plant defenses, leading to enhanced virulence in plants (21). A more detailed understanding of the genes involved in HCA degradation across different organisms may therefore inform strategies to mitigate plant disease. In addition to supporting plant health, HCAs also harbor value from human health and industrial perspectives, as they can serve as dietary antioxidants (22–24) and as precursors for a variety of commercial products. For instance, microbial strains have been engineered to convert p-coumarate into precursors for thermoplastics, flavorings, and cosmetics (25). Similarly, the bacterial degradation of ferulate, which often generates vanillin as an intermediate (26), has been exploited for the biotechnological production of commercial vanillin flavoring (27–29). Insight into the genes and pathways that modify these HCAs can thus guide technologies to generate commercially relevant products.
To date, there have been two major pathways described for the aerobic bacterial degradation of HCAs, both of which are initiated by coenzyme A (CoA) activation of the HCA carboxylate side chain to generate a hydroxycinnamoyl-CoA (Fig. 1). In both instances, the CoA addition is performed by a hydroxycinnamoyl-CoA synthase or ligase. The difference in the two described HCA pathways lies in the mechanism employed for removal of the acetate moiety, with one pathway proceeding through a β-oxidative route (30, 31) and the other through a non-β-oxidative route that generates an aldehyde intermediate (32, 33). While ferulate can be degraded through either the β-oxidative (30) or aldehyde (32–34) pathway, p-coumarate is expected to be catabolized solely through the aldehyde pathway (21, 32). The aldehyde pathways for ferulate and p-coumarate are homologous, differing only in the meta-ring substituent (ferulate, meta-OCH3; p-coumarate, meta-H) of the starting material and intermediates. Thus, after deacetylation in the aldehyde branch, the ferulate pathway generates vanillic aldehyde and then vanillate, whereas the p-coumarate pathway generates p-hydroxybenzaldehyde and then p-hydroxybenzoate. All known HCA pathways converge to form the central intermediate protocatechuate, which undergoes ring cleavage, with products ultimately feeding into the tricarboxylic acid (TCA) cycle (8). In the few strains in which the genetics of HCA catabolism has been elucidated, the genes are typically colocalized and coregulated (21, 35). For example, in Agrobacterium fabrum, all ferulate degradation genes are localized to a single catabolon (30).
FIG 1.
Select HCA degradation pathways initiated by CoA addition. The left branch exhibits the β-oxidative pathway through which ferulate is degraded. The right branch shows the aldehyde pathway through which both ferulate and p-coumarate can be processed. E-37 locus tags are provided at reactions for which there are strong homologs to functionally validated proteins and/or genome annotations. The designated proteins (NCBI RefSeq accession numbers) are as follows: SSE37_12324 (WP_005859342), SSE37_24399 (WP_005857142), SSE37_12349 (WP_005859350), SSE37_24394 (WP_005857140), SSE37_12329 (WP_005859343), SSE37_12319 (WP_005859340), SSE37_18837 (WP_040603982), and SSE37_02815 (WP_005862022). Hcs, hydroxycinnamoyl-CoA synthase; Ech, enoyl-CoA hydratase; Acd, acyl-CoA dehydrogenase; Bkt, β-ketothiolase; Adh, aldehyde dehydrogenase; PobA, p-hydroxybenzoate oxygenase; VanA, vanillate-O-demethylase. HMPHP-CoA, 4-hydroxy-3-methoxyphenyl-β-hydroxypropionyl-CoA; HPHP-CoA, 4-hydroxyphenyl-β-hydroxypropionyl-CoA; HMPKP-CoA, 4-hydroxy-3-methoxyphenyl-β-ketopropionyl-CoA. Dashed arrows indicate reactions unique to the β-oxidative pathway, light solid lines represent reactions unique to the aldehyde pathways, and bold solid lines indicate reactions shared between the two branches. Figure is adapted from Overhage et al. (34) and Lowe et al. (21).
For most bacteria with described HCA degradation pathways, the relevant CoA synthase is either reported specifically for ferulate, or more generically, for hydroxycinnamates, as many characterized CoA synthases can act upon both ferulate and p-coumarate, as well as a variety of structurally related compounds. The hydroxycinnamoyl-CoA ligase from Acinetobacter baylyi ADP1 (HcaC) can transfer CoA to p-coumarate, ferulate, and caffeate (32). Similarly, the annotated feruloyl-CoA ligase (FerA) from Sphingobium sp. strain SYK-6 has been demonstrated to convert p-coumarate, ferulate, caffeate, and sinapate (33). Although direct evidence is not yet available, the CoA ligase (Atu1416) from A. fabrum is also likely to accept both ferulate and p-coumarate based on transcriptomics data (36) and growth profiles from deletion constructs (30).
The marine bacterium Sagittula stellata E-37 has been developed as a model for the study of HCA degradation and the catabolism of other plant-derived aromatic compounds in coastal ocean environments (37–39), where the dissolved organic carbon pool is highly aromatic in nature (40). This isolate has been shown to selectively attach to and partially mineralize a synthetic form of lignin and demonstrates robust growth on a variety of aromatic monomers, including ferulate and p-coumarate (38, 39, 41). However, the pathways utilized by this strain to degrade these HCAs are unknown. As described herein, the genome of S. stellata harbors two open reading frames (ORFs) with significant homology to hydroxycinnamoyl-CoA synthases (both annotated as feruloyl-CoA synthases [Fcs]). Since Fcs proteins have been shown to have broad substrate specificities across para-substituted HCAs and are encoded by a single gene in other bacteria (33, 42), the presence of two disparately localized fcs homologs in S. stellata is an intriguing phenomenon and suggests that dedicated pathways for p-coumarate and ferulate exist in this strain. This genetic arrangement in S. stellata presents insight into the complexity of HCA catabolism in lignin-rich coastal marine environments.
RESULTS
S. stellata possesses two disparately located hydroxycinnamoyl-CoA synthase homologs.
The S. stellata genome was queried for the presence of hydroxycinnamoyl-CoA synthase homologs through alignments to functionally validated protein sequences from Acinetobacter baylyi ADP1 (GenBank accession no. AAL54850), Agrobacterium fabrum C58 (NCBI RefSeq accession no. NP_354423), and Sphingobium sp. SYK-6 (GenBank accession no. BAK67177). BLASTp alignments of the selected proteins against the S. stellata genome identified two ORF products, here referred to as Fcs-1 (WP_005859342) and Fcs-2 (WP_005857142), which showed highest sequence similarity to A. baylyi and A. fabrum Fcs proteins. Fcs-1 showed moderately higher sequence identity and expectation (E) values than both of these previously characterized Fcs proteins (identities, 55 and 40%; E values = 0 and e−147 for A. fabrum and A. baylyi, respectively) relative to Fcs-2 (identity, 40 and 36%; E values = e−140 and e−124 for A. fabrum and A. baylyi, respectively). No significant homology was evident between the validated Fcs from Sphingobium and either of the S. stellata Fcs sequences. S. stellata Fcs-1 and Fcs-2 sequences share 41% identity and 57% similarity to one another and possess conserved sequence motifs diagnostic of hydroxycinnamoyl synthases (i.e., acyl-activating enzyme [AAE] consensus, putative AMP binding site, putative active site, and putative CoA binding site motifs).
S. stellata ORFs predicted to be involved in downstream conversions for the β-oxidative branch for HCA degradation were also identified (Fig. 2 and Table 1). BLASTp alignment of the β-oxidative acyl-CoA dehydrogenase (Atu1415) from A. fabrum (30) against the S. stellata genome yielded homology to SSE37_24394 (identity, 51%; E value = e−79), annotated as a 3-hydroxy-2-methylbutyryl-CoA dehydrogenase, which lies just upstream of fcs-2. Interestingly, another acyl-CoA dehydrogenase (Atu1414) exists within the A. fabrum HCA catabolon but was shown not to be essential to ferulate degradation in that system (36). This protein demonstrates homology to the S. stellata acyl-CoA dehydrogenase SE37_12329 (identity, 55%; E value = e−138) located next to fcs-1. Last, the A. fabrum HMPKP β-ketothiolase, Atu1421, strongly aligns to S. stellata SSE37_12319 (identity, 82%; E value = e−180), annotated as a 4-hydroxyphenyl-beta-ketoacyl-CoA hydrolase, located adjacent to fcs-1.
FIG 2.
Genetic landscape of the two fcs loci in S. stellata. Locus tags for predicated HCA-related degradation genes in E-37 and associated A. fabrum homologs (denoted with Atu prefix) are provided with percent protein identity above the corresponding gene arrows. Dark-pink arrows represent genes involved in the β-oxidative and/or the aldehyde pathway described in Fig. 1. Light-pink arrows indicate genes involved only in the β-oxidative pathway. bkt, β-ketothiolase; fcs, feruloyl-CoA synthase; acd, acyl-CoA dehydrogenase; ech, enoyl-CoA hydratase. Other functionally related genes are coordinately color coded and include a predicted operon for cobalamin biosynthesis (SSE37_12299, SSE37_12304, and SSE37_12309); the dct operon (SSE37_24379, SSE37_24384, and SE37_24839), predicted to encode a C4-dicarboxylate TRAP transporter; and a portion of the box pathway (SSE37_24404, SSE37_24409, SSE37_24414, SSE37_24419, and SSE37_24424) encoding enzymes for benzoate degradation. Dark gray represents genes for which a defined role in either HCA degradation or another pathway is not predicted with the following numbers: 1 indicates SSE37_12314, a protein of unknown function (DUF 3237); 2 indicates SSE37_12334, annotated as an aldo/ketoreductase; 3 indicates SSE37_12339, annotated as a flavin adenine dinucleotide (FAD)-dependent oxidoreductase; and 4 indicates SSE37_12344, annotated as a MarR-like transcriptional regulator, as discussed in the text. The complete box operon is provided in Fig. S6.
TABLE 1.
S. stellata homologs to A. fabrum HCA catabolism proteins
| A. fabrum locus (NCBI RefSeq accession no.) | S. stellata homolog locus (NCBI RefSeq accession no.) | Putative function | Coverage (%) | Amino acid identity (%) | E value |
|---|---|---|---|---|---|
| Atu1414 (NP_354421) | SSE37_12329 (WP_005859343) | Acyl-CoA dehydrogenase | 83 | 54 | 3e−138 |
| Atu1415 (NP_354422) | SSE37_24394 (WP_005857140) | Acyl-CoA dehydrogenase | 100 | 51 | 1e−79 |
| Atu1416 (NP_354423) | SSE37_12324 (WP_005859342) | Feruloyl-CoA synthase | 98 | 55 | 0.0 |
| Atu1416 (NP_354423) | SSE37_24399 (WP_005857142) | Feruloyl-CoA synthase | 97 | 40 | 2e−140 |
| Atu1417 (NP_354425) | SSE37_12349 (WP_005859350) | Enoyl-CoA hydratase | 96 | 67 | 6e−126 |
| Atu1421 (NP_354429) | SSE37_12319 (WP_005859340) | β-Ketothiolase | 100 | 82 | 8e−180 |
The two fcs genes and several of the genes potentially involved in downstream conversions reside in two distinct genetic loci on the S. stellata chromosome (Fig. 2). S. stellata harbors ORFs with high sequence similarity to many of the gene products within the A. fabrum catabolon (Table 1), nearly all of which are within 5 kb of fcs-1. The single exception is an acyl-CoA dehydrogenase, which is adjacent to fcs-2. Interestingly, fcs-2 is immediately upstream of an operon that encodes the recently characterized benzoyl-CoA oxidation (Box) pathway, through which benzoate is degraded by this strain (S. stellata E-37) (39). Additionally, fcs-2 is just downstream from a three-gene cassette anticipated to encode a tripartite ATP-independent periplasmic (TRAP) family transporter, which consists of a substrate-binding protein (DctP; SSE37_24379) and two membrane-bound proteins (DctQM; SSE37_24384 and SSE37_24389) (43) (Fig. 2). The proximity of the TRAP gene cassette to these catabolic genes suggests that the encoded transporter may facilitate the uptake of aromatic compounds.
Though S. stellata can use vanillin as a sole carbon source (see Fig. S3 in the supplemental material), genomic evidence for the aldehyde pathway, which generates vanillin as an intermediate, is not strong in S. stellata. Homology searches to characterized vanillin dehydrogenases (i.e., LigV [GenBank accession no. BAK65381] from Sphingobium sp. SKY-6 and HcaB [CAG68567] from A. baylyi) result in moderate-to-weak similarities (maximum identities, LigV = 37%, E value ≤ e−76; HcaB = 34%, E value ≤e−82) to a number of putative aldehyde dehydrogenases encoded in the S. stellata genome, none of which are adjacent to either fcs locus.
Genetic analysis reveals overlapping but distinct roles of fcs genes in HCA degradation.
To assess the contributions of the putative fcs homologs to hydroxycinnamate catabolism in S. stellata, mutant strains lacking either or both functional copies of fcs-1 and fcs-2 were generated, and the phenotypes on a suite of substrates were determined. The three mutants (fcs-1, fcs-2, and fcs-1 fcs-2) displayed wild-type growth on the control nonaromatic and aromatic carbon sources, acetate and p-hydroxybenzoate (POB), respectively (Fig. S4A and B). In S. stellata, POB is converted to the central intermediate protocatechuate (PCA), which then undergoes ring fission (39). Hence, wild-type growth on POB is a strong indicator that the ring cleavage pathway through which all other aromatic acids tested here are funneled (37–39) is unaffected by the mutations.
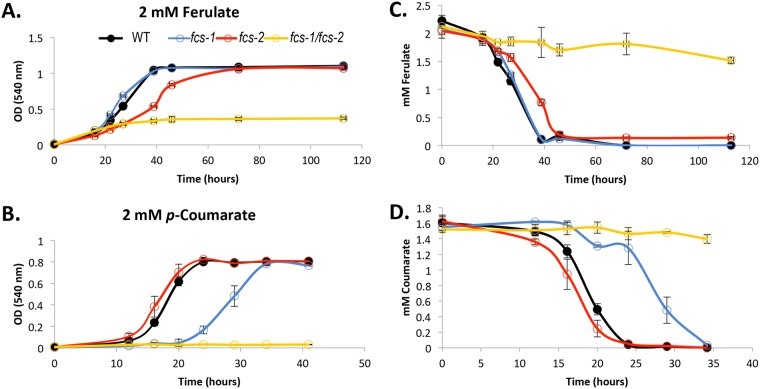
Growth curve analyses on ferulate revealed that while the fcs-1 mutant demonstrated wild-type growth, the fcs-2 mutant showed an extended lag phase (Fig. 3A) and significantly depressed growth rate (22% of wild-type rate; P ≤ 0.01; Fig. S5). Yet, the culture ultimately reached wild-type cell densities in stationary phase (Fig. 3A). The fcs-1 fcs-2 double mutant elicited a severely depressed growth phenotype on this substrate, with a maximum absorbance reading only ∼30% of that of the wild-type cultures. As ferulate imparts a maroon coloration to the medium that may inflate absorbance readings, viable counts were also performed as an additional measure of cell growth. Maximum viable cell abundances of the double mutant grown on ferulate were only 2% of those of the wild-type cultures (1.81 × 107 [± 1.56 × 106] CFU/ml versus 7.27 × 108 [± 7.64 × 107] CFU/ml, respectively). Consistent with this finding, only 30% of the provided ferulate was taken up by the double mutant. In contrast to the double mutant, ferulate concentrations were drawn below the limit of detection for all other strains (Fig. 3C).
FIG 3.
Catabolism of hydroxycinnamates by S. stellata E-37 and the fcs mutants. Growth of strains on ferulate (A) or p-coumarate (B), with corresponding concentrations of ferulate (C) or p-coumarate (D) in cell-free spent medium. Wild type is shown with black closed circles, the fcs-1 mutant with blue open circles, the fcs-2 mutant with red open circles, and the fcs-1 fcs-2 double mutant with yellow open circles. Averages from a minimum of three biological replicates are shown, along with one standard deviation from the mean.
The individual fcs mutants showed opposing phenotypes on p-coumarate. The fcs-2 mutant displayed wild-type growth, while the fcs-1 mutant strain showed a lag phase double that of wild-type cultures (Fig. 3B) and a significantly decreased exponential-growth rate (61% of wild-type rate; P ≤ 0.01; Fig. S5). Both single mutants ultimately reached wild-type cell densities on this substrate, whereas the double mutant failed to grow (Fig. 3B). The abolished growth of the double mutant on p-coumarate was corroborated by the lack of p-coumarate depletion from the medium (Fig. 3D). For all strains and substrates, the extracellular concentrations of substrates were consistent with the growth assays (Fig. 3).
Given that many aromatic acids are toxic to bacterial cells (44, 45), a parallel set of growth experiments were performed using a Biolog array, which included a tetrazolium dye that is responsive to cellular respiration-associated redox changes (46). Here, cells were incubated in 96-well plates supplemented with ferulate, p-coumarate, or control compounds (this study), in the presence of a proprietary tetrazolium-based dye, and monitored for colorimetric changes indicative of dye reduction upon cellular respiration. The Biolog assays were conducted to cross-validate the optical density (OD)-based growth studies, as measurement of respiration provides a supplemental analysis of cell growth. The general trends from these assays are consistent with absorbance-based measures of growth, with the exception of the ferulate Biolog measurements, which may be due to interference of the maroon color imparted by ferulate (Fig. S4). Regardless, the viable count and HCA depletion assays agree that the double mutant cannot effectively oxidize ferulate, while the fcs-2 mutant is moderately delayed in its processing.
Gene expression assays indicate that fcs genes are growth substrate inducible.
To complement the mutational analysis, reverse transcription-quantitative PCR (RT-qPCR) was performed to assess fcs gene expression when S. stellata was grown on ferulate or p-coumarate. Both the fcs-1 and fcs-2 genes were significantly upregulated during growth on both HCAs relative to growth on the nonaromatic substrate acetate (P < 0.005). However, the relative gene expression of fcs-1 far exceeded that of fcs-2. The fcs-1 expression levels from p-coumarate- and ferulate-grown cells were 10-fold higher than those of fcs-2 during growth on either HCA (Fig. 4).
FIG 4.

fcs gene expression for S. stellata grown on two hydroxycinnamates. Gene expression was relativized to growth on 7 mM acetate and normalized to three housekeeping genes. Blue bars represent fcs-1 expression, and red bars represent fcs-2 expression. Identical letters above bars indicate nonsignificant values, whereas nonidentical letters represent a significant difference (*, P < 0.005). Averages from a minimum of three biological and two technical replicates are shown, along with one standard deviation from the mean.
To expand our understanding of fcs gene regulation in S. stellata, additional sequence analysis was performed to identify putative transcriptional regulators. A putative MarR-type regulator (NCBI RefSeq accession no. WP_005859349, SSE37_12344) is present within the fcs-1 region (Fig. 2). Alignment of the S. stellata MarR to this protein yields very weak identity (35%). Along those same lines, there is no notable similarity between the S. stellata predicted regulatory protein and the predicted regulator of the A. fabrum hca catabolon, GntR (NCBI RefSeq accession no. NP_354412, Atu1405). Here, the S. stellata putative protein (NCBI RefSeq accession no. WP_005861787) with highest sequence similarity to GntR demonstrates low identity (30%) and poor E value (e−6). Furthermore, it is not located near either fcs gene. A single putative regulator (SSE37_24449) is found within the fcs-2 region. This gene is located within and cotranscribed with the box operon but is not anticipated to be cotranscribed with fcs-2 given the intergenic distance (>500 bp) (Fig. S6).
Identification of a transporter for hydroxycinnamates in S. stellata.
Though Fcs-1 appears to play a more dominant role in p-coumarate degradation and Fcs-2 in ferulate degradation, our results suggested overlapping specificities, implying compensatory roles for each protein when provided a nonpreferred HCA substrate. We next sought to determine whether this apparent relaxation of specificity was extended to proteins involved in the uptake of these compounds.
We focused our efforts on the dctPQM cassette located adjacent to the fcs-2 gene (Fig. 2). Initial RT-qPCR studies of ferulate- and p-coumarate-grown cells showed dctP gene expression to be elevated 13-fold (±2-fold) and 23-fold (±14-fold), respectively, relative to acetate-grown cultures. To functionally determine the role of this putative aromatic transporter, dctP (SSE37_24379) was interrupted using insertional mutagenesis. The dctP mutant was unable to grow on either ferulate or p-coumarate (Fig. S7), supporting its indispensable role in HCA transport. Partial restoration of activity was evident with a wild-type dctP-complemented strain (Fig. S7).
DISCUSSION
Despite the abundance of hydroxycinnamates (HCAs) in lignin-rich environments and the demonstrated importance of bacteria in the recycling of this material, relatively little is known of the bacterial catabolic pathways used to remineralize these compounds. Using the model marine heterotrophic bacterium Sagittula stellata E-37, we provide compelling evidence for the presence of multiple HCA degradation pathways with overlapping substrate specificities. We argue that this genetic arrangement allows the strain to be poised to utilize these compounds in nature, where they are found in complex mixtures of aromatics.
Our data support a primary role for fcs-1 in p-coumarate catabolism and fcs-2 in ferulate catabolism in S. stellata, with evidence of overlapping functionalities between the two enzymes encoded by these genes. The ability of each single mutant to ultimately reach wild-type cell densities on nonoptimal substrates suggests a compensatory function for each of their gene products. The concept of overlapping substrate specificity for this class of enzymes is not novel, as many aromatic CoA ligases have been demonstrated to transform a variety of structurally related compounds. As previously mentioned, the hydroxycinnamoyl-CoA ligases from A. baylyi (HcaA) (32), Sphingobium sp. SYK-6 (FerA) (33), and A. fabrum (Atu1416) (30, 36) demonstrate broad substrate specificity. Although these well-documented CoA ligases convert multiple substrates, these strains do not possess multiple hydroxycinnamoyl-CoA ligase homologs, nor is there evidence for compensatory function. Accordingly, single fcs gene knockouts were sufficient to prevent HCA utilization in both A. fabrum and Sphingobium spp. (30, 33).
The expanded substrate range of the two Fcs proteins from S. stellata is further corroborated by transcriptional analyses of the wild-type strain, which show that both fcs-1 and fcs-2 are upregulated in cultures grown on either HCA. However, transcriptional responses to growth on HCAs were different, perhaps in part due to differences in their basal (noninduced) expression levels. S. stellata grown with acetate as the sole carbon source had 5.2× (±0.9×) more fcs-2 transcripts than fcs-1 transcripts. This difference in basal gene expression suggests that these genes are under different transcriptional regulation. Consequently, S. stellata appears to be better poised to rapidly oxidize ferulate with Fcs-2. If substantiated, these findings could indicate that S. stellata is primed to respond to ferulate as a growth substrate.
The difference in expression of fcs-1 and fcs-2 in S. stellata suggests distinct regulation of the two genes. This is perhaps intuitive given the physical separation of the two fcs genes within the S. stellata chromosome. There are annotated regulators within both fcs genomic regions that could potentially serve this function; however, convincing genomic evidence is lacking. In A. baylyi, a MarR-type regulator (GenBank accession no. AAP78949) is responsible for regulating an hca operon (32). While a putative MarR-type regulator (SSE37_12344) is located in the S. stellata fcs-1 region, it shares little sequence similarity with the A. baylyi characterized protein. A single putative regulator (SSE37_24449) is found within the fcs-2 region but is not predicted to regulate HCA degradation based on gene expression assays. This ORF (SSE37_24449) is predicted to encode a transcriptional regulator for benzoate catabolism and has been accordingly designated BoxR. SSE37_24449 shares 44% (E value = e−68) sequence identity with two transcriptional regulators of benzoate catabolism in Azoarcus sp. strain CIB (BoxR [Ga0098266_114713] and BzdR [Ga0098266_111630]) (47, 48), the bacterium for which the novel aerobic box pathway was first described (49). Finally, SSE37_24449 contains the Walker-A motif [consensus sequence, GLRGAGK(T/S)], which has been proposed to specifically interact with benzoyl-CoA (47). Thus, there is currently no evidence to support the involvement of SSE37_24449 or SSE37_12344 in the direct genetic regulation of HCA catabolism in S. stellata.
The apparent relaxed substrate specificity for the HCA catabolic proteins appears to extend to proteins involved in transport. The TRAP transporter gene dctP (SSE37_24379) is required for growth on both ferulate and p-coumarate. The upregulation of dctP in S. stellata cultures grown on p-coumarate or ferulate provides additional evidence that the products of the dctPQM cassette act on both p-coumarate and ferulate. The broad specificity of TRAP family members is well documented and includes aromatic acids (43). In Comamonas sp. strain DJ-12, the transport of 4-chlorobenzoate and related compounds is mediated by a TRAP family transporter (50). The Rhodopseudomonas palustris TRAP substrate-binding protein TarP has been shown to bind not only ferulate and p-coumarate but also caffeate and cinnamate (51). No other aromatic acid transporters have yet been identified in S. stellata. and very few genes with sequence similarity to transporter proteins have been identified in aromatic compound catabolism gene clusters in this strain (39).
The ability of the fcs double mutant to sustain limited growth on ferulate is intriguing. It is conceivable that S. stellata is able to access the methoxy side chain of ferulate, which is absent in p-coumarate, to support restricted growth. The spectrophotometric assay indicates the double mutant is capable of taking up ∼30% of the provided substrate, though cellular growth is not consistent with complete utilization of this amount of ferulate. This could occur through removal of the entire methoxy group to generate p-coumarate, an unusable product for the double mutant, or through removal of the methyl group to yield caffeate (as observed in plants [52]), a substrate S. stellata is unable to catabolize (39). Although documentation of methoxy utilization from lignin-derived aromatics is typically observed with anaerobic methanogens or acetogens (53, 54), S. stellata harbors a putative vanillate-O-demethylase (SSE37_02815) that could presumably remove the methyl group from ferulate. The energy and biomass derived from a C1 methyl group would be considerably less than that of C10 ferulate, which is consistent with the growth phenotype of the mutant. Furthermore, although results presented here indicate that S. stellata predominantly degrades ferulate via a β-oxidative mechanism, it is feasible that additional parallel not-yet-characterized pathway(s) for ferulate degradation exist in this strain and could explain this restricted growth phenotype.
While this study suggests a dominant role of fcs-1 for p-coumarate utilization and fcs-2 for ferulate utilization, an important ecological finding is the ability of both of these genes to be upregulated by its noncognate HCA substrate. The cross-substrate utilization may reflect the diverse substrate pools to which the strain is adapted. The eastern U.S. coastal salt marshes from which the strain was isolated (55) are rich in decaying plant matter, principally derived from the cordgrass Spartina alterniflora, which is rich in ferulate and p-coumarate (56). Indeed, plant detritus is the principle source of dissolved organic carbon in these territories (57). These coastal marshes are also dominated by members of the Roseobacter lineage, to which S. stellata belongs (58). The ability to degrade aromatic compounds is common among lineage members, as is the presence of multiple (≤6) ring-cleaving pathways (59). Roseobacters, in general, and S. stellata, in particular, appear to be uniquely adapted to accessing lignin-derived carbon. S. stellata is able to mineralize synthetic lignin (38) and is adapted to growth on mixtures of aromatic compounds. The strain demonstrates a synergistic growth response when provided mixtures of two lignin degradation products, benzoate and p-hydroxybenzoate (39). This phenomenon has been shown for other roseobacters (39) and indicates a positive relationship between substrate complexity and aromatic compound utilization in this group of bacteria. In this context, the multisubstrate specificity of the two hydroxycinnamoyl-CoA synthases encoded by S. stellata seems conducive to its lifestyle, as it appears to have evolved an arsenal of tools to degrade the wide breadth of aromatics in its surroundings.
These insights into HCA catabolism of the marine bacterium S. stellata unveil the modularity and complexity of approaches for aromatic compound degradation within this organism. Through a continued understanding of the reactions used to transform lignin-derived compounds, there is potential for uncovering novel physiologies, pathways, and enzymes that could be of industrial use, as many valuable products can be generated from lignin (2) and its HCA derivatives (25–27). The ease of handling and the expanding genetic tractability of S. stellata and other roseobacters (60) offer an exciting opportunity for future engineering applications.
MATERIALS AND METHODS
Strains and growth conditions.
Sagittula stellata E-37 was previously isolated from pulp mill effluent and enriched on Indulin AT, a commercially available Kraft lignin generated from pulp mills, as described by Gonzalez et al. (38). Unless otherwise noted, wild-type S. stellata and any mutant derivatives were routinely maintained at 30°C in YTSS medium (per liter, 2.5 g yeast extract, 4 g tryptone, 15 g sea salts [Sigma-Aldrich, St. Louis, MO]). Escherichia coli strains used for cloning and conjugation experiments were maintained at 37°C in Luria-Bertani broth (per liter, 10 g tryptone, 5 g yeast extract, 10 g NaCl). Antibiotics were added to the E. coli growth medium to maintain selective pressure at 50 μg/ml kanamycin or 10 μg/ml tetracycline. S. stellata growth assays were routinely performed in marine basal medium (MBM) containing 1.5% (wt/vol) Sigma sea salts, 225 nM K2HPO4, 13.35 μM NH4Cl, 71 mM Tris-HCl (pH 7.5), 68 μM Fe-EDTA, trace metals and vitamins (38), and carbon (2 mM aromatic and 10 mM acetate, unless otherwise noted). Strains were incubated at 30°C, in the dark, with shaking. All glassware was combusted in an ashing furnace (type F62700; Barnstead Thermolyne) for 4 to 24 h prior to use to eliminate trace carbon. Seeding densities were ∼1 × 106 CFU/ml, and no-carbon-added controls were run in parallel for comparison. Growth was monitored using a spectrophotometer at 540 nm. All strains used in this study are listed in Table 2.
TABLE 2.
Plasmids and strains used in this study
| Plasmid or strain | Descriptiona | Source or reference |
|---|---|---|
| Plasmids | ||
| pCR2.1-TOPO | TA plasmid for cloning | Invitrogen |
| pRMJ1 | Plasmid harboring sacB-kanR cassette for marker exchange mutagenesis | 63 |
| pARO180 | Mobilizable suicide plasmid for marker exchange mutagenesis | D. Park (ATCC 77123) |
| pARO180_12343 | pARO180 containing fused flanking regions of fcs-1 (SSE37_12324) cloned into XbaI and HindIII sites of the MCS | This study |
| pARO180_12324_SK | pARO180_12324 with sacB-kanR from pRMJ1 inserted into the middle of the fcs-1 insert through designed SalI site | This study |
| pKNOCK-Km | Mobilizable suicide plasmid harboring kanamycin resistance gene; used for site-directed mutagenesis via chromosomal integration | 64 (Addgene 46262) |
| pKNOCK-Km_24399 | pKNOCK-Km with small internal region (231 bp) of fcs-2 cloned into BamHI and XhoI sites of the MCS | This study |
| pKNOCK-Tc | Mobilizable suicide plasmid harboring tetracycline resistance gene; used for site-directed mutagenesis via chromosomal integration | 64 (Addgene 46259) |
| pKNOCK-Tc_24399 | pKNOCK-Tc with small internal region (231 bp) of fcs-2 cloned into the BamHI and XhoI sites of the MCS | This study |
| pKNOCK-Km_24379 | pKNOCK-Km with 292-bp internal region of dctP cloned into the HindIII and BamHI sites of the MCS | This study |
| pRK415 | Broad-host-range plasmid for DNA cloning in Gram-negative bacteria used for dctP complementation | 66 |
| pRK415_24379 | pRK415 containing a 1.7-kb HindIII-BamHI DNA fragment that includes the region 579 bp upstream of dctP start codon and 83 bp downstream of dctP stop codon | This study |
| Strains | ||
| E-37 | Wild-type Sagittula stellata E-37 | 58 |
| E-37 fcs-1::sacB-kanR | E-37 fcs-1 mutant where fcs-1 (SE37_12324) is replaced with sacB-kanR from pARO180_12324_SK | This study |
| E-37 fcs-2::pKNOCK-Km | E-37 fcs-2 mutant where fcs-2 (SSE37_24399) is interrupted by pKNOCK-Km_24399 | This study |
| E-37 fcs-1::sacB-kanR fcs-2::pKNOCK-Tc | E-37 fcs-1 fcs-2 double mutant generated by interrupting fcs-2 (SSE37_24399) with pKNOCK-Tc_24399 in strain E-37 fcs-1::sacB-kanR | This study |
| E-37 dctP::pKNOCK-Km | E-37 dctP mutant wherein dctP (SSE37_24379) was interrupted by pKNOCK-Km_24379 | This study |
| E-37 dctP-pKNOCK-Km_dctP::pRK415 | E-37 dctP mutant complemented with pRK415_24379 | This study |
| E-37 dctP-pKNOCK-Km_pRK415 | E-37 dctP mutant transformed with empty pRK415 vector | This study |
| E. coli TOP10 | Cloning strain for pCR2.1-TOPO plasmid | Invitrogen |
| E. coli JM109 | Subcloning and transformation strain | Invitrogen |
| E. coli S17-1 | Mating strain for marker exchange mutagenesis | ATCC 47055 |
| E. coli BW20767 | Mating strain for pKNOCK mutagenesis, derived from S17-1 | 65 (ATCC 47084) |
MCS, multiple cloning site.
Generation of S. stellata mutants.
A S. stellata fcs-1 (SSE37_12324) mutant strain was produced using marker exchange mutagenesis following modifications of previously described procedures (61–63). Briefly, a 402-bp region upstream and a 585-bp region downstream of fcs-1 was amplified to generate “A” and “B” amplicons, respectively. During PCR amplification, a 21-bp scar sequence was added through the internal primers, generating complementary appendages for overlap extension PCR. PCR mixtures for each A and B reaction included 1× GoTaq buffer (Promega, Madison, WI), 200 μM deoxynucleotide triphosphates (Promega), 6 μM internal primer, 0.6 μM external primer, 0.025 U/μl GoTaq DNA polymerase (Promega), and 1 to 2 ng/μl S. stellata genomic DNA. Thermocycling conditions consisted of a 3-min denaturation at 95°C, followed by 31 cycles of 30 s at 95°C, 30 s at 56°C, 30 s at 72°C, and a final extension for 5 min at 72°C. The A and B amplicons were subsequently used as the template DNA during overlap extension PCR to generate a fused “AB” product. This PCR was performed with 1× FailSafe buffer F (Epicentre, Madison, WI), 6 μM external primer A, 6 μM external primer B, 0.05 U/μl FailSafe enzyme mix (Epicentre), 1 to 2 ng/μl amplicon A, and 1 to 2 ng/μl amplicon B. The thermocycling conditions were the same as used previously, except with a 10-min final extension. The resulting product was cloned into pCR2.1-TOPO (Invitrogen, Carlsbad, CA). Positive clones were selected on LB-kanamycin (50 μg/ml) and verified by sequence analysis. The AB insert was then subcloned into the mobilizable suicide plasmid pARO180 (ATCC 77123), through shared HindIII and XbaI sites, generating plasmid pARO180_12324, and transformed into chemically competent E. coli JM109. Positive clones were selected on LB-ampicillin (50 μg/ml) agar and verified through amplification and sequencing. The sacB-kanR cassette from pRMJ1 (63) was inserted into the middle of the AB insert at the SalI site, generating plasmid pARO180_12324_SK. The complete A-sacB-kanR-B vector construct was then transformed into the mating strain, E. coli S17-1. The suicide vector was delivered to S. stellata through biparental mating with E. coli S17-1(pARO180_12324_SK) on a YTSS agar plate. S. stellata transconjugants were enriched from the mating mixture by requiring growth on 5 mM p-hydroxybenzoate (a restrictive substrate for E. coli) and 50 μg/ml kanamycin. Allelic replacement was confirmed by PCR amplification across the chromosomal region of interest using primers 12324_AsacF/12324_BkmR (Table 3) and sequencing of the PCR product. PCR for this amplification was performed using the LongRange PCR kit (Qiagen, Valencia, CA), according to the manufacturer's instructions, with the following thermocycling conditions: initial denaturation at 93°C for 3 min, followed by 30 cycles of 93°C denaturation for 15 s, 57°C annealing for 30 s, 68°C elongation for 12 min, and no final extension.
TABLE 3.
Primers used in this study
| Primer name | Sequencea | Functionb |
|---|---|---|
| 12324_A_ex | GATCACTGCGCCGATCTT | Primer pair amplifies A region (402 bp upstream of 12324) with 21-bp scar SalI site appended to the 3′ end; A_ex primer is also used with B_ex for crossover PCR to generate the A-scar-B product |
| 12324_A_in | gattcgaggagcgatagagctGTCGACTTCTCCTCCTGGCGTTTTAG | |
| 12324_B_ex | GGAGCCCAACAGCATCAT | Primer pair amplifies B region (585 bp downstream of 12324) with 21-bp scar appended to the 5′ end; B_ex primer is also used with A_ex for crossover PCR to generate the A-scar-B product |
| 12324_A_in | agctctatcgctcctcgaatcGACCGGCGCCCGGTT | |
| 12324_Asac_F | GGATCAGGATGTCGTCGTTC | Primer pair binds E-37 DNA upstream and downstream of fcs-1; used to verify replacement of gene with sacB-kanR cassette |
| 12324_Bkm_R | CAGCTTCTCCTCGATTCGCT | |
| 24399_int_F | TGGTGTTTTATGCAGGCGCG | Primer pair amplifies 231-bp internal region of fcs-2 (SSE37_24399) used for cloning into pKNOCK plasmids |
| 24399_int_R | TTTCGCAGCGCATGTCTTCG | |
| pKNOCKKm_752_F | ACGGCTGACATGGGAATTCC | Primer pair binds around MCS of pKNOCK-Km to amplify inserts off plasmid |
| pKNOCKKm_901_R | GCGGAATTAATTCGACGCGTC | |
| pKNOCKTc_425_F | GAATTCCCCTCCACCGCGG | Primer pair binds around MCS of pKNOCK-Tc to amplify inserts off plasmid |
| pKNOCKTc_558_R | TGATCAAGCTGACGCGTCCT | |
| 24399_884_F | GAACGCTGGCCTTCAACGTG | Primer pair binds E-37 DNA upstream and downstream of the 231-bp internal region of fcs-2; used to verify insertion of pKNOCK plasmids in fcs-2 of E-37 |
| 24399_1383_R | GAAATCCTCCGAAATCCGCCC | |
| 24379_423_F | TCTGGAAGGCATGAAGATCC | Primer pair amplifies 292-bp region of dctP (SSE37_24379) used for cloning into pKNOCK-Km |
| 24379_714_R | ATCGATCACCTCCTGCAGAT | |
| 24379_367966_F | TCTAGAAGCTTTGTTTGATGGATCAACGCACT | Primer pair amplifies 1.7-kb HindIII-BamHI DNA fragment which encompasses the region 579 bp upstream of dctP start codon and 83 bp downstream of dctP stop codon |
| 24379_369669_R | TGCGTAGGATCCATCAGGATCAGGAACGTCAGC | |
| 24379_690_F | AGAGGATCTGCAGGAGGTGA | Primer pair amplifies 188-bp region of dctP (SSE37_24379) used for quantifying dctP expression in WT E-37 on hydroxycinnamic acids |
| 24379_877_R | ACGTCTCGTAGATCGGGTTG | |
| 12324_562_F | CTGACAGCGACACACCTGAC | Amplifies 181-bp region of (SSE37_12324) for quantifying fcs-1 expression in WT E-37 and fcs-2 mutant |
| 12324_742_R | CCTTCAGGAAGGTGAAGCAC | |
| 24399_1249_F | CTCAACGACCCGAAGAAGAC | Amplifies 187-bp region of fcs-2 (SSE37_24399) for quantifying fcs-2 expression in WT E-37 and fcs-1 mutant |
| 24399_1435_R | ACAATTCCAGACGCAGGTTC | |
| 15096_340_F | GCCCATATCTGGTTCCTCAA | Primer pair amplifies 159-bp region of rpoC (SSE37_15096) for use as housekeeping gene 1 of 3 in RT-qPCR |
| 15096_498_R | TTCCTCTTCGGTCAGCATCT | |
| map_13553_620_F | GCATGTTCTTCACCATCGAG | Primer pair amplifies 166-bp region of map (SSE37_13553) for use as housekeeping gene 2 of 3 in RT-qPCR |
| map_13553_785_R | GCGGGAGAGAGGGTAAAGAT | |
| alaS_ 05000 _97_F | GATCCGACGCTTATGTTCGT | Primer pair amplifies 151-bp region of alaS (SSE37_05000) for use as housekeeping gene 3 of 3 in RT-qPCR |
| alaS_ 05000_247_R | TGTAACCGACGTTGTCCAGA |
The 21-bp scar sequences are in lowercase. The SalI site is underlined.
WT, wild type.
The fcs-2 (SSE37_24399) mutant was generated using insertional mutagenesis with the pKNOCK-Km plasmid (64). A 231-bp internal region of SSE37_24399 (bp positions 983 to 1213) was cloned into pCR2.1-TOPO, subcloned into pKNOCK-Km to generate plasmid pKNOCK-Km_24399, and then introduced into the E. coli mating strain BW20767 (65). Conjugation of BW20767(pKNOCK-Km_24399) with wild-type S. stellata was performed as described above. Single-crossover homologous recombination resulted in integration of the entire plasmid into the fcs-2 gene. The fcs-1 fcs-2 double mutant was generated by cloning the same 231-bp fragment of fcs-2 into pKNOCK-Tc (64) (making pKNOCK-Tc_24399), and biparental mating of the E. coli BW20767 harboring this plasmid with the fcs-1 mutant strain of S. stellata. Different plasmids were used for chromosomal integration of fcs-1(pARO180) and fcs-2(pKNOCK) to eliminate the chance of crossover between homologous sections of the plasmids during generation of the double knockout.
A dctP (SSE37_24379) mutant was generated using insertional mutagenesis with the pKNOCK-Km vector harboring a 292-bp internal fragment (positions 423 to 714 bp) of dctP and following procedures outlined above. To confirm the role of SSE37_24379, complementation of the dctP with the broad-host-range plasmid pRK415 (66) harboring the wild-type dctP gene was attempted. The phenotypes on different substrates were determined by growth assays on HCAs and their intermediates.
All chromosomal disruptions were confirmed by PCR amplification and sequencing of junction sites. All plasmids and primers used in generation of mutants are listed in Tables 2 and 3, respectively.
Biolog phenotypic microarrays of fcs mutants.
Custom phenotypic microarrays were performed to assay for redox activity of strains when grown on defined carbon sources (10 mM acetate and 2 mM each aromatic monomer [p-hydroxybenzoate, ferulate, and p-coumarate]). All fcs mutants and wild-type S. stellata strains were precultured in MBM with 10 mM acetate and supplemented with kanamycin and tetracycline, as necessary. Assays were performed in 96-well flat-bottom plates (Costar) containing 1× MBM, 1× Biolog dye mix G (Hayward, CA), 107 cells/ml S. stellata, and a single carbon source. Plates were incubated at 30°C in an OmniLog reader (Biolog), where digital colorimetric readings were captured every 30 min to monitor the intensity of dye reduction over time, recorded in OmniLog units/time.
RNA isolation and reverse transcription.
For total RNA extraction, strains were grown on 7 mM acetate or 2 mM HCA. Total RNA was extracted using the RNeasy minikit (Qiagen), with slight modification; cells were lysed by vortexing at maximum speed for 10 min with 0.2 g of low-binding 200-μm zirconium beads (OPS Diagnostics, LLC, Lebanon, NJ). Genomic DNA was removed using the Turbo DNA-free kit (Ambion, Austin, TX), as per the manufacturer's instruction. cDNA synthesis was performed with Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen) using 500 ng total RNA, as described in the product manual.
Gene expression analyses.
RT-qPCR was performed using 1× SYBR Premix Ex Taq (Perfect Real Time) (TaKaRa Bio, Inc., Otsu, Japan) in 25-μl volumes consisting of 12.5 μl of 1× SYBR premix, 10 μl cDNA, and 1.25 μl forward and 1.25 μl reverse primers (10 μM). Genes of interest were normalized to three previously validated housekeeping genes, rpoC, map, and alaS (39). Quantitative PCR (qPCR) amplification included a 95°C denaturation for 3 min, followed by 40 cycles of 95°C for 20 s, 57°C for 20 s, and 72°C extension for 15 s, and a final extension at 72°C for 5 min. Melt curves were obtained between 50 and 100°C at 1°C per s with readings every 1°C. cDNA was diluted with 10 mM Tris (pH 8.0), and endpoint PCR was performed prior to RT-qPCR to ensure that the threshold cycle (CT) values for each gene and replicate were between 15 and 30 cycles. Efficient genomic DNA removal (CT values >5 cycles) was confirmed by including no-RT controls. Technical and biological replicates were included in triplicate for each gene. RT-qPCR analyses were performed using qBase (67) to normalize and relativize gene transcripts.
Analysis of substrate concentrations.
The concentrations of p-coumarate and ferulate were monitored in cell-free supernatants from S. stellata cultures spectrophotometrically. The wavelengths at which each substrate absorbed maximally in minimal medium were empirically determined by performing wavelength scans from 200 to 700 nm, with 0.002% sample (1 ml total volume, diluted with sterile Milli-Q water). Molar extinction coefficients for each substrate in the minimal medium were calculated to be 0.21 liters · mol−1 · cm−1 for ferulate and 0.40 liters · mol−1 · cm−1 for p-coumarate. To quantify extracellular concentrations of substrates, culture aliquots were passaged through a 0.22-μm filter, and absorbances at 283 nm (p-coumarate) and 308 nm (ferulate) were monitored with a Beckman DU 800 UV-Vis spectrophotometer. Concentrations were calculated from 10-point standard curves (0.3 to 3.0 mM), with r2 values of 0.97 for ferulate and 0.99 for p-coumarate (Fig. S1). Full (200 to 700 nm) wavelength scans were performed for all cultures. The shape of the absorbance curves provides additional evidence of the specificity of the approach (Fig. S2).
Genome analyses.
Protein sequences from organisms with experimental validation of function were used in homology searches against the S. stellata genome. These searches were performed using the BLASTp algorithm at NCBI.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Elizabeth Fozo (UTK) for providing guidance on transcriptional analysis of the box operon. This research was supported by a grant from the National Science Foundation (OCE-1357242). Additionally, A.M.F. and C.A.G. were supported as part of the Center for Direct Catalytic Conversion of Biomass to Biofuels (C3Bio), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, award number DE-SC0000997.
A.M.F., C.A.G., and A.B. developed initial project objectives and experimental design. A.M.F. generated the fcs mutants and M.J.C. constructed the dctP mutant. A.M.F. and M.J.C. performed mutant growth characterizations. M.J.C. performed RT-qPCR assays, with statistical support from C.A.G. A.M.F. led fcs data analysis and manuscript preparation, with support from M.J.C. and A.B.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02027-18.
REFERENCES
- 1.Laskar DD, Yang B, Wang H, Lee J. 2013. Pathways for biomass-derived lignin to hydrocarbon fuels. Biofuels Bioprod Biorefin 7:602–626. doi: 10.1002/bbb.1422. [DOI] [Google Scholar]
- 2.Ragauskas AJ, Beckham GT, Biddy MJ, Chandra R, Chen F, Davis MF, Davison BH, Dixon RA, Gilna P, Keller M, Langan P, Naskar AK, Saddler JN, Tschaplinski TJ, Tuskan GA, Wyman CE. 2014. Lignin valorization: improving lignin processing in the biorefinery. Science 344:1246843. doi: 10.1126/science.1246843. [DOI] [PubMed] [Google Scholar]
- 3.Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, Marita JM, Hatfield RD, Ralph SA, Christensen JH, Boerjan W. 2004. Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem Rev 3:29–60. doi: 10.1023/B:PHYT.0000047809.65444.a4. [DOI] [Google Scholar]
- 4.Bugg TD, Ahmad M, Hardiman EM, Rahmanpour R. 2011. Pathways for degradation of lignin in bacteria and fungi. Nat Prod Rep 28:1883–1896. doi: 10.1039/c1np00042j. [DOI] [PubMed] [Google Scholar]
- 5.Kuwahara M, Glenn JK, Morgan MA, Gold MH. 1984. Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169:247–250. doi: 10.1016/0014-5793(84)80327-0. [DOI] [Google Scholar]
- 6.Tien M, Kirk TK, Bull C, Fee JA. 1986. Steady-state and transient-state kinetic studies on the oxidation of 3,4-dimethoxybenzyl alcohol catalyzed by the ligninase of Phanerochaete chrysosporium Burds. J Biol Chem 261:1687–1693. [PubMed] [Google Scholar]
- 7.Kirk TK, Farrell RL. 1987. Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol 41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- 8.Harwood CS, Parales RE. 1996. The beta-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol 50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs G. 2008. Anaerobic metabolism of aromatic compounds. Ann N Y Acad Sci 1125:82–99. doi: 10.1196/annals.1419.010. [DOI] [PubMed] [Google Scholar]
- 10.Díaz E, Jimenez JI, Nogales J. 2013. Aerobic degradation of aromatic compounds. Curr Opin Biotechnol 24:431–442. doi: 10.1016/j.copbio.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Hartley RD, Ford CW. 1989. Phenolic constituents of plant cell walls and wall biodegradability, p 137–145. In Lewis NG, Paice MG (ed), Plant cell wall polymers, vol 399 American Chemical Society, Washington, DC. [Google Scholar]
- 12.Dixon RA, Achnine L, Kota P, Liu C-J, Reddy MSS, Wang L. 2002. The phenylpropanoid pathway and plant defence-a genomics perspective. Mol Plant Pathol 3:371–390. doi: 10.1046/j.1364-3703.2002.00131.x. [DOI] [PubMed] [Google Scholar]
- 13.Kroon PA, Williamson G. 1999. Hydroxycinnamates in plants and food: current and future perspectives. J Sci Food Agric 79:355–361. doi:. [DOI] [Google Scholar]
- 14.Naczk M, Shahidi F. 2006. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal 41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Torres-Mancera MT, Cordova-Lopez J, Rodriguez-Serrano G, Roussos S, Ramirez-Coronel MA, Favela-Torres E, Saucedo-Castaneda G. 2011. Enzymatic extraction of hydroxycinnamic acids from coffee pulp. Food Technol Biotechnol 49:369–373. [Google Scholar]
- 16.Sun RC, Sun XF, Zhang SH. 2001. Quantitative determination of hydroxycinnamic acids in wheat, rice, rye, and barley straws, maize stems, oil palm frond fiber, and fast-growing poplar wood. J Agric Food Chem 49:5122–5129. doi: 10.1021/jf010500r. [DOI] [PubMed] [Google Scholar]
- 17.Ralph J, Hatfield RD, Quideau S, Helm RF, Grabber JH, Jung HJG. 1994. Pathway of p-coumaric acid incorporation into maize lignin as revealed by NMR. J Am Chem Soc 116:9448–9456. doi: 10.1021/ja00100a006. [DOI] [Google Scholar]
- 18.Ralph J, Hatfield RD, Grabber JH, Jung H-JG, Quideau S, Helm RF. 1998. Cell wall cross-linking in grasses by ferulates and diferulates, p 209–236. In Lewis NG, Sarkanen S (ed), Lignin and lignan biosynthesis, vol 697 American Chemical Society, Washington, DC. [Google Scholar]
- 19.de Oliveira DM, Finger-Teixeira A, Mota TR, Salvador VH, Moreira-Vilar FC, Molinari HB, Mitchell RA, Marchiosi R, Ferrarese-Filho O, dos Santos WD. 2015. Ferulic acid: a key component in grass lignocellulose recalcitrance to hydrolysis. Plant Biotechnol J 13:1224–1232. doi: 10.1111/pbi.12292. [DOI] [PubMed] [Google Scholar]
- 20.Macoy DM, Kim W-Y, Lee SY, Kim MG. 2015. Biotic stress related functions of hydroxycinnamic acid amide in plants. J Plant Biol 58:156–163. doi: 10.1007/s12374-015-0104-y. [DOI] [Google Scholar]
- 21.Lowe TM, Ailloud F, Allen C. 2015. Hydroxycinnamic acid degradation, a broadly conserved trait, protects Ralstonia solanacearum from chemical plant defenses and contributes to root colonization and virulence. Mol Plant Microbe Interact 28:286–297. doi: 10.1094/MPMI-09-14-0292-FI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fresco P, Borges F, Diniz C, Marques MPM. 2006. New insights on the anticancer properties of dietary polyphenols. Med Res Rev 26:747–766. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- 23.Razzaghi-Asl N, Garrido J, Khazraei H, Borges F, Firuzi O. 2013. Antioxidant properties of hydroxycinnamic acids: a review of structure-activity relationships. Curr Med Chem 20:4436–4450. doi: 10.2174/09298673113209990141. [DOI] [PubMed] [Google Scholar]
- 24.Teixeira J, Gaspar A, Garrido EM, Garrido J, Borges F. 2013. Hydroxycinnamic acid antioxidants: an electrochemical overview. Biomed Res Int 2013:251754. doi: 10.1155/2013/251754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vargas-Tah A, Gosset G. 2015. Production of cinnamic and p-hydroxycinnamic acids in engineered microbes. Front Bioeng Biotechnol 3:116. doi: 10.3389/fbioe.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar N, Pruthi V. 2014. Potential applications of ferulic acid from natural sources. Biotechnol Rep 4:86–93. doi: 10.1016/j.btre.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priefert H, Rabenhorst J, Steinbuchel A. 2001. Biotechnological production of vanillin. Appl Microbiol Biotechnol 56:296–314. doi: 10.1007/s002530100687. [DOI] [PubMed] [Google Scholar]
- 28.Plaggenborg R, Overhage J, Loos A, Archer JA, Lessard P, Sinskey AJ, Steinbuchel A, Priefert H. 2006. Potential of Rhodococcus strains for biotechnological vanillin production from ferulic acid and eugenol. Appl Microbiol Biotechnol 72:745–755. doi: 10.1007/s00253-005-0302-5. [DOI] [PubMed] [Google Scholar]
- 29.Muheim A, Lerch K. 1999. Towards a high-yield bioconversion of ferulic acid to vanillin. Appl Microbiol Biotechnol 51:456–461. doi: 10.1007/s002530051416. [DOI] [Google Scholar]
- 30.Campillo T, Renoud S, Kerzaon I, Vial L, Baude J, Gaillard V, Bellvert F, Chamignon C, Comte G, Nesme X, Lavire C, Hommais F. 2014. Analysis of hydroxycinnamic acid degradation in Agrobacterium fabrum reveals a coenzyme A-dependent, beta-oxidative deacetylation pathway. Appl Environ Microbiol 80:3341–3349. doi: 10.1128/AEM.00475-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otani H, Lee YE, Casabon I, Eltis LD. 2014. Characterization of p-hydroxycinnamate catabolism in a soil actinobacterium. J Bacteriol 196:4293–4303. doi: 10.1128/JB.02247-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parke D, Ornston LN. 2003. Hydroxycinnamate (hca) catabolic genes from Acinetobacter sp. strain ADP1 are repressed by HcaR and are induced by hydroxycinnamoyl-coenzyme A thioesters. Appl Environ Microbiol 69:5398–5409. doi: 10.1128/AEM.69.9.5398-5409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masai E, Harada K, Peng X, Kitayama H, Katayama Y, Fukuda M. 2002. Cloning and characterization of the ferulic acid catabolic genes of Sphingomonas paucimobilis SYK-6. Appl Environ Microbiol 68:4416–4424. doi: 10.1128/AEM.68.9.4416-4424.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overhage J, Priefert H, Steinbuchel A. 1999. Biochemical and genetic analyses of ferulic acid catabolism in Pseudomonas sp. strain HR199. Appl Environ Microbiol 65:4837–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattes TE, Alexander AK, Richardson PM, Munk AC, Han CS, Stothard P, Coleman NV. 2008. The genome of Polaromonas sp. strain JS666: insights into the evolution of a hydrocarbon- and xenobiotic-degrading bacterium, and features of relevance to biotechnology. Appl Environ Microbiol 74:6405–6416. doi: 10.1128/AEM.00197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baude J, Vial L, Villard C, Campillo T, Lavire C, Nesme X, Hommais F. 2016. Coordinated regulation of species-specific hydroxycinnamic acid degradation and siderophore biosynthesis pathways in Agrobacterium fabrum. Appl Environ Microbiol 82:3515–3524. doi: 10.1128/AEM.00419-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchan A, Gonzalez JM, Moran MA. 2005. Overview of the marine roseobacter lineage. Appl Environ Microbiol 71:5665–5677. doi: 10.1128/AEM.71.10.5665-5677.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez JM, Mayer F, Moran MA, Hodson RE, Whitman WB. 1997. Sagittula stellata gen. nov., sp. nov., a lignin-transforming bacterium from a coastal environment. Int J Syst Bacteriol 47:773–780. doi: 10.1099/00207713-47-3-773. [DOI] [PubMed] [Google Scholar]
- 39.Gulvik CA, Buchan A. 2013. Simultaneous catabolism of plant-derived aromatic compounds results in enhanced growth for members of the Roseobacter lineage. Appl Environ Microbiol 79:3716–3723. doi: 10.1128/AEM.00405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moran MA, Hodson RE. 1994. Dissolved humic substances of vascular plant origin in a coastal marine environment. Limnol Oceanogr 39:762–771. doi: 10.4319/lo.1994.39.4.0762. [DOI] [Google Scholar]
- 41.Buchan A, Collier LS, Neidle EL, Moran MA. 2000. Key aromatic-ring-cleaving enzyme, protocatechuate 3,4-dioxygenase, in the ecologically important marine Roseobacter lineage. Appl Environ Microbiol 66:4662–4672. doi: 10.1128/AEM.66.11.4662-4672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitra A, Kitamura Y, Gasson MJ, Narbad A, Parr AJ, Payne J, Rhodes MJC, Sewter C, Walton NJ. 1999. 4-Hydroxycinnamoyl-CoA hydratase/lyase (HCHL)–an enzyme of phenylpropanoid chain cleavage from Pseudomonas. Arch Biochem Biophys 365:10–16. doi: 10.1006/abbi.1999.1140. [DOI] [PubMed] [Google Scholar]
- 43.Mulligan C, Fischer M, Thomas GH. 2011. Tripartite ATP-independent periplasmic (TRAP) transporters in bacteria and archaea. FEMS Microbiol Rev 35:68–86. doi: 10.1111/j.1574-6976.2010.00236.x. [DOI] [PubMed] [Google Scholar]
- 44.Sikkema J, de Bont JAM, Poolman B. 1994. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem 269:8022–8028. [PubMed] [Google Scholar]
- 45.Sikkema J, de Bont JAM, Poolman B. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59:201–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borglin S, Joyner D, DeAngelis KM, Khudyakov J, D'Haeseleer P, Joachimiak MP, Hazen T. 2012. Application of phenotypic microarrays to environmental microbiology. Curr Opin Biotechnol 23:41–48. doi: 10.1016/j.copbio.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Barragán MJL, Blazquez B, Zamarro MT, Mancheno JM, Garcia JL, Diaz E, Carmona M. 2005. BzdR, a repressor that controls the anaerobic catabolism of benzoate in Azoarcus sp. CIB, is the first member of a new subfamily of transcriptional regulators. J Biol Chem 280:10683–10694. [DOI] [PubMed] [Google Scholar]
- 48.Valderrama JA, Durante-Rodriguez G, Blazquez B, Garcia JL, Carmona M, Diaz E. 2012. Bacterial degradation of benzoate: cross-regulation between aerobic and anaerobic pathways. J Biol Chem 287:10494–10508. doi: 10.1074/jbc.M111.309005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gescher J, Eisenreich W, Worth J, Bacher A, Fuchs G. 2005. Aerobic benzoyl-CoA catabolic pathway in Azoarcus evansii: studies on the non-oxygenolytic ring cleavage enzyme. Mol Microbiol 56:1586–1600. doi: 10.1111/j.1365-2958.2005.04637.x. [DOI] [PubMed] [Google Scholar]
- 50.Chae JC, Zylstra GJ. 2006. 4-Chlorobenzoate uptake in Comamonas sp. strain DJ-12 is mediated by a tripartite ATP-independent periplasmic transporter. J Bacteriol 188:8407–8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salmon RC, Cliff MJ, Rafferty JB, Kelly DJ. 2013. The CouPSTU and TarPQM transporters in Rhodopseudomonas palustris: redundant, promiscuous uptake systems for lignin-derived aromatic substrates. PLoS One 8:e59844. doi: 10.1371/journal.pone.0059844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ni J, Tao F, Du HQ, Xu P. 2015. Mimicking a natural pathway for de novo biosynthesis: natural vanillin production from accessible carbon sources. Sci Rep 5:13670. doi: 10.1038/srep13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kato S, Chino K, Kamimura N, Masai E, Yumoto I, Kamagata Y. 2015. Methanogenic degradation of lignin-derived monoaromatic compounds by microbial enrichments from rice paddy field soil. Sci Rep 5:14295. doi: 10.1038/srep14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sembiring T, Winter J. 1990. Demethylation of aromatic compounds by strain B10 and complete degradation of 3-methoxybenzoate in co-culture with Desulfosarcina strains. Appl Microbiol Biotechnol 33:233–238. [Google Scholar]
- 55.González JM, Whitman WB, Hodson RE, Moran MA. 1996. Identifying numerically abundant culturable bacteria from complex communities: an example from a lignin enrichment culture. Appl Environ Microbiol 62:4433–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bergbauer M, Newell SY. 1992. Contribution to lignocellulose degradation and DOC formation from a salt marsh macrophyte by the ascomycete Phaeosphaeria spartinicola. FEMS Microbiol Lett 86:341–348. doi: 10.1111/j.1574-6968.1992.tb04826.x. [DOI] [Google Scholar]
- 57.Moran MA, Hodson RE. 1990. Contributions of degrading Spartina alterniflora lignocellulose to the dissolved organic carbon pool of a salt marsh. Mar Ecol Prog Ser 62:161–168. doi: 10.3354/meps062161. [DOI] [Google Scholar]
- 58.González JM, Moran MA. 1997. Numerical dominance of a group of marine bacteria in the alpha-subclass of the class Proteobacteria in coastal seawater. Appl Environ Microbiol 63:4237–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newton RJ, Griffin LE, Bowles KM, Meile C, Gifford S, Givens CE, Howard EC, King E, Oakley CA, Reisch CR, Rinta-Kanto JM, Sharma S, Sun S, Varaljay V, Vila-Costa M, Westrich JR, Moran MA. 2010. Genome characteristics of a generalist marine bacterial lineage. ISME J 4:784–798. doi: 10.1038/ismej.2009.150. [DOI] [PubMed] [Google Scholar]
- 60.Piekarski T, Buchholz I, Drepper T, Schobert M, Wagner-Doebler I, Tielen P, Jahn D. 2009. Genetic tools for the investigation of Roseobacter clade bacteria. BMC Microbiol 9:265. doi: 10.1186/1471-2180-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lidbury I, Murrell JC, Chen Y. 2014. Trimethylamine N-oxide metabolism by abundant marine heterotrophic bacteria. Proc Natl Acad Sci U S A 111:2710–2715. doi: 10.1073/pnas.1317834111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stibitz S. 1994. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol 235:458–465. doi: 10.1016/0076-6879(94)35161-9. [DOI] [PubMed] [Google Scholar]
- 63.Jones RM, Williams PA. 2003. Mutational analysis of the critical bases involved in activation of the AreR-regulated sigma54-dependent promoter in Acinetobacter sp. strain ADP1. Appl Environ Microbiol 69:5627–5635. doi: 10.1128/AEM.69.9.5627-5635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alexeyev MF. 1999. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of Gram-negative bacteria. Biotechniques 26:824–826. doi: 10.2144/99265bm05. [DOI] [PubMed] [Google Scholar]
- 65.Metcalf WW, Jiang W, Daniels LL, Kim SK, Haldimann A, Wanner BL. 1996. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 66.Keen NT, Tamaki S, Kobayashi D, Trollinger D. 1988. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 67.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.