Abstract
Background
Philip Morris International (PMI) currently claims that its heated tobacco product, IQOS, reduces health risk by reducing users’ exposure to harmful and potentially harmful constituents present in tobacco smoke. Given the tobacco industry’s long history of misrepresenting and obfuscating research, independent assessment of PMI’s claims is important. Analysis of Accord, a failed but strikingly similar precursor to IQOS, may help contextualise PMI’s claims in its Modified Risk Tobacco Product (MRTP) application.
Methods
We analysed previously secret internal Philip Morris (PM) and PMI documents, public communications and MRTP application.
Results
PM marketed Accord as a ‘cleaner’ tobacco product in an attempt to address smokers’ growing health concerns without making explicit health claims. While PM communications asserted that Accord reduced users’ exposure to harmful constituents, company scientists and executives consistently stressed to both regulators and the public that such reductions did not render Accord safer. IQOS’s design and marketing are similar to Accord’s. On the basis of aerosol chemistry data, IQOS reduces user exposure to some compounds compared with Accord but raises them for others.
Discussion
IQOS appears to be a variant of Accord without consistent improvements in exposure to aerosol toxic compounds. In contrast to PM’s past claims for Accord, PMI now claims in its MRTP application that IQOS reduces health risk. This shift in stance is likely not the result of any toxicological difference between Accord and IQOS, but rather a change in the social and regulatory landscape permitting these claims.
Keywords: advertising and promotion, tobacco industry documents, toxicology, non-cigarette tobacco products
Background
The tobacco industry has developed heated tobacco products since the 1960s.1 When these ‘safer’ offerings have been marketed, consumers have generally rejected the products’ poor taste, smell and user experience.2 As of May 2018, however, a heated tobacco product from Philip Morris International (PMI), IQOS, had won at least moderate consumer acceptance in several of the 31 countries in which it was available.3 By early 2018, IQOS had captured nearly 15% of the national tobacco market share in Japan, 2 years after its introduction.4 Despite the continued predominance of conventional cigarettes to company profit,3 PMI publicly frames IQOS as presaging the company’s supposed departure from the cigarette business altogether.5
In promoting IQOS, PMI has attempted to foster a perception of the product as reduced risk. The company’s public communications and warning label statements claim that switching completely to IQOS is a safer alternative to smoking cigarettes.6 In an attempt to court favourable regulation, taxation and exemptions from smoke-free ordinances for IQOS, PMI has begun promoting the product’s purported benefits in meetings with national health authorities.7 In December 2016, PMI submitted a multi-million page Modified Risk Tobacco Products (MRTP) application to the US Food and Drug Administration (FDA).8 If approved, PMI will be able to market IQOS in the USA as a reduced risk alternative to conventional cigarettes.
Given the tobacco industry’s well-documented history of misrepresenting and obfuscating its research,2 independent assessment of PMI’s claims is important. While PMI’s internal data and business strategy on IQOS are largely unknown, internal Philip Morris (PM) documents discussing Accord—a failed, but similar heat-not-burn precursor to IQOS—are available in public archives. We compared available documents detailing product design, exposure data and safety claims PM made for Accord to data and claims submitted as part of the IQOS MRTP application. Our aims were to compare product design characteristics; determine if IQOS exposure levels were demonstrably and consistently improved compared with Accord; and learn how PM understood the extent to which reductions in exposure to harmful constituents reduced harm to users.
Methods
Between October 2013 and January 2016, we searched industry documents (available through the Truth Tobacco Industry Document Library; https://industrydocuments.library.ucsf.edu/tobacco/) detailing tobacco companies’ development of various heat-not-burn tobacco product prototypes. Between January 2017 and May 2018, this dataset was expanded with additional iterative searches9–11 focused specifically on Accord, with initial keyword searches including "Accord market*,” "Accord research," "Accord consumer," "Accord science," and related terms drawn from earlier searches, such as "electrically heated cigarette smoking system" (EHCSS) and "EHCSS." We then conducted snowball searches to locate related documents using reference (Bates) numbers, file locations, dates and individuals mentioned in pertinent documents, and by refining subsequent searches with Boolean operators and year and publication type filters. Iterative searches were repeated until keywords and documents yielded only previously viewed documents, suggesting saturation.
To ensure documents’ internal consistency, all documents were organised thematically and chronologically, and relevant documents were compared with relevant sections of PMI’s MRTP application available on the FDA’s website.8 Triangulation with online search engines, news coverage, public statements made by PMI (most often found at http://www.pmi.com) and internal PMI documents obtained by Reuters (accessible at https://www.documentcloud.org/public/search/projectid:_2033738) generated data that helped resolve and contextualise questions raised by the documents. Having reviewed over 1,000 documents, this analysis is based on a final collection of 200 documents.
Results
Accord origins and specifications
Through the late 1980s and early 1990s, public health consensus on the negative health effects and addictiveness of smoking led to smoke-free policies and the broader social denormalisation of smoking.12 In response to these pressures, RJ Reynolds introduced its ‘clean smoke’ heated tobacco product, Premier, in 1988.13 Although similar to a conventional cigarette, Premier heated tobacco, instead of burning (combusting) it, producing an aerosol for inhalation.14 15 Industry scientists hypothesised that such products presented a lower health risk to users: because tobacco was not combusted, smokers might beexposed to fewer respiratory irritants and carcinogens.1 Marketed as a ‘smokeless’, ‘cleaner’ cigarette, Premier was widely rejected by consumers and pulled from shelves within 4 months of its release.
In response to Premier, PM began developing reduced-risk tobacco products of its own.2 13 16 A 1990 presentation introduced Project Beta, a battery-operated device that would heat rather than burn tobacco.17 Through Project Beta, PM developed the heat-not-burn product, Accord (internally referred to as an electrically heated smoking system (EHCSS)).18 Accord comprised a short, low-tar cigarette (in 3 and 6 mg tar versions) and a battery-powered lighter into which the user inserted the short cigarette. On the user’s puffing, the tobacco in the cigarette would heat, generating a tobacco-flavoured aerosol for inhalation. Retailing at US$77 (inflation-adjusted to 2018), Accord was packaged for sale as part of a kit that included Accord’s special cigarettes (sold at prices comparable to regular cigarettes), the product’s heating device and an instructional video (table 1).16
Table 1.
Component comparison: Accord and IQOS
| Accord | IQOS | |
| Product description | '… [Accord is] an electrically heated cigarette smoking system, comprised of a lighter/heater, which supplies power to the system, and a cigarette designed to be used only with the lighter/heater … activation occurs as the smoker takes a puff. The flow of the puff is detected by a sensor, which then activates the blade-heating cycle. The electronic system in the lighter/heater synchronizes puffing and energy to the blades. The result is that the cigarette delivers eight puffs to the smoker on demand, one for each blade.'36 | ’To operate … the user inserts a Tobacco Stick into the holder and turns on the device by means of a switch. This initiates the heating of the tobacco via the heating blade inserted into the tobacco plug. The tobacco neither ignites nor burns. The electronically controlled heating, in combination with the uniquely processed tobacco, prevents combustion from occurring. The holder supplies heat to the Tobacco Stick through the heating blade for a fixed period of approximately six min and allows up to fourteen puffs to be taken during that time.'61 |
| Health claims | ’The Accord product has not been proven to be safer. Substantial reductions in certain harmful compounds have not been proven to lead to a reduction in smoking-related diseases.'41 | '[IQOS] heats tobacco but does not burn it. This significantly reduces the production of harmful and potentially harmful chemicals. Scientific studies have shown that switching completely from cigarettes to [IQOS] can reduce the risks of tobacco-related diseases.'61 |
| Tobacco material | Tobacco, glycerol, water, ammonium magnesium phosphate, pectin, beta-cyclodextrin | Tobacco, glycerol, water, guar gum, cellulose, and propylene glycol |
| Cigarette configuration | 62 mm (overall length) 32 mm (tobacco plug) |
45 mm (overall length) 12 mm (tobacco plug) |
| Tobacco weight (mg/cig) | 407.6 | 314 |
| Tar (mg/cig) | 3.0 | 19.4 |
| Nicotine (mg/cig) | 0.2 | 1.29 |
| Filters | A low-efficiency filter (30 mm), containing a segment of activated carbon-in-paper | A polylactic acid filter (18 mm) and a low-density cellulose acetate mouthpiece filter similar to the filter of a conventional cigarette |
| Tube | Hollow acetate tube | Hollow acetate tube |
| Paper | Outer paper Tipping paper |
Outer paper Tipping paper |
| Retail price | US$77 (inflation adjusted to 2018) | US$110 |
| Heater description | ’The heater is equipped with an array of eight blades made from an iron-aluminide alloy, one blade for each of the eight possible puffs per cigarette …. activation occurs as the smoker takes a puff. The flow of the puff is detected by a sensor, which then activates the blade-heating cycle.'18 | ’The Holder heats the tobacco using a ceramic blade, which is pushed into the tobacco plug [upon insertion]. The Holder has a small battery, which stores enough energy for a single experience (ie, complete use of one Tobacco Stick). The power to the heating element is controlled by an embedded electronic system, which ensures that the temperature follows a pre-defined heating profile for each puff regardless of the user’s puffing behaviors. The Holder needs to be recharged prior to each Tobacco Stick use. The Holder is activated by a simple button and its status is conveyed through an interface that includes a colored LED.'61 |
| Peak temperature (°C) | 500 | 310–350 |
| Heating blade thickness (mm) | 8 | 0.36 |
| Puffs per charge | 13 cigarettes (eight puffs each) | 14 puffs per cigarette or 6 minutes of use (whichever comes first), after which the Holder must be recharged in the portable case before a new tobacco stick can be used. Portable case can charge Holder 20 times before needing re-charge. |
Accord marketing
In October 1998, PM introduced Accord in 16–20 stores in Richmond, Virginia (the location of a major PM manufacturing facility), expanding to 120 stores within 3 months.19 In the same year, PM released Accord in Osaka, Japan, under the name ‘Oasis’. The tobacco industry has long marketed its ‘cleaner’ (eg, low-tar) products in Japan due to perceptions that Japanese smokers value cleanliness and are more willing than consumers in other countries to embrace new technologies.13 20 During Accord’s first three years on the market, consumer awareness remained limited, and sales and retention were low.21 Dual use of Accord with conventional cigarettes was also high: 86% of those who smoked Accord used the product for between one-quarter and one-half of their tobacco use; and only 9% of Accord users used the product for 75% or more of their tobacco use.22 PM attributed this ‘situationa[l]/occasiona[l]’ use to the product’s poor taste, higher price than conventional cigarettes, unfamiliar operating system and unconventional appearance.21 22
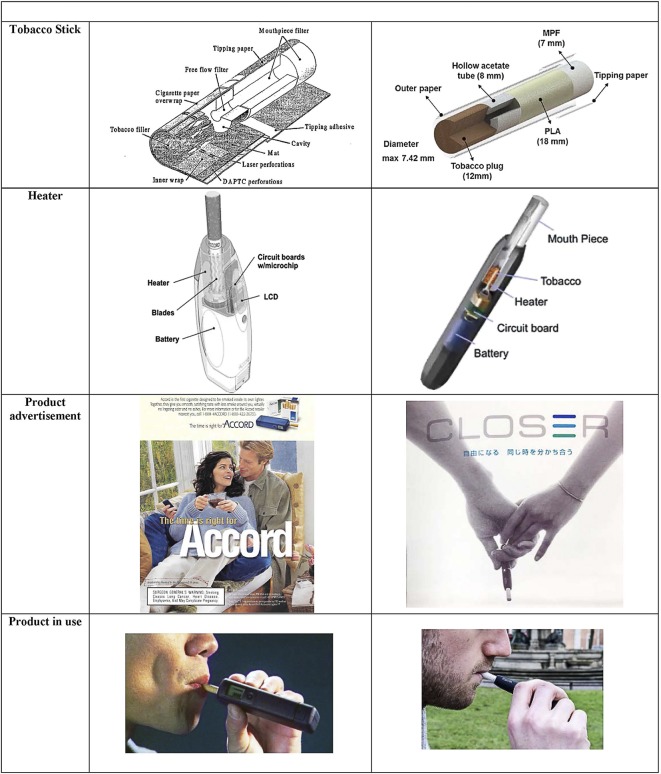
PM characterised Accord smokers as favouring product characteristics related to ‘hygiene or consideration of others,'23 and attributed the little success that Accord enjoyed to its perception as a ‘cleaner’ product that would not irritate others.21 To attract hygiene-conscious consumers, one Accord advertisement read, ‘Less smoke around you. Virtually no lingering odor. And no ashes’, all of which rendered Accord ‘a whole new way to smoke'.24 Another ad (figure 1) showed a couple sitting together on the same lounge chair while the man holds an Accord device, the woman seemingly unperturbed by any foul smell or smoke.
Figure 1.
Visual comparison of Accord and IQOS tobacco stick, heating device structure, advertising and product demonstration. Photo credit for Product in Use images: www.vaping360.com and WGBH Educational Foundation.
While PM emphasised Accord’s ‘cleanliness’ in consumer communications,25 the company also went to great lengths to clarify that such ‘cleanliness’ did not render the product safer than conventional cigarettes. In an advertisement accompanying the Accord Kit, PM stated that while ‘Accord reduces certain harmful smoke compounds you inhale … [(such] reductions … have not been proven to lead to a reduction in smoking-related diseases'.26 After asserting that Accord reduces carbon monoxide exposure by 98% compared with an ultra-light cigarette, PM asked rhetorically in the same brochure, ‘Are we saying that Accord is a "safer" cigarette? No.'26 The brochure also stated that ’public health authorities do not endorse either smoking fewer cigarettes or switching to lower-yield brands or a cigarette that heats rather than burns tobacco as a satisfactory way of reducing risk'.26 27 This is consistent with past and current research showing that the extent to which reduced exposure leads to reduced harm is unclear.28 29 Despite these clarifications, a 2003 PM presentation to the company’s ‘New Products Committee’ reported that on average 11% of consumers exposed to Accord advertisements believed Accord to be less harmful than other cigarettes.30
Outside of PM, research on Accord’s safety was limited. Two independent studies compared Accord smokers’ CO intake and heart rate to those of cigarette smokers, and found both levels to be lower in the Accord users.31 32 However, nicotine uptake for Accord smokers was slower than conventional cigarettes, leading smokers to ‘compensate’; that is, inhale more deeply to extract more nicotine, thereby negating any health benefits of lower tar cigarettes.31
The significance of these findings, however, was undercut by the product’s scant adoption. By 2003, PM had spent over $400 million in operating expenses and almost $70 million (inflation-adjusted to 2018) in capital expenses in developing subsequent versions33 of Accord.34 One PM study found that both Japanese and American consumers rejected Accord (although acceptance was higher in Japan) because of its taste (‘hard to draw, perceived harshness, not enough taste, not enough puffs per cigarette’) and inconvenience (‘charging time, cigarettes per charge’).35 Accord also required smokers to adopt a new routine (eg, buying a new brand of cigarettes, recharging the battery pack), while the device itself required ‘enhanced consumer support, product maintenance, spare parts, technology acceptance and product education’, which most users found burdensome.22 PM discontinued Accord in 2006 after 8 years on the market.
PM’s pursuit of normalisation
Accord was designed as a potentially reduced exposure product (PREP), with PM clarifying in external company communications that reduced exposure did not indicate reduced risk.36 While PM contracted an advertising company to draft harm reduction advertisements for Accord in the USA, these advertisements were ultimately not used,12 perhaps because tobacco companies were legally barred from making reduced risk or reduced exposure claims while Accord was available.37 PM marketed the product instead as a low-smoke alternative,16 likely hoping to imply to consumers that Accord was safer than conventional cigarettes.
In 2000, as part of efforts to improve the company’s beleaguered image, PM began lobbying for legislation that would grant the FDA authority over tobacco.38 In 2001, 3 years after PM introduced Accord, the Institute of Medicine (IoM, now National Academies of Sciences, Engineering and Medicine) conditionally endorsed PREPs as potentially capable of reducing user risk.39 Believing that reduced risk products could help ‘normalise’ the company,27 40 PM echoed IoM policy recommendations in arguing that potential legislation should create separate classifications for reduced exposure products and reduced risk products. This distinction would enable PM to make reduced exposure claims for products like Accord, without having to wait for long-term evidence finding exposure reduction sufficient to indicate risk reduction.27 The proposed legislation failed to pass Congress in 2004.
In 2007, a year after PM discontinued Accord, Kenneth Podraza, Vice President of Research and Development at PM USA, wrote to the Surgeon General in an effort to gain government endorsement of Accord. ‘In the absence of FDA regulation’, Podraza stated that PM ‘now turn[ed] to you for guidance’, asking that the Office of the Surgeon General determine if Accord is a PREP,41 likely in the hopes that the Surgeon General’s designation of Accord as reduced exposure would generate increased consumer acceptance of either Accord (should it be reintroduced), or of future, similar products. The tobacco industry has long viewed third-party endorsements of industry products as crucial to those products’ success.2 42 Podraza attributed Accord’s commercial failure to both consumer rejection and the company’s ‘inability to communicate a potential reduced exposure message'.41
Reiterating PM’s previous communications on Accord, Podraza clarified that while Accord may be a PREP it nonetheless ’has not been proven safer [as] substantial reductions in certain harmful compounds have not been proven to lead to a reduction in smoking-related diseases'.41 Podraza concluded his letter stating that PM would continue to develop future potentially reduced-risk products that smokers would hopefully accept.41 We found no evidence that the Surgeon General responded to Podraza. In 2008, PM briefly test-marketed a near-identical product, ‘Heatbar’ in Switzerland and Australia.16 43 44
IQOS and Accord product design
In 2014, PMI introduced a new heated tobacco product, IQOS, in Italy and Japan. IQOS’s moderate success in several markets as of 2018 is at least partly attributable to the current social and regulatory landscape. When Accord was available, public health authorities were unwilling to deem safe, new, unpopular products designated as ‘cleaner’ by the industry.2 Since 2007, however, as the popularity of electronic cigarettes (e-cigarettes) increased,45–47 several prominent public health organisations48–51 and health authorities52 53 have promoted e-cigarettes as safer alternatives to cigarettes. In the USA, the 2009 Family Smoking Prevention and Tobacco Control Act granted the FDA authority to regulate all tobacco products.54 As part of this legislation, tobacco manufacturers could, for the first time, market preapproved products as ‘modified risk tobacco products’.
In addition to its distinct context, IQOS has several notable design differences from Accord. IQOS cigarettes have more nicotine and more than six times as much tar per cigarette as Accord cigarettes. IQOS’s HeatSticks are shorter than Accord’s cigarettes (45 mm vs 62 mm), as is its tobacco plug (12 mm vs 32 mm). IQOS cigarettes also have less tobacco per cigarette than Accord (314 mg vs 407.6 mg), are burned at a lower temperature (~350°C vs 500°C) and the product kit is approximately US$40 more expensive (inflation adjusted to 2018) than Accord’s (table 1).
The products also share a number of similarities. Like Accord, IQOS maintains the core technology of heating rather than burning tobacco, which purportedly lowers users’ exposure to harmful or potentially harmful constituents (HPHCs)—constituents linked to the most serious effects of tobacco use (eg, cancer, cardiovascular disease, respiratory effects, addiction). Both products work by activating a heating blade (eight iron-aluminide alloy blades for Accord and one ceramic blade for IQOS), which then warm(s) the adjacent tobacco, and both products’ electronic systems control the temperature of the blade and delimit the amount of puffs the user takes per cigarette (figure 1).
IQOS and Accord advertising
IQOS’s marketing has also resembled Accord’s. A 2016 internal training for employees managing IQOS’s social media presence revealed that, like Accord, IQOS is partially targeted at the hygiene-conscious, particularly ‘those who want to reduce risks to their young families'.55 PMI alerted employees that, as occurred with Accord, consumers may be turned off by the product’s ‘learning curve’, different ‘taste and satisfaction levels’ and the required time commitment, estimated at 3 weeks, to become accustomed to the product experience.55 Nonetheless, PMI stated in the same document that IQOS would ‘change the way legal-age smokers smoke for the better'.55
PMI also expects a number of indirect benefits to accrue to the company if consumers perceive IQOS to be safer than cigarettes. This is consistent with PM’s hopes for earlier reduced risk products.27 40 A 2014 internal PMI document entitled ‘10 year Corporate Affairs Objectives and Strategies’ frames IQOS as a key component in ‘normalising’ PMI’s business more broadly, transforming the company into a ‘trusted and indispensable partner … bringing solutions to the table'.56 In gaining the trust of the public and regulators, PMI hoped to both regain access to broader regulatory discussions from which it is currently excluded and reverse the trend of ‘PMI/industry de-normalization … [so as] to drive future growth'.56
Such normalisation efforts will be most successful if smokers switch completely to IQOS. Nonetheless, research on corporate social responsibility programmes has shown that tobacco companies can use purported gestures of goodwill (such as developing ‘safer’ products) to increase access to policymakers and generate support for industry activity regardless of a given programme’s effectiveness.38 57 58 In IQOS’s case, the mere manufacture of a potentially safer product may be enough to earn PMI influence.59 60
Comparing IQOS and Accord aerosol chemistry
In December 2016, PMI submitted a multi-million-page MRTP application to the FDA for the company to market IQOS in the USA with reduced-risk claims.8 While PM consistently clarified that reductions in exposure did not reduce user risk, PM’s spin-off company, PMI, now claims that such reductions in exposure do indeed reduce user risk, asserting that ‘switching completely [from cigarettes to IQOS] presents less risk of harm than continuing to smoke cigarettes'.61
Internal documents and statements by PMI scientists contradict these reduced risk claims about IQOS. In October 2015, an internal newsletter for Philip Morris Japan stated that while ‘the ‘Tobacco Vapor’ generated by the use of iQOS contains significantly lower levels of harmful or potentially harmful compounds’, PMI has nonetheless ‘not reached the point where we can say that it is ‘less harmful for adult smokers and those around them'62 (figure 2). In 2018, four former PMI scientists and researchers that worked on IQOS also claimed that the product’s reduction of certain compounds does not necessarily render IQOS safer.63
Figure 2.
Excerpt from an internal October 2015 Philip Morris Japan newsletter. To clarify employee understanding of IQOS, a mock employee asks: '… iQOS has a less harmful impact on health, right?' The company expert replies, ’No no no! While these [ambient air reductions] are important results, we have not reached the point where we can say that it is “less harmful” for both adult smokers and those around them.'74
To assess one measure on which PMI bases IQOS’s claims of reduced exposure and thus reduced risk, we compared aerosol chemistry data from PM’s Scientific Data Summary (SDS) of Accord with information provided in PMI’s MRTP application for IQOS. PM assembled the SDS to detail Accord’s product specifications and clinical and non-clinical research findings. An ‘evolving document … intended for scientific and regulatory discussions',36 PM compiled the report from 2002 to 2006 to support reduced-risk claims for the Accord,64 perhaps anticipating US governmental regulation of tobacco and the creation of a regulatory mechanism through which manufacturers could make reduced-exposure and/or reduced-risk claims for preapproved products.27 Despite detailing reductions in HPHC exposure compared with reference cigarettes, PM stated in later versions of the SDS that the company made neither a reduced exposure claim for Accord,36 nor a reduced risk claim, stating that ‘reduc[ing] exposure to potentially harmful smoke constituents [did] not … establis[h] whether the product decreases the hazard of smoking.'36
In both PM’s SDS for Accord and PMI’s MRTP application for IQOS, industry scientists quantified levels of HPHCs in the products’ mainstream aerosol. PMI frames IQOS as safer than conventional cigarettes partly because levels of 58 constituents (PMI-58) were lower in IQOS mainstream aerosol relative to 3R4F reference cigarette mainstream smoke. The MRTP application for IQOS does not report data on 18 compounds included in the SDS for Accord, many of which are nitrosamines and polycyclic aromatic hydrocarbons, all of which are known toxicants (table 2).
Table 2.
Compounds listed in Philip Morris’ Scientific Data Summary for Accord but not Philip Morris International’s modified risk tobacco product application for IQOS
| Polycyclic aromatic hydrocarbons | Benzo[b]fluoanthene, Benzo[k]fluoanthene, Benzo[j]fluoanthene, Ideno[1,2,3 cd]pyrene, Dibenz[a,h]pyrene, Dibenz[a,l]pyrene, Dibenz[a,e]pyrene, 5-methylchrysene |
| N-nitrosamines | N-Nitrosodimethylamine (NDMA), N-Nitrosoethylmethylamine (NEMA), N-Nitrosodiethylamine (NDEA), N-Nitrosodi-n-propylamine (NDPA), N-Nitrosodi-n-butylamine (NDBA), N-Nitrosopyrrolidine (NPYR), N-Nitrosopiperidine (NPIP) |
| Aliphatic nitrogen compounds | 2-nitropropane |
| Aromatic amines | o-anisidine, 2-naphthylamine |
The MRTP application presents percent reduction of HPHC yields from IQOS compared with that of 3R4F reference cigarette on a per cigarette/stick basis as well as normalised by nicotine yields (per nicotine basis). The SDS for Accord provides only absolute HPHC yields. In addition, because Accord and IQOS were compared to different reference cigarettes (2R4F and 3R4F, respectively), which have different smoke constituent yields, the percent reductions of Accord and IQOS to their respective reference cigarettes are not comparable. Thus, we compared constituent yields from Accord and IQOS on a per stick basis, using values reported in the SDS for Accord and MRTP for IQOS (table 3). Based on levels of 25 constituents measured in both Accord and IQOS mainstream aerosol, 8 constituents appeared to be higher in Accord emissions while 17 appeared to be higher in IQOS emissions. When normalized by the weight of tobacco in the product, IQOS exposures were higher for 12 constituents and lower for 13 compared to Accord.
Table 3.
Comparison of Accord and IQOS constituent yields on a per cigarette basis based on data from Philip Morris' Scientific Data Summary and Philip Morris International’s modified risk tobacco product application
| Constituent | IQOS (abs. value) |
Accord (abs. value) |
3R4F (abs. value) |
2R4F (abs. value) |
IQOS (% of 3R4F) |
Accord (% of 2R4F) |
Ratio IQOS/Accord (abs. values) |
| 1,3-Butadiene (μg) | 0.207 | 2.2 | 89.2 | 36.7 | 0.23 | 5.99 | 0.09 |
| 4-Aminobiphenyl (ng) | 7.8 | 0.113 | 3.21 | 1.24 | 243.0 | 9.1 | 69.0 |
| Acetaldehyde (μg) | 192 | 114 | 1602 | 670 | 12.0 | 17.0 | 1.68 |
| Acetamide (μg) | 2.96 | 0.592 | 13 | 4.72 | 22.8 | 12.5 | 5.00 |
| Acrolein (μg) | 8.32 | 16.2 | 158 | 61 | 5.3 | 26.6 | 0.51 |
| Acrylonitrile (μg) | 0.145 | 0.415 | 21.2 | 15.1 | 0.68 | 2.75 | 0.35 |
| Benz[a]anthracene (ng) | 2.65 | <0.13 | 28.4 | 10.8 | 9.3 | <1.20 | >20.4 |
| Benzene (μg) | 0.452 | 0.413 | 77.3 | 53.7 | 0.58 | 0.77 | 1.09 |
| Benzo[a]pyrene (ng) | 0.736 | <0.13 | 13.3 | 7.75 | 5.5 | <1.68 | >5.66 |
| Carbon Monoxide (mg) | 0.347 | 0.564 | 29.4 | 14.3 | 1.18 | 3.94 | 0.62 |
| Catechol (μg) | 14 | 4.53 | 84.1 | 45.9 | 16.6 | 9.87 | 3.09 |
| Formaldehyde (μg) | 14.1 | 7.41 | 79.4 | 18.6 | 17.8 | 39.8 | 1.90 |
| Isoprene (μg) | 6.55 | 35.4 | 891 | 386 | 0.74 | 9.17 | 0.19 |
| Lead (ng) | 2.23 | <0.676 | 31.2 | 12 | 7.15 | <5.63 | >3.30 |
| Nicotine (mg) | 1.29 | 0.21 | 1.74 | 0.934 | 74.1 | 22.5 | 6.14 |
| Nitrogen oxides (μg) | 14.2 | 28.6 | 538 | 298 | 2.64 | 9.60 | 0.50 |
| NNK (ng) | 7.8 | <12 | 244.7 | 150 | 3.19 | <8.00 | >0.65 |
| NNN (ng) | 10.1 | 15.2 | 271 | 166 | 3.73 | 9.16 | 0.66 |
| o-Toluidine (ng) | 1.1 | 0.773 | 96.2 | 56.6 | 1.14 | 1.37 | 1.42 |
| Phenol (μg) | 1.47 | <0.01 | 15.6 | 8.27 | 9.42 | <0.12 | >147.0 |
| Proprionaldehye (μg) | 10.8 | 4.94 | 109 | 54.7 | 9.91 | 9.03 | 2.19 |
| Styrene (μg) | 0.577 | 0.176 | 13.9 | 5.85 | 4.15 | 3.01 | 3.28 |
| Toluene (μg) | 1.42 | 1.26 | 129 | 80.4 | 1.10 | 1.57 | 1.13 |
| Tar (mg) | 19.4 | 2.27 | 25 | 10.3 | 77.6 | 22 | 8.55 |
| Total particulate matter (mg) | 30.2 | 3.56 | 41.4 | 12.6 | 72.9 | 28.3 | 8.48 |
For all ratio values that are under one, IQOS has less of the given compound than Accord on a per stick basis. For all values that are over one, IQOS has more of the given compound than Accord on a per stick basis. IQOS reduces levels of 8 constituents compared with Accord, but raises them for 17 others. When normalized by the weight of tobacco in the product, IQOS exposures were higher for 12 constituents and lower for 13 compared to Accord.
Discussion
While Accord was a commercial failure, IQOS is situated in a distinct social and regulatory landscape, which may increase its chances of success. The decline of cigarette consumption in developed markets, the increased popularity of e-cigarettes, select public health endorsements of e-cigarettes and the creation of a legal mechanism in the USA through which manufacturers can now make reduced risk claims may all have increased both consumers’ willingness to try new tobacco products, and PMI’s willingness to designate new products as safer than conventional cigarettes. In 2017, PMI announced the establishment of a US$1 billion foundation dedicated to partnering with public health and promoting PMI’s portfolio of reduced harm products, chiefly IQOS.65
Despite PMI’s claims of IQOS’s novelty,66 the product appears to be a successor of Accord on the basis of product design, marketing and aerosol chemistry. Both products work by inserting a modified cigarette into a holder that heats, rather than burns tobacco. Both products’ marketing implies reduced harm relative to cigarettes. While PMI claims that IQOS is reduced risk largely because it reduces users’ exposure to harmful constituents, IQOS does not drastically improve users' exposure to toxic compounds than relative to Accord. PMI’s claims for IQOS’s comparative safety on the basis of reduced exposure are further undermined by internal documents from PMI’s parent company: as late as 2007, PM executives and scientists claimed that reductions in exposure did not render Accord safer than conventional cigarettes.
One limitation of this study is that the Truth Tobacco Industry Documents Library is fragmented and incomplete, as it primarily comprises documents released through litigation. As a result, we may have missed documents relevant to our analysis, including information contained in the archive’s many ‘restricted’ documents, which the industry protects under attorney/client privilege.67 Given these limitations, there may be unreported differences in product design, marketing and aerosol chemistry between Accord and IQOS that could have informed our analysis. Similarly, we were only able to analyse one part of PMI’s MRTP application. Nonetheless, the results of this analysis suggest that PMI’s new claims of reduced health risk for IQOS are more likely the product of the current regulatory environment rather than a substantive improvement in the tobacco product’s harm profile compared with its precursor, Accord. Scientific evidence supporting reduced harm claims based solely on reduced exposure remains in.28 29
To prove that IQOS is safer than combustible cigarettes, PMI must show that IQOS ’significantly reduce[s] harm and the risk of tobacco-related disease to individual tobacco users and benefit[s] the health of the population as a whole taking into account both users of tobacco products and persons who do not currently use tobacco products'.68 Studies related to health effects range from lab-based smoking machine studies to assess constituent concentrations in products and aerosols/smoke; in vitro and in vivo toxicology studies; clinical and human pharmacology studies to examine the subjective and health effects of product use in lab and ambulatory settings; and epidemiological studies to examine the longer-term effects of these products relative to smoking. Though PMI has conducted several of these studies with favourable results, studies must also address effects on youth uptake, and product appeal to former-smokers and never-smokers to determine population-level health effects. Given the lack of long-term data on the individual and population health effects of IQOS, we remain sceptical of PMI’s claims of reduced risk through the use of predictive models.
Other independent research on IQOS has also contested IQOS’s claims of comparative safety.69–72 In analysing PMI’s MRTP application, independent researchers have noted that PMI’s conclusions of reduced population-level risk are based on data that factor in neither concerns of gateway effects for youth,73 nor secondhand smoke and dual use.74 Despite public communications about targeting solely adult smokers who would not otherwise quit,75 PMI has so far marketed IQOS much like an upscale tech gadget,6 offered primarily in countries with declining smoking prevalence and rising cessation rates.76
At the individual level, IQOS appears to cause damage to endothelial function, and the liver and the immune system at levels comparable to conventional cigarettes.77 78 In addition to showing toxicology yields from only a limited range of HPHC’s present in IQOS’s aerosol,79 22 of the constituents in the MRTP application had yields more than 200% higher than those present in conventional cigarettes, while another 7 had yields more than 1000% higher.79 PMI’s medical tests on human subjects also demonstrate ‘no statistically detectable difference between IQOS and conventional cigarettes for 23 of the 24 biomarkers of potential harm'.80 In January 2018, the FDA’s Tobacco Products Scientific Advisory Committee concluded that PMI had not proven IQOS to be safer than conventional cigarettes.81
This paper joins these analyses in casting doubt on PMI’s health claims for IQOS. Based on product specifications, marketing and aerosol chemistry, IQOS appears to represent less of a technical breakthrough than it does an attempt to capitalise on a social and regulatory landscape more favourable than a similar precursor product’s. When the regulatory environment prohibited reduced risk claims, PMI’s parent company consistently stated that reduced exposure did not mean reduced risk. The FDA should take PM at its word.
What this paper adds.
What is already known on this subject
Philip Morris International (PMI) claims that IQOS reduces users’ risk by reducing their exposure to harmful and potentially harmful constituents.
From 1998 to 2006, PMI’s parent company, Philip Morris (PM), marketed a strikingly similar heated tobacco product, Accord, with little commercial success.
What important gaps in knowledge exist on this topic
Independent assessment of PMI’s health claims for IQOS important, given the tobacco industry’s long history of misrepresented and manipulated research.
Analysis of internal communications surrounding Accord may help shed light onto PMI’s understanding of IQOS.
What this paper adds
PM scientists and executives consistently stated that Accord reduced users’ exposure to harmful constituents but that these reductions did not render Accord safer than conventional cigarettes.
IQOS’s design and marketing are similar to Accord’s.
We found that when comparing the aerosol chemistry test results between Accord and IQOS there was not a consistent reduction in exposure to toxicants, calling into question PMI’s current safety claims for IQOS, which are made on the basis of reduced exposure.
Footnotes
Contributors: Conceptualisation, data curation, investigation, writing, original draft: JE and LMD. Formal analysis, writing, review and editing: JE, LMD, GSH and PML. Funding acquisition, resources, supervision and validation: PML. Methodology: JE, LMD and PML.
Funding: This study was funded by Division of Cancer Prevention, National Cancer Institute (R01 CA87472).
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All of the documents included in this study are publicly available through the Truth tobacco documents library at https://www.industrydocumentslibrary.ucsf.edu/tobacco/.
References
- 1. Risi S. On the Origins of the Electronic Cigarette: British American Tobacco’s Project Ariel (1962–1967). American Journal of Public Health 2017:e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Proctor RN. Golden holocaust: Origins of the cigarette catastrophe and the case for abolition. Berkeley, CA: University of California Press, 2011:1–737. [Google Scholar]
- 3. Rossolillo N, 2018.. What? Philip morris wants out of the cigarette business? https://www.fool.com/investing/2018/01/11/what-philip-morris-wants-out-of-the-cigarette-busi.aspx.
- 4. Trefis Team, 2018. iQOS Expected to be a key growth driver for philip morris https://www.nasdaq.com/article/iqos-expected-to-be-a-key-growth-driver-for-philip-morris-cm916377.
- 5. Philip Morris International, 2017. Designing a smoke-free future. https://www.pmi.com/who-we-are/designing-a-smoke-free-future (accessed 13 Feb 2017).
- 6. Kim M. Philip Morris International introduces new heat-not-burn product, IQOS, in South Korea. Tobacco control 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bansal P, Lasseter T, Wilson D, et al. , 2018. How Philip Morris is selling regulators on its hot new smoking device. https://www.reuters.com/article/us-tobacco-iqos-marketing-specialreport/special-report-how-philip-morris-is-selling-regulators-on-its-hot-new-smoking-device-idUSKBN1EF1H8.
- 8. Center for Tobacco Products. Philip Morris Products S.A. Modified Risk Tobacco Product (MRTP) Applications. Food and Drug Administration, 2017. https://www.fda.gov/TobaccoProducts/Labeling/MarketingandAdvertising/ucm546281.htm.
- 9. Malone RE, Balbach ED. Tobacco industry documents: treasure trove or quagmire? Tob Control 2000;9:334–8. 10.1136/tc.9.3.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balbach ED, Gasior RJ, Barbeau EM. Tobacco industry documents: comparing the Minnesota Depository and internet access. Tob Control 2002;11:68–72. 10.1136/tc.11.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carter SM. Tobacco document research reporting. Tob Control 2005;14:368–76. 10.1136/tc.2004.010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kessler D. A question of intent: A great American battle with a deadly industry. PublicAffairs 2002. [Google Scholar]
- 13. Ling PM, Glantz SA. Tobacco industry consumer research on socially acceptable cigarettes. Tob Control 2005;14:e3 10.1136/tc.2005.011239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. RJ Reynolds, 1988. Premier Overview http://legacy.library.ucsf.edu/tid/dom64d00 (accessed 12 May 2014).
- 15. Reynolds RJ, 1986. It’s the World’s Cleanest Cigarette. http://legacy.library.ucsf.edu/tid/rsz94d00 (accessed 12 May 2014).
- 16. Rees V, Connolly G, Patil PA. Potentially reduced exposure tobacco products. Boston (MA): Harvard School of Public Health, 2008. [Google Scholar]
- 17. Philip Morris, Beta Board Speech. 1990.. http://legacy.library.ucsf.edu/tid/vch47c00 (accessed 21 Oct 2013).
- 18. Deposition of Peter J. Lipowicz. RJ Reynolds Records; 2010. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/xjcl0154 [accessed Nov 27 2017]. [Google Scholar]
- 19. Philip Morris USA, 2001. Tobacco Related Technology Review http://legacy.library.ucsf.edu/tid/bhe26j00 (accessed 18 Mar 2015).
- 20. Assunta M and Chapman S. A “clean cigarette” for a clean nation: a case study of Salem Pianissimo in Japan. Tobacco control 2004;13:ii58–ii62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Philip Morris, 2001. Accord Ownership https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/jxlx0179 (accessed 18 Jan 2018).
- 22. Philip Morris, 2001. Accord Update https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/jrlx0179 (accessed 18 Jan 2018).
- 23. Philip Morris, 2001. Tobacco Related Technology Review https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/rsbf0218 (accessed 27 Nov 2017).
- 24. Philip Morris, 2002. Accord Marketing Brochure https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/pplp0183 (accessed 18 Jan 2018).
- 25. Philip Morris, 1998. Accord Positioning Alternatives Brochure Revisions. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/qkvl0159 (accessed 18 Jan 2018).
- 26. Philip Morris, 2004. Accord Kit Brochure Draft https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/lnkw0151 (accessed 18 Jan 2018).
- 27. McDaniel PA, Malone RE. Understanding Philip Morris’s pursuit of US government regulation of tobacco. Tob Control 2005;14:193–200. 10.1136/tc.2004.009878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hackshaw A, Morris JK, Boniface S, et al. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ 2018;360:j5855 10.1136/bmj.j5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation 2005;111:2684–98. 10.1161/CIRCULATIONAHA.104.492215 [DOI] [PubMed] [Google Scholar]
- 30. Philip Morris, 2003. Accord Marketing Presentation https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/njxm0218 (accessed 18 Jan 2018).
- 31. Buchhalter AR, Eissenberg T. Preliminary evaluation of a novel smoking system: effects on subjective and physiological measures and on smoking behavior. Nicotine Tob Res 2000;2:39–43. [DOI] [PubMed] [Google Scholar]
- 32. Breland AB, Buchhalter AR, Evans SE, et al. Evaluating acute effects of potential reduced-exposure products for smokers: clinical laboratory methodology. Nicotine Tob Res 2002;4 Suppl 2:131–40. 10.1080/1462220021000032780 [DOI] [PubMed] [Google Scholar]
- 33. Philip Morris, 2004. EHCSS Program Update http://legacy.library.ucsf.edu/tid/fop92g00 (accessed 23 Oct 2013).
- 34. Philip Morris, 2003. Accord JLI Presentation Philip morris records https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/xtlv0151 (accessed 16 Jan 2018).
- 35. Philip Morris, 2003. Accord Presentation https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/xtlv0151 (accessed 12 Dec 2017).
- 36. Schorp MK, 2006. Scientific Data Summary "EHCSS-K3/K6" Cigarettes https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/xnxd0180 (accessed 27 Nov 2017).
- 37. Givel M, Glantz SA. The "global settlement" with the tobacco industry: 6 years later. Am J Public Health 2004;94:218–24. 10.2105/AJPH.94.2.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang JS, Malone RE. "Working to shape what society’s expectations of us should be": Philip Morris' societal alignment strategy. Tob Control 2008;17:391–8. 10.1136/tc.2008.026476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bondurant S, Wallace R, Shetty P, et al. Clearing the smoke: assessing the science base for tobacco harm reduction: National Academies Press, 2001. [PubMed] [Google Scholar]
- 40. Philip Morris, 2001. Nationwide poll on opinions toward FDA regulation https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/#id=mxwk0057 (accessed 28 Jun 2018).
- 41. Podraza K. Philip Morris Records, 2007. PM USA Letter to Surgeon General. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/nrkv0219 (accessed 27 Nov 2017).
- 42. Elias J, Ling PM. Invisible smoke: third-party endorsement and the resurrection of heat-not-burn tobacco products. Tob Control 2018;27(Suppl1):s96–s101. 10.1136/tobaccocontrol-2018-054433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tobacco Products. Heatbar, 2018. Tobacco Products. http://www.tobaccoproducts.org/index.php/Heatbar.
- 44. Brown D, Attachment U, 2008. Philip morris records. https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/kykh0219 (accessed 08 May 2018).
- 45. King BA, Alam S, Promoff G, et al. Awareness and ever-use of electronic cigarettes among U.S. adults, 2010-2011. Nicotine Tob Res 2013;15:1623–7. 10.1093/ntr/ntt013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Regan AK, Promoff G, Dube SR, et al. Electronic nicotine delivery systems: adult use and awareness of the ’e-cigarette' in the USA. Tob Control 2013;22:19–23. 10.1136/tobaccocontrol-2011-050044 [DOI] [PubMed] [Google Scholar]
- 47. Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new ’tobacco' industry? Tob Control 2011;20:81 10.1136/tc.2010.038562 [DOI] [PubMed] [Google Scholar]
- 48. ASH. ASH endorses RCP call for a tobacco harm reduction strategy. London: Action on Smoking and Health, 2007. [Google Scholar]
- 49. England PH. E-cigarettes: a new foundation for evidence-based policy and practice. London: Public Health England, 2015. [Google Scholar]
- 50. Royal College of Physicians. Nicotine Without Smoke: Tobacco Harm Reduction. London: Royal College of Physicians, 2016. [Google Scholar]
- 51. Truth Initiative, 2017. Truth initiative supports tobacco harm minimization effort. https://truthinitiative.org/news/truth-initiative-supports-tobacco-harm-minimization-effort (accessed 8 May 2018).
- 52. UK National Health Service, 2018. E-cigarettes https://www.nhs.uk/smokefree/help-and-advice/e-cigarettes (accessed 8 May 2018).
- 53. Kennedy RD, Awopegba A, De León E, et al. Global approaches to regulating electronic cigarettes. Tob Control 2017;26:440–5. 10.1136/tobaccocontrol-2016-053179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. H.R.1256 - Family Smoking Prevention and Tobacco Control Act 2009. 111th Congress ed. U.S.A.
- 55. Philip Morris International, 2016. 2016 iQOS brand voice guidelines. Reuters https://www.documentcloud.org/documents/4331963-IQOS-Brand-Voice-Guidelines.html (15 Jan 2018).
- 56. Philip Morris International, 2014. 10-year corporate affairs objectives, strategies. Reuters https://www.documentcloud.org/documents/4333395-10-Year-Corporate-Affairs-Objectives-and.html (accessed 31 Jan 2018).
- 57. Fooks GJ, Gilmore AB, Smith KE, et al. Corporate social responsibility and access to policy élites: an analysis of tobacco industry documents. PLoS Med 2011;8:e1001076 10.1371/journal.pmed.1001076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McDaniel PA, Malone RE. The role of corporate credibility in legitimizing disease promotion. Am J Public Health 2009;99:452–61. 10.2105/AJPH.2008.138115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Philip Morris International, 2018. Our manifesto: Designing a smoke-free future. https://www.pmi.com/who-we-are/designing-a-smoke-free-future (accessed 8 May 2018).
- 60. Philip Morris International, 2018. Who we are: Our goal and strategies. https://www.pmi.com/who-we-are/our-goal-and-strategies (accessed 8 May 2018).
- 61. Philip Morris International, 2018. Who we are: Our goal and strategies https://www.pmi.com/who-we-are/our-goal-and-strategies (accessed 8 May 2018).
- 62. Philip Morris Japan. iQOS National Expansion Post-launch Report: Reuters: The Philip Morris Files, 2015. https://www.documentcloud.org/documents/4331981-iQOS-Japan-Newsletter.html (accessed 24 Jan 2018). [Google Scholar]
- 63. Bansal P, Lasseter T, Wilson D, et al. 2017. Philip Morris' search for a cigarette no more harmful than a cup of coffee https://www.reuters.com/article/tobacco-iqos-science-risk/philip-morris-search-for-a-cigarette-no-more-harmful-than-a-cup-of-coffee-idUSL1N1OK0C0.
- 64. Philip Morris, 2002. EHCSS Basis for a Prep Claim - Scientific Data Summary https://www.industrydocumentslibrary.ucsf.edu/tobacco/docs/rscp0151 (accessed 16 Jan 2018).
- 65. Yach D. Foundation for a smoke-free world. The Lancet 2017;390:1807–10. 10.1016/S0140-6736(17)32602-8 [DOI] [PubMed] [Google Scholar]
- 66.McGrath Thomas. 2017. Offering adult smokers a portfolio of potentially less harmful products https://www.youtube.com/watch?v=r_k0sxL3eiQ.
- 67. LeGresley EM, Muggli ME, Hurt RD. Playing hide-and-seek with the tobacco industry. Nicotine Tob Res 2005;7:27–40. 10.1080/14622200412331328529 [DOI] [PubMed] [Google Scholar]
- 68. Food and Drug Administration. Guidance for Industry - Modified Risk Tobacco Product Applications: U.S. Department of Health and Human Services, 2012. [Google Scholar]
- 69. Auer R, Concha-Lozano N, Jacot-Sadowski I, et al. Heat-Not-Burn Tobacco Cigarettes: Smoke by Any Other Name. JAMA Intern Med 2017;177:1050–2. 10.1001/jamainternmed.2017.1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nabavizadeh P, Liu J, Ibrahim S, et al. Abstract 16035: Impairment of Endothelial Function by Inhalation of Heat-Not-Burn Tobacco Aerosol. Circulation 2017;136:A16035. [Google Scholar]
- 71. Glantz SA, 2018. Philip Morris hides data in plain sight on dangers of new heat-not-burn product http://theconversation.com/philip-morris-hides-data-in-plain-sight-on-dangers-of-new-heat-not-burn-product-87636.
- 72. Kopel E, Davidovitch N, Levine H. Using all means to protect public health in israel from emerging tobacco products. Am J Public Health 2017;107:1599–600. 10.2105/AJPH.2017.304016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McKelvey K, Popova L, Kim M, et al. IQOS labelling will mislead consumers. Tob Control 2018;27(Suppl1):s48–s54. 10.1136/tobaccocontrol-2018-054333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moazed F, Chun L, Matthay MA, et al. Assessment of industry data on pulmonary and immunosuppressive effects of IQOS. Tob Control 2018;27(Suppl1):s20–s25. 10.1136/tobaccocontrol-2018-054296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Philip Morris International, 2017. Harm reduction equation. https://www.pmi.com/glossary-section/glossary/harm-reduction-equation (accessed 2 Oct 2017).
- 76. Bilano V, Gilmour S, Moffiet T, et al. Global trends and projections for tobacco use, 1990-2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet 2015;385:966–76. 10.1016/S0140-6736(15)60264-1 [DOI] [PubMed] [Google Scholar]
- 77. Nabavizadeh P, Liu J, Havel CM, et al. Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob Control 2018;27(Suppl1):s13–s19. 10.1136/tobaccocontrol-2018-054325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chun L, Moazed F, Matthay M, et al. Possible hepatotoxicity of IQOS. Tob Control 2018;27(Suppl1):s39–s40. 10.1136/tobaccocontrol-2018-054320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Helen G, Jacob III P, Nadrone N, et al. IQOS: examination of Philip Morris International’s claim of reduced exposure. Tob Control 2018;27(Suppl1):s30–s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Glantz SA. PMI’s own in vivo clinical data on biomarkers of potential harm in Americans show that IQOS is not detectably different from conventional cigarettes. Tob Control 2018;27(Suppl1):s9–s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Edney A, 2018. Tobacco device hits snag as experts question disease dangers. https://www.bloomberg.com/news/articles/2018-01-25/high-tech-tobacco-gadget-hits-snag-in-push-to-disrupt-smoking.