Abstract
We examined the effect of a combination of astaxanthin (AX) supplementation, repeated heat stress, and intermittent reloading (IR) on satellite cells in unloaded rat soleus muscles. Forty-nine male Wistar rats (8-week-old) were divided into control, hind-limb unweighting (HU), IR during HU, IR with AX supplementation, IR with repeated heat stress (41.0–41.5 °C for 30 min), and IR with AX supplementation and repeated heat stress groups. After the experimental period, the antigravitational soleus muscle was analyzed using an immunohistochemical technique. Our results revealed that the combination of dietary AX supplementation and heat stress resulted in protection against disuse muscle atrophy in the soleus muscle. This protective effect may be partially due to a higher satellite cell number in the atrophied soleus muscle in the IR/AX/heat stress group compared with the numbers found in the other groups. We concluded that the combination treatment with dietary AX supplementation and repeated heat stress attenuates soleus muscle atrophy, in part by increasing the number of satellite cells.
Keywords: Antioxidant astaxanthin, Disuse muscle atrophy, Heat stress, Satellite cell
1 Introduction
Skeletal muscle is defined by a net balance between protein accumulation and degradation. Decreased protein synthesis and increased protein degradation both contribute to the loss of muscle protein (Schiaffino et al., 2013). Although the protein synthesis rate rapidly declines following hind-limb unloading, disuse-induced muscle atrophy is mainly due to an increase in the protein degradation rate in rat skeletal muscles (Powers et al., 2007). Moreover, recent reports have demonstrated that satellite cells (SCs) are required for muscle mass maintenance and muscle regrowth following muscle atrophy (Brooks and Myburgh, 2014).
SCs, which are located between the basement and plasma membranes in myofibers, contribute to muscle fiber hypertrophy (Egner et al., 2016) and are necessary for skeletal muscle regeneration following muscle injury (Dumont et al., 2015). Many studies have demonstrated that the number of SCs is decreased by muscle catabolic conditions such as disuse and aging (Shefer et al., 2006; le Grand and Rudnicki, 2007; Brooks and Myburgh, 2014). Indeed, recent evidence has demonstrated that SC loss is correlated with muscle fiber atrophy following 14 d of bed rest in healthy middle-aged adults (Arentson-Lantz et al., 2016). This may be due to apoptosis of SCs as well as myonuclei in situations of disuse (Matsuba et al., 2009). Thus, maintenance of the SC number and activity is an important countermeasure during disuse to counter skeletal muscle atrophy.
Numerous strategies have been proposed to prevent the reduction and inactivation of SCs during muscle atrophy. Mechanical load is the most logical countermeasure to increase the number of, or activate, SCs during disuse but when the time and frequency of reloading is limited, it is not always sufficient to prevent atrophy. Therefore, a more effective countermeasure is needed that would result in a preventive effect. Of the tested strategies, whole-body heat stress, applied to inactive muscles, is a particularly effective method for preventing muscular atrophy (Naito et al., 2000; Yoshihara et al., 2015) and facilitating regeneration (Kojima et al., 2007; Oishi et al., 2009), and evidence has demonstrated that heat stress activates SCs in injured rodent skeletal muscles (Kojima et al., 2007; Oishi et al., 2009). Notably, intermittent (repeated) heat stress during hind-limb disuse reduced oxidative stress in atrophied soleus muscle (Selsby and Dodd, 2005; Yoshihara et al., 2015); therefore, repeated treatment with heat stress may be effective tools for the maintenance of the number of SCs during skeletal muscle disuse. Furthermore, antioxidant supplementation also shows preventive effects against muscular atrophy (Powers, 2014); however, its effect on SCs remains unknown. Especially, astaxanthin (AX), a potent antioxidant, has attracted attention as an effective method to counter disuse muscle atrophy. Indeed, we previously demonstrated that dietary AX administration attenuates the unloading-induced soleus muscle atrophy (Yoshihara et al., 2017); however, the effect of AX on SCs during muscle atrophy is still unknown. Based on these data, a combination of these treatments may be a more effective countermeasure for preventing disuse-induced muscular atrophy than either treatment alone.
Therefore, the purpose of this study was to examine the effect of a combinational treatment including intermittent reloading, potent antioxidant AX supplementation, and repeated heat stress, prior to and/or during the phase of unloading, on the number of SCs in the atrophied soleus muscle.
2 Methods
2.1 Experimental animals
Forty-nine male Wistar rats (age: 8 weeks; weight: ((261.3±1.4) g) were used. The rats were housed in a climate-controlled room (24.0±1.0) °C, 50%–60% relative humidity, 12-h:12-h light-dark photoperiod) and were given a standard rat chow (CE-2 powder, CLEA Japan, Inc., Japan) and water ad libitum. The rats were randomly divided into the following six groups: (1) control (CON, n=8); (2) hind-limb unweighting (HU, n=9); (3) intermittent reloading (IR) during HU (HU+IR, n=8); (4) IR with AX administration during HU (HU+IR+AX, n=8); (5) IR with heat stress (HS) during HU (HU+IR+HS, n=8); and (6) IR with HS during HU plus AX administration (HU+IR+AX+HS, n=8). This study was approved by the Yamaguchi University Animal Care Committee and followed the guiding principles for the care and use of animals set forth by the Physiological Society of Japan.
2.2 Astaxanthin supplementation
The diet for the two AX administration groups was prepared by mixing AX (BioAstin, Toyo Koso Kagaku Co., Ltd., Japan) at 0.04% (w/w) with CE-2 powder. The rats in the AX groups were fed the AX diet for three weeks, beginning at two weeks before the unloading. The rats in the CON, HU, HU+IR, and HU+IR+HS groups were fed a control diet with CE-2 powder, which did not contain AX, during the experimental period.
2.3 Hind-limb unloading
All the groups, except CON, were unloaded for seven days, as described previously (Sugiura et al., 2005). Briefly, a tail cast was applied to the distal one-third of the tail and attached to a hook on the ceiling of the cage. The height of the hook was adjusted at an inclination of approximately 35° in a head-down orientation. The rat was free to move around the cage on its front feet.
2.4 Intermittent reloading
During HU, the HU+IR, HU+IR+AX, HU+IR+HS, and HU+IR+AX+HS groups were released from the unweighting for 1 h every other day to allow daily activities. The reloading was performed during the heating phase described below.
2.5 Heat treatment
One day before the unloading and during IR (for 1 h), the HU+IR+HS and HU+IR+AX+HS groups were placed in a heat chamber (IS-2400, Advantec, Tokyo, Japan) for 30 min (41.0–41.5 °C). The mean rectal temperature of the heat-treated rats was (41.2±0.2) °C at the end of the heat treatment.
2.6 Muscle sampling
After the experimental period, all rats were anesthetized with ether and the left soleus muscles were removed, weighed, rapidly frozen in liquid nitrogen, and stored at −80 °C until histological analysis.
2.7 Histochemical and immunohistochemical analyses
Cross-sections (8 μm) of the mid-belly region of the soleus muscle were cut using a cryostat, then air-dried and stored at −20 °C until further analysis. The sections were then rehydrated in phosphate-buffered saline (PBS) with 0.1% Tween 20 (PBS-T) and fixed in 4% paraformaldehyde at room temperature for 15 min. After several washes with PBS-T, the sections were fixed in methanol at 8 °C for 15 min. After washing, the sections were blocked with a 1% (0.01 g/ml) blocking reagent (Roche Diagnostics, Penzberg, Germany) in 1% (0.01 g/ml) maleic acid buffer (0.1 mol/L maleic acid and 0.15 mol/L NaCl, pH 7.5) at room temperature for 30 min. After washing, the sections were immunoreacted with anti-paired box protein 7 (Pax7) and anti-laminin (Dako Japan, Tokyo, Japan) antibodies and diluted 1:500 in the blocking reagent at 37 °C for 1 h. After several washes in PBS-T, the sections were incubated with secondary cyanine 3-conjugated anti-mouse IgG1 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and fluorescein isothiocyanate-conjugated anti-rabbit IgG (Sigma-Aldrich, St. Louis, MO, USA) antibodies diluted 1:250. After several washes in PBS-T, the nuclei were stained by a 15-min incubation in a solution of 4',6-diamidino-2-phenylindole (DAPI, 1.0 mg/ml; Sigma-Aldrich). After washes in PBS-T, the sections were dried at room temperature and cover-slipped using Dako Fluorescent Mounting Medium (S3023, Dako, Carpinteria, CA, USA).
2.8 Imaging and analyses of muscular sections
Fluorescent microscopy (Eclipse E400; Nikon, Tokyo, Japan) was used to determine the number of myonuclei and SCs and the cross-sectional area (CSA) of muscle fibers. The microscopy was equipped with fluorescent cubes to visualize Pax7-positive cells (600–660 nm), laminin (520 nm), and DAPI-positive nuclei (435–485 nm). Images of the stained sections at ×200 magnification were taken with a digital camera (DC 120; Leica, Wetzlar, Germany) connected to the microscope. In each section, five different fields, each containing 80–120 fibers, were chosen randomly for counting DAPI-positive nuclei, myonuclei, and SCs. Pax7-negative/DAPI-positive and Pax7-positive/DAPI-positive nuclei that were located within the laminin-positive basal membrane were counted as myonuclei and SCs, respectively. The numbers of myonuclei and SCs per section and fiber number were then calculated. The percentage of SCs was also calculated in each section as follows: SC number/(myonuclear number+SC number)×100%. In addition, we measured muscle fiber CSA in the soleus muscle to evaluate muscle fiber atrophy. Briefly, the CSA of 200–600 muscle fibers in each rat was determined using Scion Image software (National Institutes of Health (NIH)) by tracing the outline of individual fibers (detected by laminin staining). The mean fiber CSA was then calculated by dividing the total area by the total number of fibers and is expressed as μm2.
2.9 Statistical analysis
Values are presented as the mean±standard error (SE). Some animals that had unexpected reloading during the hind-limb unloading period (one animal from each of the HU+IR and HU+IR+AX+HS groups) or a remarkably high body weight (one animal from the CON group) were excluded from the data analysis because of their effect on the independent variables of measurements. Differences were detected using one-way analysis of variance (ANOVA). When the results of the ANOVA were significant, group differences were determined using the Holm-Sidak’s post hoc test. P-values of <0.05 were considered significant.
3 Results
3.1 Body weight, soleus muscle weight, relative soleus muscle weight, and fiber CSA
Table 1 shows the body weights, soleus muscle weights, relative soleus muscle weights, and soleus muscle fiber CSA after seven days of unloading. The body weight of the HU+IR+HS group was significantly lower than that of the CON group, but no differences were observed among the five HU groups. Although the seven days of HU resulted in a significant reduction of muscle mass (both absolute and relative) and fiber size compared with the CON group, the absolute weight in the HU+IR+AX+HS group and the relative weight in the HU+IR+AX, HU+IR+HS, and HU+IR+AX+HS groups were significantly higher than that of the HU group. Moreover, the hind-limb unloading-induced reduction of soleus muscle fiber CSA was significantly suppressed in the HU+IR+HS and HU+IR+AX+HS groups.
Table 1.
Body weight, soleus muscle weight, and myofiber cross-sectional area (CSA)
| Group | Body weight (g) | Soleus weight (mg) | Soleus weight/body weight (mg/kg) | Soleus CSA (μm2) |
| CON (n=7) | 409.6±6.8a | 163.6±6.7a | 399.2±13.2a | 3056.4±158.3a |
| HU (n=9) | 383.7±10.3a,b | 112.9±4.1b | 295.7±12.5b | 1737.9±84.9b |
| HU+IR (n=7) | 383.1±7.6a,b | 126.1±5.0b,c | 328.6±9.2b,c | 2257.3±171.8b,c |
| HU+IR+AX (n=8) | 370.6±14.1a,b | 130.3±5.7b,c | 353.3±16.1c,d,e | 2160.0±166.0b,c |
| HU+IR+HS (n=8) | 360.4±8.9b | 131.6±3.4b,c | 365.6±7.7d,e | 2549.0±158.9a,c |
| HU+IR+AX+HS (n=7) | 381.3±12.1a,b | 136.5±7.3c | 357.9±14.9d,e | 2375.2±197.1a,c |
The values are expressed as mean±SE. CON, control; HU, hind-limb unweighting; HU+IR, intermittent reloading during HU; HU+IR+AX, IR during HU plus AX administration; HU+IR+HS, IR with heat stress (HS) during HU; HU+IR+AX+HS, IR with HS during HU plus AX administration. Different lowercase letters (a, b, c, d, e) are significantly different from each other according to Holm-Sidak’s post hoc test
3.2 Myonuclear numbers
Table 2 shows the changes in the numbers of myonuclei and myofibers in the soleus muscle after seven days of unloading. The numbers of DAPI-positive nuclei per section significantly decreased in the HU, HU+IR+AX, HU+IR+HS, and HU+IR+AX+HS groups compared with that in the CON group, and the numbers in the HU+IR+AX, HU+IR+HS, and HU+IR+AX+HS groups were significantly lower than those in the HU and HU+IR groups. The number of myofibers per section significantly increased in the HU group compared with that in the CON group, but not in the HU+IR, HU+IR+AX, HU+IR+HS, or HU+IR+AX+HS group. The numbers of myonuclei per fiber significantly decreased in the HU, HU+IR+AX, HU+IR+HS, and HU+IR+AX+HS groups compared with the CON group; however, that in the HU+IR was significantly higher than those in the other experimental groups.
Table 2.
Changes in the number of 4',6-diamidino-2-phenylindole (DAPI)-positive nuclei and myofibers, and the ratio of DAPI-positive nuclei relative to the number of myofibers
| Group | Number of DAPI-positive nuclei per section | Number of myofiber per section | Number of DAPI-positive nuclei/number of myofiber per fiber |
| CON (n=7) | 329.5±17.6a | 94.1±3.4a | 3.5±0.2a |
| HU (n=9) | 269.5±11.4b | 138.3±2.0b | 2.0±0.1b |
| HU+IR (n=7) | 289.4±9.9a,b | 114.3±9.5a,b | 2.6±0.2c |
| HU+IR+AX (n=8) | 190.8±11.0c | 125.2±9.2a,b | 1.5±0.1b,d |
| HU+IR+HS (n=8) | 176.1±10.9c | 105.1±5.5a | 1.7±0.1b,d |
| HU+IR+AX+HS (n=7) | 181.4±12.3c | 121.5±8.0a,b | 1.5±0.1b,d |
The values are expressed as mean±SE. Different lowercase letters (a, b, c, d) are significantly different from each other according to Holm-Sidak’s post hoc test
3.3 Satellite cell numbers
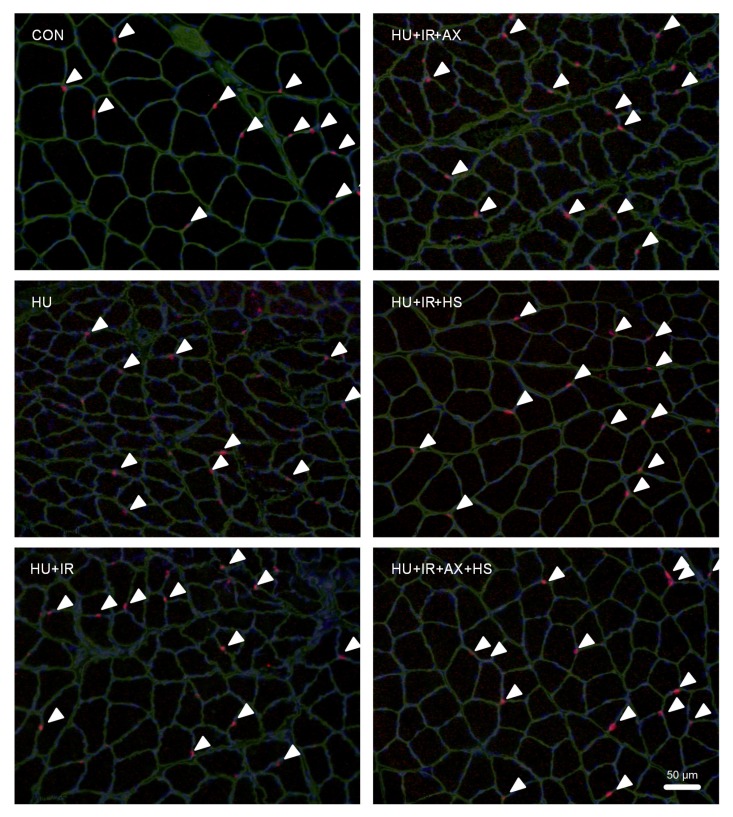
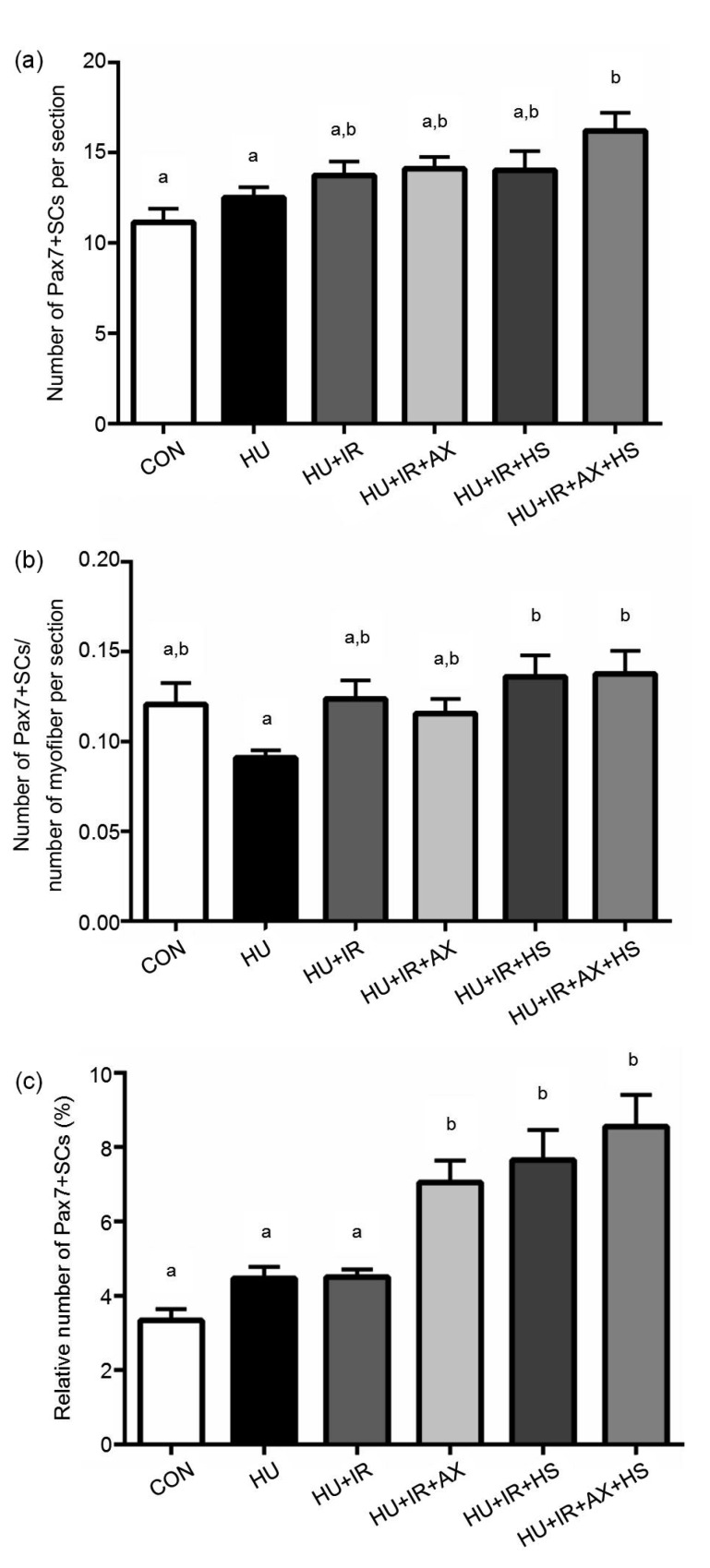
SCs were identified using triple staining for Pax7 (SCs), laminin (basal membrane), and DAPI (nucleus) (Fig. 1). Seven days of HU did not alter the number of SCs per section and myofiber, nor the percentage of SCs, although the SC number per myofiber tended to be lower (17.1%) in the HU than in the CON group (Fig. 2). However, the number of SCs per section in the HU+IR+AX+HS group was significantly higher than those in the CON and HU groups (Fig. 2a). The SC number per myofiber also significantly increased in the HU+IR+HS and HU+IR+AX+HS groups compared with the HU group (Fig. 2b). Moreover, the percentages of SCs in the HU+IR+AX, HU+IR+HS, and HU+IR+AX+HS groups were significantly higher than those in the CON, HU, and HU+IR groups (Fig. 2c).
Fig. 1.
Representative images of immunofluorescent staining for Pax7-positive satellite cells (SCs) after seven days of unloading in each group
The arrows indicate the SCs positive to triple staining for Pax7 (red), laminin (green), and 4',6-diamidino-2-phenylindole (DAPI; blue) (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
Fig. 2.
Numbers of Pax7-positive SCs per section (a) and myofiber (b) and percentage of SCs relative to the number of DAPI-positive nuclei (c)
The values are expressed as mean±SE. CON, n=7; HU, n=9; HU+IR, n=7; HU+IR+AX, n=8; HU+IR+HS, n=8; HU+IR+AX+HS, n=7. Bars with the different lowercase letters (a, b) are significantly different from each other according to Holm-Sidak’s post hoc test
4 Discussion
The main finding of this study was the demonstration that the combination of antioxidant AX supplementation, HS, and IR increased the number of Pax7-positive SCs. It has been well established that SCs play important roles in the repair and regeneration of damaged skeletal muscles (Brooks and Myburgh, 2014; Dumont et al., 2015) and are therefore required for muscle mass maintenance. In contrast, atrophic conditions lead to a reduction in the number of SCs (Shefer et al., 2006; le Grand and Rudnicki, 2007) and the loss of SCs is associated with muscle fiber atrophy following 14 d of bed rest in humans (Arentson-Lantz et al., 2016). In the present study, we found that the relative number of Pax7-positive SCs increased in the AX-supplemented and heat-stressed groups compared with those in the hind-limb-unloaded group. Gao et al. (2015) demonstrated that HS promotes the growth of cultured Lantang swine skeletal muscle SCs. HS activates SCs and accelerates the regeneration of damaged muscle fibers in rat skeletal muscle after bupivacaine injection (Oishi et al., 2009). Therefore, HS-induced activation of SCs plays an important role in the suppression of soleus muscle atrophy. Moreover, it is possible that increased anti-oxidative capacity contributes to the maintenance of SCs. A previous study reported that oxidative stress induced by 1 mmol/L H2O2 treatment reduced the number of SCs in human skeletal muscles (Renault et al., 2002). AX is a red carotenoid pigment with potent antioxidant and anti-inflammatory properties (Dose et al., 2016). We have previously demonstrated that dietary AX administration attenuates unloading-induced soleus muscle atrophy via inhibition of oxidative stress (Yoshihara et al., 2017); however, the effect of AX on SCs during muscle atrophy is still unknown. Fulle et al. (2005) reported that oxidative injury, derived from a decrease in anti-oxidative capacity, may negatively affect the ability of aging SCs to repair muscle. Our data indicated that supplementation of the antioxidant AX contributed to the maintenance of a redox status during disuse-induced muscle atrophy and thereby the number of SCs was preserved. This suggests that AX supplementation and HS are effective in the maintenance of SCs in the atrophied soleus muscle. Interestingly, the number of SCs was greater and the decline in both absolute soleus muscle weight and fiber CSA was suppressed only in the combinational treatment group, while IR was not sufficient to prevent atrophy. Therefore, the combination of antioxidant AX supplementation and environmental HS is an effective countermeasure for suppressing the SC decline in atrophied rat skeletal muscles.
Unfortunately, the specific mechanisms responsible for the AX supplementation-and HS-induced SC activation during skeletal muscle atrophy are currently unknown. Nonetheless, it appears likely that the SC activation may be due to the suppression of both myonuclear apoptosis and increased oxidative stress (Matsuba et al., 2009). A previous study demonstrated that AX treatment abolished the generation of 6-hydroxydopamine-induced reactive oxygen species (ROS) and apoptosis in SH-SY5Y cells (Ikeda et al., 2008). We have previously demonstrated that dietary AX supplementation prior to and during HU attenuates soleus muscle atrophy via suppression of myonuclear apoptosis and ROS production (Yoshihara et al., 2017). Moreover, protein kinase B (Akt)/mechanistic target of rapamycin (mTOR) kinase/p70S6K kinase activation plays a key role in facilitating SC activation. Gao et al. (2015) have demonstrated that HS promotes the growth of cultured Lantang swine skeletal muscle SCs via the activation of the Akt/mTOR/p70S6K signaling pathway. In addition, Uehara et al. (2004) have shown that HS induced a significant increase in p-p70S6K expression after heat exposure at 41 °C, suggesting that HS promotes SC growth via the Akt/mTOR/p70S6K pathway. These data suggest that the combination of antioxidant AX supplementation and environmental HS may contribute to the suppression of unloading-induced oxidative stress, myonuclear apoptosis, and Akt/mTOR/p70S6K signaling deactivation, thereby maintaining the number of SCs.
It has been demonstrated that the number of SCs in the rat skeletal muscle is decreased by HU and limb immobilization (Schultz et al., 1994; le Grand and Rudnicki, 2007). In the present study, the relative number of Pax7-positive SCs decreased by 17.1% after the seven-day unloading; however, the difference between the HU and CON groups was not significant. This may be due to the fact that the decline in the number of SCs is a very early event in disuse muscle atrophy. Many studies have indicated that the number of SCs decreases during the early stages (within 24–72 h) of disuse-induced skeletal muscle atrophy. This suggests that marked changes in SCs might have occurred in an earlier phase of disuse muscle atrophy in our study. More recent studies have indicated that changes in myonuclear numbers are not always observed during disuse and that a loss of SCs with disuse muscle atrophy is not a consistent finding after 14 d of immobilization (Snijders et al., 2014) or 28 d of bed rest (Brooks et al., 2010). Thus, further investigations are required to evaluate the significance of the combinational treatment with antioxidant AX supplementation and HS for SC activation during disuse muscle atrophy.
5 Conclusions
The present study demonstrated, for the first time, that a combinational treatment with dietary AX supplementation, repeated HS, and IR attenuates soleus muscle atrophy, in part by increasing the number of SCs.
Acknowledgments
We would like to thank Editage (http://www.editage.jp) for English language editing.
Footnotes
Project supported by the Japan Society for the Promotion of Science (JSPS) KA-KENHI (Nos. 20500578 and 17K01765) and the Ministry of Education, Culture, Sports, Science and Technology (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities
Compliance with ethics guidelines: Toshinori YOSHIHARA, Takao SUGIURA, Nobuyuki MIYAJI, Yuki YAMAMOTO, Tsubasa SHIBAGUCHI, Ryo KAKIGI, Hisashi NAITO, Katsumasa GOTO, Daijiro OHMORI, and Toshitada YOSHIOKA declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Arentson-Lantz EJ, English KL, Paddon-Jones D, et al. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J Appl Physiol. 2016;120(8):965–975. doi: 10.1152/japplphysiol.00799.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks NE, Myburgh KH. Skeletal muscle wasting with disuse atrophy is multi-dimensional: the response and interaction of myonuclei, satellite cells and signaling pathways. Front Physiol, 5:99. 2014 doi: 10.3389/fphys.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks NE, Cadena SM, Vannier E, et al. Effects of resistance exercise combined with essential amino acid supplementation and energy deficit on markers of skeletal muscle atrophy and regeneration during bed rest and active recovery. Muscle Nerve. 2010;42(6):927–935. doi: 10.1002/mus.21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dose J, Matsugo S, Yokokawa H, et al. Free radical scavenging and cellular antioxidant properties of astaxanthin. Int J Mol Sci. 2016;17(1):103. doi: 10.3390/ijms17010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumont NA, Bentzinger CF, Sincennes MC, et al. Satellite cells and skeletal muscle regeneration. Compr Physiol. 2015;5(3):1027–1059. doi: 10.1002/cphy.c140068. [DOI] [PubMed] [Google Scholar]
- 6.Egner IM, Bruusgaard JC, Gundersen K. Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development. 2016;143(16):2898–2906. doi: 10.1242/dev.134411. [DOI] [PubMed] [Google Scholar]
- 7.Fulle S, Di Donna S, Puglielli C, et al. Age-dependent imbalance of the antioxidative system in human satellite cells. Exp Gerontol. 2005;40(3):189–197. doi: 10.1016/j.exger.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Gao CQ, Zhao YL, Li HC, et al. Heat stress inhibits proliferation, promotes growth, and induces apoptosis in cultured lantang swine skeletal muscle satellite cells. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(6):549–559. doi: 10.1631/jzus.B1400339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda Y, Tsuji S, Satoh A, et al. Protective effects of astaxanthin on 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J Neurochem. 2008;107(6):1730–1740. doi: 10.1111/j.1471-4159.2008.05743.x. [DOI] [PubMed] [Google Scholar]
- 10.Kojima A, Goto K, Morioka S, et al. Heat stress facilitates the regeneration of injured skeletal muscle in rats. J Orthop Sci. 2007;12(1):74–82. doi: 10.1007/s00776-006-1083-0. [DOI] [PubMed] [Google Scholar]
- 11.le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19(6):628–633. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuba Y, Goto K, Morioka S, et al. Gravitational unloading inhibits the regenerative potential of atrophied soleus muscle in mice. Acta Physiol. 2009;196(3):329–339. doi: 10.1111/j.1748-1716.2008.01943.x. [DOI] [PubMed] [Google Scholar]
- 13.Naito H, Powers SK, Demirel HA, et al. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol. 2000;88(1):359–363. doi: 10.1152/jappl.2000.88.1.359. [DOI] [PubMed] [Google Scholar]
- 14.Oishi Y, Hayashida M, Tsukiashi S, et al. Heat stress increases myonuclear number and fiber size via satellite cell activation in rat regenerating soleus fibers. J Appl Physiol. 2009;107(5):1612–1621. doi: 10.1152/japplphysiol.91651.2008. [DOI] [PubMed] [Google Scholar]
- 15.Powers SK. Can antioxidants protect against disuse muscle atrophy? Sports Med. 2014;44(S2):155–165. doi: 10.1007/s40279-014-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102(6):2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- 17.Renault V, Thornell LE, Butler-Browne G, et al. Human skeletal muscle satellite cells: aging, oxidative stress and the mitotic clock. Exp Gerontol. 2002;37(10-11):1229–1236. doi: 10.1016/S0531-5565(02)00129-8. [DOI] [PubMed] [Google Scholar]
- 18.Schiaffino S, Dyar KA, Ciciliot S, et al. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280(17):4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 19.Schultz E, Darr KC, Macius A. Acute effects of hindlimb unweighting on satellite cells of growing skeletal muscle. J Appl Physiol. 1994;76(1):266–270. doi: 10.1152/jappl.1994.76.1.266. [DOI] [PubMed] [Google Scholar]
- 20.Selsby JT, Dodd SL. Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol. 2005;289(1):R134–R139. doi: 10.1152/ajpregu.00497.2004. [DOI] [PubMed] [Google Scholar]
- 21.Shefer G, van de Mark DP, Richardson JB, et al. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294(1):50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snijders T, Wall BT, Dirks ML, et al. Muscle disuse atrophy is not accompanied by changes in skeletal muscle satellite cell content. Clin Sci (Lond) 2014;126(8):557–566. doi: 10.1042/CS20130295. [DOI] [PubMed] [Google Scholar]
- 23.Sugiura T, Abe N, Nagano M, et al. Changes in PKB/Akt and calcineurin signaling during recovery in atrophied soleus muscle induced by unloading. Am J Physiol. 2005;288(5):R1273–R1278. doi: 10.1152/ajpregu.00688.2004. [DOI] [PubMed] [Google Scholar]
- 24.Uehara K, Goto K, Kobayashi T, et al. Heat-stress enhances proliferative potential in rat soleus muscle. Jpn J Physiol. 2004;54(3):263–271. doi: 10.2170/jjphysiol.54.263. [DOI] [PubMed] [Google Scholar]
- 25.Yoshihara T, Sugiura T, Yamamoto Y, et al. The response of apoptotic and proteolytic systems to repeated heat stress in atrophied rat skeletal muscle. Physiol Rep. 2015;3(10):e12597. doi: 10.14814/phy2.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshihara T, Yamamoto Y, Shibaguchi T, et al. Dietary astaxanthin supplementation attenuates disuse-induced muscle atrophy and myonuclear apoptosis in the rat soleus muscle. J Physiol Sci. 2017;67(1):181–190. doi: 10.1007/s12576-016-0453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]