Abstract
Objective: Keloids are exuberant cutaneous scars that form due to abnormal growth of fibrous tissue following an injury. The primary aim of this study was to assess the efficacy and mechanism of hyperbaric oxygen therapy (HBOT) to reduce the keloid recurrence rate after surgical excision and radiotherapy. Methods: (1) A total of 240 patients were randomly divided into two groups. Patients in the HBOT group (O group) received HBOT after surgical excision and radiotherapy. Patients in the other group were treated with only surgical excision and radiotherapy (K group). (2) Scar tissue from recurrent patients was collected after a second operation. Hematoxylin and eosin (H&E) staining was used to observe keloid morphology. Certain inflammatory factors (interleukin-6 (IL-6), hypoxia-inducible factor-1α (HIF-1α), tumor necrosis factor-α (TNF-α), nuclear factor κB (NF-κB), and vascular endothelial growth factor (VEGF)) were measured using immunohistochemical staining. Results: (1) The recurrence rate of the O group (5.97%) was significantly lower than that of the K group (14.15%), P<0.05. Moreover, patients in the O group reported greater satisfaction than those in the K group (P<0.05). (2) Compared with the recurrent scar tissue of the K group, the expression levels of the inflammatory factors were lower in the recurrent scar tissue of the O group. Conclusions: Adjunctive HBOT effectively reduces the keloid recurrence rate after surgical excision and radiotherapy by improving the oxygen level of the tissue and alleviating the inflammatory process.
Keywords: Keloid, Hyperbaric oxygen therapy, Surgical excision, Radiotherapy, Recurrence rate
1. Introduction
Keloids and hypertrophic scars are fibroproliferative disorders of the skin that result from the abnormal healing of injured or irritated skin (Ogawa and Akaishi, 2016). Keloids appear as firm, well-demarcated nodules or tumors with shiny surfaces and irregular borders. Mass coarse collagen fibers are observed via microscopy.
The most frequent precipitating event of keloid formation is trauma, including surgery, piercing, lacerations, and abrasions. Minor burns and vaccinations are also associated with keloids. The highest incidence of keloids occurs during the second and third decades of life, although they also occur in children and the elderly (Datubo-Brown, 1990; Niessen et al., 1999). Some studies have shown that the tissue surrounding a keloid is in a hypoxic state (Balestri et al., 2013). Inflammatory factors and vascular endothelial growth factors (VEGFs) are also involved in the pathologic process of keloid formation (Arno et al., 2014; Alexandrescu et al., 2016).
Hyperbaric oxygen therapy (HBOT) is an established technology that has been used for more than 40 years. In HBOT, patients receive oxygen treatment in a compression chamber under a pressure greater than one atmosphere absolute (ATA) of 100% oxygen (O'Reilly et al., 2011) to alleviate their hypoxic condition. HBOT has been used to treat skin disease and infection. In plastic surgery, HBOT is regarded as a successful adjunctive therapy for promoting wound healing, reducing inflammatory reactions, and improving flap survival (Zhang et al., 2007; Demirtaş et al., 2014).
Many keloid treatment methods have been reported. The most frequent problem associated with treatment is the high recurrence rate. In this study, we investigated whether adjunctive HBOT reduces the high recurrence rate of keloids after surgical excision and radiotherapy.
2. Materials and methods
2.1. Grouping
This clinical study was conducted between January 2012 and November 2016. The Institutional Review Board of Peking Union Medical College Hospital (Beijing, China) approved the surgical protocols.
The patients were randomly divided into two groups: patients who came to the clinic on odd months received HBOT (O group), while those who came to the clinic on even months did not (K group). A total of 134 patients, 33 males and 101 females, comprised the O group and their ages ranged from 16 to 52 years (mean (26.10±0.58) years). They received HBOT after surgical excision and radiotherapy. A total of 106 patients, 32 males and 74 females, comprised the K group and their ages ranged from 17 to 58 years (mean (28.06±0.92) years). These patients were treated with surgical excision and radiotherapy without HBOT. Patients with systemic diseases that are contraindications for HBOT were excluded from this study. The keloids were located on the chest or shoulder of the patients. The patients had received one or more non-surgical treatments, such as hormone injections, silicone sheeting, or herb ointment, in another hospital before surgery, but all such treatments had to have been terminated three months prior to surgery.
Recurrent patients from the above groups were included in the following two groups: recurrent patients from the O group (8 patients) were placed in the R-O group and recurrent patients from the K group (15 patients) were placed in the R-K group.
All relevant information was provided to the patients prior to the study. Informed consent was provided by all patients. All patient information was formally recorded.
2.2. Scoring method
A self-designed questionnaire, including sex, age, treatment time, scale of keloid, site, pigmentation, vascularity, pliability, height of keloid, and degree of satisfaction with treatment, was used to collect information before and after treatment. The Vancouver scar scale (Bloemen et al., 2009) was used to evaluate the keloid condition of all the patients before surgery. No significant differences were found between the K and O groups in regard to age, sex, keloid site, dimensions or Vancouver scar scale score before surgery (P>0.05).
Patient follow-up evaluations were conducted by an experienced doctor from our center who was blinded to the detailed treatment of each patient. The median follow-up time was 20.5 months for the O group and 21.0 months for the K group, with no significant difference between these groups. The treatment results were graded as follows: fully cured (the patient has an ordinary scar at the surgical site without obvious itching or pain), partially cured (the patient has a hypertrophic scar on a portion of the surgical site with occasional itching or pain), and keloid recurrence (the patient has a keloid scar on a portion of or over the entire surgical site with obvious itching and pain). Photographs of each patient were taken at the initial follow-up appointment and the evaluation was completed at the same time. The patients completed the aesthetic satisfaction questionnaire at each follow-up visit. The questionnaire was completed anonymously. Aesthetic satisfaction opinion was graded as excellent, good, or dissatisfied. The patients made their decision using their own judgment, and the clinic members did not provide suggestions during the follow-up visits. The Vancouver scar scale was used to evaluate patients in the O and K groups.
2.3. Surgical method
An incision line was marked on the normal skin 2 mm from the edge of the keloid. Surgery was performed under local anesthesia or local anesthesia plus sedation with conventional disinfection and a sterile operation sheet. A cut was made deep into the skin and subcutaneous tissue along the marked line, and all the keloid tissues were removed. Subcutaneous tissue blunt dissection was performed. Layered suturing was used to reduce the suture tension, and the incision was closed with double layer continuous intradermal sutures with 3–0 monofilament nylon. If the keloid was too large to sew up without tension, then a flap transplantation or skin graft was performed and the incision was closed with 5–0 monofilament nylon with interrupted sutures. Routine postoperative nursing care was provided. The sutures were removed 14 d after the surgery. The same group of doctors performed all the operations.
For the patients who had keloid recurrence confirmed, a second surgery was carried out at any time after the patients gave consent for another surgical treatment and the radiotherapy doctor thought that another round of radiotherapy was safe (this usually takes place one year after the first radiotherapy treatment). The recurred keloid was usually small. It was removed and the wound was closed directly.
2.4. Radiotherapy method
Patients whose wounds were primary closed or closed with a skin flap usually received radiotherapy on postoperative days 1 and 7 (900 cGy each). If some complications occurred, such as wound infection, wound dehiscence, or skin flap necrosis, the second round of radiotherapy was postponed until the complicated wound healed. In patients whose wounds were closed with skin grafts, the incisions were first made around the edge of the keloids (pre-cut method) the day before the keloids were excised, and the initial round of radiotherapy was carried out the next day followed by keloid excision and skin grafting on the same day. The second round of radiotherapy was carried out when the skin graft had survived and sutures were removed.
2.5. Hyperbaric oxygen therapy
Patients in the O group usually received HBOT after the operation for 120 min once daily for two weeks after which time the skin graft or flap had survived well and the sutures had been removed. If complications occurred, HBOT was continued until the wound healed. The operating parameters were as follows: air pressure was increased uniformly for 30 min to 0.2 MPa (2 ATAs) and then maintained. Patients received mask oxygen inhalation (100% oxygen concentration) for 60 min continuously. After that, the air pressure was reduced uniformly for 30 min.
2.6. Blood perfusion evaluation of patients with recurrent keloids or hypertrophic scars
Patients with recurrent keloids or hypertrophic scars after the initial operation received a keloid tissue blood perfusion evaluation using a laser Doppler flowmeter (LDF; Perimed AB, Stockholm, Sweden) and laser speckle contrast analysis (LSCA; Perimed AB, Stockholm, Sweden). The distance between the laser scanning probe and the patient was 15 cm. The image acquisition rate was 3 Hz, and the entire process lasted for 3 min. The ambient temperature was maintained between 22 and 25 °C. The vascular flow was measured using perfusion units (PUs).
2.7. Scar tissue collection and hematoxylin and eosin staining
Scar tissue was collected after the second operation from patients with recurrent keloids or hypertrophic scars following the first treatment. After being fixed in a 10% formalin solution for 48 h, tissue specimens were embedded in paraffin, sectioned, and placed on a slide. Histological characteristics were observed via hematoxylin and eosin (H&E) staining.
2.8. Scar tissue immunohistochemical study
Scar tissues were collected in the same way and then studied via immunohistochemical staining. In this process, paraffin-embedded sections were dewaxed, rehydrated, and incubated with 3% H2O2 for 10 min to block endogenous catalase. The slides were heated in a citrate buffer (CB) solution to 90 °C for 4 min and 40 °C for 10 min to retrieve the antigen. To block nonspecific staining, slides were incubated with normal goat serum at 37 °C for 30 min. Sections were placed in a humidified chamber at 4 °C overnight (12–16 h) with anti-interleukin-6 (anti-IL-6, 1:400), anti-hypoxia-inducible factor-1α (anti-HIF-1α, 1:200), anti-tumor necrosis factor-α (anti-TNF-α, 1:200), anti-nuclear factor κB (anti-NF-κB, 1:200), and anti-VEGF (1:200) antibodies (all purchased from Abcam, Cambridge, UK). The primary antibody was marked using horseradish peroxidase-conjugated secondary antibody (ZSGB-BIO, Beijing, China). After incubation, the slides were flushed with phosphate-buffered saline (PBS) and then stained with 3,3'-diaminobenzidine (DAB) and hematoxylin. Brown pigment, representing the presence of antibodies combined with antigens, was detected via optical microscopy with a computer-controlled digital camera and imaging software. The positive cells were measured by estimating the color and the shade: brown staining implies a positive expression area and the shade of brown denotes the expression level of the target protein.
2.9. Statistical analyses
Student’s t-test and a chi-square test were used to analyze the data via SPSS Ver. 22.0 (SPSS, Inc., Chicago, IL, USA). The significance level of the test was P≤0.05.
3. Results
3.1. Evaluation analysis
A total of 240 patients with keloids were included in this study. In the O group (a total of 134 patients), 119 patients were fully cured, 7 were partially cured, and 8 showed keloid recurrence (efficacy rate, 94.03%; recurrence rate, 5.97%). In the K group (a total of 106 patients), 80 patients were fully cured, 11 were partially cured, and 15 showed keloid recurrence (efficacy rate, 85.85%; recurrence rate, 14.15%; Table 1).
Table 1.
Comparison of the cure rates between the two patient treatment groups
| Group | Fully cured (%) | Partially cured (%) | Recurrence rate (%) | Efficacy rate (%) |
| O | 88.81 (119) | 5.22 (7) | 5.97 (8) | 94.03 (126) |
| K | 75.47 (80) | 10.38 (11) | 14.15 (15) | 85.85 (91) |
|
| ||||
| P | <0.05 | <0.05 | <0.05 | <0.05 |
O: the group that received HBOT; K: the group that did not receive HBOT. The numbers in parentheses represent the number of patients
The aesthetic satisfaction results of the O group showed that 115 patients rated the aesthetic results as excellent and 10 rated the results as good (satisfaction rate, 93.28%). The results of the K group showed that 79 patients rated the aesthetic results as excellent and 7 rated the results as good (satisfaction rate, 81.13%; Table 2).
Table 2.
Comparison of satisfaction between the two patient treatment groups
| Group | Excellent (%) | Good (%) | Dissatisfaction (%) |
| O | 85.82 (115) | 7.46 (10) | 6.72 (9) |
| K | 74.53 (79) | 6.60 (7) | 18.87 (20) |
|
| |||
| P | <0.05 | <0.05 | <0.05 |
O: the group that received HBOT; K: the group that did not receive HBOT. The numbers in parentheses represent the number of patients
The Vancouver scar scale scores of the partially cured plus recurrent keloids were 1.267±0.267 in the O group and 3.375±1.375 in the K group.
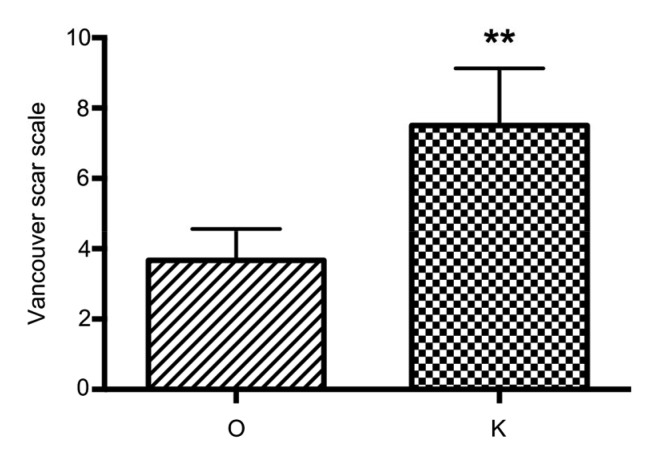
Tables 1 and 2 show that the efficacy and satisfaction rates of the O group were higher than those of the K group, and these differences were significant (P<0.05). The recurrence rate of the O group was lower than that of the K group (P<0.05). Moreover, more patients were satisfied with the aesthetic results in the O group than in the K group (P<0.05). Fig. 1 shows that the Vancouver scar scale score of the partially cured plus recurrent keloids in the O group was lower than that in the K group (P<0.05). Recurred keloids were more serious in the K group than in the O group (Figs. 2 and 3).
Fig. 1.
Vancouver scar scale score of partially cured plus recurrent keloids in the O and K groups
Higher scores denote keloids that are more serious. Data are expressed as mean±standard deviation (SD; O group, n=15; K group, n=26). ** P<0.01, vs. the O group
Fig. 2.
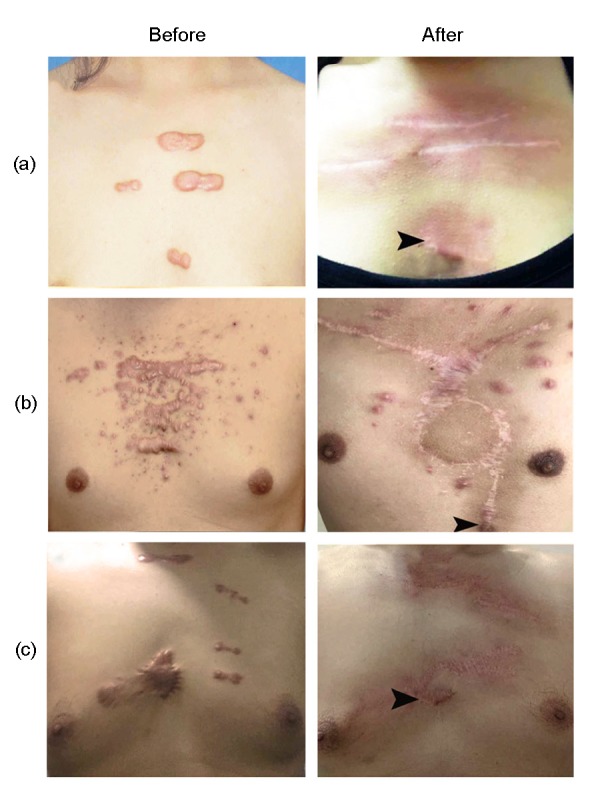
Keloid recurrence in the O group
Small partial recurrences were observed (black arrowheads). (a) Surgical excision with primary closure was performed on a 27-year-old female with keloids caused by breast acne; (b) Surgical excision with internal thoracic perforator vessel skin flap was performed on a 35-year-old male with keloids caused by mass acne; (c) Surgical excision with primary closure was performed on a 42-year-old male with keloids caused by trauma
Fig. 3.
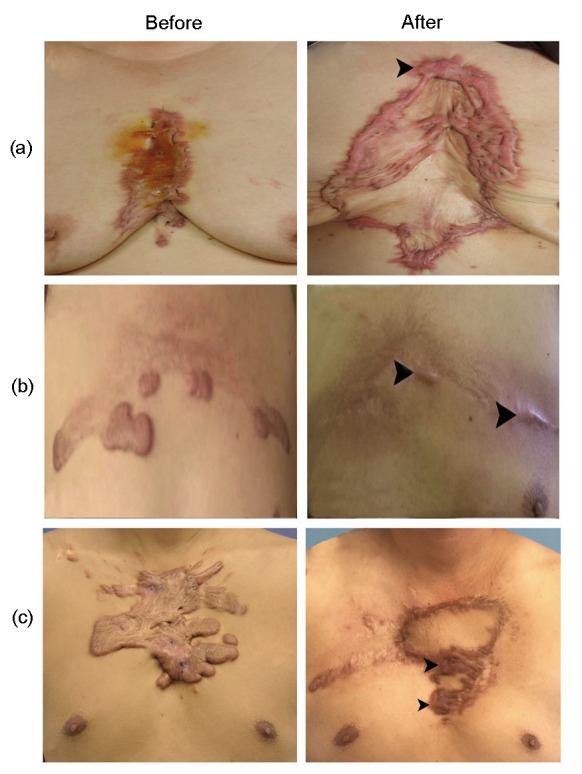
Keloid recurrence in the K group
A more serious keloid recurrence was observed in the K group than in the O group. Keloid recurrence was noted over almost all of the incision sites in many of the patients (black arrowheads). The recurrence time was shorter than that in the O group. (a) A 65-year-old female underwent keloid removal and skin graft for keloids caused by acne; (b) Surgical excision with primary closure was performed on a 52-year-old male with keloids caused by acne; (c) Surgical excision with superficial circumflex iliac artery perforator skin flap transplantation was performed on a 31-year-old male with keloids caused by acne
3.2. Keloid tissue blood perfusion evaluation
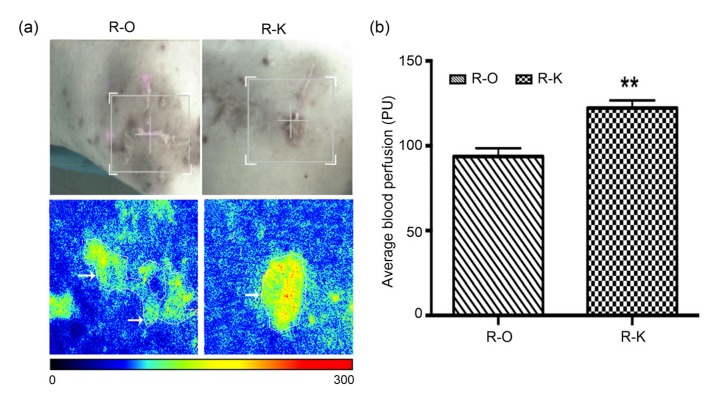
The average blood perfusion of the keloid tissues in the R-K group was (122.1±1.2) PU (ml/(100 g∙min)), which was higher than that in the R-O group ((93.7±1.8) PU, P<0.01; Fig. 4).
Fig. 4.
Average blood perfusion of the keloid tissue in recurrent patients
(a) The blood perfusion detecting image; (b) Average blood perfusion of the R-O and R-K groups (R-O: recurrent patients from the O group; R-K: recurrent patients from the K group). The white arrows indicate keloid blood perfusion. Red represents high blood perfusion. Data are expressed as mean±SD (R-O group, n=8; R-K group, n=15). ** P<0.01, vs. the R-O group (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
3.3. Histological analysis
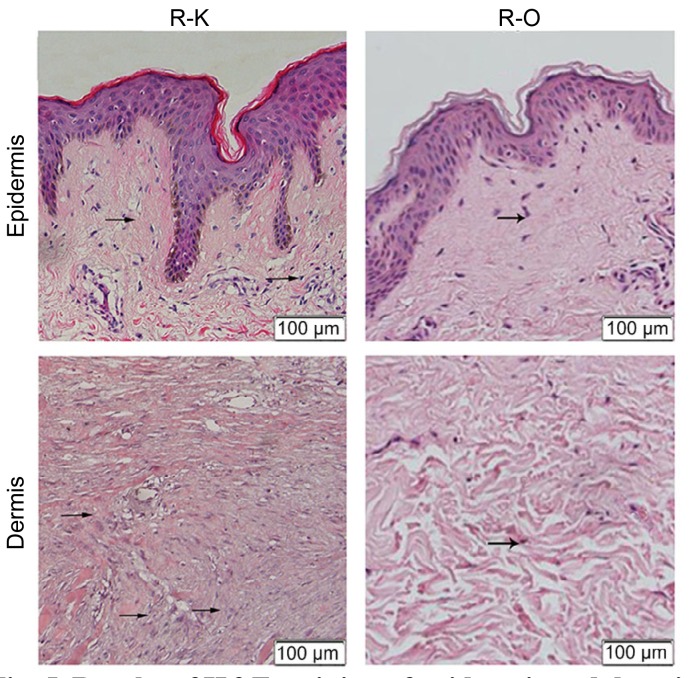
H&E-stained tissues were used to confirm the pathological status of the recurred keloids. Many fibroblasts and a large amount of inflammatory infiltration could be observed in the K group. Thick and extremely compact collagen fibrils also appeared disordered. These pathological changes were less obvious in the O group (Fig. 5).
Fig. 5.
Results of H&E staining of epidermis and dermis in the R-O and R-K groups
Numerous fibroblasts and a large amount of inflammatory infiltration can be observed. Thick and extremely compact collagen fibrils appear disordered. Arrows represent inflammatory cells
3.4. Immunohistochemical evaluation
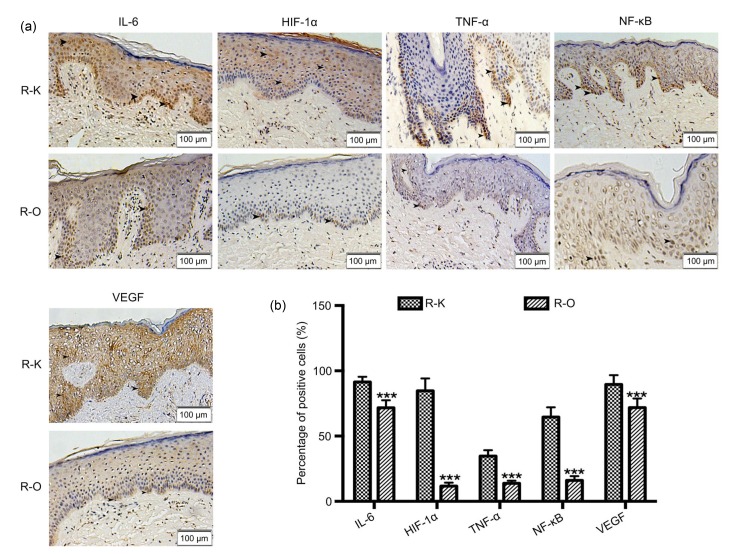
A qualitative analysis of the expression of IL-6, HIF-1α, TNF-α, NF-κB, and VEGF was conducted via an immunohistochemical study. According to the results, these inflammatory factors showed a higher expression in the R-K group than in the R-O group (Fig. 6a). Table 3 and Fig. 6b show the percentage of positively stained cells. Significant differences were found between the K and O groups for all the inflammatory factors (P<0.001).
Fig. 6.
Immunohistochemical studies of all factors
(a) Immunohistochemical studies of IL-6, HIF-1α, TNF-α, NF-κB, and VEGF in the R-K and R-O groups. In the R-K group, the expression of all these inflammatory factors was higher than that in the R-O group. (b) Percentage of positively stained cells. Data are expressed as mean±SD (R-O group, n=8; R-K group, n=15). *** P<0.001, vs. the R-K group
Table 3.
Percentage of positive cells in the R-K and R-O groups
| Group | Percentage of positive cells (%) |
||||
| IL-6 | HIF-1α | TNF-α | NF-κB | VEGF | |
| R-K | 91.40±1.03 | 84.67±2.47 | 34.67±1.17 | 64.53±1.93 | 89.53±1.86 |
| R-O | 71.63±2.06 | 11.63±0.96 | 13.75±0.75 | 16.00±1.16 | 71.75±2.48 |
|
| |||||
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
R-K: recurrent patients from the group not given HBOT; R-O: recurrent patients from the group given HBOT. Data are expressed as mean±SD (R-O group, n=8; R-K group, n=15)
4. Discussion
Keloids influence patients both psychologically and physically. The most important histological manifestation of keloids is the numerous cytoplasm-rich fibroblasts with clear nucleoli in the papillary and reticular layers of the dermis. The mass consists of coarse collagen fibers in a disorderly arrangement.
Inflammatory factors have been suggested as having a relationship with keloid formation, especially IL-6 (Arno et al., 2014). Chen et al. (2003) and Dong et al. (2013) found that proinflammatory factors, such as IL-6 and TNF-α, are upregulated in keloid tissues. Furthermore, previous results have shown that VEGFs appear to play important roles in the pathogenesis of keloids (Alexandrescu et al., 2016). Makino et al. (2008) found that NF-κB plays an important role in the regulation of inflammation, immune response, and cell proliferation. The activation of the NF-κB pathway is likely closely linked to abnormal cell proliferation and extracellular matrix (ECM) production in keloid fibroblasts. However, HIF-1α is highly expressed in keloid tissue because of its continuous exposure to hypoxia, which promotes the adaptation of the cells to a hypoxic microenvironment (Dhani et al., 2015).
HBOT is widely used in clinical practice. In plastic surgery, HBOT is regarded as a successful adjunctive therapy to promote wound healing, reduce inflammatory reactions, and improve flap survival (Zhang et al., 2007; Demirtaş et al., 2014). Keloid incisions are often vulnerable to infection after radiotherapy. HBOT can improve local blood circulation and wound healing by increasing the blood oxygen capacity and tension of the body. Based on this capacity, we innovatively applied adjunctive HBOT to keloid treatment and achieved satisfactory results.
Keloids might be the result of chronic inflammation in the reticular dermis. Hypoxia influences the early inflammatory phase of wound healing. Furthermore, hypoxia induces inflammation through HIF-1α (Manresa et al., 2014). HBOT involves breathing pure oxygen in a pressure chamber at greater than one ATA (Wang et al., 2014). The pressure increases the level of oxygen in the blood plasma, which affects the immune system, wound healing, and vascular tone (O'Reilly et al., 2011). HBOT effectively promotes wound healing and reduces inflammatory reactions (Zhang et al., 2007; Demirtaş et al., 2014). According to our immunohistochemical studies, expression of the detected inflammatory factors (IL-6, HIF-1α, TNF-α, NF-κB, and VEGF) was lower in the R-O group than in the R-K group. These results suggest that HBOT depresses the inflammatory reaction associated with incision recovery among patients with keloids after operation. Furthermore, recent studies have shown that keloids are the result of chronic inflammation in the reticular dermis (Ogawa, 2017). Therefore, HBOT alleviates inflammation and decreases the recovery rate.
Accumulating evidence suggests that a hypoxic microenvironment is associated with keloids through the abnormally large number of occluded microvessels; moreover, hypoxia might play a crucial role in keloid pathogenesis (Zhang et al., 2003). The level of HIF-1α is consistently higher in freshly biopsied keloid tissues than in their associated normal skin borders, which provides direct evidence of the local hypoxic state in keloids. Liu et al. (2016) found that the perfusion values of keloids and the adjacent skin were significantly higher than those of nonadjacent skin. Early and timely HBOT intervention can greatly improve the condition of tissues because HIF-1α is inhibited (Ueda et al., 2004; Yang et al., 2014). Our study found that the blood perfusion of keloid tissue in the R-O group was lower than that in the R-K group. This finding might indicate that HBOT increases the oxygen content, but decreases the blood perfusion of keloid tissues. This effect might influence HIF-1α expression, thereby reducing the keloid recurrence rate.
Many methods of keloid treatment have been reported, such as intralesional injections of steroids, cryotherapy, laser therapy, topical imiquimod application, bleomycin, and 5-fluorouracil as well as interferon α, β, and γ application (Goldenberg and Luber, 2013). However, surgery is the gold standard to treat keloids of any size because it reduces the mass of the lesion (de Felice et al., 2015). Current operative methods often include the combination of excision followed by a reconstructive procedure (Berman et al., 2007).
High recurrence rates have been reported in keloid surgical treatment without adjunctive therapy. Froelich et al. (2007) found recurrence rates ranging from 45% to 100% in their literature review and suggested that adjuvant treatment after keloid surgery should be mandatory. The most reported adjuvant treatments are intralesional injections and radiotherapy. Park et al. (2013) examined 79 patients treated with complete surgical excision and full-thickness skin grafting followed by corticosteroid injections. A total of 17 (21.5%) patients experienced recurrence after surgery.
Radiotherapy after surgery is a prevalent keloid treatment method. It can effectively reduce recurrence, but recurrence rates remain high in some reports. Kovalic and Perez (1989) and Perez et al. (2001) reported a recurrence rate of 33% in their studies. This result is identical to that obtained by Darzi et al. (1992). Doornbos et al. (1990) treated 203 keloids, and the recurrence rate was 23.5%. Ragoowansi et al. (2003) used a single full fraction of 10 Gy after surgery and achieved a recurrence rate of 16%. Emad et al. (2010) enrolled 26 patients with a total of 76 keloids; of these, 19 patients with 44 keloids underwent surgical excision combined with immediate 12-Gy irradiation, and the recurrence rate was 18.2%. In Germany, Kutzner et al. (2003) used total doses between 10 and 20 Gy and achieved a recurrence rate of 11.4%.
In our study, adjunctive HBOT after surgical excision and radiotherapy effectively reduced the keloid recurrence rate. The overall recurrence rate was 5.97%, which was lower than that associated with surgical excision and radiotherapy, for which the recurrence rate was 14.15%. This result is also lower than those obtained by Kutzner et al. (2003), Ragoowansi et al. (2003), and Emad et al. (2010) who used radiotherapy as the only postoperative auxiliary treatment method. Significant differences were also found between the two groups in regard to the satisfaction ratings and the Vancouver scar scale score. The Vancouver scar scale is used to evaluate scar appearance. The satisfaction rate is based on not only scar appearance, but also the relief of symptoms, such as itching and pain. The obvious differences between these groups demonstrated the effect of HBOT on keloid treatment. HBOT might reduce inflammatory reactions and hypoxia during wound healing after surgical procedures.
HBOT is less harmful and more feasible than other techniques, such as intralesional injection or laser therapy. Patients need only to stay in an HBOT room to avoid additional burdens (e.g. painful intralesional injections and silicon gel that is not easy to wear). Furthermore, HBOT is economical, which makes it more likely to be accepted by patients. However, certain features of the human body must also be considered when evaluating the recurrence rate. The keloids of the patients registered in this study occurred on the chest or shoulder. These two areas were selected because they are both on the torso, so the common location could prevent biases incurred by body features. In our study, we did not include keloids on other parts, such as the face, ear, or limbs, because differences in blood supply and activity in various body parts of the patients may cause high variability in postoperative recovery and recurrence. We will investigate the recurrence rate of keloids on other parts of the body in the future.
5. Conclusions
HBOT effectively reduces the recurrence rate of keloids after surgical excision and radiotherapy by reducing the inflammatory reaction and relieving tissue hypoxia during the wound healing process. However, additional research must be conducted regarding its mechanisms of action.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81471885) and the Beijing Natural Science Foundation (No. 7172172), China
Contributors: You-bin WANG conceived the study. Ming-zi ZHANG, Wei-zhong LIANG, Hao LIU, Xin-hang DONG, and Xiao-jun WANG were responsible for data management and statistical analyses. All authors were responsible for the interpretation of the data. Ke-xin SONG and Shu LIU wrote the paper and all authors read and approved the final manuscript.
Compliance with ethics guidelines: Ke-xin SONG, Shu LIU, Ming-zi ZHANG, Wei-zhong LIANG, Hao LIU, Xin-hang DONG, You-bin WANG, and Xiao-jun WANG declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for whom identifying information is included in this article.
References
- 1.Alexandrescu D, Fabi S, Yeh LC, et al. Comparative results in treatment of keloids with intralesional 5-FU/kenalog, 5-FU/verapamil, enalapril alone, verapamil alone, and laser: a case report and review of the literature. J Drugs Dermatol. 2016;15(11):1442–1447. [PubMed] [Google Scholar]
- 2.Arno AI, Amini-Nik S, Blit PH, et al. Effect of human Wharton’s jelly mesenchymal stem cell paracrine signaling on keloid fibroblasts. Stem Cells Transl Med. 2014;3(3):299–307. doi: 10.5966/sctm.2013-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balestri R, Misciali C, Zampatti C, et al. Keloidal basal cell carcinoma: should it be considered a distinct entity? J Dtsch Dermatol Ges. 2013;11(12):1196–1198. doi: 10.1111/ddg.12168. [DOI] [PubMed] [Google Scholar]
- 4.Berman B, Perez OA, Konda S, et al. A review of the biologic effects, clinical efficacy, and safety of silicone elastomer sheeting for hypertrophic and keloid scar treatment and management. Dermatol Surg. 2007;33(11):1291–1303. doi: 10.1111/j.1524-4725.2007.33280.x. [DOI] [PubMed] [Google Scholar]
- 5.Bloemen MCT, van der Veer WM, Ulrich MMW, et al. Prevention and curative management of hypertrophic scar formation. Burns. 2009;35(4):463–475. doi: 10.1016/j.burns.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Fu XB, Sun XQ, et al. Analysis of differentially expressed genes in keloids and normal skin with cDNA microarray. J Surg Res. 2003;113(2):208–216. doi: 10.1016/S0022-4804(03)00188-4. [DOI] [PubMed] [Google Scholar]
- 7.Darzi MA, Chowdri NA, Kaul SK, et al. Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. Br J Plast Surg. 1992;45(5):374–379. doi: 10.1016/0007-1226(92)90008-L. [DOI] [PubMed] [Google Scholar]
- 8.Datubo-Brown DD. Keloids: a review of the literature. Br J Plast Surg. 1990;43(1):70–77. doi: 10.1016/0007-1226(90)90047-4. [DOI] [PubMed] [Google Scholar]
- 9.de Felice B, Guida M, Boccia L, et al. Ingenol mebutate treatment in keloids. BMC Res Notes, 8:466. 2015 doi: 10.1186/s13104-015-1429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demirtaş A, Azboy I, Bulut M, et al. The effect of hyperbaric oxygen therapy on fracture healing in nicotinized rats. Ulus Travma Acil Cerrahi Derg. 2014;20(3):161–166. doi: 10.5505/tjtes.2014.52323. [DOI] [PubMed] [Google Scholar]
- 11.Dhani N, Fyles A, Hedley D, et al. The clinical significance of hypoxia in human cancers. Semin Nucl Med. 2015;45(2):110–121. doi: 10.1053/j.semnuclmed.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Dong XL, Mao SL, Wen H. Upregulation of proinflammatory genes in skin lesions may be the cause of keloid formation (Review) Biomed Rep. 2013;1(6):833–836. doi: 10.3892/br.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doornbos JF, Stoffel TJ, Hass AC, et al. The role of kilovoltage irradiation in the treatment of keloids. Int J Radiat Oncol Biol Phys. 1990;18(4):833–839. doi: 10.1016/0360-3016(90)90405-9. [DOI] [PubMed] [Google Scholar]
- 14.Emad M, Omidvari S, Dastgheib L, et al. Surgical excision and immediate postoperative radiotherapy versus cryotherapy and intralesional steroids in the management of keloids: a prospective clinical trial. Med Princ Pract. 2010;19(5):402–405. doi: 10.1159/000316381. [DOI] [PubMed] [Google Scholar]
- 15.Froelich K, Staudenmaier R, Kleinsasser N, et al. Therapy of auricular keloids: review of different treatment modalities and proposal for a therapeutic algorithm. Eur Arch Otorhinolaryngol. 2007;264(12):1497–1508. doi: 10.1007/s00405-007-0383-0. [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg G, Luber AJ. Use of intralesional cryosurgery as an innovative therapy for keloid scars and a review of current treatments. J Clin Aesthet Dermatol. 2013;6(7):23–26. [PMC free article] [PubMed] [Google Scholar]
- 17.Kovalic JJ, Perez CA. Radiation therapy following keloidectomy: a 20-year experience jeffrey. Int J Radiat Oncol Biol Phys. 1989;17(1):77–80. doi: 10.1016/0360-3016(89)90373-8. [DOI] [PubMed] [Google Scholar]
- 18.Kutzner J, Schneider L, Seegenschmiedt MH. Radiotherapy of keloids. Patterns of care study-results. Strahlenther Onkol. 2003;179(1):54–58. doi: 10.1007/s00066-003-1023-2. [DOI] [PubMed] [Google Scholar]
- 19.Liu QL, Wang XJ, Jia YH, et al. Increased blood flow in keloids and adjacent skin revealed by laser speckle contrast imaging. Lasers Surg Med. 2016;48(4):360–364. doi: 10.1002/lsm.22470. [DOI] [PubMed] [Google Scholar]
- 20.Makino S, Mitsutake N, Nakashima M, et al. DHMEQ, a novel NF-kappaB inhibitor, suppresses growth and type I collagen accumulation in keloid fibroblasts. J Dermatol Sci. 2008;51(3):171–180. doi: 10.1016/j.jdermsci.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Manresa MC, Godson C, Taylor CT. Hypoxia-sensitive pathways in inflammation-driven fibrosis. Am J Physiol Regul Integr Comp Physiol. 2014;307(12):R1369–R1380. doi: 10.1152/ajpregu.00349.2014. [DOI] [PubMed] [Google Scholar]
- 22.Niessen FB, Spauwen PHM, Schalkwijk J, et al. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104(5):1435–1458. doi: 10.1097/00006534-199910000-00031. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18(3):606. doi: 10.3390/ijms18030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa R, Akaishi S. Endothelial dysfunction may play a key role in keloid and hypertrophic scar pathogenesis– keloids and hypertrophic scars may be vascular disorders. Med Hypotheses. 2016;96:51–60. doi: 10.1016/j.mehy.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 25.O'Reilly D, Linden R, Fedorko L, et al. A prospective, double-blind, randomized, controlled clinical trial comparing standard wound care with adjunctive hyperbaric oxygen therapy (HBOT) to standard wound care only for the treatment of chronic, non-healing ulcers of the lower limb in patients with diabetes mellitus: a study protocol. Trials, 12:69. 2011 doi: 10.1186/1745-6215-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park TH, Park JH, Chang CH. Clinical features and outcomes of foot keloids treated using complete surgical excision and full thickness skin grafting followed by corticosteroid injections. J Foot Ankle Res, 6:26. 2013 doi: 10.1186/1757-1146-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez CA, Lockett MA, Young G. Radiation therapy for keloids and plantar warts. Front Radiat Ther Oncol. 2001;35:135–146. doi: 10.1159/000061273. [DOI] [PubMed] [Google Scholar]
- 28.Ragoowansi R, Cornes PG, Moss AL, et al. Treatment of keloids by surgical excision and immediate postoperative single-fraction radiotherapy. Plast Reconstr Surg. 2003;111(6):1853–1859. doi: 10.1097/01.PRS.0000056869.31142.DE. [DOI] [PubMed] [Google Scholar]
- 29.Ueda K, Yasuda Y, Furuya E, et al. Inadequate blood supply persists in keloids. Scand J Plast Reconstr Surg Hand Surg. 2004;38(5):267–271. doi: 10.1080/02844310410029552. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Chen DD, Chen G. 2014. [Google Scholar]
- 31.Yang Y, Zhang YG, Lin GA, et al. The effects of different hyperbaric oxygen manipulations in rats after traumatic brain injury. Neurosci Lett. 2014;563:38–43. doi: 10.1016/j.neulet.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Zhang QZ, Wu YD, Ann DK, et al. Mechanisms of hypoxic regulation of plasminogen activator inhibitor-1 gene expression in keloid fibroblasts. J Invest Dermatol. 2003;121(5):1005–1012. doi: 10.1046/j.1523-1747.2003.12564.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang T, Gong W, Li Z, et al. Efficacy of hyperbaric oxygen on survival of random pattern skin flap in diabetic rats. Undersea Hyperb Med. 2007;34(5):335–339. [PubMed] [Google Scholar]