Abstract
Background. The relationships between motor impairment of the affected leg, postural control asymmetry, and impaired body sway control after stroke are not well understood. Objective. To examine the relationship between motor impairment of the affected leg and reduced contribution of this leg to body sway control (ie, dynamic control asymmetry [DCA]) and to determine the relationships between impaired body sway control, DCA, and weight-bearing asymmetry (WBA). Methods. We assessed quiet-standing balance with eyes open in 70 persons with a unilateral supratentorial chronic stroke using 2 force plates. Center-of-pressure (COP) velocity was calculated for both feet together in the anteroposterior (AP) and mediolateral (ML) directions as a measure of body sway control. Bilateral AP COP velocities were used to calculate an index for DCA and weight borne on each side to calculate WBA. Fugl-Meyer assessment of the lower extremity (FMA-LE; range: 0-28) served as a measure of affected-leg motor impairment. Results. All participants with FMA-LE <24 showed pronounced DCA, but this was also true for 21% of those with FMA ⩾24. Higher DCA values were related to more WBA (rs = 0.496; P < .001), and less ML sway control (rs = 0.268; P = .025). AP sway control was not significantly related to either DCA or WBA. Conclusions. Even clinically well-recovered stroke survivors with (near) maximal FMA-LE scores may show clear postural asymmetry in terms of the relative contribution of the affected leg to body sway control. WBA seems to be an effective compensatory mechanism to optimize the contribution of the less-affected leg to balance, particularly in the AP direction.
Keywords: stroke, postural balance, weight-bearing, hemiparesis
Introduction
It is well known that a unilateral supratentorial stroke may affect the control of standing balance in several ways. Many posturographic studies have reported impaired body sway control as exemplified by increased center-of-pressure (COP) movements in the anteroposterior (AP) and mediolateral (ML) directions.1-3 In addition, some degree of weight-bearing asymmetry (WBA) in favor of the less-affected leg is observed in many patients with stroke.4-7 Although both abnormalities may improve during the subacute phase after stroke, patients with a moderate to severe paresis often show persistently impaired body sway control as well as persistent WBA (on average about 10% more weight being borne on the less-affected leg) in the chronic phase (ie, >6 months poststroke).8 More recent studies in patients with unilateral supratentorial stroke that used dual-plate posturography have further revealed that, in kinetic terms, the less-affected leg contributes much more to standing balance than the affected leg.9 In other words, after a unilateral supratentorial stroke, both legs do not contribute equally to the generation of COP movements or ankle torques. Remarkably, this so-called dynamic control asymmetry (DCA) shows little tendency to diminish during rehabilitation.8-10 Together, these results suggest that although postural sway control and affected-leg weight bearing improve over the course of rehabilitation,8 the contribution of the affected leg to standing balance control often remains poor in the chronic phase poststroke.9 Asymmetry in the contribution of each leg to standing balance is also reflected in a reduced between-limb temporal synchronization (ie, cross-correlation) of COP movements. Recently, Mansfield et al11 investigated quiet standing balance in the subacute phase of stroke and found that between-limb synchronization was indeed reduced. This synchronization was moderately associated with motor scores (Chedoke-McMaster Stroke Assessment) of the affected leg and foot (rs = 0.4),12 but the relationship between leg motor impairment and DCA is still unknown. In addition, whether persisting DCA may be present in the chronic phase after stroke even in well-recovered individuals has yet to be established.
Persistent DCA after unilateral supratentorial stroke together with improvements in postural sway control suggest that the less-affected leg compensates for the loss of selective motor control on the affected side to maintain standing balance. This compensation may be so effective that body sway control does not necessarily differ between patients with mild paresis and those with relatively severe paresis. Typically, patients with more severe paresis tend to show a greater degree of DCA and WBA than those with limited motor impairments, without significant group differences in overall COP movements.10 Yet patients who show a fairly symmetric weight distribution may still exhibit DCA in favor of the less-affected leg.9
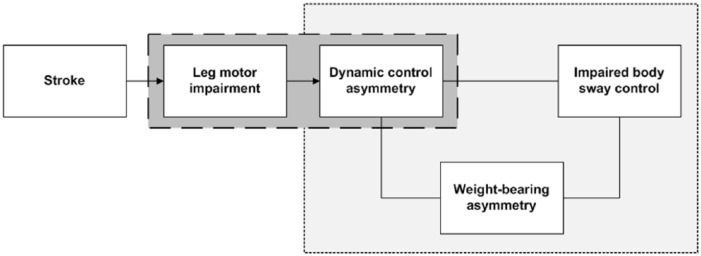
To gain better insight into the effects of unilateral supratentorial stroke on the various characteristics of standing balance control, we conducted a cross-sectional study in the chronic phase after unilateral supratentorial stroke and included community-dwelling independent walkers with a wide range of motor impairments of the affected leg. Our first aim was to examine the relationship of motor impairment of the affected leg, as assessed with the Fugl-Meyer assessment (FMA) with DCA (Figure 1). We hypothesized that the relationship between leg motor impairment and DCA would be nonlinear—that is, even patients with clinically no impairment or only very limited leg motor impairment may show a substantial degree of DCA as a result of loss of fine lower-leg motor control needed to execute ankle strategies. Our second aim was to determine the mutual relationships between WBA, DCA, and body sway control during quiet standing (Figure 1). We hypothesized that the relationship between DCA and WBA would be positive and statistically significant, but that the relationship between DCA and body sway control would be statistically nonsignificant because of a modulating effect of WBA.
Figure 1.
Conceptual framework of the presumed mutual relationships between unilateral supratentorial stroke, motor impairment on the affected side, dynamic control asymmetry in favor of the less affected leg, weight-bearing asymmetry, and impaired body sway control.
Methods
Participants
A total of 70 persons in the chronic phase (>6 months) after a unilateral supratentorial stroke were included. Potential participants were recruited from the outpatient departments of Rehabilitation and Neurology at Radboud University Medical Center, from the outpatient clinics of affiliated rehabilitation centers, and through advertisements in local newspapers. The medical record of each individual was checked before participation in the case of any uncertainty about the stroke location. Participants had to be able to stand and walk independently on a regular surface without supervision (Functional Ambulation Categories [FAC] score ⩾4).13 All participants had normal or corrected to normal vision. We excluded potential participants if they suffered from neurological (except stroke), musculoskeletal, or cognitive (Mini Mental State Examination < 24)14 impairments as well as individuals who used psychotropic medication. Table 1 shows relevant participant characteristics, including the FMA lower-extremity score without the coordination domain (FMA-LE; range: 0-28) as a measure of leg motor impairment15; the Motricity Index lower-extremity score (MI-LE; range: 0-100) as a measure of leg strength16; Quantitative Vibration Threshold (QVT) of the affected lateral malleolus and hallux as a reliable and sensitive measure of deep sensibility17,18; and the 10-m walking test at comfortable walking speed, the Timed Up and Go test,19 and the Berg Balance Scale20,21 as functional measures. Written informed consent was obtained from all participants. The protocol was approved by the Medical Ethical Board of the region Arnhem-Nijmegen (NL33977.091.10), and all procedures were conducted in accordance with the Declaration of Helsinki.
Table 1.
Clinical Characteristics of Participants (n = 70).
| Mean (SD) or Percentage | |
|---|---|
| Age (years) | 63.6 (8.2) |
| Sex (male/female, percentage male) | 77.1% |
| Months since stroke | 56.2 (46.7) |
| Stroke type (ischemic/hemorrhagic, percentage ischemic) | 85.3% |
| Affected hemisphere (left/right, percentage left) | 42.9% |
| MMSE | 28.5 (1.5) |
| FMA-LE | 25.0 (4.2) |
| MI-LE | 79.9 (15.1) |
| QVT-lateral malleolus | 4.7 (2.1) |
| QVT-hallux | 4.2 (2.4) |
| FAC (4/5, percentage FAC 5) | 95.7% |
| Comfortable walking speed (m/s) | 1.0 (0.3) |
| Timed Up and Go test (s) | 12.2 (7.8) |
| Berg Balance Scalea | 51.8 (5.6) |
Abbreviations: FAC, Functional Ambulation Categories; FMA-LE, Fugl-Meyer Assessment—lower extremity, affected side (range: 0-28); MI-LE, Motricity Index—lower extremity, affected side (range: 0-100); MMSE, Mini Mental State Examination (range: 0-30); QVT-hallux, Quantitative Vibration Threshold—hallux, affected side (range: 0-8); QVT-lateral malleolus, QVT-lateral malleolus, affected side (range: 0-8).
Berg Balance Scale, range: 0-56.
Experimental Setup and Assessment Protocol
During the balance assessment, participants stood barefoot on 2 force plates (AMTI, Watertown, NY) with their arms alongside their trunk. Each foot was positioned on one force plate against a fixed foot frame, so that the medial sides of the heels were 8.4 cm apart and the toes were oriented outward at a 9° angle from the sagittal midline.22 Participants were instructed to stand as still as possible on a firm surface with their eyes open for 30 s. Participants were instructed to look straight ahead at a fixation cross mounted on the opposite wall at a distance of 8.1 m. Two trials were recorded for each participant.
Data Collection and Analysis
Force-plate data were recorded at 1000 Hz and were low-pass filtered with a dual-pass, second-order Butterworth filter with a cutoff frequency of 6 Hz. For each trial, the first 5 seconds were removed to prevent the influence of starting effects. In addition, the last 5 seconds were discarded to reduce any influence of loss of focus on the task (standing still) at the end of each trial. This resulted in 20 s of data available for analysis. Previous research has shown that 20 to 30 s quiet standing registrations yield optimal test-retest reliability.23 As a measure of body sway control in the AP and ML directions, we calculated the root mean square (RMS) of the overall COP velocity (ie, taking 2 feet together without correction for toeing-out angle). To assess the symmetry in the use of ankle strategies, COP movements in the AP direction were also calculated for each leg separately, corrected for toeing-out angle because of the different orientation of each foot in the coordinate system (left foot 9° rotated to the left; right foot 9° rotated to the right with respect to the sagittal midline). To express the degree of DCA in the AP direction, we calculated a symmetry index (SI) according to the following formula24:
A SI of zero represented an equal contribution of both legs to the overall AP COP velocity. Positive and negative SI values indicated a larger contribution of the less-affected and the affected leg to the overall AP COP velocity, respectively. If the SI fell outside the normal range observed in healthy control individuals (ie, −66% to 66%),22,25 DCA was considered to be significant. WBA was defined as the difference between 50% and the percentage of total body weight borne on the affected leg. A value of 0% indicated an equal weight distribution between both legs. Positive WBA values indicated a larger amount of weight on the less-affected leg, whereas negative WBA values corresponded to more weight on the affected leg.
Statistical Analysis
To determine whether the FMA-LE score was predictive for DCA, we calculated the positive and negative predictive values (PPVs and NPVs) and their corresponding 95% CIs for several cutoff points of the FMA-LE score (ie, 22 to 27). The PPV was defined as the percentage of participants with a FMA-LE score below or equal to the cutoff point who had significant DCA (ie, an abnormally high SI >66%). The NPV was defined as the percentage of participants with a FMA-LE score above the cutoff point who had no DCA (ie, a normal SI between −66% and 66%). To also examine other contributors to DCA, we conducted a forward stepwise logistic regression analysis with DCA (normal vs abnormal) as the dependent variable and FMA-LE score, MI-LE score, QVT of the affected lateral malleolus, QVT of the affected hallux, affected body side, and age as independent variables. To examine the relationship between DCA, WBA, and body sway control, we performed linear regression analyses and calculated Spearman correlation coefficients between each pair of postural characteristics. All statistical analyses were performed in SPSS (version 22.0). P values <.05 were considered statistically significant.
Results
Participants showed, on average, a relatively high level of balance and gait capacities based on FAC, gait speed, Timed Up and Go test, and Berg Balance Scale (Table 1). The DCA in favor of the less-affected leg fell outside the normal range in favor of the less-affected leg (SI > 66%) in 28 participants (40%). There was on average 4.4% WBA in favor of the less-affected leg, but a large between-subjects variance existed (Table 2).
Table 2.
Posturographic Characteristics of Participants (n = 70).
| Mean (SD; range) | |
|---|---|
| Body sway control (AP; RMS AP COP velocity; mm/s) | 13.7 (5.4; 5.6-28.2) |
| Body sway control (ML; RMS ML COP velocity; mm/s) | 7.3 (3.7; 2.4-22.3) |
| Dynamic control asymmetry (AP; symmetry index [AP]) | 48.9 (52.9; −56.4 to 159.7) |
| Weight-bearing asymmetry (50% − Percentage of body weight on affected leg) | 4.4 (7.8; −9.9 to 28.7) |
Abbreviations: AP, anteroposterior direction; ML, mediolateral direction; RMS COP velocity, root mean square velocity of center-of-pressure movements.
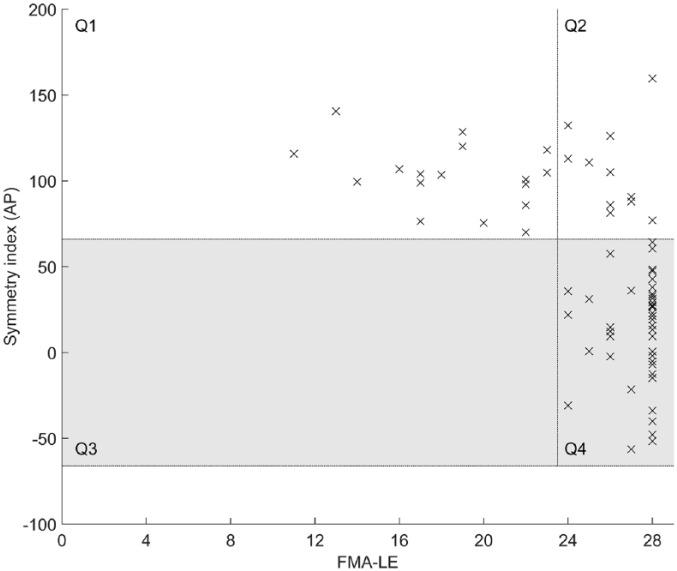
To provide insight into the relationship between motor impairment of the affected leg and DCA, Table 3 shows the PPVs and NPVs for several FMA-LE values with regard to DCA. The sum of the PPV and NPV was highest for FMA-LE score 23. All participants who scored <24 points on the FMA-LE had abnormally high SI values (PPV value 100%; Figure 2, Q1). Of the 53 participants who scored ⩾24 points on the FMA-LE, 42 had normal SI values (NPV value 79%; Figure 2, Q4). However, 11 participants (21%) with such a high FMA-LE score still showed abnormally high SI values, indicating DCA (Figure 2, Q2).
Table 3.
PPV and NPV of the FMA-LE With Respect to Dynamic Control Asymmetry.
| FMA-LE | PPV (%) [95% CI] | NPV (%) [95% CI] |
|---|---|---|
| 22 | 100 | 76; [66-86] |
| 23 | 100 | 79; [69-89] |
| 24 | 86; [78-94] | 81; [72-90] |
| 25 | 80; [71-89] | 82; [73-91] |
| 26 | 71; [60-82] | 89; [82-96] |
| 27 | 67; [56-78] | 94; [88-100] |
Abbreviations: FMA-LE, Fugl-Meyer Assessment—lower extremity; NPV, negative predictive value; PPV, positive predictive value.
Figure 2.
Dynamic control asymmetry (expressed as a symmetry index) plotted against leg motor impairment (expressed as Fugl-Meyer Assessment—lower extremity [FMA-LE] score). The gray area represents the normal range of symmetry indices obtained from healthy individuals.22,25 Q1 and Q2 represent participants with SI values >66% (ie, in favor of the less-affected leg) and FMA-LE scores <24 points (Q1) or ⩾24 points (Q2). None of our participants with a FMA-LE score <24 points had a SI value within the normal range for healthy individuals (gray area; Q3). Participants in Q4 had a SI value within the normal range and a FMA-LE score ⩾24 points. The vertical line represents the distinction between a FMA-LE score <24 (ie, moderate to severe leg motor impairment) and a FMA-LE score ⩾24 points (ie, mild leg motor impairment).
Abbreviation: AP, anteroposterior direction.
Furthermore, the results of the forward stepwise logistic regression analysis showed that besides the FMA-LE score (Nagelkerke R2 = 62.6%; P < .001), only QVT of the affected hallux significantly contributed to the overall explained DCA variance (Nagelkerke R2 = 69.0%; FMA-LE P < .001; QVT-hallux P = .024).
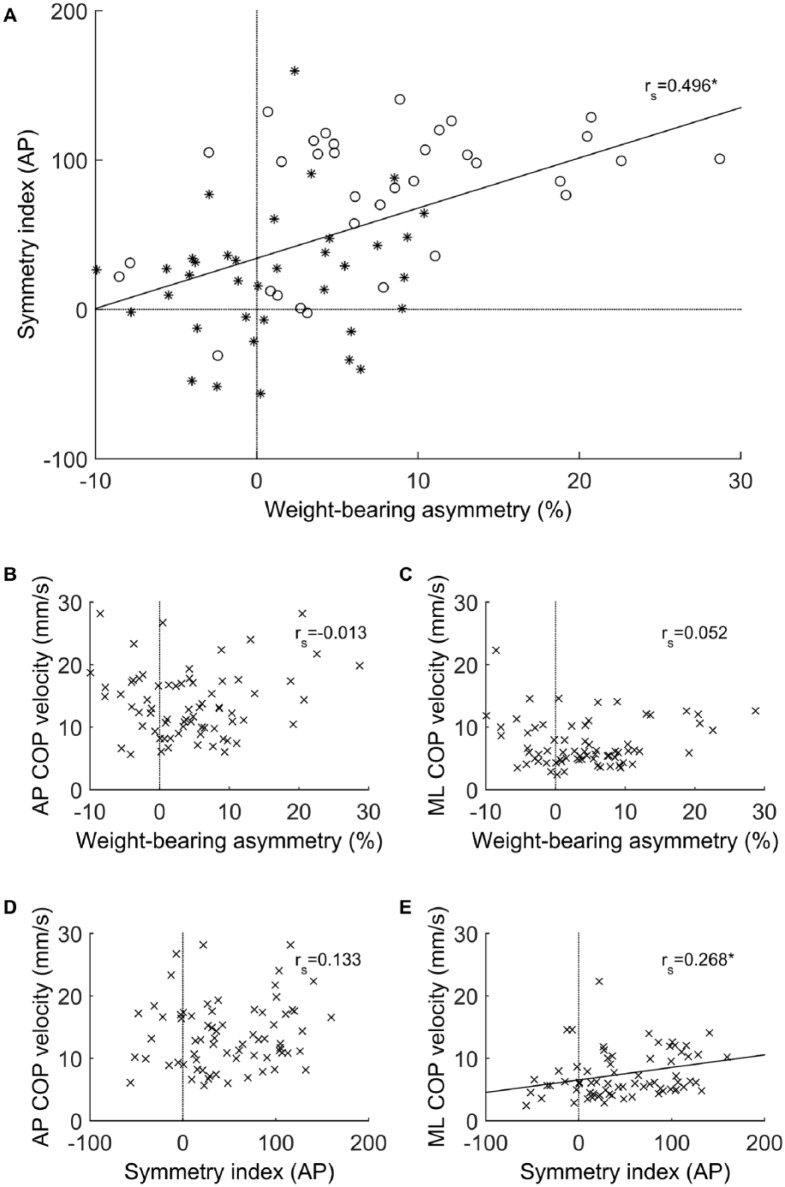
Figure 3 provides insight into the mutual relationships between DCA, WBA, and body sway control. A significant and moderately strong correlation between DCA and WBA was found (rs = 0.496; P < .001); however, body sway control in the AP direction did not show a significant correlation with either DCA (rs = 0.133; P = .273) or WBA (rs = −0.013; P = .917). Body sway control in the ML direction, on the other hand, showed a significant yet rather weak correlation with DCA (rs = 0.268; P = .025), but not with WBA (rs = 0.052; P = .670).
Figure 3.
Upper panel: dynamic control asymmetry in the AP direction (expressed as a symmetry index) in relation to weight-bearing asymmetry (expressed as 50% − Percentage of body weight borne on the affected leg). Asterisks represent individuals with FMA-LE score ⩾27, and circles represent individuals with FMA-LE score <27 (A). Midpanel: body sway control in AP (B) and ML (C) directions in relation to weight-bearing asymmetry. Lower panel: body sway control in AP (D) and ML (E) directions in relation to dynamic control asymmetry in the AP direction.
Abbreviations: AP, anteroposterior; COP, center of pressure; FMA-LE, Fugl-Meyer Assessment—lower extremity; ML, mediolateral.
Discussion
In this study, we evaluated the effects of a unilateral supratentorial stroke on various characteristics of standing balance control. Confirming our first hypothesis, the results demonstrated that even individuals in the chronic phase after stroke with clinically no impairment or only very limited leg motor impairment can show substantial asymmetry in the relative contribution of each leg to body sway control (DCA). This finding suggests that the relationship between leg motor impairment and this dynamic aspect of postural asymmetry may indeed be nonlinear. Furthermore, as hypothesized, we found a significant relationship between DCA and static control asymmetry in terms of weight bearing (WBA), indicating that persons with DCA bear more weight on their less-affected leg. Coherent with previous findings of others,10,12 we found that DCA was weakly associated with body sway control in the ML direction, but not with body sway control in the AP direction.
Previous studies have shown that people with stroke recover 33% to 88% of their initial leg motor impairments within 3 to 4 months after stroke.26,27 Despite these relatively large improvements, a substantial degree of impairment in the motor control of the affected leg may persist in the chronic phase after stroke. In line with this notion, 17 of our participants (24%) who showed a substantial degree of affected-leg motor impairment (ie, FMA-LE < 24) all showed significant DCA. This finding is in accordance with previous studies reporting that greater leg motor impairment results in a lower relative contribution of the affected leg to body sway control compared with the less-affected leg.10,28 Based on logistic regression analysis, it can be concluded that this relationship is independent of affected body side or age, but to some degree (6.4% additionally explained variance) influenced by loss of deep sensibility (ie, vibration sense). Whereas substantial motor impairment always resulted in poorer DCA, the opposite was not necessarily true. In all, 21% of participants with good leg motor recovery (ie, FMA-LE ≥ 24) demonstrated significant DCA. These results imply that the functional impact of a relatively mild paresis, based on clinical examination, on the control of standing balance may be underestimated, even though DCA was not significantly associated with impaired body sway control. This lack of association is most likely caused by the efficacy of compensatory control by the less-affected leg.
Indeed, the absence of a correlation between DCA and AP body sway control and the presence of an association between DCA and WBA corroborate the notion that the ankle strategies executed by the less-affected leg compensate for the loss of ankle control by the affected leg, which compensation may be facilitated by a certain degree of WBA in favor of the less-affected leg. Notably, a strategy of more than 10% WBA was observed only in favor of the less-affected leg and nearly always in participants (except 1 individual) with a FMA-LE score <27. As a result, DCA and WBA are to some extent associated, whereas their respective associations with AP body sway control are lost because of the efficacy of these compensatory mechanisms. This notion is in accordance with previous studies in subacute stroke patients.10-12 It is also coherent with results obtained in healthy individuals who showed increased contribution of the more loaded leg when being forced toward an asymmetric weight distribution.22 The underlying mechanism is probably that increasing the load on one leg also increases the efficacy of the corrective COP movements on this body side because it enhances the corresponding ankle torques. This compensatory mechanism is most likely less effective in the ML sway direction.
In contrast with the AP direction, we did find a weak association (24% covariance) between DCA and ML body sway control, which is in agreement with previous work by Mansfield et al.11,12 They reported that reduced between-limb temporal synchronization in COP movements in subacute stroke patients was related to impaired ML body sway control (rs = 0.3), WBA (rs = 0.2), and clinical balance scores (Berg Balance Scale; rs = 0.3), whereas no associations could be demonstrated with AP sway control.12 This pattern of results supports the idea that DCA may be less effective for compensating impaired ML body sway control compared with AP body sway control. In fact, there are several explanations for this notion. First, ML body sway control during quiet standing is mostly controlled by a so-called weight-shifting strategy that does not require the generation of corrective ankle torques but merely the generation of hip torques (abduction/adduction) in the frontal plane. Second, ML body sway control after stroke is relatively severely affected compared with AP body sway control,8 which besides sensorimotor impairments may be a result of disturbances in the perception of the postural and visual vertical.29 Hence, DCA and ML body sway control probably both reflect the severity of stroke and more so than AP sway control.
Strengths and Limitations
In our study, we determined DCA based on COP movements solely in the AP direction for 2 reasons. First, motor impairment of the affected leg (FMA-LE) was based on testing the selective use of affected ankle plantarflexors and dorsiflexors that are working in the sagittal plane. Second, as mentioned above, ankle mechanisms in the ML direction hardly contribute to body sway control during quiet 2-legged standing. A slightly different approach for estimating the kinetic contribution of the affected leg to body sway control is the calculation of ankle torques (instead of COP movements) that integrate the amount of weight bearing.9 By focusing on COP movements, however, we were able to study the modulating effect of WBA on the relation between DCA and body sway control, which cannot be identified from an integrated ankle torque measure.
As in most posturographic studies, participants stood at a standardized stance width, which could have influenced the results. However, stance width predominantly influences ML sway control, whereas it has only limited influence on COP movements in the AP direction30 and, thus, on our main outcome measures (ie, DCA and AP sway control). Moreover, we focused on the relationships between several posturographic characteristics rather than on their absolute values. Therefore, we believe that a possible influence of the selected standardized stance width on our results remains very limited.
Although we included participants with a wide range of leg motor impairments after stroke, a fairly skewed distribution toward well-recovered individuals was present (76% had a FMA-LE score ⩾ 24). Because all severely affected participants (FMA-LE score < 24) showed DCA, more participants with severe stroke would probably not have changed our results.
In this study, we compared the DCA values of our participants with those of healthy individuals obtained from a previous study of our group.22 Preferably, we had included an age-matched healthy control group in the current study, but the similar experimental conditions did not justify the extra effort and burden on participants.
Ideally, we had attempted a longitudinal study starting in the subacute phase after stroke to be better able to study causal relationships based on time-dependent analysis. However, many subacute patients with a hemiparesis are not able to stand safely and independently without aids or support until several weeks after stroke, as we observed in a previous study.8 Nevertheless, such a study would be warranted based on the results of the current study to further substantiate the presumed causalities.
In conclusion, even clinically well-recovered stroke survivors with (near) maximal FMA-LE scores may still show subtle impairments in distal leg motor control leading to DCA (in terms of an asymmetric kinetic contribution of each leg to standing balance). This dynamic postural control asymmetry does not seem to hamper AP body sway control during quiet 2-legged standing, presumably because of compensation by the less-affected ankle that may be further facilitated by concomitantly adopting some degree of static postural control asymmetry (in terms of WBA). The clinical implication of this knowledge is that one may easily overestimate “well-recovered” stroke survivors with regard to their capacity to perform complex balance skills that require single-leg balance control—for example, during regular walking, stair climbing, hopping, jumping, getting on and off a bike, and so on. Clinicians could assess whether a lower kinetic contribution of the affected leg to standing balance is present by using dual-plate posturography. Another, yet speculative, clinical implication may be that AP body sway control will be much more seriously affected once a patient suffers a second contralateral (or bilateral) stroke, even if the severity of the paraparesis would appear to be mild. In such cases, use of ankle strategies for controlling AP body sway may be seriously affected, forcing patients to rely on alternative, but less efficient, hip strategies. This notion needs to be corroborated by future research.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JMBR was funded by an IMDI Move On Research Grant (104003014) of The Netherlands Organisation for Health Research and Development. VW reports a grant from Netherlands Organization for Scientific Research (Veni Research Grant 916.10.106). ACHG reports an unrestricted grant from Ipsen Pharmaceutica and Merz.
References
- 1. Dickstein R, Abulaffio N. Postural sway of the affected and nonaffected pelvis and leg in stance of hemiparetic patients. Arch Phys Med Rehabil. 2000;81:364-367. [DOI] [PubMed] [Google Scholar]
- 2. Mizrahi J, Solzi P, Ring H, Nisell R. Postural stability in stroke patients: vectorial expression of asymmetry, sway activity and relative sequence of reactive forces. Med Biol Eng Comput. 1989;27:181-190. [DOI] [PubMed] [Google Scholar]
- 3. Corriveau H, Hébert R, Raîche M, Prince F. Evaluation of postural stability in the elderly with stroke. Arch Phys Med Rehabil. 2004;85:1095-1101. [DOI] [PubMed] [Google Scholar]
- 4. Bohannon RW, Larkin PA. Lower extremity weight bearing under various standing conditions in independently ambulatory patients with hemiparesis. Phys Ther. 1985;65:1323-1325. [DOI] [PubMed] [Google Scholar]
- 5. Caldwell C, Macdonald D, Macneil K, McFarland K, Turnbull GI, Wall JC. Symmetry of weight distribution in normals and stroke patients using digital weigh scales. Physiother Theory Pract. 1986;2:109-116. [Google Scholar]
- 6. Dickstein R, Nissan M, Pillar T, Scheer D. Foot-ground pressure pattern of standing hemiplegic patients: major characteristics and patterns of improvement. Phys Ther. 1984;64:19-23. [DOI] [PubMed] [Google Scholar]
- 7. Sackley CM. The relationships between weight-bearing asymmetry after stroke, motor function and activities of daily living. Physiother Theory Pract. 1990;6:179-185. [Google Scholar]
- 8. de Haart M, Geurts AC, Huidekoper SC, Fasotti L, van Limbeek J. Recovery of standing balance in postacute stroke patients: a rehabilitation cohort study. Arch Phys Med Rehabil. 2004;85:886-895. [DOI] [PubMed] [Google Scholar]
- 9. van Asseldonk EH, Buurke JH, Bloem BR, et al. Disentangling the contribution of the paretic and non-paretic ankle to balance control in stroke patients. Exp Neurol. 2006;201:441-451. [DOI] [PubMed] [Google Scholar]
- 10. Roerdink M, Geurts AC, de Haart M, Beek PJ. On the relative contribution of the paretic leg to the control of posture after stroke. Neurorehabil Neural Repair. 2009;23:267-274. [DOI] [PubMed] [Google Scholar]
- 11. Mansfield A, Danells CJ, Inness E, Mochizuki G, McIlroy WE. Between-limb synchronization for control of standing balance in individuals with stroke. Clin Biomech (Bristol, Avon). 2011;26:312-317. [DOI] [PubMed] [Google Scholar]
- 12. Mansfield A, Mochizuki G, Inness EL, McIlroy WE. Clinical correlates of between-limb synchronization of standing balance control and falls during inpatient stroke rehabilitation. Neurorehabil Neural Repair. 2012;26:627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L. Clinical gait assessment in the neurologically impaired: reliability and meaningfulness. Phys Ther. 1984;64:35-40. [DOI] [PubMed] [Google Scholar]
- 14. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198. [DOI] [PubMed] [Google Scholar]
- 15. Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16:232-240. [DOI] [PubMed] [Google Scholar]
- 16. Collin C, Wade D. Assessing motor impairment after stroke: a pilot reliability study. J Neurol Neurosurg Psychiatry. 1990;53:576-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pestronk A, Florence J, Levine T, et al. Sensory exam with a quantitative tuning fork: rapid, sensitive and predictive of SNAP amplitude. Neurology. 2004;62:461-464. [DOI] [PubMed] [Google Scholar]
- 18. van der Linden MH, van der Linden SC, Hendricks HT, van Engelen BG, Geurts AC. Postural instability in Charcot-Marie-Tooth type 1A patients is strongly associated with reduced somatosensation. Gait Posture. 2010;31:483-488. [DOI] [PubMed] [Google Scholar]
- 19. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142-148. [DOI] [PubMed] [Google Scholar]
- 20. Flansbjer UB, Blom J, Brogardh C. The reproducibility of Berg Balance Scale and the Single-leg Stance in chronic stroke and the relationship between the two tests. PM R. 2012;4:165-170. [DOI] [PubMed] [Google Scholar]
- 21. Mao HF, Hsueh IP, Tang PF, Sheu CF, Hsieh CL. Analysis and comparison of the psychometric properties of three balance measures for stroke patients. Stroke. 2002;33:1022-1027. [DOI] [PubMed] [Google Scholar]
- 22. Anker LC, Weerdesteyn V, van Nes IJ, Nienhuis B, Straatman H, Geurts AC. The relation between postural stability and weight distribution in healthy subjects. Gait Posture. 2008;27:471-477. [DOI] [PubMed] [Google Scholar]
- 23. Le Clair K, Riach C. Postural stability measures: what to measure and for how long. Clin Biomech (Bristol, Avon). 1996;11:176-178. [DOI] [PubMed] [Google Scholar]
- 24. Marigold DS, Eng JJ. The relationship of asymmetric weight-bearing with postural sway and visual reliance in stroke. Gait Posture. 2006;23:249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geurts AC, Boonstra TA, Voermans NC, Diender MG, Weerdesteyn V, Bloem BR. Assessment of postural asymmetry in mild to moderate Parkinson’s disease. Gait Posture. 2011;33:143-145. [DOI] [PubMed] [Google Scholar]
- 26. Smith MC, Byblow WD, Barber PA, Stinear CM. Proportional recovery from lower limb motor impairment after stroke. Stroke. 2017;48:1400-1403. [DOI] [PubMed] [Google Scholar]
- 27. Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke. 2006;37:2348-2353. [DOI] [PubMed] [Google Scholar]
- 28. Genthon N, Rougier P, Gissot AS, Froger J, Pelissier J, Perennou D. Contribution of each lower limb to upright standing in stroke patients. Stroke. 2008;39:1793-1799. [DOI] [PubMed] [Google Scholar]
- 29. Barra J, Chauvineau V, Ohlmann T, Gresty M, Pérennou D. Perception of longitudinal body axis in patients with stroke: a pilot study. J Neurol Neurosurg Psychiatry. 2007;78:43-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Day BL, Steiger MJ, Thompson PD, Marsden CD. Effect of vision and stance width on human body motion when standing: implications for afferent control of lateral sway. J Physiol. 1993;469:479-499. [DOI] [PMC free article] [PubMed] [Google Scholar]