Abstract
Nanomaterials offer unique advantages as drug-delivery vehicles for cancer therapeutics. For immuno-oncology applications, cancer nanomedicine should be developed beyond drug-delivery platforms. A greater emphasis on actively modulating host anticancer immunity using nanomaterials provides new avenues for developing novel cancer therapeutics.
Keywords: Nanomedicine, cancer immunotherapy, cancer vaccine, drug delivery, nanotechnology
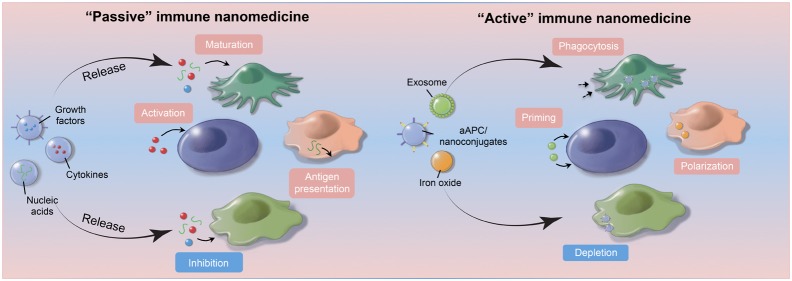
Recent successes in cancer immunotherapy have generated significant interest in harnessing the body’s immune system to fight cancer.1 Numerous strategies have been investigated to improve the effectiveness while minimizing toxicities of cancer immunotherapy.2 Traditionally used as passive vehicles for delivering conventional cancer therapeutics, nanomaterials are also being explored to boost host anticancer immunity.3 Different nanoformulations of antigens, cytokines, chemokines, nucleotides, and Toll-like receptor agonists targeting various immune cells have been successfully demonstrated in many preclinical settings, producing promising results.3 However, in these instances, nanomedicine mostly serves as a delivery vehicle to enable the more-efficient and more-targeted transport of immunostimulatory agents to help mount antitumor immune responses.
In this perspective, we examine how nanomedicine can progress beyond solely providing delivery platforms for immunotherapy and how different nanomaterials can be designed to possess intrinsic immunomodulatory properties that can help mount antitumor immune responses. This new class of immune nanomedicines can selectively regulate important signaling pathways within distinctive immune cell populations through their material compositions, geometries, or surface modifications to generate potent antitumor effects. Thus, whether through the design of more-efficient delivery devices for immunomodulating agents or the engineering of sophisticated nanoconstructs that can selectively regulate immune cell functions, immune nanomedicine represents an exciting opportunity to develop effective strategies that may one day significantly improve cancer treatment.
Nanomedicines as Delivery Platforms for Immunomodulating Agents
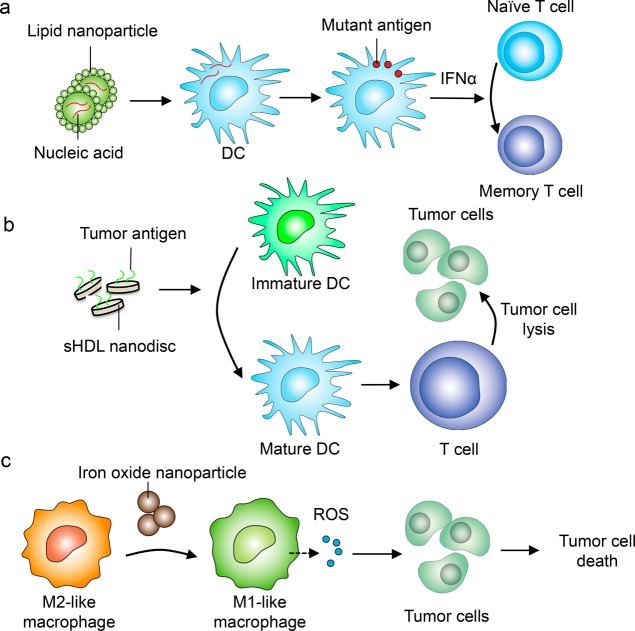
Synthetic and naturally derived nanoparticles possess unique physical and chemical properties that make them well-suited as drug-delivery platforms. Nanoformulations of conventional chemotherapeutic agents provide ways to modify the pharmacokinetic and pharmacodynamic properties of cytotoxic drugs without altering their tumoricidal activities.4 Nanoparticles synthesized using different material compositions can encapsulate anticancer drugs within their inner cores. The surface of nanoparticles can be further modified to dock targeting moieties such as antibodies, peptides, or recombinant proteins that further enhance the selective accumulation of drugs within tumor tissues.5 These unique advantages of nanomaterials have also been adopted for immuno-oncology applications. Both polymeric and lipid-based nanoparticles have been engineered to efficiently deliver antigens or viral peptides to antigen-presenting cells to stimulate memory T cell responses against tumors.6,7 Self-assembled nanoparticles, including those derived from viruses, can exhibit remarkable ability to generate potent immune responses against poorly immunogenic tumors by increasing the production of inflammatory cytokines such as interleukin (IL)-2 and interferon (IFN) -γ within activated leukocytes.8 Nanoparticles can also be used to deliver specific cytokines, growth factors, or a cocktail of immune stimulants to boost immune cell functions.9 With recent developments in genome editing, there have been growing interest in using nanoparticles to deliver nucleic acids such as siRNA for transcriptional modification or cas9 mRNA to repair specific disease-associated genes in vivo. For example, synthetic DNA nanocarriers were able to reprogram circulating T cells into antitumor phenotype by inserting leukemia-targeting chimeric antigen receptor (CAR) genes into the nucleus of these T cells.10 This in vivo programing strategy using nanocarriers offers great potential to overcome many technical limitations associated with current CAR T cell therapy, such as extensive effort associated with ex vivo expansion of isolated T cells from individual patients.10 Similarly, lipid nanoparticles assembled with specified compositions were recently shown to protect RNA sequences from extracellular ribonuclease degradation and promote their efficient uptake by professional antigen-presenting cells to express tailor-designed antigenic peptides in vivo.11 Using RNA sequences that encode viral or mutant neo-antigens, these nanoparticle constructs induce strong antitumor effector and memory T-cell responses through potent IFNα activation (Figure 1a).12 Remarkably, early clinical data from the first three melanoma patients treated with these lipid nanoparticle–RNA constructs showed strong IFNα and antigen-specific antitumor responses,12 thus offering significant hope that such a system can be readily translated to the clinic.
Figure 1.
Examples of nanomedicine strategies to enhance antitumor immune responses. (a) Lipid-based nanoparticles encapsulated with nucleic acids such as RNA encoding for mutant antigens can be designed to home to professional antigen-presenting cells (APCs) such as dendritic cells (DCs). The translation and cross-presentation of the mutant antigens by DCs then primes antitumor memory T cell responses.12 (b) Nanomedicine can also help improve the efficacy of cancer vaccines. Synthetic high-density lipoprotein (sHDL) nanoparticles decorated with tumor antigens can promote the more-efficient delivery to APCs in lymphoid tissues, resulting in improved DC maturation and T cell-mediated tumor killing.7 (c) Beyond delivery, nanoparticles themselves can also promote antitumor immune cell phenotypes. Iron oxide nanoparticles, for example, can polarize tumor-associated macrophages from a protumor M2-like to an antitumor M1-like phenotype, which releases reactive oxygen species (ROS) to induce tumor cell killing.23
In addition to allowing the delivery of multiple cargos, nanoparticles are advantageous for immune targeting because of their preferential uptake by innate immune cells, such as monocytes, macrophages, and dendritic cells within the body.4,5 The surfaces of synthetic nanoparticles present an ideal substrate for serum proteins, including albumin, apolipoproteins, and complements to bind, forming a biological corona that then interacts with multiple receptors (e.g., scavenger and complement receptors) expressed by professional phagocytes.5 For traditional cancer nanomedicine, such nonspecific uptake by phagocytic cells is not desirable because it reduces the availability of encapsulated drugs to tumor tissues. For immune nanomedicine, however, this physiological process may, in fact, be beneficial. Nanoparticles designed with unique composition and surface-charge profiles can selectively home to lymphoid organs such as the spleen and produce desired immunomodulatory effects. These unique properties make nanoparticle an ideal candidate for antitumor vaccine delivery. In particular, delivery of antitumor vaccine adjuvants via nanoformulation can enhance their potency while reducing side effects by limiting systemic distribution of adjuvants and prolonging their activity in draining lymph nodes.13 Delivery of adjuvants and antigens can also benefit from inherent lymph node-targeting of endogenous proteins. For example, lipid micellar nanoparticles composed of amphiphilic adjuvant molecules have been designed to readily dissociate and bind to albumin upon in vivo administrations.14 This “hitchhiking” strategy allows for markedly improved delivery to antigen-presenting cells and elicitation of robust antitumor T cell responses.14 Alternatively, nanomaterials designed to mimic endogenous proteins have been utilized for cancer immunotherapy. High-density lipoprotein-mimicking nanodiscs “personalized” with tumor-specific neo-antigens have been shown to dramatically improve lymph-node targeting of neo-antigens, generating strong polyfunctional, neo-antigen-specific CD8 and CD4 T cell immunity that eliminated established tumors in combination with immune checkpoint blockade (Figure 1b).7 These studies are paving the way for improving cancer immunotherapy with personalized nanomedicine.
Alternatively, nanoparticles can take advantages of existing cancer therapies to improve tumor-directed delivery. For example, perivascular macrophages can results in enhanced intratumoral delivery of nanotherapeutics through microvascular burst following local tumor irradiation.15 In this case, nanomedicine designed to accumulate in perivascular tumor-associated macrophages would also benefit from the extravasation of these immune cells into the tumor tissue. These unique characteristics of nanomedicine will be highly valuable for immuno-oncology.
Nanomedicine as Immune-Modulating Agents
Although most of the research on nanomaterial–immune cell interactions has focused on studying the toxic effects of nanoparticles,16 an area of intense interest is to harness the immunomodulating properties of nanomaterials to treat patients with cancer or autoimmune diseases. Liposomal or polymeric nanoparticles can be engineered to mimic the biological interactions between antigen-presenting cells and T cells or to act as specific subcellular granules to promote antitumor immunity.17 Similarly, nanomedicine can be engineered to rely on immune cells to specially target and attack different types of tumors. Nanoparticles loaded with chemotherapeutic agents were delivered into neutrophils, which are then recruited to resection bed of brain tumors by postsurgical inflammatory cytokines to release the drugs that reduce local recurrences.18 This immune-cell-based tumor targeting strategy also applies to other innate immune cell subtypes. For example, directly conjugating anti-PD-L1 antibodies onto the plasma membrane of platelets, which accumulate in the resection cavity after tumor surgery, can significantly facilitate the delivery of the antibody to the surgical bed, leading to reduced local and distant recurrence risks and prolonged survival in tumor-bearing mice.19 Nano- and microparticles can also be designed to directly mimic the functions of immune cell subsets. For example, polydimethylsiloxane (PDMS) particles modified with activating antibodies to CD3 and CD28 resulted in enhanced expansion of CD4+ and CD8+ T cells in vitro.20 Similarly, multivalent synthetic dendritic cells made of polymeric nanoparticles greatly increased the efficiency of T cell activation.21 Although still early in development, these studies highlighted the importance of nanoparticle size, shape, rigidity and surface level, density, and spatial organization pattern of major histocompatibility complex (MHC) as well as co-stimulatory molecules contribute to generating T cell responses. This point was further reinforced by a recent study that demonstrated that the sizes of nanoparticles used as substrate for artificial antigen presenting cells is critical in T cell activation.22 Therefore, if nanoparticle or nanoparticle cluster is below certain threshold size, then even increased ligand density may not be sufficient to properly stimulate a T cell response.22
Another emerging area of research in immune cancer nanomedicine is the identification or engineering of nanoconstructs that can modulate specific steps along the immune activation cascade. For example, ferumoxytol, an iron oxide nanoparticle formulation approved by the United States Food and Drug Administration for the treatment of anemia, was recently shown to induce the polarization of tumor-associated macrophages toward the pro-inflammatory M1-like phenotype and promotes reactive oxygen species (ROS)-mediated tumor cell killing (Figure 1c).23 Systemic injection of ferumoxytol drastically reduced tumor growth of orthotopically implanted MMTV-PyMT breast tumors and significantly inhibited the formations of metastatic lesions in the liver and lung.23 It is unclear, however, what molecular mechanisms are involved in the phenotypic and functional changes within macrophages that were caused by ferumoxytol. Nanoparticles can also be engineered to improve both tumor delivery and to produce enhanced antitumor immune responses. Magnetic nanoparticles consist of therapeutic fucoidan-dextran were modified with PD-L1 inhibitors and T cell activators (anti-CD3 and anti-CD28) to generate a multifunctional complex (termed IO@FuDex3).24 The magnetic core of the nanocomplex allowed in vivo magnetic navigation to improve tumor targeting and minimize off-target effect, while the simultaneous integration of PD-L1 inhibitor and T cell activator further augmented antitumor immune responses.24
Beyond the modification of nanomaterial compositions, the surfaces of nanoparticles can also be engineered to enhance tumor cell phagocytosis and antigen presentation by macrophages. Using a bispecific multivalent nanoengager design, receptor-targeted clearance of HER2-positive breast cancer cells by macrophages can be induced through pro-phagocytic signaling mediated by calreticulin.25 The bispecific nanoengager system recruits phagocytes to recognize and take up receptor-positive cancer cells and subsequently process them and present tumor-associated antigens to T cells.25
In addition to improving the potency of exogenously administered molecular adjuvants, nanoparticles can also potentiate endogenous “danger signals” released by tumor cells. Tumor cells are known to undergo immunogenic cell death (ICD) upon treatment with certain chemotherapeutic agents.26 To harness their therapeutic potential, whole tumor cells undergoing ICD have been decorated with adjuvant-loaded nanoparticles, promoting the co-delivery of exogenous and endogenous danger signals.27 Alternatively, the direct tumor-targeted delivery of ICD-inducers may also play major roles in cancer immunotherapy.28 Nanodiscs tethered with an ICD-inducing agent were recently shown to improve their pharmacokinetic profiles and tumor accumulation, leading to robust upregulation of immunostimulatory danger signals within tumors and potent T cell responses against neo-antigens, tumor-associated antigens, and intact whole tumor cells.29 Moreover, some nanoparticles exhibit intrinsic properties to induce ICD. For example, photothermal therapy (PTT) using gold nanoshells was shown to effectively ablate local tumors, releasing endogenous immunostimulatory molecules and promoting subsequent activation of dendritic cells.30 Even stronger antitumor immune responses can be achieved by combination therapies. For example, PTT in combination with ICD-inducing chemotherapeutic agents or immunomodulating agents have been shown to synergistically elicit systemic antitumor immunity that can eliminate local tumors directly treated with PTT as well as untreated, disseminated tumors in multiple tumor models.31,32 Similarly, nanoparticles have also been used to induce ICD by combining chemotherapy and photodynamic therapy, or to capture tumor-associated antigens released by radiotherapy as a way to prime more potent antitumor T cell responses when combined with anti-PD-L1 antibody.33,34 As cancer immunotherapies such as immune checkpoint inhibitors are increasingly being used for localized cancers in the frontline settings often concurrently with other therapeutic modalities, these studies further demonstrate nanomedicine’s potential for combinational immunotherapy for cancer treatments.
However, generation of the optimal antitumor immune responses will also require reducing the intrinsic immune suppressive signals that exist within a tumor. Investigations into the use of inorganic nanocrystals such as magnesium silicide (Mg2Si) to induce changes in local intratumoral partial O2 concentration or identification of materials that can scavenge excess immune suppressive extracellular ions (e.g., potassium), are already underway, and represents potential new strategies to reprogramming the tumor microenvironment to improve cancer immunotherapies.35
Future Outlook
Despite its great promises, the clinical translation of nanomedicine to treat human cancers has been slow. Recent successes of cancer immunotherapy have provided new opportunities and insights into exploring the unique properties of nanomedicines to enhance antitumor immune responses. Compared with other bioengineered materials such as hydrogels or implants, which have demonstrated ability to modulate local immune responses and offer great promises for cell therapy,36,37 nanomedicine can be engineered to target specific tissues and induce systemic antitumor immunity. Because we have made significant progress in understanding how nanomaterials interact with biological system over the past decade, the emergence of immune nanomedicine poses new questions and challenges. For example, large-scale reproducible production of immuno-nanomedicine that is compliant with chemistry, manufacturing and controls (CMC), and good manufacturing practice (GMP) will likely require the development of manufacturing processes.38 Furthermore, majority of the preclinical evidence highlighting immune nanomedicine’s clinical potential are limited to small rodent studies. Studies in larger mammals that are more reflective of human physiology can provide valuable information before clinical trials take place. Regardless of these challenges, it is foreseeable that immune nanomedicine will play a more -rominent role in the development of cancer medicine. A better understanding of how the immune system interacts with engineered nanomaterials will enable researchers to design optimal immune nanomedicines that are both effective and safe.
Acknowledgments
This work was supported by the Mayo Clinic Center for Regenerative Medicine (B.Y.S.K.), the National Institute of Neurological Disorders and Stroke Grant R01 NS104315 (B.Y.S.K.), the Florida Center for Brain Tumor Research & Accelerate Brain Cancer Cure (B.Y.S.K.), Cancer Prevention and Research Institute of Texas CPRIT (RR180017; W.J.), the National Natural Science Foundation of China (grant no. 81572500), and the Hunan Young Talent (grant no. 2016RS3036). The authors thank C. Wogan of MD Anderson Cancer Center’s Division of Radiation Oncology for editorial assistance.
Author Contributions
◆ Z.L. and W.J. contributed equally
The authors declare no competing financial interest.
References
- Mellman I.; Coukos G.; Dranoff G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvistborg P.; Yewdell J. W. Enhancing responses to cancer immunotherapy. Science 2018, 359, 516–517. 10.1126/science.aar6574. [DOI] [PubMed] [Google Scholar]
- Jiang W.; von Roemeling C. A.; Chen Y.; Qie Y.; Liu X.; Chen J.; Kim B. Y. S. Designing nanomedicine for immuno-oncology. Nat. Biomed. Eng. 2017, 1, 0029. 10.1038/s41551-017-0029. [DOI] [Google Scholar]
- Blanco E.; Shen H.; Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S.; Tavares A. J.; Dai Q.; Ohta S.; Audet J.; Dvorak H. F.; Chan W. C. W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- Moon J. J.; Suh H.; Bershteyn A.; Stephan M. T.; Liu H.; Huang B.; Sohail M.; Luo S.; Um S. H.; Khant H.; et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011, 10, 243–251. 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai R.; Ochyl L. J.; Bahjat K. S.; Schwendeman A.; Moon J. J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizotte P. H.; Wen A. M.; Sheen M. R.; Fields J.; Rojanasopondist P.; Steinmetz N. F.; Fiering S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol. 2016, 11, 295–303. 10.1038/nnano.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q.; Yin Y.; Shang L.; Wu T.; Zhang D.; Kong M.; Zhao Y.; He Y.; Tan S.; Guo Y.; Zhang Z. Tumor Microenvironment Responsive Nanogel for the Combinatorial Antitumor Effect of Chemotherapy and Immunotherapy. Nano Lett. 2017, 17, 6366–6375. 10.1021/acs.nanolett.7b03186. [DOI] [PubMed] [Google Scholar]
- Smith T. T.; Stephan S. B.; Moffett H. F.; McKnight L. E.; Ji W.; Reiman D.; Bonagofski E.; Wohlfahrt M. E.; Pillai S. P. S.; Stephan M. T. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 2017, 12, 813–820. 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberli M. A.; Reichmuth A. M.; Dorkin J. R.; Mitchell M. J.; Fenton O. S.; Jaklenec A.; Anderson D. G.; Langer R.; Blankschtein D. Lipid Nanoparticle Assisted mRNA Delivery for Potent Cancer Immunotherapy. Nano Lett. 2017, 17, 1326–1335. 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz L. M.; Diken M.; Haas H.; Kreiter S.; Loquai C.; Reuter K. C.; Meng M.; Fritz D.; Vascotto F.; Hefesha H.; Grunwitz C.; Vormehr M.; Hüsemann Y.; Selmi A.; Kuhn A. N.; Buck J.; Derhovanessian E.; Rae R.; Attig S.; Diekmann J.; Jabulowsky R. A.; Heesch S.; Hassel J.; Langguth P.; Grabbe S.; Huber C.; Türeci Ö.; Sahin U. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- Lynn G. M.; Laga R.; Darrah P. A.; Ishizuka A. S.; Balaci A. J.; Dulcey A. E.; Pechar M.; Pola R.; Gerner M. Y.; Yamamoto A.; Buechler C. R.; Quinn K. M.; Smelkinson M. G.; Vanek O.; Cawood R.; Hills T.; Vasalatiy O.; Kastenmüller K.; Francica J. R.; Stutts L.; Tom J. K.; Ryu K. A.; Esser-Kahn A. P.; Etrych T.; Fisher K. D.; Seymour L. W.; Seder R. A. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat. Biotechnol. 2015, 33, 1201–1210. 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Moynihan K. D.; Zheng Y.; Szeto G. L.; Li A. V.; Huang B.; Van Egeren D. S.; Park C.; Irvine D. J. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 2014, 507, 519–522. 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A.; Chandra R.; Cuccarese M. F.; Pfirschke C.; Engblom C.; Stapleton S.; Adhikary U.; Kohler R. H.; Mohan J. F.; Pittet M. J.; Weissleder R. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci. Transl Med. 2017, 9, eaal0225. 10.1126/scitranslmed.aal0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier H.; Lacotte S.; Pastorin G.; Marega R.; Wu W.; Bonifazi D.; Briand J.-P.; Prato M.; Muller S.; Bianco A. Functionalized Carbon Nanotubes Are Non-Cytotoxic and Preserve the Functionality of Primary Immune Cells. Nano Lett. 2006, 6, 3003–3003. 10.1021/nl068003i. [DOI] [PubMed] [Google Scholar]
- Gao W.; Fang R. H.; Thamphiwatana S.; Luk B. T.; Li J.; Angsantikul P.; Zhang Q.; Hu C-M. J.; Zhang L. Modulating Antibacterial Immunity via Bacterial Membrane-Coated Nanoparticles. Nano Lett. 2015, 15, 1403–1409. 10.1021/nl504798g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J.; Zhao Z.; Zhang L.; Xue L.; Shen S.; Wen Y.; Wei Z.; Wang L.; Kong L.; Sun H.; Ping Q.; Mo R.; Zhang C. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017, 12, 692–700. 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- Wang C.; Sun W.; Ye Y.; Hu Q.; Bomba H. N.; Gu Z. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 2017, 1, 0011. 10.1038/s41551-016-0011. [DOI] [Google Scholar]
- Lambert L. H.; Goebrecht G. K. E.; De Leo S. E.; O’Connor R. S.; Nunez-Cruz S.; Li T. D.; Yuan J.; Milone M. C.; Kam L. C. Improving T Cell Expansion with a Soft Touch. Nano Lett. 2017, 17, 821–826. 10.1021/acs.nanolett.6b04071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammink R.; Mandal S.; Eggermont L. J.; Nooteboom M.; Willems P. H. G. M.; Tel J.; Rowan A. E.; Figdor C. G.; Blank K. G. Controlling T-Cell Activation with Synthetic Dendritic Cells Using the Multivalency Effect. ACS Omega 2017, 2, 937–945. 10.1021/acsomega.6b00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey J. W.; Vicente F. P.; Howard G. P.; Mao H. Q.; Schneck J. P. Biologically Inspired Design of Nanoparticle Artificial Antigen-Presenting Cells for Immunomodulation. Nano Lett. 2017, 17, 7045–7054. 10.1021/acs.nanolett.7b03734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanganeh S.; Hutter G.; Spitler R.; Lenkov O.; Mahmoudi M.; Shaw A.; Pajarinen J. S.; Nejadnik H.; Goodman S.; Moseley M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C. S.; Lin Y. J.; Lee R.; Lai Y. H.; Cheng H. W.; Hsieh C. H.; Shyu W. C.; Chen S. Y. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 2018, 13, 746. 10.1038/s41565-018-0146-7. [DOI] [PubMed] [Google Scholar]
- Yuan H.; Jiang W.; von Roemeling C. A.; Qie Y.; Liu X.; Chen Y.; Wang Y.; Wharen R. E.; Yun K.; Bu G.; Knutson K.; Kim B. Y. S. Multivalent Bi-Specific Nano-Bioconjugate Engager for Targeted Cancer Immunotherapy. Nat. Nanotechnol. 2017, 12, 763–769. 10.1038/nnano.2017.69. [DOI] [PubMed] [Google Scholar]
- Galluzzi L.; Buque A.; Kepp O.; Zitvogel L.; Kroemer G. Nat. Rev. Immunol. 2017, 17, 97–111. 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Kuai R.; Xu Y.; Ochyl L. J.; Irvine D. J.; Moon J. J. Immunogenic Cell Death Amplified by Co-localized Adjuvant Delivery for Cancer Immunotherapy. Nano Lett. 2017, 17, 7387–7393. 10.1021/acs.nanolett.7b03218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.; Liu X.; Liao Y.-P.; Salazar F.; Sun B.; Jiang W.; Chang C. H.; Jiang J.; Wang X.; Wu A. M.; et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat. Commun. 2017, 8, 1811. 10.1038/s41467-017-01651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai R.; Yuan W.; Son S.; Nam J.; Xu Y.; Fan Y.; Schwendeman A.; Moon J. J. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Science Adv. 2018, 4, eaao1736. 10.1126/sciadv.aao1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear A. S.; Kennedy L. C.; Young J. K.; Perna S. K.; Mattos Almeida J. P.; Lin A. Y.; Eckels P. C.; Drezek R. A.; Foster A. E. Elimination of Metastatic Melanoma Using Gold Nanoshell-Enabled Photothermal Therapy and Adoptive T Cell Transfer. PLoS One 2013, 8, e69073. 10.1371/journal.pone.0069073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J.; Son S.; Ochyl L. J.; Kuai R.; Schwendeman A.; Moon J. J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1074. 10.1038/s41467-018-03473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.; Xu L.; Liang C.; Wang C.; Peng R.; Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.; Duan X.; Guo N.; Chan C.; Poon C.; Weichselbaum R. R.; Lin W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat. Commun. 2016, 7, 12499. 10.1038/ncomms12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Y.; Roche K. C.; Tian S.; Eblan M. J.; McKinnon K. P.; Caster J. M.; Chai S.; Herring L. E.; Zhang L.; Zhang T.; DeSimone J. M.; Tepper J. E.; Vincent B. G.; Serody J. S.; Wang A. Z. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 2017, 12, 877–882. 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Ni D.; Liu Y.; Yao H.; Bu W.; Shi J. Magnesium silicide nanoparticles as a deoxygenation agent for cancer starvation therapy. Nat. Nanotechnol. 2017, 12, 378–386. 10.1038/nnano.2016.280. [DOI] [PubMed] [Google Scholar]
- Cheung A. S.; Zhang D. K. Y.; Koshy S.; Mooney D. J. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat. Biotechnol. 2018, 36, 160–169. 10.1038/nbt.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. T.; Moffett H. F.; Stephan S. B.; Opel C. F.; Dumigan A. G.; Jiang X.; Pillarisetty V. G.; Pillai S. P. S.; Wittrup K. D.; Stephan M. T. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J. Clin. Invest. 2017, 127, 2176–2191. 10.1172/JCI87624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Kantoff P. W.; Wooster R.; Farokhzad O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]