Abstract
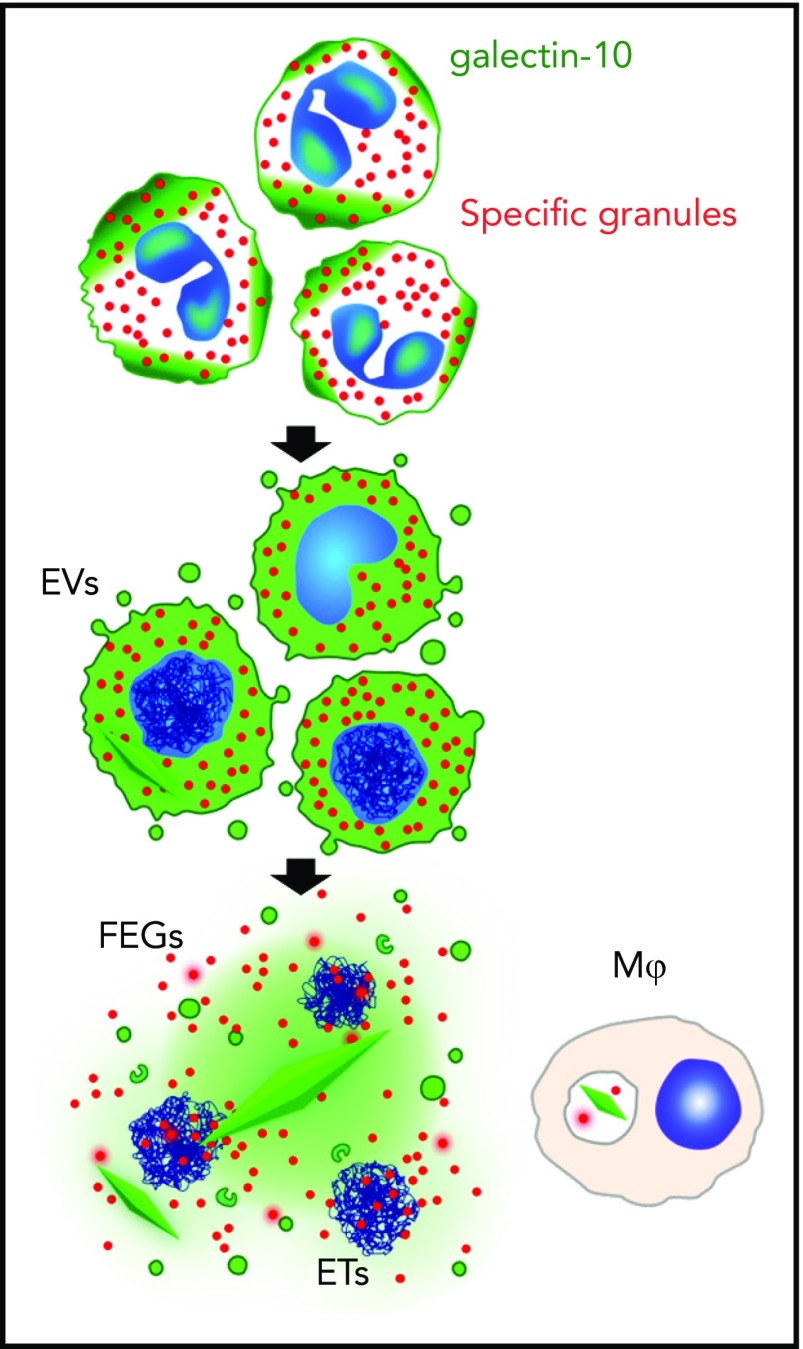
In this issue of Blood, Ueki et al elegantly demonstrate the active formation of Charcot-Leyden crystals (CLCs) during eosinophil cytolysis.1 After confirming the association of CLC deposition with eosinophilic inflammation and eosinophil plasma membrane disruption in tissue sections by light and electron microscopy, Ueki et al used a combination of sophisticated imaging techniques, including immunofluorescent time-lapse photography, to follow the course of CLC formation in vitro in response to a variety of stimuli that induce eosinophil extracellular trap death (EETosis). The association of CLC with the disintegration of eosinophils was proposed as early as the 1940s,2 and agents, such as Aerosol MA, which disrupt the integrity of eosinophils, were subsequently shown to promote CLC formation in vitro in the surrounding media.3 Ueki et al provide the first definitive evidence that CLC formation is energy-dependent and closely tied to the process of EETosis (see figure).
EETosis mediates galectin-10 crystallization. The graphic shows the temporal course of galectin-10 crystallization in tissues. During stimuli-elicited EETosis, loss of regulated intracellular localization of galectin-10 occasionally causes galectin-10 crystallization in the cytoplasm before cell lysis. Galectin-10 is released by plasma membrane disintegration, which may result in extracellular crystallization by increasing local concentrations. Some galectin-10 is also budded from the plasma membrane within enveloped EVs. Thus, FEG, ETs, and galectin-10–containing EV were associated with varied sizes of CLC. Tissue macrophages can also take up galectin-10 and/or small CLC. ET, extracellular trap; EV, extracellular vesicle; FEG, free extracellular granule. See supplemental Figure 8 in the article by Ueki et al that begins on page 2183.
Although the presence of CLC in tissues was first described in the late 1800s, the mechanism of formation and function of these crystals are only beginning to be understood. Charcot-Leyden protein (galectin-10) composes up to 10% of the total protein in the eosinophil, and although galectin-10 has been demonstrated in T cells,4 CLC formation appears to be restricted to human eosinophils and basophils.5 Moreover, tissue deposition of CLC has been described exclusively in tissues and body fluids at sites of eosinophilic inflammation. Recent studies have begun to shed light on the functional roles of Charcot-Leyden protein in eosinophilic inflammation. Initially thought to be a lysophospholipase on the basis of gel chromatography studies, the observed increase in lysophospholipase activity was subsequently shown to be due to binding of galectin-10 to a lysophospholipase inhibitor.6 Galectin-10 has also been implicated in eosinophil lineage development7 and, more recently, in eosinophil regulation of T-cell proliferation.8 The role, if any, of crystal formation in these scenarios is unknown, however.
Extracellular crystals, including calcium oxalate and monosodium urate crystals, can deposit in tissues and trigger an inflammatory response resulting in tissue damage. They have also been shown to induce regulated cell death (necroptosis) in a caspase-independent manner that involves receptor-interacting serine-threonine kinase 3 and mixed lineage kinase domain-like pseudokinase in vitro and in vivo.9 These data suggest that Charcot-Leyden crystals may contribute to eosinophil-induced tissue damage in a manner that is distinct from that of the secondary eosinophil granules, previously shown by Ueki et al to be associated with eosinophil extracellular DNA traps.10
In summary, the work of Ueki et al clearly demonstrates that CLC formation is an active, energy-dependent process that is associated with eosinophil cytolysis. Moreover, the energy dependence of this process supports the hypothesis that CLC formation is not merely a by-product of eosinophil destruction, and provides a first and essential step in understanding the function of CLC in eosinophilic inflammation. Whether the primary role of CLC is cytotoxic or is more intricately involved in immunoregulatory processes remains to be elucidated.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Ueki S, Tokunaga T, Melo RCN, et al. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood. 2018;132(20):2183-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samter M. Charcot-Leyden crystals; a study of the conditions necessary for their information. J Allergy. 1947;18(4):221-230. [DOI] [PubMed] [Google Scholar]
- 3.Ayres WW, Starkey NM. Studies on Charcot-Leyden crystals. Blood. 1950;5(3):254-266. [PubMed] [Google Scholar]
- 4.Kubach J, Lutter P, Bopp T, et al. Human CD4+CD25+ regulatory T cells: proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood. 2007;110(5):1550-1558. [DOI] [PubMed] [Google Scholar]
- 5.Ackerman SJ, Weil GJ, Gleich GJ. Formation of Charcot-Leyden crystals by human basophils. J Exp Med. 1982;155(6):1597-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackerman SJ, Liu L, Kwatia MA, et al. Charcot-Leyden crystal protein (galectin-10) is not a dual function galectin with lysophospholipase activity but binds a lysophospholipase inhibitor in a novel structural fashion. J Biol Chem. 2002;277(17):14859-14868. [DOI] [PubMed] [Google Scholar]
- 7.Abedin MJ, Kashio Y, Seki M, Nakamura K, Hirashima M. Potential roles of galectins in myeloid differentiation into three different lineages. J Leukoc Biol. 2003;73(5):650-656. [DOI] [PubMed] [Google Scholar]
- 8.Lingblom C, Andersson J, Andersson K, Wennerås C. Regulatory eosinophils suppress T cells partly through galectin-10. J Immunol. 2017;198(12):4672-4681. [DOI] [PubMed] [Google Scholar]
- 9.Mulay SR, Desai J, Kumar SV, et al. Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat Commun. 2016;7:10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueki S, Melo RCN, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121(11):2074-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]