Abstract
A new reference material was characterized for 229Th molality and thorium isotope amount ratios. This reference material is intended for use in nuclear forensic analyses as an isotope dilution mass spectrometry spike. The reference material value and expanded uncertainty (k = 2) for the 229Th molality is (1.1498 ± 0.0016) × 10−10 mol g−1 solution. The value and expanded uncertainty (k = 2) for the n(230Th)/n(229Th) ratio is (5.18 ± 0.26) × 10−5 and the n(232Th)/n(229Th) ratio is (3.815 ± 0.092) × 10−4.
Keywords: Thorium, Isotope dilution mass spectrometry, Nuclear forensics, Radiochronometry, Reference material
1. Introduction
The United States Department of Homeland Security’s Domestic Nuclear Detection Office (DNDO) has an ongoing program to enhance capabilities for nuclear forensic measurements by funding the production, characterization, and certification of new reference materials. As part of this effort, DNDO has supported the production of a 229Th molality (mol g−1) reference material that is traceable to the S.I. and has a certified thorium isotopic composition.
Analyses performed to ascertain the provenance of nuclear materials discovered in association with illicit activities or otherwise outside of normal safeguards control, are commonly referred to as nuclear forensic measurements. Results from these measurements could potentially be used in a court of law and/or be subject to scrutiny by an unreceptive audience. Accordingly, nuclear forensics data must be scientifically defensible and must meet established data quality standards for use as evidence in legal proceedings (Leggitt et al., 2009). To meet these requirements, it is imperative that appropriate reference materials be available for development of nuclear forensic analysis methods, quality assurance, and measurement traceability.
Uranium in various forms is the nuclear material most frequently encountered in association with illicit activity (Zaitseva, 2010). Measuring the model purification age of a uranium material is particularly useful for nuclear forensics but the utility of age data is contingent upon the precision and accuracy of the analyses performed to calculate ages. There are multiple radiochronometers that can potentially be used to measure the model purification age of uranium but the most robust and commonly applied is the 234U-230Th parent-daughter system (Stanley, 2012). A major difficulty and source of uncertainty for the 234U-230Th radiochronometer is accurately measuring the amount of 230Th daughter isotope produced from radioactive decay of the 234U parent. The amount of 230Th or relative proportions of 234U and 230Th have been measured by methods such as externally calibrated inductively coupled plasma mass spectrometry (ICP-MS) (Varga et al., 2010) and alpha spectrometry (Moorthy and Kato, 1994; Wallenius et al., 2002; Essex et al., 2013). More commonly, 230Th is measured by isotope dilution mass spectrometry (IDMS) using an isotopically enriched 229Th material as a spike (e.g. Lamont and Hall, 2005; Williams and Gaffney, 2011). These 229Th spikes can be “in-house” standards or certified 229Th radioactivity standards such as Standard Reference Material (SRM) 4328C (NIST, 2013). To use an “in-house” isotopic spike, an analyst must expend considerable effort producing, calibrating, and, if possible, establishing traceability. Alternatively, the use of certified radioactivity standards necessitates a conversion from massic activity to molality using the 229Th half-life. This results in relatively large uncertainties for the molality value (~ 1.0% for SRM 4328C) and requires the user to independently characterize the thorium isotopic composition of the reference material. The 229Th reference material described in this report will allow laboratories to perform precise and traceable measurements of thorium amount by IDMS using a well-characterized spike solution that is specifically developed for determination of thorium occurring at trace concentrations. Production and characterization of the 229Th reference material has been a collaborative effort involving several US laboratories including the National Institute of Standards and Technology (NIST), New Brunswick Laboratory (NBL), Argonne National Laboratory (ANL), Lawrence Livermore National Laboratory (LLNL), and Los Alamos National Laboratory (LANL) as well as the Commissariat à l′énergie atomique et aux énergies alternatives (CEA) and the Institut de Physique du Globe de Paris (IPGP), in France.
2. Experimental
Initial work for this project included the preparation of 229Th reference material units, a 232Th isotopic spike, thorium isotopic composition samples, and IDMS samples. Follow-on activities included the production of two new thorium spike solutions and preparation of additional IDMS samples. Labware used for production of the reference material and preparation of the initial set of analysis samples consisted primarily of new PFA and FEP Teflon1 bottles and vials. Other materials included HDPE funnels and disposable pipettes. This labware was cleaned for ultra-low contamination chemistry in a class-100 cleanroom at NBL. New FEP and PFA Teflon labware for the follow-on set of thorium spikes and IDMS samples were clean for ultra-low contamination work at ANL.
The ampoules for the reference material units were custom made by Quartz Scientific, Inc. (USA) using semiconductor grade quartz glass. Each 229Th unit consists of a 3 mL aliquot of stock solution encapsulated in a quartz ampoule by flame sealing. Prior to filling, the quartz ampoules were subjected to an aggressive cleaning procedure that included two successive soakings in a 1.7 mol L−1 nitric acid solution prepared from Optima high-purity nitric acid (Fisher Scientific, USA) and 18.2 MΩ deionized water (Milli-Q, EMD Millipore, USA). After the second soak, the ampoules were repeatedly rinsed with 18.2 MΩ deionized water and placed in a laboratory oven to dry.
Preparation of the reference material stock solution, unit production, and creation of characterization subsamples for the initial IDMS analyses were performed within a radiochemistry laboratory at NIST. Two fume hoods within the production lab were cleared of material and decontaminated to the extent possible. In addition, a portable recirculating HEPA-filtered laminar flow workbench (LFR-1200, Salare Inc., USA) was purchased specifically for this project and used as the primary work area for solution handling and sample preparation. The second set of IDMS spikes and samples were prepared in newly renovated laboratory space at ANL. This work was primarily performed in a new ducting HEPA-filtered laminar flow hood within a recently refurbished laboratory.
The reagents used for the project work at NIST were 1 mol L−1 HNO3 and a mixed acid solution of 0.3 mol L−1 HNO3 and 0.005 mol L−1 HF. These reagents were prepared using Optima ultrapure HNO3, Optima HF (Fisher Scientific, USA), and 18.2 MΩ deionized water. Aliquots of the HNO3-HF mixed solution were dispensed to precleaned containers and provided to the mass spectrometry labs for analysis along with the characterization samples to assess potential blank contributions. The primary reagents for the work performed at Argonne were Ultrex II ultra-pure HNO3 (Avantor, USA) and Optima HF. These acids were used in concentrated form and diluted to produce a 3 mol L−1 HNO3 solution, a 1 mol L−1 HNO3 solution, and a 0.3 mol L−1 HNO3 − 0.005 mol L−1 HF mixed solution.
Due to the large range in the size of containers and mass of solutions necessary for production of the stock solution and the IDMS reverse spike solution, 3 different balances were used at NIST; a XP205 readable to 0.01 mg (Mettler Toledo, USA), an AE240 readable to 0.1 mg (Mettler Toledo, USA) and a large-capacity (3 kg) Jupiter 3000 readable to 0.1 mg, (Voland, Japan). The ANL sample preparation work required the use of a XPE205 balance readable to 0.01 mg (Mettler Toledo, USA) and a MS3002SE 2-place balance readable to 0.01 g (Mettler Toledo, USA). All balances were within their annual calibration periods and were checked using calibrated weights to assess accuracy, repeatability, and linearity. Simulated weighing experiments were performed at both labs to assess the reproducibility of mass determinations for Teflon containers and for the disposable pipettes used for subsampling. Laboratory temperature, atmospheric pressure, and humidity conditions were monitored during preparation of spikes analysis sample. These data were used to calculate appropriate buoyancy corrections for thorium metal and for the thorium solutions.
The stock solution for the 229Th reference material was prepared from a concentrated master or “M” solution (Table 1) that was previously characterized for 229Th massic activity as part of the production project for NIST activity standard SRM 4328C (Fitzgerald et al., 2010). Two 5-mL ampoules of the 229Th M solution were transferred to a FEP Teflon stock bottle using a disposable flat bottom HDPE pipette (Canus Plastics, Canada). The flat-bottomed disposable pipette was repeated weighed on the XP205 balance after the M solution had been drawn up and again after the solution was dispensed. The mass of the transferred M solution was accurately determined by taking the difference of the pipette weights. (Note: The disposable pipette transfer method was used for all subsequent subsamples for which an accurate mass was needed. A new pipette was used for each solution.) Once the M solution was transferred to the stock bottle, it was diluted with 550 mL of 1 mol L−1 HNO3 to produce a stock solution with an approximate thorium molality 1.15 × 10−10 mol g−1.
Table 1.
Thorium starting materials.
| Material | Known value | Prepared Form | As prepared value |
|---|---|---|---|
| 229Th “M” Solution | (10.90 ± 0.03) | 229Th reference material | (194.4 ± 1.1) |
| (1 mol L−1 HNO3) | kBq 229Th g−1 | (1 mol L−1 HNO3) | Bq g−1 |
| SRM 4342A | (40.83 ± 0.16) | No additional preparation | |
| (1 mol L−1 HNO3) | Bq 230Th g−1 | ||
| NP-TH-1 CB-2–20 | (35.450 ± 0.004) | Dilution 2 | (1.12821 ± 0.00045) × 10−10 |
| (dilute HNO3) | mg Th | (1 mol L−1 HNO3) | mol g−1 |
| NP-TH-1 | (573.80 ± 0.13) | Dilution 2 A | (1.46811 ± 0.00081) × 10−10 |
| (Th metal) | mg Th | (0.3 mol L−1 HNO3–0.005 mol L−1 HF) | mol g−1 |
| NP-TH-1 | (390.78 ± 0.09) | Dilution 2B | (1.06774 ± 0.00058) × 10−10 |
| (Th metal) | mg Th | (0.3 mol L−1 HNO3–0.005 mol L−1 HF) | mol g−1 |
Thorium materials used for production and characterization of the 229Th reference material including starting materials for production of the reference material stock solution, IDMS isotopic tracers, and quality assurance samples. As-Prepared values were decay corrected to 15 September 2012. Uncertainties are expanded uncertainties (U = k uc) with a coverage factor (k) of 2.
The 229Th stock solution was dispensed into the quartz ampoules using a MICROLAB 510B Series Dispenser (Hamilton Company, UK) outfitted with a new 5 mL capacity micro-liter syringe and new 12 gauge Teflon tubing for solution uptake and solution dispensing. Prior to use, the syringe and tubing were cleaned by flushing the apparatus with high-purity 1.7 mol L−1 HNO3 and allowing it to stand with the cleaning solution for 24 h. The dispenser was then thoroughly flushed with 18.2 MΩ deionized water. Immediately prior to filling the reference material ampoules, 15 mL of the 229Th stock solution was dispensed to displace any water and to precondition the system. A total of 180 ampoules were filled with 3 mL of the 229Th stock solution and each ampoule was flame sealed immediately after filling to minimize the potential for evaporation.
The starting material for the 232Th reverse IDMS spike used for the initial set of molality measurements was a NIST thorium primary calibration solution, NP-TH-1-CB-2–20 (NIST, 2000a). The calibration solution was prepared from a high purity “Ames” thorium metal (NP-Th-1, NIST, 2000b) and consisted of a dilute nitric acid solution with (35.450 ± 0.004) mg of thorium at mass a fraction of 1 mg g−1 solution. The NP-TH-1-CB-2–20 solution was quantitatively transferred from the original 60-mL HDPE bottle into a pre-weighed 125-mL FEP Teflon stock bottle. To produce a thorium reverse spike solution with an appropriate thorium molality of approximately 1.1 × 10−10 mol g−1, it was necessary to dilute the NP-TH-1-CB-2–20 solution to a total volume of 120 mL and then perform 2 serial dilutions. The resulting reverse spike solution was designated Dilution 2 (Table 1). Subsamples of Dilution 2 ranging from 0.5 to 3.0 g were transferred to a series of Teflon vials (6 mL) and Teflon bottles (30-mL and 60-mL bottles). Subsamples from 11 separate ampoules of the 229Th reference material, ranging from 1.5 to 3 g, were then added to selected bottles and vials to create IDMS samples with nominal n(232Th)/n(229Th) ratios ranging from 0.5 to 1.2. The remaining Dilution 2 subsamples were prepared for isotopic composition analysis or mixed with SRM 4342A for QC analyses (see below).
Following production of the Dilution 2 solution, an acid leach test was performed on the HDPE bottle for NP-TH-1-CB-2–20 to assess the amount of thorium remaining in the container. Thorium IDMS results from analyses performed at LLNL indicated that less than 0.004% of the thorium was remaining after the quantitative transfer and instrumental neutron activation analysis of the original bottle showed that only about 2.3 ng thorium was retained in or on the plastic.
The NIST 230Th counting standard SRM 4342A (NIST, 2007) was used as a quality control (QC) sample for the project. Fresh units of SRM 4342A were opened and subsamples of the solution were combined directly with aliquots of the Dilution 2 232Th reverse spike and a single ampoule of the 229Th reference material solution. A 230Th molality of (2.327 ± 0.020) × 10−10 mol g−1 was calculated for SRM 4342A using the certified massic activity and the 230Th half-life of (75381 ± 295) years from Meadows et al. (1980). More recent half-life determinations have been published for 230Th (i.e. Cheng et al., 2000, 2013). These values indicate a somewhat longer half-life but the measured values are directly dependent upon the half-life of 238U and an assumption of secular equilibrium between 238U, 234U, and 230Th in natural materials. The Meadows et al. (1980) determination was based on measurements of thorium amount versus measured activity and is the value that is accepted by the evaluated National Nuclear Data Center (NNDC, 2011) data base.
Each mass spectrometry analysis laboratory was also provided with a full unit of the reference material was that was transferred to a Teflon sample container. These samples were prepared for measurement of the amount and isotopic composition of uranium in the 229Th solution. Finally, a single unit of the 229Th reference material was analyzed at NIST by isotope dilution alpha spectrometry to verify that the massic activity of the reference material solution was consistent with the massic activity value derived from the quantitative dilution of the M solution.
A systematic bias in measurements of the 229Th molality could go unrecognized if only a single preparation of 232Th reverse spike was used for IDMS analyses. To address this possibility, 2 new 232Th spike solutions and additional IDMS analyses samples were prepared at ANL. The new spikes were prepared directly from a high purity thorium metal starting material obtained from NIST, NP-Th-1 (NIST, 2000a). The thorium was stored in a sealed glass tube and consisted of a single multi-crystalline cylinder of metal with a mirror finish and no signs of oxidation. After opening the glass tube, the metal was immediately cut into 2 pieces using a new wire snip that was carefully wiped down with methanol. The pieces were rinsed with acetone, allowed to dry, carefully weighed in separate plastic weighing boats, and transferred to separate pre-cleaned 1-L PFA Teflon bottles. The weighing boats were then re-weighed to determine the mass of thorium transferred to each bottle. Approximately 50 mL of ultra-pure concentrated HNO3 and a few drops of HF were added to the bottle. As dissolution of thorium metal progressed a white precipitate formed (ThF4). This precipitate was dissolved using a mixture of ultra-pure HNO3 and 0.3 mol L−1 Puratronic H3BO3 (Alfa Aesar, USA). The resulting solution was dried and the boron was driven off as trimethyl borate (B(OCH3)3) by repeated dissolutions and drying with ultra-pure Optima methanol (Fisher Scientific, Fair Lawn, NJ). Finally, the material was dissolved in 50 milliliters of 3 mol L−1 HNO3 and diluted to approximately 500 mL with additional 3 mol L−1 HNO3. This work was performed within the same PFA Teflon bottles used for the dissolution of the thorium metal pieces to assure that no material was lost due to handling or transfers.
After complete dissolution of the Th, two serial dilutions of each solution were performed using a 0.3 mol L−1 HNO3–0.005 mol L−1 HF mixed acid solution. Subsamples from the final dilutions, designated as Dilutions 2A and 2B (Table 1), were transferred to 60-mL FEP Teflon bottles and mixed with subsamples from 3 ampoules of the 229Th reference material and a single ampoule of SRM 4342A for QC analyses. Subsamples of the Dilution 2A and 2B solutions were also prepared to verify the isotopic composition of the reverse spikes. The analysis samples were then shipped to LANL and LLNL for thorium mass spectrometric analysis.
3. Isotope amount-ratio and molality measurements
Thorium mass spectrometric analyses at LANL, LLNL, and IPGP included isotopic composition measurements of the IDMS mixes and the 229Th reference material solution. The thorium isotopic composition of Dilutions 2, 2A, and 2B, and the SRM 4342A solution were also measured for use in IDMS calculations. Each analysis scheme included specifications to: 1) perform IDMS isotopic analyses in parallel with the isotopic composition measurements; 2) use a uranium isotopic Certified Reference Material (CRM) for instrument mass bias corrections; 3) perform measurements at ~ 3 V signal intensities for the most abundant isotope; and 4) perform isotopic measurements of each sample in duplicate.
All 3 labs performed isotopic measurements on magnetic-sector ICP-MS instruments equipped with multiple Faraday collectors and ion counting capabilities. The thorium isotope amount ratios, n(232Th)/n (229Th) or n(230Th)/n(229Th), for the IDMS mixes were measured using Faraday cups. The isotopic composition for 229Th solution and the Dilution 2, 2A, and 2B solutions were measured using a combination of Faraday cups for the primary isotope and secondary electron multipliers (SEM) for the low-abundance isotopes. Table 2 is a summary of the instrument and analysis parameters for the mass spectrometry measurements.
Table 2.
Th Mass spectrometry analysis information.
| Laboratory | LANL | LLNL | IPGP-CEA |
|---|---|---|---|
| Instrument | ThermoFisher Neptune MC-ICP-MS |
Nu-Plasma HR MC-ICP-MS |
ThermoFisher Neptune MC-ICP-MS (at IPGP) |
| Collectors | 9 Faraday, 7 Channeltron, 1 SEM w/ RPQ |
5 Faraday, 3 SEMs (2 w/ RPQ) |
9 Faraday, 1 SEM w/RPQ |
| Introduction System | Stable Signal Spray Chamber | Cetac Aridus II | APEX HF (ESI) |
| Nebulizer | PFA-FTST Element Scientific | Cetac PFA Nebulizer | PFA Micro-flow Nebulizer |
| Nebulizer Gas Flow | 0.9 L/min | 0.94 L/min | Not Reported |
| Solution Uptake | 250 µL/min | 120 µL/min | 50 µL/min |
| Mass Bias CRM | CRM U010 (NBL, 2008a) | CRM U010 (NBL, 2008a) | CRM U500 (NBL, 2008b) |
| Quality Control CRM | IRMM-035 (IRMM, 1997) CRM 112A (NBL, 2010) |
CRM U005A (NBL, 2008c) | CRM U100 (NBL, 2008d) |
| Corrections Applied | Detector Baseline Instrument Background Peak Tailing Detector Gains Mass Bias Hydride Correction |
Detector Baseline Instrument Background Peak Tailing Detector Gains Mass Bias |
Detector Baseline Instrument Background Peak Tailing Detector Gains Mass Bias Hydride Correction |
Summary of instrumental parameters and analysis conditions for thorium isotopic ratio measurements. MC-ICP-MS is Multi-Collector Inductively Coupled Plasma Mass Spectrometry.
4. Results
The mass spectrometry measurement results for characterization of the thorium isotope amount ratios for the 229Th reference material are shown in Fig. 1. The measured n(230Th)/n(229Th) ratios for the 229Th reference material have a weighted mean value of 5.18 × 10−5 with a standard deviation of 0.023 × 10−5. The results from IPGP-CEA collaboration appears to be systematically lower than the other labs and Analysis of Variance (ANOVA) indicates a statistically significant laboratory-to-laboratory bias between the data sets (F statistic 19.22, F critical 3.52). The data also indicate a statistically significant sample-to-sample bias for the n(230Th)/n(229Th) ratio measurements (F statistic 3.44, F critical 2.76) but this appears to be related to the between-laboratory bias as sample-to-sample data, with IPGP-CEA measurements excluded, are very consistent (F statistic 0.37, F critical 3.33). The measured n(232Th)/n(229Th) ratios have a weighted mean value of 3.815 × 10−4 with a standard deviation of 0.115 × 10−4. No statistically significant bias in the n(232Th)/n(229Th) ratio data is observed either between laboratories (F statistic 1.97, F critical 3.59) or between samples (F statistic 0.62, F critical 2.91).
Fig. 1.
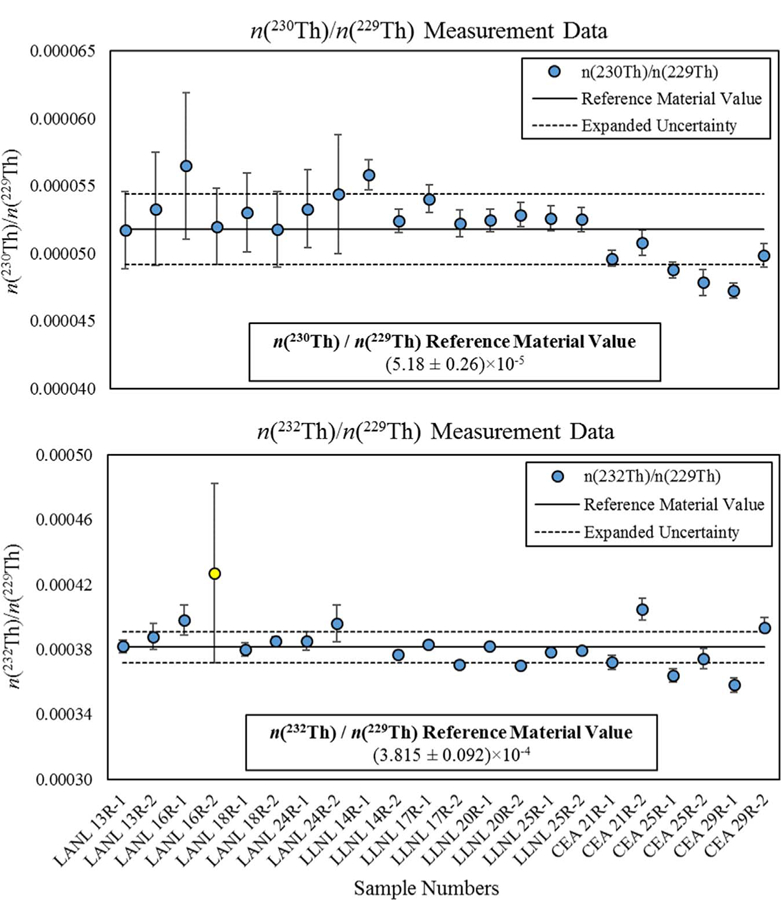
Thorium isotope amount ratio results for the 229Th reference material. Error bars are expanded uncertainties (U = k uc) for individual measurements as reported by the analysis laboratories. The solid lines are the reference material values based on weighted means of the mass spectrometry measurements. Dashed lines bracket the expanded uncertainty interval for the reference material values (k = 2). Data for n(232Th)/n(229Th) measurements of LANL 16R-2 (yellow symbol) and LLNL 14R-1 (outside of plot area) were identified as spurious analytical results by the mass spectrometry laboratories and were not incorporated into the weighed mean for the reference material value or statistical evaluations of the data. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The thorium IDMS molality measurement results for the reference material (Fig. 2, Table 3) are nearly identical for the Dilution 2, 2A, and 2B 232Th reverse spike solutions, yielding a weighted mean value of 1.1498 × 10−10 mol 229Th g−1 solution and a standard deviation of only 0.0009 × 10−10. ANOVA for the data indicate no statistically significant bias between the 3 spike solutions (F statistic = 2.29, F critical of 3.80) or the 5 samples for which multiple measurements were made (F statistic = 2.55, F critical of 4.53) but a marginal laboratory-to-laboratory difference was indicated between the 3 labs that performed mass spectrometric analyses (F statistic = 3.91, F critical of 3.59). The measured 229Th molality values are also well within the uncertainties of the verification IDMS measurements made using SRM 4342 A as a 230Th spike solution (see Table 3). For comparison purposes, published half-lives were to estimate 229Th molality values from massic activity. Although some of these values appear to be systematically biased relative to the IDMS-measured values, the two activity-based values that were calculated using half-lives determined by direct activity-versus-concentration methods (i.e. Goldstein et al., 1989 and Varga et al., 2014 “Method A”) have uncertainties that encompass the reference material value.
Fig. 2.
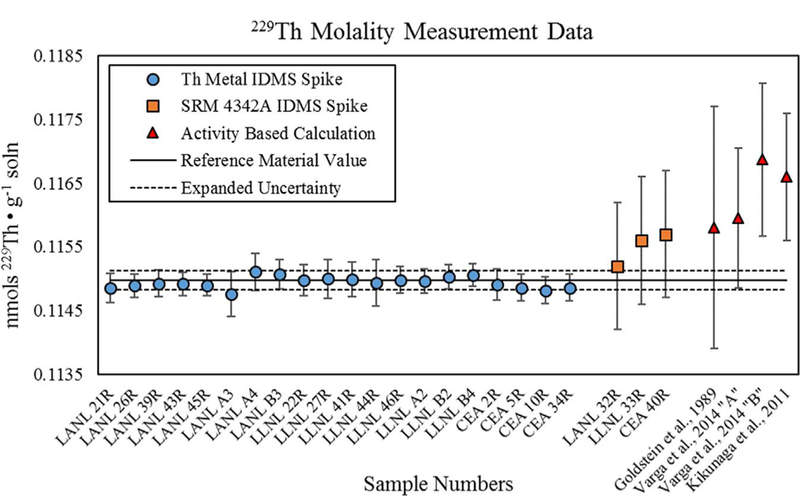
Molality measurement results for 229Th. Error bars are expanded uncertainties (U = k uc) calculated for individual IDMS molality measurements. The solid line is the reference material value based on the weighted mean of IDMS molality measurements made using Dilution 2, 2A, and 2B spikes. The dashed lines bracket an expanded uncertainty interval for the reference material value (k = 2). Data for IDMS measurements made using SRM 4342A as a 230Th spike are shown for verification purposes. The estimated massic activity for the 229Th reference material and published 229Th half-lives were used to calculate “Activity Based” values for 229Th molality.
Table 3.
Thorium molality measurement data.
| Sample number | Analysis lab | Measured 229Th Molality (mol g−1) | Ampoule number | IDMS spike |
|---|---|---|---|---|
| 21R | LANL | (1.1485 ± 0.0023) × 10−10 | 151 | Dilution 2 |
| 22R | LLNL | (1.1498 ± 0.0024) × 10−10 | Dilution 2 | |
| 26R | LANL | (1.1489 ± 0.0018) × 10−10 | 126 | Dilution 2 |
| 27R | LLNL | (1.1500 ± 0.0031) × 10−10 | Dilution 2 | |
| 34R | CEA | (1.1486 ± 0.0021) × 10−10 | Dilution 2 | |
| 39R | LANL | (1.1493 ± 0.0021) × 10−10 | 72 | Dilution 2 |
| 41R | LLNL | (1.1499 ± 0.0027) × 10−10 | 173 | Dilution 2 |
| 43R | LANL | (1.1492 ± 0.0026) × 10−10 | 49 | Dilution 2 |
| 44R | LLNL | (1.1494 ± 0.0037) × 10−10 | 67 | Dilution 2 |
| 45R | LANL | (1.1490 ± 0.0017) × 10−10 | Dilution 2 | |
| 46R | LLNL | (1.1498 ± 0.0021) × 10−10 | 176 | Dilution 2 |
| 2R | CEA | (1.1491 ± 0.0024) × 10−10 | 41 | Dilution 2 |
| 5R | CEA | (1.1486 ± 0.0021) × 10−10 | 4 | Dilution 2 |
| 10R | CEA | (1.1482 ± 0.0021) × 10−10 | 82 | Dilution 2 |
| A2 | LLNL | (1.1497 ± 0.0019) × 10−10 | 17–06-1 | Dilution 2A |
| A3 | LANL | (1.1476 ± 0.0035) × 10−10 | Dilution 2A | |
| A4 | LANL | (1.1511 ± 0.0029) × 10−10 | 17–06-2 | Dilution 2A |
| B4 | LLNL | (1.1506 ± 0.0018) × 10−10 | Dilution 2B | |
| B2 | LLNL | (1.1503 ± 0.0019) × 10−10 | 18–02-1 | Dilution 2B |
| B3 | LANL | (1.1507 ± 0.0029) × 10−10 | Dilution 2B | |
| 32R | LANL | (1.152 ± 0.010) × 10−10 | 96 | SRM 4342A |
| 33R | LLNL | (1.156 ± 0.010) × 10−10 | SRM 4342A | |
| 40R | CEA | (1.157 ± 0.010) × 10−10 | SRM 4342A | |
| 229Th Half-life Reference | Activity Based 229Th Molality (mol g−1) | 229Th Half-life Value (years) | ||
| Goldstein et al. (1989) | (1.158 ± 0.019) × 10−10 | 7880 ± 120 | ||
| “A” Varga et al. (2014) | (1.160 ± 0.011) × 10−10 | 7889 ± 64 | ||
| “B” Varga et al. (2014) | (1.169 ± 0.012) × 10−10 | 7952 ± 72 | ||
| Kinkunaga et al., 2011 | (1.166 ± 0.010) × 10−10 | 7932 ± 55 |
Summary of measured 229Th molality values for the reference material. Uncertainties are expanded uncertainties (U = k uc) with a coverage factor (k) of approximately 2. Reference material samples that were spiked with Dilution 2 and SRM 4342A were prepared and analyzed in 2012. Ampoule numbers associated with these samples represent filling order number. The samples spiked with Dilutions 2A and 2B were prepared and analyzed in 2015. Ampoule numbers for the later analyses represent unit identification numbers. Activity based molality values for the 229Th reference material were calculated using half-lives from cited publications and assuming a massic activity of (194.4 ± 1.1) Bq g−1. Half-lives for the italicized data were determined using methods that are dependent on half-live values of other isotopes. Half-lives for un-italicized values are based on direct thorium amount-versus-activity measurements.
IDMS quality control measurements of SRM 4342 A using Dilutions 2, 2 A, and 2B have a mean value of (2.3175 ± 0.0011) × 10−10 mol 230Th g−1 (k = 2). The measured QC data indicate no statistically significant bias between the 3 IDMS reverse spikes (F statistic 0.057, F, critical 5.14) and the measured molality is consistent with a molality value of (2.327 ± 0.020) × 10−10 mol 230Th g−1 calculated from the massic activity of SRM 4342 A using the 230Th half-life (Meadows et al., 1980).
The highest thorium blanks measured for acid solutions used during the production of the reference material units were 2.5 × 10−16 mol g−1 for 229Th, 1.3 × 10–17 mol g−1 for 230Th, and 3.5 × 10−15 mol g−1 for 232Th. These values correspond to a thorium sample-to-blank ratio of over 30,000. The 229Th reference material samples provided for uranium analysis had an insignificant quantity of residual uranium that was primarily comprised of 233U and 238U with a n(233U)/n(238U) ratio of 0.65 ± 0.16 (k = 2) and a maximum uranium molality of approximately 4.2 × 10–15 mol g−1.
The results for the 229Th activity measurements made by isotope dilution alpha spectrometry indicate a massic activity of (195.9 ± 3.8) Bq g−1. This value is essentially indistinguishable from the massic activity of (194.4 ± 1.1) Bq g−1 (decay corrected to September of 2012) which was calculated based on the quantitative dilution of the M solution used to produce the 229Th reference material units.
5. Discussion
Detailed requirements for production of a certified reference material are outline in ISO Guide 34 (2009) and ISO Guide 35 (2006). These requirements include evaluation of material stability and homogeneity, metrological traceability, reproducibility, and the assignment of GUM compliant measurement uncertainties. The production and characterization project for the 229Th reference material was planned and executed specifically to meet these requirements and provide the documentation necessary to certify the target attributes.
It is anticipated that the 229Th reference material units will retain their integrity over the expected lifetime of the batch 10–20 years). A unit of the 229Th reference material is comprised of 3 mL of 1 M HNO3 stored in a flame-sealed semiconductor grade quartz ampoule. This is a stable configuration that should prevent any contamination of the reference material or change in concentration due to evaporation. Analytical data appear to confirm this expectation. The reference material units were produced in November of 2011 and primary characterization measurements were made during 2012. Additional units were then opened for IDMS molality measurements in June of 2015. After 3 years, no change was observed in the molality of the solutions contained in the ampoules.
Homogeneity of the 229Th units is of paramount importance for use as a reference material. The units were prepared from a single master solution, so it is assumed that the material dispensed to the individual units essentially identical. There is, however, the potential for differential evaporation and contamination to occur after the solution was transferred to individual ampoules. The project incorporated measures to minimize the potential for these effects, including the use of custom-made quartz ampoules, an aggressive cleaning of the ampoules with high purity reagents, use of an efficient and high-precision dispenser, production of all sample units in a single work period, and the rapid sealing of the ampoules in parallel with the filling effort.
Despite careful production efforts, there is still the potential for unit-to-unit variability. To identify and estimate the extent of any variability, 8 randomly selected units of the reference material were analyzed for thorium isotope amount ratios and 14 units were measured for 229Th molality. ANOVA performed to identify statistically significant bias in the measurement result for the 229Th reference material indicated no sample-to-sample bias with the exception of the n(230Th)/n (229Th) isotope amount-ratio. As noted above, it appears that a bias in the n(230Th)/n(229Th) isotopic ratio measurements from one lab appears to be the driver for a marginal sample-to-sample bias indicated in the statistical analysis of the data. Furthermore, the n(232Th)/n(229Th) results do not indicate any statistically significant bias between samples or measurement laboratories. Similarly, the 229Th molality data do not indicate any sample-to-sample bias or a bias associated with the 3 different 232Th IDMS spikes utilized. Therefore, the data display some limited variability associated with measurement laboratories but indicate that 229Th spike units are homogeneous with respect to thorium isotope amount ratios and molality. The variability observed between laboratories is incorporated into measurement uncertainty models for the reference material attribute.
To establish traceability for the characterized reference material attributes it is necessary to demonstrate that “the result can be related to a reference through a documented unbroken chain of calibrations, each contributing to the measurement uncertainty” (JCGM, 2012). The attributes for this material are the 229Th isotope molality and the n(230Th)/n(229Th) and n(232Th)/n(229Th) isotope amount ratios. Fig. 3 summarizes traceability relationships for these attributes showing direct linkages to certified reference materials and primary calibration materials produced by the National Institute of Standards and Technology.
Fig. 3.
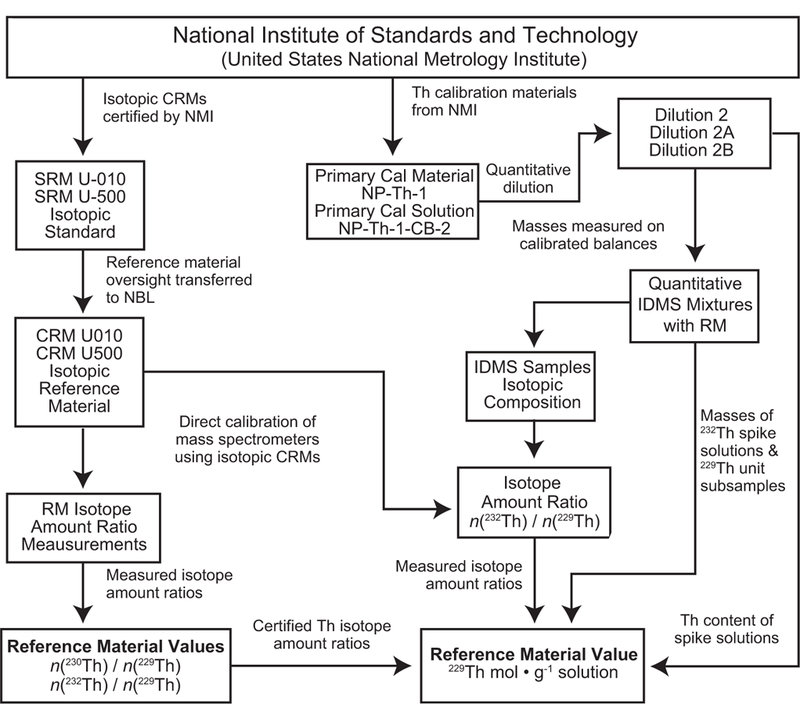
Schematic showing the metrological traceability relationships of characterized thorium isotope amount ratio values and the 229Th molality value for the reference material (RM).
All measurements of thorium isotope amount ratios were made by magnetic sector mass spectrometry on multi-collector inductively coupled plasma mass spectrometers that were calibrated with CRM U500 or U010. Uranium isotope amount ratio CRMs were used for thorium mass bias corrections due to the large uncertainties (U > 0.6%) and extreme Th isotopic ratios (n(230Th)/n(232Th) < 0.000012) of the available certified reference materials (Raptis et al., 1998). Although Ball et al. (2008) reported a systematic bias between the mass fractionation behavior of thorium and uranium in multi-collector ICP-MS, several contemporary studies did not observe any systematic discrepancies for thorium multi-collector ICP-MS measurements that were corrected for mass bias using uranium isotopic CRMs (Hoffmann et al., 2007; Mason and Henderson, 2010; and Cheng et al., 2013). Mason and Henderson (2010) could not discern a difference in multi-collector ICP-MS fractionation behavior of the elements and concluded that the relative error in thorium isotopic ratio measurements resulting from the use of uranium CRMs for mass bias corrections is not more than 0.05%. Accordingly, it is assumed the mass bias correction factors for the thorium isotope amount ratios are accurate within the resolution of measurements. An uncertainty component was, however, incorporated into the model for the characterized attributes to cover any unrecognized bias due to the use of uranium CRMs for estimating the Th mass bias corrections.
Hoffmann et al. (2007) and Ball et al. (2008) observed that Faraday-SEM detector inter-calibration yield factors can differ by up to 1% for uranium and thorium. The isotopic ratio measurements for n(230Th)/n (229Th) and n(232Th)/n(229Th) in unspiked samples were made using a combination of SEM and Faraday collectors and yield calibrations were performed using uranium CRMs. The available data are not sufficient to rule out this bias or to quantify a correction, so the uncertainty models for these ratios incorporate a component to account for potential differences in SEM-Faraday yield factors for uranium and thorium.
Isotope dilution mass spectrometry is a primary method for making traceable measurement of molality, assuming measurement inputs are appropriately constrained (De Bièvre and Peiser, 1993; Milton and Quinn, 2001). The primary variables for the IDMS calculation are measured isotope amount-ratios, the molality of the isotopic spike, and the masses of the spike and sample aliquots. As described above, the thorium isotope amount ratio measurements are traceable. The molality of thorium IDMS spikes used for this study are traceable through NIST Primary Calibration Material NP-TH-1 (NIST, 2000a, 2000b) and mass measurements were made on calibrated balances that were checked during project activities using certified weight sets.
There is sufficient isotope amount ratio and molality data to demonstrate the measured attributes for the 229Th reference material can be replicated under reproducibility measurement conditions (as defined in JCGM, 2012). Analyses for the n(230Th)/n(229Th) and n(232Th)/n (229Th) ratios were independently performed by 3 laboratories, LANL, LLNL, and CEA, specifically to assess reproducibility. Although the measurement instruments used by the labs are the same in principle (multi-collector ICP-MS), the mass spectrometers were from two manufactures, two different CRMs were used to calibrate instruments, and analyses were performed by different analysts using independently developed analytical schemes and data reduction algorithms. The resulting n(232Th)/n(229Th) isotope amount ratios for all 3 labs indicate no statistically significant bias. Although the n(230Th)/n(229Th) data do indicate a statistically significant bias, this is due to data from a single lab and the uncertainty model for the n(230Th)/n(229Th) value incorporates the observed variability.
The 229Th molality of the reference material was determined using three separate preparations of a 232Th reverse IDMS spike. The 232Th spike solutions were created at 2 different laboratories with one spike solution being prepared 3 years prior to the other two spikes. The isotopic measurements of the IDMS solution mixes were performed at 3 laboratories in two distinct analytical campaigns. No statistically significant bias in the molality measurements is observed between the three different spike preparations nor between the analyzed 229Th units. The 229Th molality of the reference material is also independently confirmed by IDMS measurements using the 230Th counting standard SRM 4342 A as a spike (Table 3).
The 229Th IDMS molality measurements for the 229Th reference material were also compared to molality values calculated using the 229Th massic activity and published half-lives. Although the activity-based molality values are systematically higher than the measured values (Fig. 2), the reference material value is within the uncertainties of the molality calculated from half-life from Goldstein et al. (1989) and one of measurements from Varga et al. (2014), here referred to as Varga “Method A”. However, the second half-life measurement from Varga et al. (2014), i.e. Varga “Method B”, and the half-life cited by Kikunaga et al. (2011) yield 229Th molality values that do not overlap with the reference material value. The bias between IDMS and activity-based molality values could, potentially, be attributed to factors such as an erroneous assay value for the high purity thorium used to make the 232Th IDMS spikes or by a massic activity that is lower than the value calculated for the 229Th reference material solution but neither of these possibilities is consistent with available data. A thorium metal assay of over 100% would be necessary to raise the IDMS values to an average of the activity-based values and measurements of the reference material activity confirm the value calculated from the quantitative dilution. It is beyond the scope of this report resolve the observed discrepancy between the measured 229Th molality for the reference material and some of the values calculated using published half-lives. The characterization and QC data for this study is, however, sufficiently robust to show that the measured 229Th molality value for the reference material is traceable and reproducible.
6. Attribute values and measurement uncertainty
A weighted mean was calculated for the characterized attributes of the 229Th reference material using a DerSimonian-Laird (DSL) procedure (DerSimonian and Laird, 1986; Rukhin, 2009). Uncertainty models were developed in accordance with the ISO Guide for the Expression of Uncertainty in Measurement (JCGM et al., 2008) and NIST Technical Note 1297 (Taylor and Kuyatt, 1994) using the GUM Workbench software developed by Metrodata (2009).
The DSL procedure was used to fit a one-way Gaussian random effects model to the data using a laboratory-to-laboratory factor. This procedure also yields an uncertainty for the values, as observed, that incorporates within-laboratory as well as between-laboratory variability. The uncertainties were computed using a parametric bootstrap method that uses Monte Carlo simulation assuming Gaussian distributions for the measurement and factor-level random errors. Symmetric expanded uncertainties were then obtained from the Monte Carlo sampling distributions for each quantity along with the standard uncertainty. Then, coverage factors and effective degrees of freedom are back-calculated assuming the results follow a Student’s t distribution when computing the effective degrees of freedom. The resulting standard uncertainties and effective degrees of freedom were input to the uncertainty models and combined with Type B uncertainty components. A coverage factor of 2 was applied to combined standard uncertainties to achieve an approximate 95% level of confidence (Table 4).
Table 4.
229Th Reference Material Attribute Uncertainty Budgets.
| Components | Comment | Type | ui% |
|---|---|---|---|
| n (230Th)/n (229Th) Isotope Amount Ratio | |||
| Measured n(230Th)/n (229Th) |
Standard uncertainty of measurement data |
A | 2.4 |
| Calibration CRM(s) | Standard uncertainty of calibration CRMs for MC-ICP-MS measurements |
B | 0.041 |
| Detector Yield Calibration |
Uncertainty for SEM-Faraday collector inter-calibration |
B | 0.50 |
| Th Blank Correction | Reagent and instrument blank uncertainty |
B | 0.085 |
|
229Th Hydride Correction |
229ThH+ correction uncertainty | B | 0.097 |
| Relative combined standard uncertainty (uc) | 2.5 | ||
| n(230Th)/n(229Th) | Expanded Uncertainty (k = 2) | ||
| 5.18 × 10−5 | 0.26 × 10−5 (Relative: 5.0%) | ||
| n(232Th)/n(229Th) Isotope Amount Ratio | |||
| Measured n(232Th)/n (229Th) |
Standard uncertainty of measurement data |
A | 1.1 |
| Calibration CRM(s) | Standard uncertainty of calibration CRMs for MC-ICP-MS measurements |
B | 0.041 |
| Detector Yield Calibration |
Uncertainty for SEM-Faraday collector inter-calibration |
B | 0.50 |
| Th Blank Correction | Reagent and instrument blank uncertainty |
B | 0.12 |
| Relative combined standard uncertainty (uc) | 1.2 | ||
| n(232Th)/n(229Th) | Expanded Uncertainty (k = 2) | ||
| 3.815 × 10−4 | 0.092 × 10−4 (Relative: 2.4%) | ||
| 229Th Molality | |||
| Measured 229Th Molality |
Standard uncertainty of measurement data |
A | 0.043 |
| Molality 232Th Spikes | Standard uncertainties of IDMS spikes | B | 0.015 |
| Calibration CRM(s) | Standard uncertainties of calibration CRMs for MC-ICP-MS measurements |
B | 0.042 |
| Mass Bias Correction | Uncertainty for use of uranium CRM for thorium mass bias correction |
B | 0.025 |
| Aliquot Weighing | Uncertainty for potential evaporation during sample weighing. |
B | 0.015 |
| Relative combined standard uncertainty (uc) | 0.068 | ||
| 229Th Molality (mol g−1) | Expanded Uncertainty (k = 2) | ||
| 1.1498 × 10−10 | 0.0016 × 10−10 (Relative: 0.14%) | ||
Uncertainty budgets for the 229Th reference material attributes. Values are shown for a reference date of 15 September 2012. Only the most significant uncertainty components are shown.
7. Conclusion
Measurements performed for this study demonstrated that prepared units of the 229Th reference material are homogeneous and that they should be stable for a sufficient duration. Attribute values for the 229Th isotope molality and thorium isotope amount ratios of the reference material are traceable, reproducible, and have appropriate measurements uncertainties. Accordingly, the 229Th reference material is a suitable reference standard for use as a thorium isotope dilution mass spectrometry spike. This new reference material will enhance capabilities to make traceable high-precision measurements of radiogenic 230Th due to a 7-fold decrease in uncertainty of the 229Th molality relative to existing activity standards.
HIGHLIGHTS.
New thorium-229 isotopic reference material was characterized.
Thorium-229 molality was measured by isotope dilution mass spectrometry.
Multiple thorium-232 isotopic spikes were prepared from high purity metal.
Thorium isotope amount ratios were measured with 3 multi-collector ICP-MS instruments.
Uncertainty budgets are provided for characterized reference material attributes.
Acknowledgements
Project activities at ANL, LANL, LLNL, NBL, and NIST were supported by the United States Department of Homeland Security Domestic Nuclear Detection Office. Support for project activities at the IPGP was provided by CEA. Considerable assistance in data evaluation and uncertainty analysis was provided by Michael Soriano at NBL and William Guthrie and Andrew Rukhin at NIST. Also, the assistance of Donald Graczyk at ANL was instrumental to the successful preparation of the Th metal solutions.
Footnotes
Certain commercial equipment, instruments, software, or materials are identified in this paper to foster understanding. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
References
- Ball L, Sims KWW, Schwieters J, 2008. Measurement of 234U/238U and 230Th/232Th in volcanic rocks using the Neptune MC-ICP-MS. J. Anal. At. Spectrom 23, 173–180. [Google Scholar]
- Cheng H, Edwards RL, Hoff J, Gallup CD, Richards DA, Asmerom Y, 2000. the half-lives of uranium-234 and thorium-230. Chem. Geol 169, 17–33. [Google Scholar]
- Cheng H, Edwards RL, Shen CC, Polyak VJ, Asmerom Y, Woodhead J, Hellstrom J, Wang Y, Kong X, Spötl C, Wang X, Alexander EC, 2013. Improvements in 230Th dating, 230Th and 234U half-life values, and U-Th isotopic measurements by multi-collector inductively coupled plasma mass spectrometry. Earth Planet. Sci. Lett 371–372, 82–91. [Google Scholar]
- De Bièvre P, Peiser HS, 1993. Basic equations and uncertainties in isotope-dilution mass spectrometry for traceability to SI of values obtained by this primary method. Fresenius J. Anal. Chem 359, 523–525. [Google Scholar]
- DerSimonian R, Laird N, 1986. Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188. [DOI] [PubMed] [Google Scholar]
- Essex RM, Bowers D, Croatto P, Hui N, Orlandini K, 2013. Certified reference materials for U radiochronometric measurements. In: Proceedings of the 54th Annual INMM Meeting, 2009. [Google Scholar]
- Fitzgerald R, Collé R, Laureano-Pérez L, Pibida L, Hammond MM, Nour S, Zimmerman BE, 2010. A new primary standardization of 229Th. Appl. Radiat. Isot 68, 1303–1308. [DOI] [PubMed] [Google Scholar]
- Goldstein SJ, Murrell MT, Williams RW, 1989. Half-life of 229Th. Phys. Rev. C 40–6, 2793–2795. [DOI] [PubMed] [Google Scholar]
- Hoffmann DL, Prytulak J, Richards DA, Elliot T, Coath CD, Smart PL, Scholz D, 2007. Procedures for accurate U and Th isotope measurements by precision MC-ICPMS. Int. J. Mass Spectrom 264, 97–109. [Google Scholar]
- IRMM, 1997. Certified Isotopic Reference Material IRMM-035. Institute for Reference Materials and Measurements, Geel, Belgium. [Google Scholar]
- ISO, 2006. Certification of Reference Materials – General and Statistical Principals. International Organization for Standardization, ISO GUIDE; 35:2006 (E). [Google Scholar]
- ISO, 2009. Quality System Guidelines for the Production of Reference Materials. International Organization for Standardization, ISO GUIDE; 34:2009 (E). [Google Scholar]
- JCGM-Joint Committee for Guides in Metrology, 2008. Evaluation of measurement data – guide to the expression of uncertainty in measurement. JCGM 100 (E/F). [Google Scholar]
- JCGM-Joint Committee for Guides in Metrology, 2012. International vocabulary of metrology -Basic and general concepts and terms (VIM). JCGM 200. [Google Scholar]
- Kikunaga H, Suzuki T, Nomura M, Mitsugashira T, Shinohara A, 2011. Determination of the half-life of the ground state of 229Th by using 232U and 233U decay series. Phys. Rev. C 84, 014316. [Google Scholar]
- Lamont SP, Hall G, 2005. Uranium age determination by measuring 230Th/234U ratio. J. Radioanal. Nucl. Chem 264, 423–427. [Google Scholar]
- Leggitt J, Inn K, Goldberg S, Essex R, LaMont S, Chase S, 2009. Nuclear Forensics-Metrological basis for legal defensibility. J. Radioanal. Nucl. Chem 282, 997–1001. [Google Scholar]
- Mason, Andrew J, Henderson GM, 2010. Correction of multi-collector-ICP-MS instru-mental biases in high-precision uranium-thorium chronology. Int. J. Mass Spectrom 295, 26–35. [Google Scholar]
- Meadows JW, Armani RL, Callis EL, Esserling AM, 1980. Half-life of 230Th. Phys. Rev. C 22, 750–754. [Google Scholar]
- Metrodata GmbH, 2009. GUM Workbench. Weil am Rhein, Germany. [Google Scholar]
- Milton MJT, Quinn TJ, 2001. Primary methods for the measurement of amount of substance. Metrologia 38, 289–296. [Google Scholar]
- Moorthy AR, Kato WY, 1994. HEU Age Determination. Brookhaven National Laboratory, Brookhaven, NY, USA: (BNL-60690 SSN 94–22). [Google Scholar]
- NBL, 2008a. Certificate of Analysis CRM U010 Uranium Isotopic Standard NBL Program Office, Argonne, IL: (Certificate available at: 〈https://science.energy.gov/nbl/certified-reference-materials/prices-and-certificates〉). [Google Scholar]
- NBL, 2008b. Certificate of Analysis CRM U500 Uranium Isotopic Standard NBL Program Office, Argonne, IL: (Certificate available at: 〈https://science.energy.gov/nbl/certified-reference-materials/prices-and-certificates〉). [Google Scholar]
- NBL, 2008c. Certificate of Analysis CRM U050-A Uranium Isotopic Standard NBL Program Office, Argonne, IL: (Certificate available at: 〈https://science.energy.gov/nbl/certified-reference-materials/prices-and-certificates〉). [Google Scholar]
- NBL, 2008d. Certificate of Analysis CRM U100 Uranium Isotopic Standard NBL Program Office, Argonne, IL: (Certificate available at: 〈https://science.energy.gov/nbl/certified-reference-materials/prices-and-certificates〉). [Google Scholar]
- NBL, 2010. Certificate of Analysis CRM 112-A Uranium (normal) Metal Assay Isotopic Standard NBL Program Office, Argonne, IL; (Certificate available at: 〈https://science.energy.gov/nbl/certified-reference-materials/prices-and-certificates〉). [Google Scholar]
- NIST, 2000a. Preparation of Th Primary Calibration Solutions Report of Analysis 839.01–00-596 National Institute of Standards and Technology, Gaithersburg, MD. [Google Scholar]
- NIST, 2000b. Assay of NIST Primary Calibration Material NP-TH-1. Report of Analysis National Institute of Standards and Technology, Gaithersburg, MD. [Google Scholar]
- NIST, 2007. Standard Reference Material 4342A Thorium-230 Radioactivity Standard National Institute of Standards and Technology, Gaithersburg, MD: (Certificate available at: 〈https://www.nist.gov/srm〉). [Google Scholar]
- NIST, 2013. Standard Reference Material 4328C Thorium-229 Radioactivity Standard National Institute of Standards and Technology, Gaithersburg, MD: (Certificate available at: 〈https://www.nist.gov/srm〉). [Google Scholar]
- NNDC, 2011. Nuclear Wallet Cards National Nuclear Date Center, Brookhaven National Laboratory; 〈www.nndc.bnl.gov〉. [Google Scholar]
- Raptis K, Mayer K, Hendricks F, De Bièvre P, 1998. Preparation and certification of new thorium isotopic reference materials. Fresenius J. Anal. Chem 361, 400–403. [Google Scholar]
- Rukhin AL, 2009. Weighted means statistics in interlaboratory studies. Metrologia 46, 323–331. [Google Scholar]
- Stanley FE, 2012. a beginner’s guide to uranium chronometry in nuclear forensics and safeguards. J. Anal. Spectrom http://dx.doi.org/10.1039/c2ja30182b.
- Taylor BN, Kuyatt CE, 1994. Guideline for Evaluating and Expressing the Uncertainty of NIST Measurement Results National Institute of Standards and Technology, Gaithersburg, MD: (Technical Note 1297). [Google Scholar]
- Varga Zsolt, Wallenius M, Mayer K, 2010. Age determination of uranium samples by inductively coupled plasma mass spectrometry using direct measurements and spectral deconvolution. J. Anal. At. Spectrom 25, 1958–1962. [Google Scholar]
- Varga Zsolt, Nicholl A, Mayer K, 2014. Determination of the 229Th half-life. Phys. Rev. C 89, 064310. [Google Scholar]
- Wallenius M, Morgenstern A, Apostolidis C, Mayer K, 2002. Determination of the age of highly enriched uranium. Anal. Bioanal. Chem 374, 379–384. [DOI] [PubMed] [Google Scholar]
- Williams RW, Gaffney AM, 2011. 230Th-234U model ages of some uranium standard reference materials. Proc. Radiochim. Acta 1, 31–35. [Google Scholar]
- Zaitseva L, 2010. Nuclear Trafficking: 20 Years in Review. Contribution to World Federation of Scientists, Erice, France. [Google Scholar]


