Abstract
Long-lasting changes of brain function in response to experience rely on diverse forms of activity-dependent synaptic plasticity. Chief among them are long-term potentiation and long-term depression of neurotransmitter release, which are widely expressed by excitatory and inhibitory synapses throughout the central nervous system and can dynamically regulate information flow in neural circuits. This review article explores recent advances in presynaptic long-term plasticity mechanisms and contributions to circuit function. Growing evidence indicates that presynaptic plasticity may involve structural changes, presynaptic protein synthesis, and transsynaptic signaling. Presynaptic long-term plasticity can alter the short-term dynamics of neurotransmitter release, thereby contributing to circuit computations such as novelty detection, modifications of the excitatory/inhibitory balance, and sensory adaptation. In addition, presynaptic long-term plasticity underlies forms of learning and its dysregulation participates in several neuropsychiatric conditions, including schizophrenia, autism, intellectual disabilities, neurodegenerative diseases, and drug abuse.
Keywords: LTP, LTD, structure, function, homeostatic, modulation, presynaptic
INTRODUCTION
The cellular basis of learning and memory is one of the greatest unsolved mysteries in neuroscience. Memory is believed to be stored in neuronal engrams, or specific subsets of synaptically-connected, coactive neurons, which are dynamically assembled via activity-dependent strengthening or weakening of synapses (Buzsaki 2010, Poo et al. 2016). Understanding how memories are preserved in the presence of various forms of plasticity is of great interest (Chaudhuri & Fiete 2016, Deneve et al. 2017). By expressing multiple forms of activity-dependent synaptic plasticity in space and time, individual neuronal responses can be optimized to allow for storage, transmission and recall of information (Costa et al. 2017). Synaptic plasticity therein endows networks with the robustness, redundancy and flexibility to independently adapt to changes in the environment.
Presynaptic plasticity is one form of plasticity generally defined as activity dependent modulation of neurotransmitter release. It can regulate circuit function over different timescales. Many studies have focused on short term presynaptic plasticity, which lasts for seconds to minutes (Regehr 2012), but much less is known about presynaptic changes that occur over hours, days, or even longer. Presynaptic long-term potentiation (preLTP) and presynaptic long-term depression (preLTD) of neurotransmitter release typically persist for hours, are widely expressed in the central nervous system (CNS) by both excitatory and inhibitory synapses, and are commonly due to changes in neurotransmitter release probability (Pr) (Castillo 2012,Yang and Calakos 2013). Despite significant advancements in the molecular basis of neurotransmission, exactly how transmitter release is modified in a long-term manner remains largely unclear. Although several molecular targets have been identified, the mechanisms governing preLTP and preLTD are known to be diverse and vary by brain region and cell type. Long-lasting changes in transmitter release can also occur as a synaptic adjustment in response to network activity, and such homeostatic plasticity occurs over hours to days. Silencing and unsilencing of presynaptic terminals are additional ways by which neurons persistently change their strength, but little is known about the underlying mechanisms and functional relevance. PreLTP and preLTD coexist with mechanistically distinct forms of activity-dependent plasticity, including postsynaptic LTP and LTD, which typically involves changes in receptor numbers or properties. A key question is why some synapses undergo presynaptic plasticity while others undergo postsynaptic plasticity or both. Does the distribution of presynaptic versus postsynaptic plasticity reflect a differential computational requirement? Long-term presynaptic plasticity can contribute uniquely to circuit computations by providing a way to modify short-term synaptic dynamics (i.e. by changing Pr). Lastly, what is the specific contribution of preLTP and preLTD to behavior and brain disease? Here, we address these fundamental questions and review emerging mechanisms of presynaptic long-term plasticity with the goal of elucidating its contribution to brain function.
MOLECULAR BASIS OF PRESYNAPTIC PLASTICITY
The induction mechanisms of preLTP and preLTD typically involve transient increases of presynaptic Ca2+, activation of presynaptic receptors and metabolic cascades, and retrograde signaling from the postsynapse. These processes are highly regulated, allowing for metaplasticity, the plasticity of synaptic plasticity, and input specificity, which refers to information storage at particular subsets of synapses (for a review of induction mechanisms and their regulation, see Castillo 2012). How neurotransmitter release is changed in a long-term manner during preLTP or preLTD is less clear. The tightly packed presynaptic terminal hosts thousands of different proteins that modulate neurotransmitter release (Figure 1). Molecules involved in basal transmission or short-term plasticity, as well as plasticity-specific proteins, may also have a role in long-term plasticity. These molecules regulate many different presynaptic processes which are critical to the expression of preLTP and preLTD, such as Ca2+ signaling, and synaptic vesicle exocytosis and endocytosis.
Figure 1.
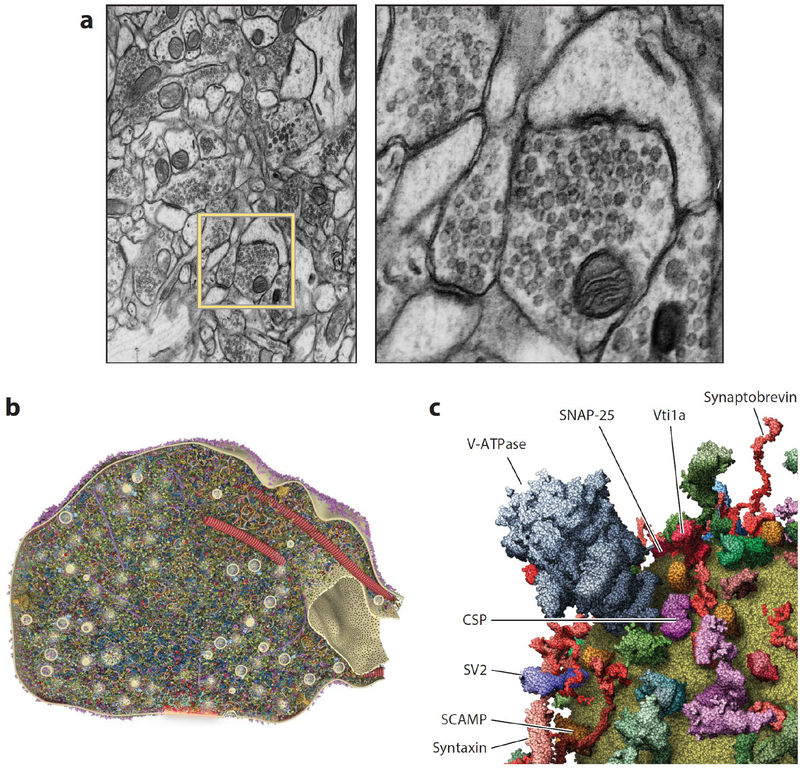
Presynaptic terminals are complex structures. (a) Transmission electron micrograph taken from rat hippocampal neuropil highlighting presynaptic terminals. The right panel is a close-up view of the yellow box in the left panel. Modified from Atlas of Ultrastructural Neurocytology on Synapse Web by Josef Spacek, Kristen Harris, and John Fiala. http://synapseweb.clm.utexas.edu/atlas. (b) Quantitative three-dimensional model of a section through an average presynaptic terminal displaying >300,000 proteins in atomic detail. The active zone is shaded red at bottom. From Wilhelm et al. (2014). (c) Three-dimensional model of a prototypical synaptic vesicle (close up) containing dozens of proteins in stoichiometrically accurate atomic detail. From Takamori et al. (2006).
Presynaptic Ca2+
Ca2+ has a critical role in neurotransmitter release and presynaptic plasticity. Presynaptic Ca2+ triggers soluble N-ethylmaleimide sensitive fusion protein (NSF) attachment protein receptor (SNARE)-mediated synaptic vesicle fusion via a low-affinity (micromolar range) process. A second, high-affinity Ca2+ sensor, distinct from the Ca2+ channel or fusion machinery, has been proposed to underlie synaptic facilitation, a seconds-long increase of transmission, in response to residual Ca2+ following repetitive activity (Jackman & Regehr 2017). Changes in action potential waveform can modulate presynaptic voltage-gated Ca2+ channel (VGCC) kinetics, Ca2+ influx, and transmitter release. Ca2+ also regulates G protein--coupled receptor (GPCR) second-messenger cascades involved in presynaptic long-term plasticity (Atwood et al. 2014). PreLTP and preLTD can rely on the recruitment of specific VGCCs. For example, hippocampal preLTP relies on N-type channels (Ahmed & Siegelbaum 2009), whereas amygdalar preLTP relies on L-type channels (Fourcaudot et al. 2009). PreLTD at the hippocampal mossy fiber (MF)-interneuron synapse involves presynaptic Gi/o-coupled metabotropic glutamate receptor 7 (mGluR7)-mediated reduction in P/Q-type Ca2+ transients (Pelkey et al. 2006). A similar mechanism for preLTD in the nucleus accumbens (NAc) relies on presynaptic Gi/o-coupled cannabinoid-1 (CB1) and mGluR2/3 receptors signaling (Mato et al. 2008, Robbe et al. 2002). Certain VGCC subtypes are tightly coupled (nanodomain) to the release machinery, whereas others are loosely coupled (microdomain) (Eggermann et al. 2011). Tight versus loose coupling is regulated by scaffolding proteins such as Rab-interacting molecules (RIMs) and RIM-binding proteins that tether VGCCs near the active zone (AZ) to ensure the fidelity of synaptic transmission (Acuna et al. 2015, Han et al. 2011). Changes in the clustering of or physical distance between VGCCs and the release site, as was recently demonstrated in the developing Calyx of Held synapse (Nakamura et al. 2015), could significantly affect the induction and expression of presynaptic long-term plasticity. Therefore, modulating presynaptic Ca2+, at the level of its influx, channel kinetics, or coupling, is an important mechanism of presynaptic plasticity. More work is needed to understand how Ca2+ microdomains are regulated by activity to impact neurotransmitter release in a long-term manner.
Release Machinery
Numerous studies have linked activity-dependent signaling to long-term changes in transmitter release (Castillo 2012, Yang and Calakos 2013). During induction, the rise in intraterminal Ca2+ or the activation of presynaptic receptors triggers a signaling cascade involving a web of kinases and phosphatases. These events can directly modify the release machinery or engage second messengers to indirectly alter release. Because cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling has a key role in multiple forms of preLTP and preLTD (Castillo 2012), many studies have focused on presynaptic PKA substrates. Among these are the AZ-scaffolding protein RIM1α, which is critical for preLTP and preLTD at excitatory and inhibitory synapses (Castillo 2012). RIM1α interacts with synaptic vesicle--associated proteins Rab3A and Rab3B, which have also been implicated in preLTP and preLTD, but it remains unclear how the Rab3/RIM1α complex is activated because its phosphorylation by PKA does not seem to be required for presynaptic long-term plasticity.
PKA phosphorylation of the synaptic vesicle protein synaptotagmin-12 is required for LTP at the MF-to-CA3 pyramidal cell synapse (MF-LTP) but not for short-term plasticity or endocannabinoid (eCB)-mediated LTD of inhibition (iLTD) (Kaeser-Woo et al. 2013), a form of presynaptic plasticity that also relies on PKA signaling (Castillo 2012). Intriguingly, genetic ablation of the PKA-anchoring protein AKAP7 from MFs abolishes chemically induced but not synaptically induced LTP (Jones et al. 2016), consistent with recent evidence that Epac2, another cAMP effector, contributes significantly to MF-LTP (Fernandes et al. 2015). Activation of presynaptic Gi/o protein--coupled mGluR2/3 and CB1 receptors, presumably by reducing cAMP/PKA activity, typically leads to long-lasting reduction in neurotransmitter release but the molecular target is unknown (Atwood et al. 2014). Phosphorylation of synaptosomal-associated protein 25 (SNAP-25) (Katayama et al. 2017) and complexin (Cho et al. 2015) is implicated in short-term presynaptic plasticity and these proteins may also be targets for long-term plasticity. In addition, it remains unclear whether changes in phosphorylation activity persist throughout preLTP and preLTD or whether other mechanisms account for the long-lasting change in neurotransmitter release. In theory, any protein that participates in the release machinery could be an activity-dependent target for presynaptic plasticity via phosphorylation or local synthesis/degradation.
The signaling cascades triggering phosphorylation events that result in presynaptic changes operate in a highly synapse-specific manner. For example, CB1 receptor-mediated depression of transmission at CA3-CA1 synapses is mediated by extracellular signal--regulated kinase (ERK) phosphorylation and subsequent degradation of Munc18, a key regulator of vesicle fusion (Schmitz et al. 2016). In contrast, at the lateral perforant path, CB1 receptors signal via β1-integrins to mediate preLTP (W. Wang et al. 2017). At hippocampal inhibitory synapses, eCB-mediated iLTD requires activation of the mammalian target of rapamycin (mTOR), rather than ERK, and presynaptic protein synthesis (Younts et al. 2016). Thus, the (de)phosphorylation events that mediate long-term changes in neurotransmitter release can depend on the brain region and synapse type. Future research should determine to what extent molecular signaling is generalizable or unique to presynaptic plasticity.
EMERGING MECHANISMS OF PRESYNAPTIC PLASTICITY
Beyond changes in presynaptic Ca2+signaling and the release machinery, recent studies have begun to reveal novel mechanisms that contribute to preLTP and preLTD. These include presynaptic changes in axonal bouton structure, protein synthesis and degradation, energy metabolism, transsynaptic cell adhesion proteins and signaling via astrocytes. The emerging picture is that long-term presynaptic plasticity is mechanistically more diverse than originally thought (Monday & Castillo, 2017).
Presynaptic Structural Plasticity
The size of the AZ and postsynaptic density (PSD) are proportional to synapse strength, suggesting that functional and structural plasticity are interdependent and structural changes are somehow coordinated across the synapse (Meyer et al. 2014, Petzoldt et al. 2016). As is the case for dendritic spines, growing evidence indicates presynaptic terminals undergo activity-dependent structural changes (Sigrist & Schmitz 2011). Presynaptic plasticity may involve the insertion or removal of AZ release sites, vesicle pool redistribution, changes in overall terminal size (Petzoldt et al. 2016), or small changes in protein clustering at the AZ (Figure 2a).
Figure 2.
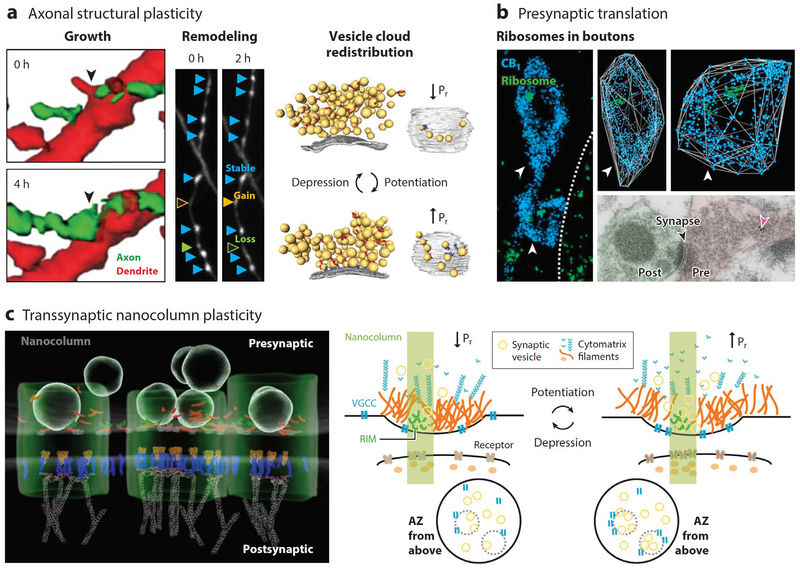
Emerging molecular mechanisms of presynaptic plasticity. (a) Different forms of axonal structural plasticity. (Left) Growth: Inhibitory axon (green) form a contact on a hippocampal pyramidal cell dendrite (red). Arrow indicates the location of a future bouton. Modified from Wierenga et al. (2008). (Middle) Remodeling: GABAergic boutons can be stable (light-blue arrowheads), gained (yellow arrowheads), or lost (orange arrowheads). Modified from Schuemann et al. (2013). Images collected from organotypic hippocampal slice cultures using time-lapse, two-photon microscopy. (Right) Vesicle cloud redistribution: Synaptic vesicle (yellow) number, density, proximity to, and priming or docking state at the AZ(gray) can contribute to Pr. Cytomatrix filaments (red and blue) may also help regulate Pr. Modified from Fernández-Busnadiego et al. (2013). Data collected from cerebrocortical synaptosomes by way of cryo-electron microscopy. (b) Ribosomes in presynaptic boutons. (Left and top-right) Dual-STORM imaging of CB1 receptors delineating two GABAergic boutons (blue) and 5.8S ribosomal rRNA (green) in acute hippocampal slices. Three-dimensional (3D) reconstructed boutons shown at right. Arrowheads indicate the two boutons. Dotted line in left panel outlines CA1 pyramidal cell soma. From Younts et al. (2016). (Bottom-right) presynaptic ribosomes (pink arrow) revealed by immunogold electron microscopy on retinal ganglion cell axon in the superior colliculus. Modified from Shigeoka et al. (2016). (c) (Left) Model of synaptic nanocolumn based on data collected by 3D-STORM revealing alignment of presynaptic and postsynaptic molecules. Shown presynaptically are synaptic vesicles, AZ proteins RIM1/2 (red) and Munc13 (green). Shown postsynaptically are glutamate receptors (orange), PSD-95 (blue), and actin (gray). The green tube highlights the nanocolumn. Modified from Tang et al. (2016). (Right) Schematic representation of the nanocolumn and its modulation. Plasticity can result from changes in presynaptic vesicle or cytomatrix filament clustering or changes in the density or proximity of VGCCs to the active zone. Adapted from Glebov et al. (2017). Abbreviations: AZ, active zone; Pr, probability of release; PSD, postsynaptic density; RIM, Rab-interacting proteins; STORM, Stochastic optical reconstruction microscopy; VGCC, voltage-gated Ca2+ channel.
Axon terminal morphological plasticity is widespread during critical periods and in adulthood (Gogolla et al. 2007, Holtmaat & Caroni 2016). At the giant hippocampal MF boutons, presynaptic morphological complexity, e.g. bouton segmentation, was correlated with increased postsynaptic response size and environmental enrichment (Galimberti et al. 2006, Gogolla et al. 2009). Similarly, high-frequency stimulation in organotypic cultures increased segmentation of the giant bouton, which depended on postsynaptic glutamate receptor activation, Ca2+ rise, and retrograde signaling via nitric oxide (NO) and arachidonic acid (Maruo et al. 2016). Persistent increases in presynaptic complexity may allow synchronization of release sites via enhanced VGCC coupling to produce long-term changes in neurotransmitter release.
Classical postsynaptic LTP in CA1 is associated with presynaptic ultrastructural changes at Schaffer collateral axons. Boutons with a single postsynaptic partner are reduced in number, and the remaining synapses are enlarged and contain fewer vesicles, suggesting a homeostatic constraint on the total amount of synaptic area in the brain (Bourne et al. 2013). The induction of LTD in hippocampal slice cultures has also been associated with presynaptic structural changes, such as fewer synaptic contacts and an increased turnover rate of presynaptic boutons (Becker et al. 2008). Moreover, a significant proportion of inhibitory axon boutons, appear, disappear, and reappear at specific locations not only in vitro (Schuemann et al. 2013) but also in vivo (Villa et al. 2016). Many factors, including synapse strength, type, location, cell identity, animal age, and network state, likely determine the relative stability of some boutons over others (Gogolla et al. 2007, Holtmaat & Caroni 2016). More work is needed to determine the precise contribution of each factor to structural plasticity.
Actin depolymerization and polymerization can drive presynaptic structural remodeling by altering the underlying cytoskeleton (Michel et al. 2015). Actin is organized around synaptic vesicles and undergoes activity-dependent remodeling such that actin is recruited to the bouton following vesicle mobilization (Sankaranarayanan et al. 2003). β-Actin and γ-actin are critical for both slow and bulk endocytosis at small CNS synapses (Wu et al. 2016), and proteins that regulate actin dynamics in an activity-dependent manner have been implicated in presynaptic plasticity (Cingolani & Goda 2008). Mammalian homolog of Diaphanous (mDia) and Rho-associated coiled-coil-containing kinase (ROCK), two Rho-regulated modulators of the actin cytoskeleton, participate in actin remodeling of the presynaptic terminals of NAc neurons as a result of social isolation (Deguchi et al. 2016). Additional actin-modulating proteins such as Profilin2 (Pilo Boyl et al. 2007) and Cyfip1 (Hsiao et al. 2016) regulate neurotransmitter release on short timescales, but their involvement in long-term plasticity is still unknown.
Axonal Proteostasis
Local synaptic protein synthesis endows remote neuronal compartments with the ability to rapidly respond and adapt to local cues (Alvarez et al. 2000). For many years, researchers thought that healthy adult mammalian CNS axons were not capable of synthesizing proteins. Indirect evidence suggested a role for protein synthesis in certain forms of preLTP and preLTD (Monday & Castillo 2017). Recent studies used super-resolution and electron microscopy to reveal ribosomes in healthy adult axon terminals (Shigeoka et al. 2016, Younts et al. 2016) (Figure 2b). Messenger RNA (mRNA) and mRNA-binding proteins have also been observed in adult axons (Akins et al. 2017). Functionally, axonal protein synthesis is required for eCB-mediated iLTD in the hippocampus (Younts et al. 2016). Activity-dependent axonal protein synthesis seems to trigger a molecular switch that regulates Pr and transmitter release. The identity of the synthesized protein(s) remains unknown, and it is unclear how widespread axonal protein synthesis is in the mature CNS.
By selective, local protein degradation, the proteasome and lysosome also have critical roles in presynaptic plasticity (Hegde 2017, Y.C. Wang et al. 2017). Selectivity of these systems can be achieved by specific localization or expression of adaptor proteins that target substrates for degradation or of the degradation machinery itself (Hegde 2017). For example, at Schaffer collaterals, activity-dependent proteasomal degradation of Munc18 was proposed to underlie CB1-mediated depression (Schmitz et al. 2016, W. Wang et al. 2017). In addition, proteasomal activation was linked to presynaptic silencing (see below) via RIM1α and Munc13–1 degradation (Jiang et al. 2010). The extent of protein degradation likely determines whether a synapse undergoes LTD, silencing, or bouton retraction or removal, but how synaptic activity leads to proteasome activation is not clear.
Transsynaptic Cell Adhesion Molecules
Transsynaptic cell adhesion molecules (CAMs) are proteinaceous bridges that were first proposed to regulate synapse specificity and formation (Dalva et al. 2007, Jang et al. 2017). Synapse formation is a unique form of plasticity that occurs throughout life, but how neurons determine synapse position is poorly understood. Growing evidence indicates that CAMs are also involved in synaptic transmission and presynaptic plasticity, likely via actin cytoskeleton rearrangements (Frias & Wierenga 2013, Jang et al. 2017). For example, the induction of presynaptic MF-LTP requires transsynaptic EphB/ephrin-B3 signaling (Contractor et al. 2002). Membrane-anchored ephrin-B ligands interact with EphB receptors bidirectionally and modulate the cytoskeleton and release machinery recruitment (Sheffler-Collins & Dalva 2012). Likewise, the transsynaptic neurexin/neuroligin complex has also been implicated in modulating Pr at mature synapses (Krueger et al. 2012). α-neurexin is essential for Ca2+-triggered transmitter release (Missler et al. 2003), and β-neurexin supports presynaptic, eCB-mediated LTP in the subiculum (Anderson et al. 2015). The transsynaptic modulation of Pr may involve neurexin signaling to Munc18, synaptotagmin, and/or VGCCs (Jang et al. 2017, Krueger et al. 2012). Similarly, postsynaptic neuroligin-2, which is expressed at GABAergic synapses (Varoqueaux et al. 2004), retrogradely regulates inhibitory synaptic transmission (Gibson et al. 2009, Jedlicka et al. 2011, Varoqueaux et al. 2004). These findings suggest transsynaptic CAMs have an integral role in regulating synaptic strength across the synaptic cleft during preLTP and preLTD.
Recent studies using super-resolution microscopy have revealed a modular organization of the synapse, in which release sites and postsynaptic receptors appear to be aligned in transsynaptic nanocolumns (Figure 2c). Correlated RIM1/2 and PSD-95 nanoclusters are modulated by N-methyl-D-aspartate receptor (NMDAR) activation, resulting in distinct pre- and postsynaptic structural changes (Tang et al. 2016). Chemical LTD disrupted the PSD-95 columnar organization, after which the RIM1/2 cluster volume markedly increased, suggesting a retrograde signal could engage some sort of presynaptic homeostatic plasticity (Tang et al. 2016). Similarly, neuronal activation promoted clustering of the AZ matrix through a retrograde mechanism likely involving eCBs (Glebov et al. 2017). Long-term blockade of neuronal activity has the opposite effect, leading to AZ scaffold unclustering as well as actin depolymerization, enrichment of AZ machinery such as P/Q-type VGCCs, and reduced AZ-PSD distance (Glebov et al. 2017). Thus, synaptic geometry can be rapidly reorganized by activity, resulting in nanoscale changes in protein localization that may affect transmission in a long-term manner. These nanocolumns reflect an additional example of bilateral coordination of pre- and postsynaptic structures.
Presynaptic Energy Metabolism
Neurotransmitter release is a highly energy-demanding process. ATP is required to maintain ion gradients, power vesicle filling and cycling, and support enzymatic activity (Harris et al. 2012). Mitochondria supply energy, buffer Ca2+, and are recruited to certain presynaptic terminals. Oxidative phosphorylation in mitochondria supplies the majority of ATP required for release (Rangaraju et al. 2014), but only approximately 40% or less of small CNS terminals contain mitochondria (Chavan et al. 2015, Smith et al. 2016). Levels of ATP can be sustained in boutons lacking mitochondria via glycolysis or axonal diffusion of mitochondrially-derived ATP (Pathak et al. 2015). Mitochondria localization may depend on synaptic energy demands because vesicle release, vesicle number, and PSD size are correlated with the presence and volume of mitochondria at terminals (Sun et al. 2013). Blocking mitochondria activity alters basal Pr, homeostatic, and synaptic plasticity (Harris et al. 2012). After inducing LTP, boutons that have mitochondria contain fewer vesicles (Smith et al. 2016), suggesting mitochondria regulate vesicle pool mobility during plasticity. Mitochondria themselves are motile, and their positioning also contributes to presynaptic homeostatic plasticity and synapse strength (Sun et al. 2013). Recent studies suggest that brain-derived neurotrophic factor (BDNF) and cannabinoids decrease presynaptic mitochondria mobility, which can enhance or depress transmitter release (Hebert-Chatelain et al. 2016, Su et al. 2014). Although energy metabolism is critical for neurotransmitter release, the activity-dependent modulation of mitochondrial localization and function as well as their role in supporting sustained changes in transmitter release deserve further attention.
Astrocytes
Astrocytes establish bidirectional interactions with neurons and have been implicated in presynaptic plasticity via gliotransmission (Min et al. 2012, Perea et al. 2009). Glutamate release from astrocytes can increase neurotransmission by activating presynaptic NMDARs (Jourdain et al. 2007) and triggers preLTP by activating presynaptic mGluRs (Perea & Araque 2007). eCBs released from neurons can activate astrocytic CB1 receptors that stimulate glutamate release from astrocytes. Astrocytic glutamate activates presynaptic NMDARs to induce neocortical preLTD (Min & Nevian 2012), whereas presynaptic group I mGluRs are engaged in hippocampal preLTP (Gómez-Gonzalo et al. 2015). Astrocytic CB1 receptors also mediate release of astrocytic D-serine which is involved in hippocampal preLTD (Andrade-Talavera et al. 2016). Given the highly interconnected astrocytic syncytium, the requirement of coincident signals, such as astrocytic CB1 engagement and postsynaptic receptor activation may help ensure input specificity of astrocyte-mediated plasticity.
ADDITIONAL FORMS OF PRESYNAPTIC PLASTICITY
Multiple forms of presynaptic plasticity can coexist in the same synapse, circuit, or brain region. In some cases, these forms of plasticity may involve similar mechanisms or timescales or they may wholly overlap with preLTP and preLTD, but how they interact remains unclear.
Presynaptic Homeostatic Plasticity
Homeostatic plasticity refers to compensatory adjustments of neuronal excitability toward a set point in the continued presence of a perturbation (Turrigiano 2012). This can be achieved via synaptic scaling and changes in intrinsic excitability and is thought to preserve computational capacity and the excitatory/inhibitory (E/I) balance of a given network. Homeostatic plasticity can also involve changes in neurotransmitter release (for a review, see Davis & Müller 2015). However, preLTP, preLTD, and presynaptic homeostatic plasticity are likely induced by distinct cues and operate over different timescales. Unlike induction of preLTP or preLTD, induction of homeostatic plasticity typically requires some long-lasting signal(s). Presynaptic homeostatic plasticity could manifest as changes in presynaptic ion channel expression, in neurotransmitter receptor surface expression and trafficking, and in the readily releasable pool (Davis & Müller 2015). Transsynaptic signaling has been implicated in this process. Given that some common mechanisms could underlie preLTP, preLTD, and homeostatic plasticity, how these forms of plasticity interact, or remain distinct, is an open question.
Presynaptic Silencing
Throughout the brain, a portion of presynaptic terminals are in a dormant state. They contain the structural hallmarks of an active terminal such as vesicles and SNARE proteins yet are release incompetent (Crawford & Mennerick 2012). Their function is unclear, but they are likely important substrates for plasticity (Atwood & Wojtowicz 1999, Voronin & Cherubini 2004). Persistent network depolarization homeostatically increases dormant glutamatergic terminals in cultured neurons (Moulder et al. 2004) in a Gi/o-dependent manner but independent of CB1-, A1 adenosine-, and GABAB- receptors (Crawford et al. 2011). Tonic CB1 receptor activation is associated with muting a subset of GABAergic terminals in the hippocampus (Losonczy et al. 2004) and cerebellar granule cells (Ramírez-Franco et al. 2014). In the latter, CB1 receptor activation silences release-competent synapses and triggers vesicle retraction from the AZ. Excitatory synaptic inputs onto motor neurons also show CB1-mediated vesicle redistribution (García-Morales et al. 2015), suggesting a common mechanism of presynaptic silencing. Like preLTD, reducing cAMP signaling increases the dormant presynaptic terminal number, which is associated with decreased RIM1 levels (Jiang et al. 2010). Conversely, unsilencing is associated with actin remodeling (Yao et al. 2006), PKA activation (Bolshakov et al. 1997, Moulder et al. 2008), and protein synthesis (Ma et al. 1999). Despite these examples and mechanistic insights, the extent, duration, and functional significance of presynaptic silencing in the brain is still unclear.
IMPLICATIONS OF PRESYNAPTIC LONG-TERM PLASTICITY FOR CIRCUIT FUNCTION
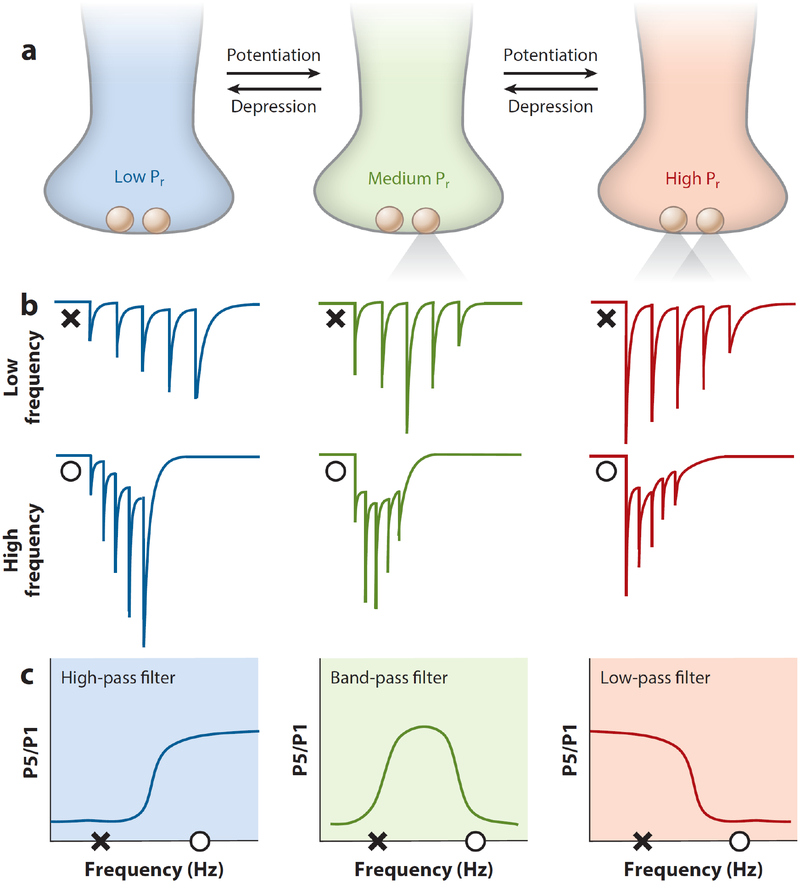
The presynaptic or postsynaptic expression locus of plasticity has important implications for circuit computation (Costa et al. 2017). Although the precise relationship between presynaptic plasticity and computation is still being explored, some principles have emerged. The timing and rate of presynaptic activation make fundamental contributions to neuronal circuit operations (Abbott & Regehr 2004). Presynapses act like filters, permitting or preventing transmission depending on activity patterns, Pr, and short-term dynamics (Abbott & Regehr 2004). In this context, long-term plasticity of transmitter release can be viewed as a modification of basal Pr and short-term release dynamics. Presynaptic long-term plasticity of basal Pr can shift synapses between low-pass, band-pass, and high-pass filtering modes (Figure 3), thus altering the computational properties of the synapse. For example, synapses with low initial Pr, such as MFCA3 synapses, function like high-pass filters that attenuate single or low-frequency signals but readily transmit information occurring in high-frequency bursts. Synapses that operate in this mode are ideally suited for novelty detection, permitting the flow of activity generated by salient information through the circuit (Lisman 1997). In contrast, synapses with high initial Pr, such as cerebellar climbing fibers, exhibit low-pass filtering characteristics, preferentially transmitting information at the onset of presynaptic activity. Synapses with intermediate initial Pr, such as most central synapses, exhibit band-pass filtering and preferentially transmit information across a normal frequency distribution. Arguably, most redistribution of synaptic efficacy because of preLTP and preLTD (Markram & Tsodyks 1996, Sjostrom et al. 2003) is likely to occur in the band-pass range, having complex and subtle effects on circuit function. For example, changes in basal Pr may subtly shift the timing of the peak postsynaptic response. This likely influences the timing and dispersion (i.e., jitter) of postsynaptic spiking, which in turn may have significant consequences on the inducibility of spike- or burst-timing-dependent plasticity.
Figure 3.
Long-term presynaptic plasticity modifies short-term plasticity and synaptic filtering. (a) PreLTP and preLTD can shift Pr of individual terminals between low, medium, and high modes. (b) Schematic EPSCs resulting from five input pulses at relatively low and high frequencies across each Pr. (c) Schematic depicting synaptic filtering of excitation across each Pr. Low-, medium-, and high-Pr synapses can respectively exhibit high-pass, band-pass, and low-pass filtering. The amplitude ratio of P5 to P1 as a function of input frequency is shown. X indicates low frequency. O indicates high frequency. Long-term presynaptic plasticity can shift the range and type of filtering at a given synapse. Abbreviations: EPSC, excitatory postsynaptic current; P5/P1, pulse 5/pulse 1; Pr, release probability; preLTD, presynaptic long-term depression; preLTP, presynaptic long-term potentiation.
Diverse forms of synaptic plasticity (e.g., short-term and long-term, presynaptic and postsynaptic, strengthening and weakening of excitatory and inhibitory transmission) combine in complex ways to affect local circuit computations. These forms of plasticity also coexist with homeostatic mechanisms to maintain circuit function despite potentially destabilizing perturbations. Presynapses from the same neuron can express different forms of plasticity that partly depend on postsynaptic identity (Larsen & Sjöström 2015). Moreover, the synaptic learning rules for inducing LTP or LTD can change depending on the state of the circuit. Neuromodulators can change overall network activity, alter plasticity induction thresholds and time windows, or even switch the plasticity sign from LTP to LTD (Froemke 2015, Hennequin et al. 2017). The coexistence of multiple forms of plasticity (e.g., presynaptic and postsynaptic) may reflect hierarchical processing of information, potentially allowing ordering of memory according to its salience (Shin et al. 2010).
Increasing Pr (e.g., preLTP of excitatory connections) increases trial-to-trial synaptic transmission reliability. For high-Pr synapses, this is nearly a one-to-one relay. In the CNS, most excitatory synapses have a lower initial Pr, and a single presynaptic spike does not lead to postsynaptic spiking, likely because the brain must both decipher and decode as well as ignore irrelevant information. The distribution of Pr across a population of inputs may be relevant for coding during sensory learning. For example, under baseline conditions before learning, auditory-evoked responses occur in the context of a noisy background of spontaneous activity, with responses comparable in size to those of other inputs (Froemke et al. 2013). Following long-term synaptic plasticity, small synaptic responses increased and large synaptic responses shrank. The average size of the sensory-evoked response remained constant, but the variance of responses decreased. These synaptic changes, which likely involve a redistribution of presynaptic weights, were correlated with improved sensory perception.
The regulation of E/I balance is one mechanism that can stabilize neuronal networks in the presence of synaptic and other forms of plasticity to preserve computational capacity. There is growing evidence that inhibitory plasticity, including preLTP and preLTD of GABA release (Castillo et al. 2011, Woodin & Maffei 2010), could have a key role in maintaining the E/I balance (Hennequin et al. 2017). In addition, inhibitory plasticity can partially disinhibit neurons, creating a window of opportunity for excitation and information flow (Basu et al. 2013, Younts et al. 2013). LTP of GABA release can occur as a result of retrograde signaling by BDNF and NO (Castillo et al. 2011), whereas LTD of GABA release is commonly mediated by retrograde eCB signaling (Castillo et al. 2012). Clearly, long-term presynaptic plasticity has emerged as a nuanced way to store, transmit and route information through circuits. Concerted efforts are needed to establish precisely how changes in presynaptic structure and function contribute to network performance and behavior.
PRESYNAPTIC LONG-TERM PLASTICITY AND BEHAVIOR
Determining the precise contribution of a given form of synaptic plasticity to brain function is extremely difficult. Establishing causality requires manipulations that specifically interfere and mimic plasticity in vivo, but such manipulations are rare. Here, we summarize a few examples that illustrate a potential role for preLTP and preLTD in behavior.
Although presynaptic forms of plasticity at the MF-CA3 synapse in vitro have been widely characterized, their relationship with cognition is not yet entirely clear. The high-pass filtering property of the MF-CA3 synapse is thought to help make patterns of neuronal activity distinct and unique to where an animal is located in the environment (Evstratova & Tóth 2014, Rebola et al. 2017). PreLTP and preLTD would have the dual effect of changing the high-pass filter cutoff frequency and signal-to-noise ratio, permitting or rejecting bursts of incoming activity. Consistent with this idea, exposure to a novel environment facilitates LTP whereas addition of prominent landmarks facilitates LTD at the MF-CA3 synapse in vivo (Hagena & Manahan-Vaughan 2011). Many of the signaling molecules that have a role in preLTP and preLTD at MFCA3 synapses in vitro also contribute to cognitive tasks, including hippocampus-dependent associative learning paradigms. For example, RIM1α knockout mice were impaired in contextual fear conditioning, whereas Rab3A knockout mice could still learn (Powell et al. 2004) but their memory precision was impaired (Ruediger et al. 2011). The cognitive effect of selective deletion of RIM1α/Rab3A from presynaptic dentate granule cells is unknown. Genetic ablation of the PKA-anchoring protein AKAP7 specifically from dentate granule cells impaired both contextual fear conditioning and MF-CA3 preLTP initiated by cAMP/PKA activation in vitro (Jones et al. 2016). There is also evidence that experience and learning lead to MF terminal structural plasticity, including expansion of the axonal field and increase in the number of release sites (Galimberti et al. 2006, Gogolla et al. 2009, Ruediger et al. 2011).
In the amygdala, preLTP and preLTD contribute to fear memory formation (Tovote et al. 2015) in a synapse-specific manner. Lateral amygdala inputs from the cortex mainly express presynaptic cAMP/PKA-dependent LTP (Huang & Kandel 1998), but coactivation of cortical and thalamic afferents triggers presynaptic NMDAR-dependent associative LTP at cortical inputs (Humeau et al. 2003). These presynaptic forms of LTP contribute to amygdala-dependent fear memory (McKernan & Shinnick-Gallagher 1997). Fear learning also induces preLTP of lateral amygdala excitatory inputs onto somatostatin inhibitory interneurons in the central amygdala, and activation of these interneurons was sufficient to drive fear responses (Li et al. 2013). In contrast, preLTP at thalamic inputs onto local amygdala inhibitory interneurons is linked with fear suppression (Bauer & LeDoux 2004), whereas fear extinction in the amygdala appears to involve eCB-mediated iLTD (Lutz 2007, Azad et al. 2004). Fear extinction is also associated with LTD at excitatory inputs from the prefrontal cortex to basolateral amygdala (Cho et al. 2013). The relationship between excitatory and inhibitory plasticity in the amygdala is complex, but one hypothesis is that inhibition controls the level of presynaptic GABAB receptor activation to regulate nonassociative forms of preLTP and generalization of fear-conditioned stimuli (Shaban et al. 2006).
Although these examples clearly indicate that presynaptic plasticity is involved in certain behaviors, more specific tools designed to interfere directly with presynaptic function are needed, for example, by optically controlling neurotransmitter accumulation inside synaptic vesicles (Rost et al. 2015) or by manipulating the molecular composition of specific presynaptic terminals, as was recently done for postsynaptic signaling molecules involved in plasticity (Hayashi-Takagi et al. 2015, Sinnen et al. 2017).
PRESYNAPTIC PLASTICITY IN DISEASE
Neuropsychiatric disorders and degenerative diseases commonly involve insults to synapse structure and function (Volk et al. 2015). Here, we describe some examples of impaired presynaptic plasticity in animal models of brain diseases and identify molecular targets of potential therapeutic relevance.
Schizophrenia
Anomalous synaptic function, due to genetic and environmental factors, likely underlies schizophrenia (Crabtree & Gogos 2014). One of the highest risk factors for schizophrenia is the 22q11 microdeletion syndrome (Earls & Zakharenko 2014). A mouse model of 22q11, Df(16)A+/−, shows an age-dependent enhancement in Pr, short-term plasticity, and LTP at hippocampal Schaffer collaterals, which coincides with deficits in spatial memory (Earls & Zakharenko 2014). Df(16)A+/− mice also exhibit decreased axonal branching in the prefrontal cortex and impaired hippocampus--prefrontal cortex oscillations (Mukai et al. 2015, Sigurdsson et al. 2010). This 22q11 mouse model also shows age-dependent deficits in social interaction, which was linked to presynaptic δ-opioid-iLTD in hippocampal CA2 (Piskorowski et al. 2016). The genes encoding neuregulin 1 (NRG1) and its receptor ErbB4 are schizophrenia susceptibility genes (Buonanno 2010). NRG1 signaling via ErB4 promotes GABA release, which impairs the E/I balance, LTP, and fear conditioning (Lu et al. 2014, Shamir et al. 2012, Woo et al. 2007). DISC1, a scaffolding protein associated with schizophrenia (Crabtree & Gogos 2014), can regulate the E/I balance via NRG1/ErbB4 signaling (Seshadri et al. 2015) and modulates presynaptic plasticity (Kvajo et al. 2011). Other schizophrenia-associated mutations affecting presynaptic long-term plasticity include microRNA-137 (Siegert et al. 2015) and RIM1α (Blundell et al. 2010). The etiology of schizophrenia involves many genes, some of which are expressed in postsynaptic compartments. Much more research is needed to untangle the relative contribution of genetic and environmental factors to synaptic dysfunction.
Autism and Intellectual Disability
Altered presynaptic plasticity has been reported in animal models of autism spectrum disorders. The most common heritable cause of autism is Fragile X syndrome, which results from FMR1 gene transcription silencing (Darnell & Klann 2013). FMRP (fragile X mental retardation protein) is an mRNA-binding protein that negatively regulates expression of mRNAs, many of which encode presynaptic proteins (Darnell & Klann 2013). FMRP is expressed in a subset of synaptic terminals (Christie et al. 2009), where it may regulate vesicle release through local protein translation (Akins et al. 2017, Broek et al. 2016). FMRP knockout mice show impaired Pr and short-term plasticity at excitatory synapses (Patel et al. 2013, Wang et al. 2014). At anterior cingulate cortical synapses, preLTP was abolished in FMRP knockout mice (Koga et al. 2015). At hippocampal inhibitory synapses, presynaptic eCB-mediated LTD is increased (Zhang & Alger 2010), whereas in NAc and the prefrontal cortex, eCB-mediated LTD is decreased (Jung et al. 2012). Alterations in the distance between group-I mGluRs and the eCB-producing enzyme diacylglycerol lipase alpha (DGLα) could explain these differences. Nontranslational roles for presynaptic FMRP, such as regulation of ion channels that control action potential waveform (Deng et al. 2013) and Ca2+ influx (Ferron et al. 2014), have also been described. Beyond FMRP, another protein for which strong links to autism have been identified is the presynaptically expressed CAM neurexin and its numerous postsynaptic docking proteins (Jang et al. 2017, Südhof 2017). While neurexin can control Pr and therefore alter presynaptic plasticity, more work is needed to establish how transsynaptic signaling is disrupted in autism.
Neurodegenerative Diseases
Patients with Alzheimer, Parkinson, or Huntington disease have toxic protein accumulations in the brain, which can trigger synapse loss, dysfunction, and cell death (Sheng et al. 2012, Waites & Garner 2011). Alzheimer’s disease is the most common cause of dementia, with β-amyloid (Aβ) protein plaque and insoluble filamentous microtubule-associated protein tau accumulations frequently observed (Forner et al. 2017, Sheng et al. 2012). Altered Aβ levels abolish presynaptic MF-LTP (Wang et al. 2008, Witton et al. 2010). The Aβ-producing enzyme presenilin is critical for short-term facilitation and LTP at CA3-CA1 synapses (Zhang et al. 2009). Aβ also interferes with eCB-mediated disinhibition and perhaps iLTD, which indirectly prevents LTP (Orr et al. 2014). Presynaptic plasticity of corticostriatal synaptic transmission is important for regulating basal ganglia activity, motor learning, and Parkinson’s and Huntington’s diseases (Kreitzer & Malenka 2008). Mutations in the Parkinson’s disease--linked gene encoding presynaptic α-synuclein results in impaired vesicle mobility and Pr (Cheng et al. 2011). Suppressing α-synuclein blocks preLTP in cultured hippocampal neurons (Liu et al. 2004). In a pharmacological model of the disease, presynaptic plasticity was impaired at corticostriatal synapses (Picconi et al. 2003). The eCB system is an attractive therapeutic target because inhibiting eCB degradation rescued preLTD at corticostriatal synapses and ameliorated motor deficits (Kreitzer & Malenka 2007).
Drugs of Abuse
PreLTP and preLTD are involved in neural adaptations induced by different drugs of abuse (Lüscher & Malenka 2011). Repeated exposure to cocaine in vivo facilitates LTP at excitatory synaptic inputs to dopaminergic ventral tegmental area (VTA) neurons, which is due in part to cocaine-induced eCB release and iLTD (Liu et al. 2005, Pan et al. 2008). Cocaine promotes presynaptic iLTP at interneurons projecting from the NAc to interneurons of the VTA that synapse onto dopaminergic VTA neurons (Bocklisch et al. 2013). These forms of long-term inhibitory plasticity in the VTA ultimately regulate dopaminergic neuron activity to control drug-seeking behavior. In contrast to cocaine, morphine and stress prevented presynaptic iLTP in the VTA and the mechanism involved NO (Nugent et al. 2007) and κ-opioid receptors (Graziane et al. 2013, Polter et al. 2014). Blocking the κ-opioid receptors may be a viable therapeutic target because it restored iLTP and prevented cocaine seeking. Single cocaine exposure and single or chronic THC exposure completely abolished eCB-mediated LTD in the NAc (Fourgeaud et al. 2004, Hoffman et al. 2003, Mato et al. 2004). Presumably this alters reward circuit output in the VTA. Moreover, drugs of abuse can engage compensatory signaling mechanisms to cope with new and unnatural constraints placed on the circuit. For example, chronic THC exposure engages homeostatic mechanisms that produce a mechanistically novel (eCB-independent) form of preLTD that is not observed in naïve brains (Mato et al. 2005). It is likely that many synapses in the brain undergo or attempt to undergo homeostatic compensatory mechanisms because of chronic drug use. The long-term consequences of these molecular changes on behavior warrants further research.
SUMMARY POINTS.
Long-term, activity-dependent increases and decreases in neurotransmitter release (preLTP and preLTD, respectively) are forms of synaptic plasticity widely expressed in the CNS that can occur at excitatory and inhibitory synapses.
Induction typically involves transient increases of presynaptic Ca2+, activation of presynaptic receptors, and retrograde or transsynaptic signaling. Molecules involved in basal neurotransmitter release or short-term plasticity may indirectly have a role in long-term plasticity (e.g., by controlling its induction).
Like dendritic spines, axons and presynaptic terminals undergo long-term experience and activity-dependent structural plasticity that are likely associated with changes in synaptic strength. Presynaptic structural plasticity may involve nanoscale changes in protein distribution at the AZ or macroscale changes such as the loss or addition of entire boutons.
New evidence supports a role for presynaptic protein synthesis in preLTP and preLTD in the mature brain. Such local protein synthesis could allow rapid response to local cues and help maintain input specificity of plasticity, but whether this is a widespread mechanism of preLTP and preLTD in the brain needs to be determined.
Emerging research suggests that long-term plasticity involves coordination between presynaptic and postsynaptic compartments. The mechanisms may include anterograde and retrograde messengers, transsynaptic CAMs, and glia.
Computationally, preLTP and preLTD can enhance circuit flexibility by altering short-term synaptic filtering dynamics and introducing correlations or decorrelations in postsynaptic activity.
New tools are needed to elucidate the function of presynaptic plasticity in behavior. The development of synapse-specific, light-sensitive or chemical-sensitive modulators of Pr would help resolve this issue.
FUTURE ISSUES.
Although several molecular targets have been identified, how exactly neurotransmitter release is changed in a long-term manner during preLTP and preLTD remains largely elusive. It is also uncertain whether preLTP or preLTD relies on specific plasticity proteins.
Given the high energy cost of maintaining release-competent presynaptic terminals, long term activity-dependent regulation of ATP availability may be a potent and efficient mechanism of controlling presynaptic strength in the long-term, but this idea has not yet been explored.
Owing mainly to a lack of selective manipulations that interfere or mimic plasticity in vivo, the precise contribution of preLTP and preLTD to behavior is mostly unknown.
Brain function relies on diverse forms of plasticity. How preLTP and preLTD interact in vivo with other plasticities (e.g., Hebbian, nonsynaptic or intrinsic, or homeostatic) is an open question.
The stability of memory in the face of molecular turnover is remarkable. How do synapses stably store information while maintaining their plastic capacity?
Presynaptic plasticity is clearly impaired in many brain diseases, but whether it is the cause or consequence of the disease is still unknown in most cases.
Acknowledgments
This work was supported by the NIH (R01-MH081935 and R01-DA17392 to P.E.C., and F31-MH114431 to H.R.M), and Marie Sklodowska-Curie International Fellowship to T.J.Y. We apologize to all investigators whose work we could not cite due to space limitations.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abbott LF, Regehr WG. 2004. Synaptic computation. Nature 431:796–803 [DOI] [PubMed] [Google Scholar]
- Acuna C, Liu X, Gonzalez A, Südhof TC. 2015. RIM-BPs mediate tight coupling of action potentials to Ca2+-triggered neurotransmitter release. Neuron 87:1234–47 [DOI] [PubMed] [Google Scholar]
- Ahmed MS, Siegelbaum SA. 2009. Recruitment of N-type Ca2+ channels during LTP enhances low release efficacy of hippocampal CA1 perforant path synapses. Neuron 63:372–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins MR, Berk-Rauch HE, Kwan KY, Mitchell ME, Shepard KA, et al. 2017. Axonal ribosomes and mRNAs associate with fragile X granules in adult rodent and human brains. Hum. Mol. Genet 26:192–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Giuditta A, Koenig E. 2000. Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog. Neurobiol 62:1–62 [DOI] [PubMed] [Google Scholar]
- Anderson GR, Aoto J, Tabuchi K, Foldy C, Covy J, et al. 2015. β-neurexins control neural circuits by regulating synaptic endocannabinoid signaling. Cell 162:593–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Talavera Y, Duque-Feria P, Paulsen O, Rodríguez-Moreno A. 2016. Presynaptic spike timing-dependent long-term depression in the mouse hippocampus. Cereb. Cortex 26:3637–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lovinger DM, Mathur BN. 2014. Presynaptic long-term depression mediated by Gi/o-coupled receptors. Trends Neurosci. 37:663–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood HL, Wojtowicz JM. 1999. Silent synapses in neural plasticity: current evidence. Learn. Mem 6:542–71 [DOI] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, et al. 2004. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J. Neurosci 24:9953–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Srinivas KV, Cheung SK, Taniguchi H, Huang ZJ, Siegelbaum SA. 2013. A corticohippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron 79: 1208–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EP, LeDoux JE. 2004. Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala. J. Neurosci 24:9507–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker N, Wierenga CJ, Fonseca R, Bonhoeffer T, Nagerl UV. 2008. LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron 60:590–97 [DOI] [PubMed] [Google Scholar]
- Blundell J, Kaeser PS, Südhof TC, Powell CM. 2010. RIM1α and interacting proteins involved in presynaptic plasticity mediate prepulse inhibition and additional behaviors linked to schizophrenia. J. Neurosci 30:5326–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocklisch C, Pascoli V, Wong JC, House DR, Yvon C, et al. 2013. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science 341:1521–25 [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Golan H, Kandel ER, Siegelbaum SA. 1997. Recruitment of new sites of synaptic transmission during the cAMP-dependent late phase of LTP at CA3-CA1 synapses in the hippocampus. Neuron 19:635–51 [DOI] [PubMed] [Google Scholar]
- Bourne JN, Chirillo MA, Harris KM. 2013. Presynaptic ultrastructural plasticity along CA3→CA1 axons during long-term potentiation in mature hippocampus. J. Comp. Neurol 521:3898–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek JAC, Lin Z, de Gruiter HM, van’t Spijker H, Haasdijk ED, et al. 2016. Synaptic vesicle dynamic changes in a model of fragile X. Mol. Autism 7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A 2010. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res. Bull 83:122–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G 2010. Neural syntax: cell assemblies, synapsembles, and readers. Neuron 68:362–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE. 2012. Presynaptic LTP and LTD of excitatory and inhibitory synapses. Cold Spring Harb. Perspect. Biol 4:a005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Chiu CQ, Carroll RC. 2011. Long-term plasticity at inhibitory synapses. Curr. Opin. Neurobiol 21:328–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. 2012. Endocannabinoid signaling and synaptic function. Neuron 76:70–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R, Fiete I. 2016. Computational principles of memory. Nat. Neurosci 19:394–403 [DOI] [PubMed] [Google Scholar]
- Chavan V, Willis J, Walker SK, Clark HR, Liu X, et al. 2015. Central presynaptic terminals are enriched in ATP but the majority lack mitochondria. PLOS ONE 10:e0125185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Vivacqua G, Yu S. 2011. The role of α-synuclein in neurotransmission and synaptic plasticity. J. Chem. Neuroanat 42:242–48 [DOI] [PubMed] [Google Scholar]
- Cho JH, Deisseroth K, Bolshakov VY. 2013. Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron 80:1491–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RW, Buhl LK, Volfson D, Tran A, Li F, et al. 2015. Phosphorylation of complexin by PKA regulates activity-dependent spontaneous neurotransmitter release and structural synaptic plasticity. Neuron 88:749–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Akins MR, Schwob JE, Fallon JR. 2009. The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J. Neurosci 29:1514–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. 2008. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci 9:344–56 [DOI] [PubMed] [Google Scholar]
- Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF. 2002. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science 296:1864–69 [DOI] [PubMed] [Google Scholar]
- Costa RP, Mizusaki BE, Sjöström PJ, van Rossum MC. 2017. Functional consequences of pre- and postsynaptic expression of synaptic plasticity. Philos. Trans. R. Soc. B 372:20160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree GW, Gogos JA. 2014. Synaptic plasticity, neural circuits, and the emerging role of altered short-term information processing in schizophrenia. Front. Synaptic Neurosci 6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Chang CY, Hyrc KL, Mennerick S. 2011. Calcium-independent inhibitory G-protein signaling induces persistent presynaptic muting of hippocampal synapses. J. Neurosci 31:979–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Mennerick S. 2012. Presynaptically silent synapses: dormancy and awakening of presynaptic vesicle release. Neuroscientist 18:216–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. 2007. Cell adhesion molecules: signalling functions at the synapse. Nat. Rev. Neurosci 8:206–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Klann E. 2013. The translation of translational control by FMRP: therapeutic targets for FXS. Nat. Neurosci 16:1530–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, Müller M. 2015. Homeostatic control of presynaptic neurotransmitter release. Annu. Rev. Physiol 77:251–70 [DOI] [PubMed] [Google Scholar]
- Deguchi Y, Harada M, Shinohara R, Lazarus M, Cherasse Y, et al. 2016. mDia and ROCK mediate actin-dependent presynaptic remodeling regulating synaptic efficacy and anxiety. Cell Rep. 17:2405–17 [DOI] [PubMed] [Google Scholar]
- Deneve S, Alemi A, Bourdoukan R. 2017. The brain as an efficient and robust adaptive learner. Neuron 94:969–77 [DOI] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, et al. 2013. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron 77:696–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earls LR, Zakharenko SS. 2014. A synaptic function approach to investigating complex psychiatric diseases. Neuroscientist 20:257–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Bucurenciu I, Goswami SP, Jonas P. 2011. Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat. Rev. Neurosci 13:7–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evstratova A, Tóth K. 2014. Information processing and synaptic plasticity at hippocampal mossy fiber terminals. Front. Cell Neurosci. 8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes HB, Riordan S, Nomura T, Remmers CL, Kraniotis S, et al. 2015. Epac2 mediates cAMP-dependent potentiation of neurotransmission in the hippocampus. J. Neurosci 35:6544–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Busnadiego R, Asano S, Oprisoreanu AM, Sakata E, Doengi M, et al. 2013. Cryo-electron tomography reveals a critical role of RIM1alpha in synaptic vesicle tethering. J Cell Biol 201: 725–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron L, Nieto-Rostro M, Cassidy JS, Dolphin AC. 2014. Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat. Commun 5:3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner S, Baglietto-Vargas D, Martini AC, Trujillo-Estrada L, LaFerla FM. 2017. Synaptic impairment in Alzheimer’s disease: a dysregulated symphony. Trends Neurosci. 40:347–57 [DOI] [PubMed] [Google Scholar]
- Fourcaudot E, Gambino F, Casassus G, Poulain B, Humeau Y, Luthi A. 2009. L-type voltage-dependent Ca2+ channels mediate expression of presynaptic LTP in amygdala. Nat. Neurosci 12:1093–95 [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. 2004. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J. Neurosci 24:6939–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias CP, Wierenga CJ. 2013. Activity-dependent adaptations in inhibitory axons. Front. Cell Neurosci 7:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC. 2015. Plasticity of cortical excitatory-inhibitory balance. Annu. Rev. Neurosci 38:195–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Carcea I, Barker AJ, Yuan K, Seybold BA, et al. 2013. Long-term modification of cortical synapses improves sensory perception. Nat. Neurosci 16:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti I, Gogolla N, Alberi S, Santos AF, Müller D, Caroni P. 2006. Long-term rearrangements of hippocampal mossy fiber terminal connectivity in the adult regulated by experience. Neuron 50:749–63 [DOI] [PubMed] [Google Scholar]
- García-Morales V, Montero F, Moreno-López B. 2015. Cannabinoid agonists rearrange synaptic vesicles at excitatory synapses and depress motoneuron activity in vivo. Neuropharmacology 92:69–79 [DOI] [PubMed] [Google Scholar]
- Gibson JR, Huber KM, Südhof TC. 2009. Neuroligin-2 deletion selectively decreases inhibitory synaptic transmission originating from fast-spiking but not from somatostatin-positive interneurons. J. Neurosci 29:13883–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebov OO, Jackson RE, Winterflood CM, Owen DM, Barker EA, et al. 2017. Nanoscale structural plasticity of the active zone matrix modulates presynaptic function. Cell Rep. 18:2715–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, Caroni P. 2007. Structural plasticity of axon terminals in the adult. Curr. Opin. Neurobiol 17:516–24 [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, Deguchi Y, Caroni P. 2009. Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber connectivities in the adult hippocampus. Neuron 62:510–25 [DOI] [PubMed] [Google Scholar]
- Gómez-Gonzalo M, Navarrete M, Perea G, Covelo A, Martín-Fernández M, et al. 2015. Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cereb. Cortex 25:3699–712 [DOI] [PubMed] [Google Scholar]
- Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. 2013. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron 77:942–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagena H, Manahan-Vaughan D. 2011. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb. Cortex 21:2442–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kaeser PS, Südhof TC, Schneggenburger R. 2011. RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron 69:304–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D. 2012. Synaptic energy use and supply. Neuron 75:762–77 [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, et al. 2015. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature 525:333–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert-Chatelain E, Desprez T, Serrat R, Bellocchio L, Soria-Gomez E, et al. 2016. A cannabinoid link between mitochondria and memory. Nature 539:555–59 [DOI] [PubMed] [Google Scholar]
- Hegde AN. 2017. Proteolysis, synaptic plasticity and memory. Neurobiol. Learn. Mem 138:98–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennequin G, Agnes EJ, Vogels TP. 2017. Inhibitory plasticity: balance, control, and codependence. Annu. Rev. Neurosci 40:557–79 [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Caulder T, Lupica CR. 2003. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J. Neurosci 23:4815–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Caroni P. 2016. Functional and structural underpinnings of neuronal assembly formation in learning. Nat. Neurosci 19:1553–62 [DOI] [PubMed] [Google Scholar]
- Hsiao K, Harony-Nicolas H, Buxbaum JD, Bozdagi-Gunal O, Benson DL. 2016. Cyfip1 regulates presynaptic activity during development. J. Neurosci 36:1564–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. 1998. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron 21:169–78 [DOI] [PubMed] [Google Scholar]
- Humeau Y, Shaban H, Bissiere S, Luthi A. 2003. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature 426:841–45 [DOI] [PubMed] [Google Scholar]
- Jackman SL, Regehr WG. 2017. The mechanisms and functions of synaptic facilitation. Neuron 94:447–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Lee H, Kim E. 2017. Synaptic adhesion molecules and excitatory synaptic transmission. Curr. Opin. Neurobiol 45:45–50 [DOI] [PubMed] [Google Scholar]
- Jedlicka P, Hoon M, Papadopoulos T, Vlachos A, Winkels R, et al. 2011. Increased dentate gyrus excitability in neuroligin-2-deficient mice in vivo. Cereb. Cortex 21:357–67 [DOI] [PubMed] [Google Scholar]
- Jiang X, Litkowski PE, Taylor AA, Lin Y, Snider BJ, Moulder KL. 2010. A role for the ubiquitin-proteasome system in activity-dependent presynaptic silencing. J. Neurosci 30:1798–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Deem J, Younts TJ, Weisenhaus M, Sanford CA, et al. 2016. Targeted deletion of AKAP7 in dentate granule cells impairs spatial discrimination. eLife 5:e20695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, et al. 2007. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci 10:331–39 [DOI] [PubMed] [Google Scholar]
- Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, et al. 2012. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat. Commun 3:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser-Woo YJ, Younts TJ, Yang X, Zhou P, Wu D, et al. 2013. Synaptotagmin-12 phosphorylation by cAMP-dependent protein kinase is essential for hippocampal mossy fiber LTP. J. Neurosci 33:9769–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama N, Yamamori S, Fukaya M, Kobayashi S, Watanabe M, et al. 2017. SNAP-25 phosphorylation at Ser187 regulates synaptic facilitation and short-term plasticity in an age-dependent manner. Sci. Rep 7:7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Liu MG, Qiu S, Song Q, O’Den G, et al. 2015. Impaired presynaptic long-term potentiation in the anterior cingulate cortex of Fmr1 knock-out mice. J. Neurosci 35:2033–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. 2007. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature 445:643–47 [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. 2008. Striatal plasticity and basal ganglia circuit function. Neuron 60:543–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, Tuffy LP, Papadopoulos T, Brose N. 2012. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr. Opin. Neurobiol 22:412–22 [DOI] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Drew LJ, Lepagnol-Bestel AM, Xiao L, et al. 2011. Altered axonal targeting and short-term plasticity in the hippocampus of Disc1 mutant mice. PNAS 108:E1349–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RS, Sjöström PJ. 2015. Synapse-type-specific plasticity in local circuits. Curr. Opin. Neurobiol 35:127–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. 2013. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci 16:332–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE. 1997. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 20:38–43 [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. 2005. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature 437:1027–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ninan I, Antonova I, Battaglia F, Trinchese F, et al. 2004. α-Synuclein produces a long-lasting increase in neurotransmitter release. EMBO J. 23:4506–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Biro AA, Nusser Z. 2004. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. PNAS 101:1362–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Sun XD, Hou FQ, Bi LL, Yin DM, et al. 2014. Maintenance of GABAergic activity by neuregulin 1-ErbB4 in amygdala for fear memory. Neuron 84:835–46 [DOI] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. 2011. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69:650–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B 2007. The endocannabinoid system and extinction learning. Mol. Neurobiol 36:92–101 [DOI] [PubMed] [Google Scholar]
- Ma L, Zablow L, Kandel ER, Siegelbaum SA. 1999. Cyclic AMP induces functional presynaptic boutons in hippocampal CA3-CA1 neuronal cultures. Nat. Neurosci 2:24–30 [DOI] [PubMed] [Google Scholar]
- Markram H, Tsodyks M. 1996. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature 382:807–10 [DOI] [PubMed] [Google Scholar]
- Maruo T, Mandai K, Takai Y, Mori M. 2016. Activity-dependent alteration of the morphology of a hippocampal giant synapse. Mol. Cell Neurosci 71:25–33 [DOI] [PubMed] [Google Scholar]
- Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ. 2004. A single in-vivo exposure to Δ9THC blocks endocannabinoid-mediated synaptic plasticity. Nat. Neurosci 7:585–86 [DOI] [PubMed] [Google Scholar]
- Mato S, Lafourcade M, Robbe D, Bakiri Y, Manzoni OJ. 2008. Role of the cyclic-AMP/PKA cascade and of P/Q-type Ca2+ channels in endocannabinoid-mediated long-term depression in the nucleus accumbens. Neuropharmacology 54:87–94 [DOI] [PubMed] [Google Scholar]
- Mato S, Robbe D, Puente N, Grandes P, Manzoni OJ. 2005. Presynaptic homeostatic plasticity rescues long-term depression after chronic Δ9-tetrahydrocannabinol exposure. J. Neurosci 25:11619–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. 1997. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390:607–11 [DOI] [PubMed] [Google Scholar]
- Meyer D, Bonhoeffer T, Scheuss V. 2014. Balance and stability of synaptic structures during synaptic plasticity. Neuron 82:430–43 [DOI] [PubMed] [Google Scholar]
- Michel K, Müller JA, Oprisoreanu AM, Schoch S. 2015. The presynaptic active zone: a dynamic scaffold that regulates synaptic efficacy. Exp. Cell Res 335:157–64 [DOI] [PubMed] [Google Scholar]
- Min R, Nevian T. 2012. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat. Neurosci 15:746–53 [DOI] [PubMed] [Google Scholar]
- Min R, Santello M, Nevian T. 2012. The computational power of astrocyte mediated synaptic plasticity. Front. Comput. Neurosci 6:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, et al. 2003. α-Neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 423:939–48 [DOI] [PubMed] [Google Scholar]
- Monday HR, Castillo PE. 2017. Closing the gap: long-term presynaptic plasticity in brain function and disease. Curr. Opin. Neurobiol 45:106–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder KL, Jiang X, Chang C, Taylor AA, Benz AM, et al. 2008. A specific role for Ca2+-dependent adenylyl cyclases in recovery from adaptive presynaptic silencing. J. Neurosci 28:5159–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder KL, Meeks JP, Shute AA, Hamilton CK, de Erausquin G, Mennerick S. 2004. Plastic elimination of functional glutamate release sites by depolarization. Neuron 42:423–35 [DOI] [PubMed] [Google Scholar]
- Mukai J, Tamura M, Fenelon K, Rosen AM, Spellman TJ, et al. 2015. Molecular substrates of altered axonal growth and brain connectivity in a mouse model of schizophrenia. Neuron 86:680–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Harada H, Kamasawa N, Matsui K, Rothman JS, et al. 2015. Nanoscale distribution of presynaptic Ca2+ channels and its impact on vesicular release during development. Neuron, 85(1), pp.145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. 2007. Opioids block long-term potentiation of inhibitory synapses. Nature 446:1086–90 [DOI] [PubMed] [Google Scholar]
- Orr AL, Hanson JE, Li D, Klotz A, Wright S, et al. 2014. β-Amyloid inhibits E-S potentiation through suppression of cannabinoid receptor 1-dependent synaptic disinhibition. Neuron 82:1334–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Hillard CJ, Liu QS. 2008. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J. Neurosci 28:1385–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, Hays SA, Bureau I, Huber KM, Gibson JR. 2013. A target cell-specific role for presynaptic Fmr1 in regulating glutamate release onto neocortical fast-spiking inhibitory neurons. J. Neurosci 33:2593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D, Shields LY, Mendelsohn BA, Haddad D, Lin W, et al. 2015. The role of mitochondrially derived ATP in synaptic vesicle recycling. J. Biol. Chem 290:22325–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Topolnik L, Lacaille JC, McBain CJ. 2006. Compartmentalized Ca2+ channel regulation at divergent mossy-fiber release sites underlies target cell-dependent plasticity. Neuron 52:497–510 [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. 2007. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317:1083–86 [DOI] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. 2009. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 32:421–31 [DOI] [PubMed] [Google Scholar]
- Petzoldt AG, Lutzkendorf J, Sigrist SJ. 2016. Mechanisms controlling assembly and plasticity of presynaptic active zone scaffolds. Curr. Opin. Neurobiol 39:69–76 [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, et al. 2003. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat. Neurosci 6:501–6 [DOI] [PubMed] [Google Scholar]
- Pilo Boyl P, Di Nardo A, Mulle C, Sassoè-Pognetto M, Panzanelli P, et al. 2007. Profilin2 contributes to synaptic vesicle exocytosis, neuronal excitability, and novelty-seeking behavior. EMBO J. 26:2991–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskorowski RA, Nasrallah K, Diamantopoulou A, Mukai J, Hassan SI, et al. 2016. Age-dependent specific changes in area CA2 of the hippocampus and social memory deficit in a mouse model of the 22q11.2 deletion syndrome. Neuron 89:163–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter AM, Bishop RA, Briand LA, Graziane NM, Pierce RC, Kauer JA. 2014. Poststress block of κ opioid receptors rescues long-term potentiation of inhibitory synapses and prevents reinstatement of cocaine seeking. Biol. Psychiatry 76:785–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM, Pignatelli M, Ryan TJ, Tonegawa S, Bonhoeffer T, et al. 2016. What is memory? The present state of the engram. BMC Biol. 14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CM, Schoch S, Monteggia L, Barrot M, Matos MF, et al. 2004. The presynaptic active zone protein RIM1α is critical for normal learning and memory. Neuron 42:143–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Franco J, Bartolomé-Martin D, Alonso B, Torres M, Sánchez-Prieto J. 2014. Cannabinoid type 1 receptors transiently silence glutamatergic nerve terminals of cultured cerebellar granule cells. PLOS ONE 9:e88594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaraju V, Calloway N, Ryan TA. 2014. Activity-driven local ATP synthesis is required for synaptic function. Cell 156:825–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Carta M, Mulle C. 2017. Operation and plasticity of hippocampal CA3 circuits: implications for memory encoding. Nat. Rev. Neurosci 18:208–20 [DOI] [PubMed] [Google Scholar]
- Regehr WG. 2012. Short-term presynaptic plasticity. Cold Spring Harb. Perspect. Biol 4:a005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. 2002. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. PNAS 99:8384–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost BR, Schneider F, Grauel MK, Wozny C, Bentz C, et al. 2015. Optogenetic acidification of synaptic vesicles and lysosomes. Nat. Neurosci 18:1845–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruediger S, Vittori C, Bednarek E, Genoud C, Strata P, et al. 2011. Learning-related feedforward inhibitory connectivity growth required for memory precision. Nature 473:514–18 [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Atluri PP, Ryan TA. 2003. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat. Neurosci 6:127–35 [DOI] [PubMed] [Google Scholar]
- Schmitz SK, King C, Kortleven C, Huson V, Kroon T, et al. 2016. Presynaptic inhibition upon CB1 or mGlu2/3 receptor activation requires ERK/MAPK phosphorylation of Munc18–1. EMBO J. 35:1236–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuemann A, Klawiter A, Bonhoeffer T, Wierenga CJ. 2013. Structural plasticity of GABAergic axons is regulated by network activity and GABAA receptor activation. Front. Neural Circuits 7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Faust T, Ishizuka K, Delevich K, Chung Y, et al. 2015. Interneuronal DISC1 regulates NRG1-ErbB4 signalling and excitatory-inhibitory synapse formation in the mature cortex. Nat. Commun 6:10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, et al. 2006. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat. Neurosci 9:1028–35 [DOI] [PubMed] [Google Scholar]
- Shamir A, Kwon OB, Karavanova I, Vullhorst D, Leiva-Salcedo E, et al. 2012. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J. Neurosci 32:2988–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler-Collins SI, Dalva MB. 2012. EphBs: an integral link between synaptic function and synaptopathies. Trends Neurosci. 35:293–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Sabatini BL, Südhof TC. 2012. Synapses and Alzheimer’s disease. Cold Spring Harb. Perspect. Biol 4:a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka T, Jung H, Jung J, Turner-Bridger B, Ohk J, et al. 2016. Dynamic axonal translation in developing and mature visual circuits. Cell 166:181–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin RM, Tully K, Li Y, Cho JH, Higuchi M, et al. 2010. Hierarchical order of coexisting pre- and postsynaptic forms of long-term potentiation at synapses in amygdala. PNAS 107:19073–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert S, Seo J, Kwon EJ, Rudenko A, Cho S, et al. 2015. The schizophrenia risk gene product miR-137 alters presynaptic plasticity. Nat. Neurosci 18:1008–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Schmitz D. 2011. Structural and functional plasticity of the cytoplasmic active zone. Curr. Opin. Neurobiol 21:144–50 [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. 2010. Impaired hippocampalprefrontal synchrony in a genetic mouse model of schizophrenia. Nature 464:763–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnen BL, Bowen AB, Forte JS, Hiester BG, Crosby KC, et al. 2017. Optogenetic control of synaptic composition and function. Neuron 93:646–60.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström PJ, Turrigiano GG, Nelson SB. 2003. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39:641–54 [DOI] [PubMed] [Google Scholar]
- Smith HL, Bourne JN, Cao G, Chirillo MA, Ostroff LE, et al. 2016. Mitochondrial support of persistent presynaptic vesicle mobilization with age-dependent synaptic growth after LTP. eLife 5:e15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacek J, Harris K, Fiala J. (2004). Atlas of Ultrastructural Neurocytology. Retrieved February 20, 2018, from http://synapseweb.clm.utexas.edu/atlas
- Su B, Ji YS, Sun XL, Liu XH, Chen ZY. 2014. Brain-derived neurotrophic factor (BDNF)-induced mitochondrial motility arrest and presynaptic docking contribute to BDNF-enhanced synaptic transmission. J. Biol. Chem 289:1213–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. 2017. Synaptic Neurexin Complexes: A Molecular Code for the Logic of Neural Circuits. Cell. 171(4), pp.745–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH. 2013. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 4:413–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, et al. 2006. Molecular anatomy of a trafficking organelle. Cell 127: 831–46 [DOI] [PubMed] [Google Scholar]
- Tang AH, Chen H, Li TP, Metzbower SR, MacGillavry HD, Blanpied TA. 2016. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature 536:210–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Luthi A. 2015. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci 16:317–31 [DOI] [PubMed] [Google Scholar]
- Turrigiano G 2012. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol 4:a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. 2004. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur. J. Cell Biol 83:449–56 [DOI] [PubMed] [Google Scholar]
- Villa KL, Berry KP, Subramanian J, Cha JW, Oh WC, et al. 2016. Inhibitory synapses are repeatedly assembled and removed at persistent sites in vivo. Neuron 89:756–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk L, Chiu SL, Sharma K, Huganir RL. 2015. Glutamate synapses in human cognitive disorders. Annu. Rev. Neurosci 38:127–49 [DOI] [PubMed] [Google Scholar]
- Voronin LL, Cherubini E. 2004. ‘Deaf, mute and whispering’ silent synapses: their role in synaptic plasticity. J. Physiol 557:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites CL, Garner CC. 2011. Presynaptic function in health and disease. Trends Neurosci. 34:326–37 [DOI] [PubMed] [Google Scholar]
- Wang H, Song L, Laird F, Wong PC, Lee HK. 2008. BACE1 knock-outs display deficits in activity-dependent potentiation of synaptic transmission at mossy fiber to CA3 synapses in the hippocampus. J. Neurosci 28:8677–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Jia Y, Pham DT, Palmer LC, Jung KM, et al. 2017. Atypical endocannabinoid signaling initiates a new form of memory-related plasticity at a cortical input to hippocampus. Cereb. Cortex 17:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Peng CZ, Cai WJ, Xia J, Jin D, et al. 2014. Activity-dependent regulation of release probability at excitatory hippocampal synapses: a crucial role of fragile X mental retardation protein in neurotransmission. Eur. J. Neurosci 39:1602–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Lauwers E, Verstreken P. 2017. Presynaptic protein homeostasis and neuronal function. Curr. Opin. Genet. Dev 44:38–46 [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Becker N, Bonhoeffer T. 2008. GABAergic synapses are formed without the involvement of dendritic protrusions. Nat Neurosci 11: 1044–52 [DOI] [PubMed] [Google Scholar]
- Wilhelm BG, Mandad S, Truckenbrodt S, Krohnert K, Schäfer C et al. 2014. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 344: 1023–8 [DOI] [PubMed] [Google Scholar]
- Witton J, Brown JT, Jones MW, Randall AD. 2010. Altered synaptic plasticity in the mossy fibre pathway of transgenic mice expressing mutant amyloid precursor protein. Mol. Brain 3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, et al. 2007. Neuregulin-1 enhances depolarization-induced GABA release. Neuron 54:599–610 [DOI] [PubMed] [Google Scholar]
- Woodin MA, Maffei AE. 2010. Inhibitory Synaptic Plasticity. New York: Springer [Google Scholar]
- Wu XS, Lee SH, Sheng J, Zhang Z, Zhao WD, et al. 2016. Actin is crucial for all kinetically distinguishable forms of endocytosis at synapses. Neuron 92:1020–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Calakos N. 2013. Presynaptic long-term plasticity. Front Synaptic Neurosci 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Qi J, Chen G. 2006. Actin-dependent activation of presynaptic silent synapses contributes to long-term synaptic plasticity in developing hippocampal neurons. J. Neurosci 26:8137–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younts TJ, Chevaleyre V, Castillo PE. 2013. CA1 pyramidal cell theta-burst firing triggers endocannabinoid-mediated long-term depression at both somatic and dendritic inhibitory synapses. J. Neurosci 33:13743–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younts TJ, Monday HR, Dudok B, Klein ME, Jordan BA, et al. 2016. Presynaptic protein synthesis is required for long-term plasticity of GABA release. Neuron 92:479–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, et al. 2009. Presenilins are essential for regulating neurotransmitter release. Nature 460:632–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Alger BE. 2010. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J. Neurosci 30:5724–29 [DOI] [PMC free article] [PubMed] [Google Scholar]