Abstract
Metacognition supports reflection upon and control of other cognitive processes. Despite metacognition occupying a central role in human psychology, its neural substrates remain underdetermined, partly due to study-specific differences in task domain and type of metacognitive judgement under study. It is also unclear how metacognition relates to other apparently similar abilities that depend on recursive thought such as theory of mind or mentalising. Now that neuroimaging studies of metacognition are more prevalent, we have an opportunity to characterise consistencies in neural substrates identified across different analysis types and domains. Here we used quantitative activation likelihood estimation methods to synthesise findings from 47 neuroimaging studies on metacognition, divided into categories based on the target of metacognitive evaluation (memory and decision-making), analysis type (judgement-related activation, confidence-related activation, and predictors of metacognitive sensitivity), and, for metamemory judgements, temporal focus (prospective and retrospective). A domain-general network, including medial and lateral prefrontal cortex, precuneus, and insula was associated with the level of confidence in self-performance in both decision-making and memory tasks. We found preferential engagement of right anterior dorsolateral prefrontal cortex in metadecision experiments and bilateral parahippocampal cortex in metamemory experiments. Results on metacognitive sensitivity were inconclusive, likely due to fewer studies reporting this contrast. Finally, by comparing our results to meta-analyses of mentalising, we obtain evidence for common engagement of the ventromedial and anterior dorsomedial prefrontal cortex in both metacognition and mentalising, suggesting that these regions may support second-order representations for thinking about the thoughts of oneself and others.
Keywords: Confidence, decision-making, mentalising, meta-analysis, metacognition, metamemory, prefrontal cortex
Introduction
Metacognition allows reflection upon and control of other cognitive processes such as perception, decision-making, and memory (Metcalfe and Shimamura, 1996). Efforts to quantify metacognition have focussed on how people judge their performance (second-order judgements) in a variety of domains (Fleming and Lau, 2014). For instance, in perceptual decision-making tasks, a first-order discrimination is made about a stimulus (e.g. orientation of a grating), followed by a second-order assessment of confidence of whether the first-order discrimination is likely to be correct. Effective metacognitive monitoring is important for behavioural control, such as when one recognises a poor decision and pursues an alternative course of action. Accounting for deficits in metacognitive function may shed light on the causes of a lack of insight into neuropsychiatric disorders and reveal possible diagnostic and therapeutic options which target metacognitive abnormalities (David et al., 2014; Koren et al., 2006).
Despite a central role for metacognition in the monitoring and control of behaviour, the relevant neurocognitive architecture supporting metacognition remains poorly understood. Initial neuropsychological studies pointed to the importance of the frontal lobe in second-order judgements about memory performance (Janowsky et al., 1989; Shimamura and Squire, 1986), with selective deficits in metacognition observed in conditions such as Korsakoff’s syndrome associated with frontal atrophy. In parallel, studies of performance monitoring identified neural signals involved in error monitoring originating in posterior medial frontal cortex (Dehaene et al., 1994; Gehring et al., 1993). Since the introduction of these seminal studies, a standard approach leverages modern neuroimaging methods to identify neural correlates of metacognitive judgements across different tasks, primarily recognition memory (metamemory), and perceptual and value-based decision-making (which we collectively refer to here as ‘metadecision’). Such research has confirmed the involvement of a frontoparietal network in metacognition (Fleming and Dolan, 2014) and begun to assign distinct computational roles to elements within this network (Bang and Fleming, 2018; Kepecs et al., 2008; Kiani and Shadlen, 2009; Miyamoto et al., 2018).
A complementary but hitherto distinct perspective on the brain basis for metacognition is provided by studies of theory of mind (ToM) – the capacity to understand others’ mental states and to appreciate that these may differ from our own. Carruthers’ interpretive sensory-access (ISA) theory proposes that self-directed metacognition relies on turning a specialised circuit for mindreading on ourselves, to indirectly infer our state of mind (Carruthers, 2009, 2011; Frith, 2012). This view is related to a recent proposal that confidence in our own actions is formed via a second-order evaluation of a coupled but distinct decision system, computationally equivalent to inferring the performance of another actor (Fleming and Daw, 2017). Indirect evidence for this view has been found in developmental studies that reveal the ability to explicitly monitor self-performance (using confidence ratings) is gained at around the same age (4–5 years old) as children begin to pass false-belief tests (Hembacher and Ghetti, 2014; Lockl and Schneider, 2007). Neuroimaging studies of mentalising have also highlighted a frontoparietal network, with meta-analyses identifying anterior dorsal medial prefrontal cortex (mPFC), bilateral temporoparietal junction (TPJ), and precuneus as key nodes (Amodio and Frith, 2006; Frith and Frith, 1999; Molenberghs et al., 2016; Schurz et al., 2014). However, given that relatively less is known about the neural basis of metacognition than ToM, whether metacognition and ToM (and mentalising more specifically) share neural substrates remains an open question (Lombardo et al., 2010; Schilbach et al., 2012; Valk et al., 2016).
When drawing inferences about the architecture of metacognition from individual studies, it is important to consider the class of second-order judgement being elicited (Schwartz and Diaz, 2014). Metacognitive judgements can be subdivided by both domain and temporal focus – retrospective or prospective (Fleming and Dolan, 2014). For instance, judgements of confidence or monitoring for errors are retrospective judgements of performance, whereas prospective judgements (typically used in metamemory tasks) include ‘feelings of knowing’ (FOKs) and ‘judgements of learning’ (JOLs) that refer to one’s future task performance. Lateral and medial aspects of prefrontal cortex (PFC) have been suggested to support retrospective and prospective judgements, respectively (Fleming and Dolan, 2014; Pannu et al., 2005). However, direct neuroimaging evidence for a distinction between different judgement types is surprisingly limited. In one of the few studies to directly compare activation related to retrospective confidence ratings and prospective FOKs, Chua et al. (2009) found that prospective judgements are associated with medial parietal and medial temporal lobe (MTL) activation, whereas retrospective judgements were related to inferior prefrontal activity. However, the same study also found that both forms of metamemory activated common regions of medial and lateral PFC, and mid-posterior areas of cingulate cortex, indicating differences may be of degree rather than of kind.
An interrelated question is whether metacognition relies on a common, domain-general resource that is recruited to evaluate performance across a variety of first-order tasks, or whether metacognition is supported by domain-specific components. Current behavioural evidence for a domain-general resource is mixed: some studies find that efficient metacognition in one task predicts good metacognition in another (Ais et al., 2016; Faivre et al., 2018; McCurdy et al., 2013; Schraw, 1996; Song et al., 2011), whereas others argue for a separation between metacognitive abilities (Baird et al., 2013; Fitzgerald et al., 2017; Garfinkel et al., 2016; Morales et al., 2018; Kelemen et al., 2000). Moreover, a correlation in behavioural measures does not necessarily mean they share the same (neural) resource, as even correlated metacognitive functions can be associated with distinct neurostructural profiles (Mccurdy et al., 2013; Rouault et al., in press). Recent neuroimaging studies have highlighted both domain-general and domain-specific neural substrates (Baird et al., 2013, 2015; Chiou et al., 2011; Fleming et al., 2014; Morales et al., 2018; Valk et al., 2016), with metamemory broadly hypothesised to recruit parietal and midline prefrontal regions, while metadecision recruits frontal regions including anterior cingulate cortex (ACC), insula, and lateral anterior prefrontal cortex (aPFC; Baird et al., 2013). However, direct comparisons between domains remain rare.
In sum, the neurocognitive architecture of metacognition remains underdetermined, partly due to study-specific differences in task domain and type of metacognitive judgement under study. Now that neuroimaging studies of metacognition are more prevalent, we have an opportunity to characterise consistencies in neural substrates of metacognition identified across different studies and task domains. In this study, we used activation likelihood estimation (ALE; Eickhoff et al., 2009) to perform meta-analyses of the current neuroimaging literature on metacognition across the two most studied domains: decision-making and memory. We also sought to analyse distinctions between different aspects of metacognitive judgements (e.g. their level and sensitivity to performance) and, within metamemory studies, their temporal focus (prospective vs retrospective). Our study thus builds on and extends a previous meta-analysis that focussed on retrospective confidence judgements about memory (White et al., 2014). Finally, we also compare the results of our meta-analysis of self-directed metacognition to networks engaged during mentalising about others.
Methods
Identifying candidate studies
Candidate studies for inclusion were initially identified from a PubMed search using the following string: (metacognition OR metamemory OR metacognitive OR ‘decision confidence’ OR ‘memory confidence’ OR ‘feeling of knowing’ OR ‘judgment of learning’ OR ‘error awareness’ OR ‘tip of the tongue’) AND (MRI OR fMRI OR ‘magnetic resonance imaging’). This string returned 169 records on 25 March 2018. The following selection criteria were used to identify studies for further evaluation: (1) studies reported in peer-reviewed journals published in English; (2) use of functional or structural MRI with associated behavioural measurements; (3) the task involved a metacognitive judgement by the subject; (4) stereotactic three-dimensional (3D) coordinates were reported from whole-brain analyses; (5) the study reported a contrast that fell into at least one of our analysis categories of interest (judgement-related activation, confidence-related activation or neural correlates of metacognitive sensitivity; refer section ‘Analysis’); and (6) the study includes data from healthy participants. Our meta-analysis differed from that of White et al. (2014) in which we required an explicit metacognitive judgement from the subject (their ‘Type B’ studies), while excluding studies which solely manipulated environmental uncertainty (their ‘Type A’ category).
From studies that met these criteria, we further limited our cohort to the two most prevalent domains in the literature: metacognition of decision-making (metadecision) and metacognition of memory (metamemory). Other less frequently studied tasks (i.e. metacognition of emotion) were excluded from analysis. Of these 169 initial results, 34 met our criteria. To ensure our search was comprehensive, we also consulted studies cited in review chapters from ‘The Cognitive Neuroscience of Metacognition’ book (Fleming and Frith, 2014) and searched the following string on Google Scholar: (metacognition OR metacognitive OR ‘error awareness’ OR ‘feeling of knowing’ OR ‘memory confidence’ OR ‘decision confidence’ OR metamemory) AND (fMRI OR MRI). These two sources resulted in an additional 13 studies that met our criteria.
Final corpus
The final corpus of 47 studies included a total of 88 analysis contrasts, 739 activation foci, and 2215 participants (see Table S1 in Supplementary Materials for full details). The number of participants in each study ranged from 11 to 191 with a mean of 47.13. One of the included studies (Hester et al., 2009) reported data collected from patient populations, but only the results from the control group were included. Coordinates reported in Talairach space were converted to Montreal Neurological Institute coordinates using the algorithm in the GingerALE software (Eickhoff et al., 2009).
Analysis
Activation-level estimation analyses were run using GingerALE (version 2.3.6) software (Eickhoff et al., 2009, 2012). The most recent instantiation of the ALE algorithm tests for clustering of peak foci from different experiments against an ALE null distribution created by randomly redistributing the same number of foci throughout the brain volume. In a typical study, the same group of subjects will contribute data to multiple statistical contrasts, and consequently, the activation patterns produced by different contrasts do not constitute independent observations. We therefore organised reported foci according to subject group (rather than contrast) and used the modified ALE algorithm to address this issue, as recommended by Turkeltaub et al. (2012). All coordinate files used in the analysis are available for download at https://github.com/metacoglab/VaccaroFleming.
Included activation foci were smoothed using a Gaussian kernel whose size depended on the sample size (larger samples result in a smaller smoothing kernel; Eickhoff et al., 2009, 2012). Multiple-comparisons correction was applied at the cluster level at a family-wise error-corrected threshold of p < .05, 5000 permutations, and a cluster-forming threshold of p < .001 uncorrected. The resulting statistical maps indicate areas of the brain where convergence between activation foci is greater than would be expected by chance (i.e. a null distribution of clusters). We followed similar methods to those used in other recent ALE studies (Garrison et al., 2013; Pollack and Ashby, 2018; Sokolowski et al., 2017). 3D statistical maps can be viewed at https://neurovault.org/collections/4238/.
Activations were labelled using a combination of group atlases and anatomical landmarks. For greater specificity in labelling clusters obtained within PFC, we also referenced coordinates against the Oxford atlases included in the FMRIB Software Library (FSL; Jenkinson et al., 2012). These atlases are derived from studies using diffusion-tensor imaging to subdivide regions sharing common connectivity fingerprints, including dorsal and ventral PFC, cingulate cortex, and orbitofrontal cortex (Neubert et al., 2014, 2015; Sallet et al., 2013).
Classification of contrasts of interest
In addition to classifying activation foci by domain (metadecision and metamemory), we also subdivided contrasts by analysis type, collapsing across domains. We identified three common contrasts of interest (Chua et al., 2014). ‘Judgment-related activity’ refers to contrasts comparing the requirement for a metacognitive judgement against a baseline or control condition. ‘Parametric effect of confidence’ refers to contrasts identifying activations that scale with the metacognitive judgement, such as a negative/positive parametric effect of JOL or confidence rating. ‘Metacognitive sensitivity’ refers to analyses identifying differences in the extent to which metacognitive judgements track objective task performance. Finally, for metamemory judgements, we also divided judgements by temporal focus (prospective and retrospective), while collapsing over contrast type. The one exception to this tripartite classification of analyses was for studies using the ‘Error Awareness Task’ (Hester et al., 2005). In this task, subjects are asked to detect each time they make an error on a go/no-go task, which is typically only achieved around 70% of the time. This feature of the task permits a contrast between ‘aware’ (reported) and ‘unaware’ (unreported) errors. We classified this contrast as both judgement and confidence related, as it reflects the deployment of a metacognitive judgement and lowered confidence in performance.
We conducted eight distinct meta-analyses: (1) all metacognition-related activations, collapsing over both domain and analysis type, (2) judgement-related activations, collapsing over domain, (3) parametric effects of confidence, collapsing over domain, (4) correlates of metacognitive sensitivity, collapsing over domain, (5) metamemory-related activations, collapsing over contrast type, (6) metadecision-related activations, collapsing over contrast type, (7) prospective metamemory-related activations, collapsing over contrast type, and (8) retrospective metamemory-related activations, collapsing over contrast type.
Comparing metacognition and ToM
To compare metacognition-related activations in our de novo meta-analysis to those associated with ToM, we obtained the ‘reverse inference’ map associated with the term ‘mentalising’ from Neurosynth (www.neurosynth.org, accessed June 2018). Neurosynth uses text-mining combined with meta-analysis to generate a large database of mappings between neural and cognitive states (Yarkoni et al., 2011). A reverse inference map displays brain regions that are preferentially related to mentalising over and above other terms in the database (i.e. that show a high posterior probability P(mentalising|activation)). The map is corrected for multiple comparisons using a false discovery rate (FDR) approach at p < 0.01. We computed the overlap between the Neurosynth mentalising map and our composite map of metacognition-related activity to examine common and distinct regional engagement. Note that while both maps are corrected for multiple comparisons across the whole-brain volume, the numerical values and thresholds are not comparable, as they are obtained via different meta-analytic methods (ALE for metacognition and multilevel kernel density analysis (MKDA) for mentalising).
Results
Composite meta-analysis of metacognition-related activity
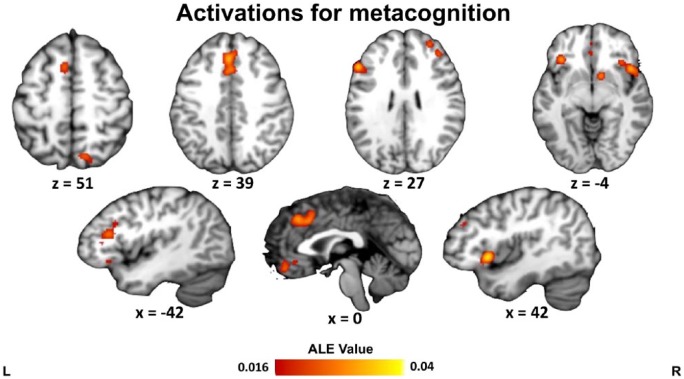
Collapsing across all 47 studies (739 foci), eight significant clusters were identified: in posterior mPFC (paracingulate gyrus/dorsal ACC), left and right insula/inferior frontal gyrus, left and right dorsolateral PFC, ventromedial PFC, right ventral striatum, and right dorsal precuneus (Table 1 and Figure 1). Notably, the activation in right dorsolateral PFC was more anterior (anterior border y = 56) than that on the left (anterior border y = 40).
Table 1.
ALE meta-analysis of all metacognition-related activations (FWE cluster-level correction p < .05; cluster-defining threshold p < .001 uncorrected, and 5000 permutations).
| Cluster | Peak coordinate (MNI) |
Volume (mm3) | Region | Maximum ALE value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1 | −2 | 30 | 38 | 5096 | L/R posterior medial frontal cortex | 0.0331 |
| 2 | 44 | 16 | 0 | 4424 | R insula/inferior frontal gyrus | 0.0398 |
| 3 | −50 | 24 | 28 | 3656 | L dorsolateral prefrontal cortex | 0.0349 |
| 4 | −36 | 28 | −6 | 1432 | L insula/inferior frontal gyrus | 0.0318 |
| 5 | 28 | 50 | 26 | 1160 | R anterior dorsolateral prefrontal cortex | 0.0245 |
| 6 | −2 | 44 | −12 | 1152 | L/R ventromedial prefrontal cortex | 0.0275 |
| 7 | 12 | −66 | 54 | 1112 | R dorsal precuneus | 0.0275 |
| 8 | 10 | 8 | −2 | 952 | R ventral striatum | 0.0290 |
L: left; R: right; ALE: activation likelihood estimation; MNI: Montreal Neurological Institute.
Figure 1.
ALE results for all studies on metacognition. Clusters are displayed in MNI standard space. Multiple-comparisons correction was applied at the cluster level at a family-wise error-corrected threshold of p < .05, 5000 permutations, and a cluster-defining threshold of p < .001 uncorrected.
Meta-analysis of judgement-related activity
We next separated activation foci by contrast type. A common distinction in the metacognition literature is between activations related to the requirement for a metacognitive judgement (judgement-related activity vs baseline/control) and those tracking judgement level (e.g. parametric effect of confidence). The analysis of judgement-related activity included 12 studies (94 foci). This analysis did not yield consistent clusters, perhaps reflecting a lack of power given that between 17–20 experiments are typically considered necessary for a well-powered neuroimaging meta-analysis (Muller et al., 2018). For completeness, in Supplementary Materials, we include an exploratory analysis of judgement-related effects at p < .001, uncorrected, minimum cluster size 200 mm3 (Table S2 and Figure S1).
Meta-analysis of parametric effects of confidence level
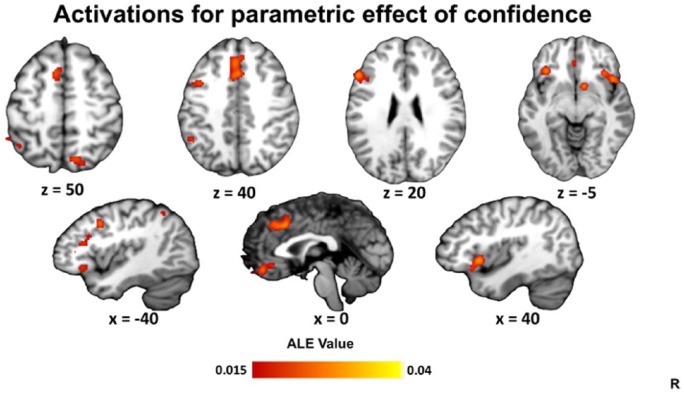
We next examined parametric contrasts for activations co-varying positively or negatively with metacognitive ratings (e.g. the level of confidence or magnitude of JOL). This analysis included 36 studies (606 foci). Nine significant clusters were identified: in posterior medial frontal cortex, left and right insula/inferior frontal gyrus, left dorsolateral PFC, ventromedial PFC, right dorsal precuneus, left lateral parietal cortex, and right ventral striatum (Table 2 and Figure 2).
Table 2.
ALE meta-analysis of parametric confidence level–related activations (FWE cluster-level correction p < .05; cluster-defining threshold p < .001 uncorrected, and 5000 permutations).
| Cluster | Peak coordinate (MNI) |
Volume (mm3) | Region | Maximum ALE value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1 | 0 | 20 | 38 | 4784 | L/R posterior medial frontal cortex | 0.0281 |
| 2 | 44 | 16 | 0 | 4480 | R insula/inferior frontal gyrus | 0.0397 |
| 3 | −50 | 24 | 28 | 3832 | L dorsolateral prefrontal cortex | 0.0348 |
| 4 | −2 | 44 | −12 | 1576 | L/R ventromedial prefrontal cortex | 0.0275 |
| 5 | −34 | 26 | −4 | 1432 | L insula/inferior frontal gyrus | 0.0272 |
| 6 | 12 | −66 | 54 | 1408 | R dorsal precuneus | 0.0273 |
| 7 | 10 | 8 | −2 | 1200 | R ventral striatum | 0.0290 |
| 8 | −42 | −54 | 48 | 1008 | L lateral parietal cortex | 0.0196 |
| 9 | −40 | 10 | 38 | 872 | L dorsolateral prefrontal cortex | 0.0248 |
L: left; R: right; ALE: activation likelihood estimation.
Figure 2.
ALE results for parametric effects of confidence. Clusters are displayed in MNI standard space. Multiple-comparisons correction was applied at the cluster level at a family-wise error-corrected threshold of p < .05, 5000 permutations, and a cluster-defining threshold of p < .001 uncorrected.
Meta-analysis of metacognitive sensitivity
Our final contrast type related to metacognitive sensitivity – the extent to which confidence effectively tracks task performance across trials. A high degree of metacognitive sensitivity is obtained when people ascribe high confidence to correct decisions, and low confidence to incorrect decisions. Because sensitivity is a property of multiple trials, it is typically analysed as a between-subjects variable.
A total of 11 studies in our corpus reported results pertaining to metacognitive sensitivity (61 foci). With cluster-correction, this analysis did not yield any consistent clusters, again consistent with a lack of power due to a limited number of studies. For completeness, in Supplementary Materials, we include an exploratory analysis of sensitivity-related effects at p < .001, uncorrected, minimum cluster size 200 mm3 (Table S2 and Figure S1).
Composite meta-analysis of metadecision-related activity
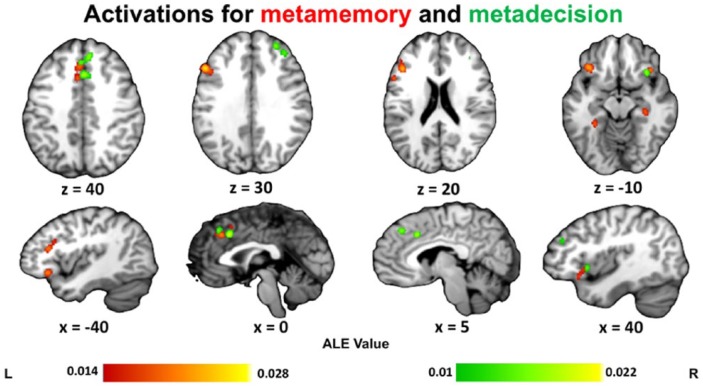
We next turned to the distinction between metacognition-related activations across domains (metadecision and metamemory) while collapsing over contrast type. For metadecision, we identified 20 studies (211 foci). Five clusters were found: one in right anterior dorsolateral PFC, two in posterior medial frontal cortex, and two in right insula/inferior frontal gyrus. In line with previous observations in the literature, we found that activations for metadecision were predominantly lateralised to the right hemisphere (Fleming and Dolan, 2014; Schmitz et al., 2004) (Table 3 and Figure 3).
Table 3.
ALE meta-analysis of metadecision-related activations (FWE cluster-level correction p < .05; cluster-defining threshold p < .001 uncorrected, and 5000 permutations).
| Cluster | Peak coordinate (MNI) |
Volume (mm3) | Region | Maximum ALE value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1 | 26 | 48 | 28 | 1336 | R anterior dorsolateral prefrontal cortex | 0.0186 |
| 2 | 6 | 38 | 42 | 1232 | L/R posterior medial frontal cortex | 0.0172 |
| 3 | 32 | 20 | −12 | 1056 | R insula | 0.0174 |
| 4 | 2 | 20 | 38 | 832 | L/R posterior medial frontal cortex | 0.0217 |
| 5 | 44 | 14 | 0 | 800 | R insula/inferior frontal gyrus | 0.0196 |
L: left; R: right; ALE: activation likelihood estimation.
Figure 3.
ALE results for all studies on metamemory (red) and metadecision (green). Clusters are displayed in MNI standard space. Multiple-comparisons correction was applied at the cluster level at a family-wise error-corrected threshold of p < .05, 5000 permutations, and a cluster-defining threshold of p < .001 uncorrected.
Composite meta-analysis of metamemory-related activity
Collapsing across contrast type, the ALE meta-analysis of metamemory included 30 studies (528 foci). Six significant clusters were identified: in left dorsolateral PFC, posterior mPFC (paracingulate gyrus), left and right insula/inferior frontal gyrus, and left and right parahippocampal gyrus (Table 4 and Figure 3).
Table 4.
ALE meta-analysis of metamemory-related activations (FWE cluster-level correction p < .05; cluster-defining threshold p < .001 uncorrected, and 5000 permutations).
| Cluster | Peak coordinate (MNI) |
Volume (mm3) | Region | Maximum ALE value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1 | −50 | 24 | 28 | 3656 | L dorsolateral prefrontal cortex | 0.0279 |
| 2 | −2 | 28 | 36 | 2128 | L/R posterior medial frontal cortex | 0.0240 |
| 3 | 44 | 18 | −2 | 2048 | R insula/inferior frontal gyrus | 0.0227 |
| 4 | 34 | −28 | −14 | 1376 | R parahippocampal gyrus | 0.0232 |
| 5 | −36 | 26 | −8 | 1224 | L insula/inferior frontal gyrus | 0.0245 |
| 6 | −28 | −38 | −14 | 808 | L parahippocampal gyrus | 0.0217 |
L: left; R: right; ALE: activation likelihood estimation.
Meta-analysis comparing prospective and retrospective metamemory judgements
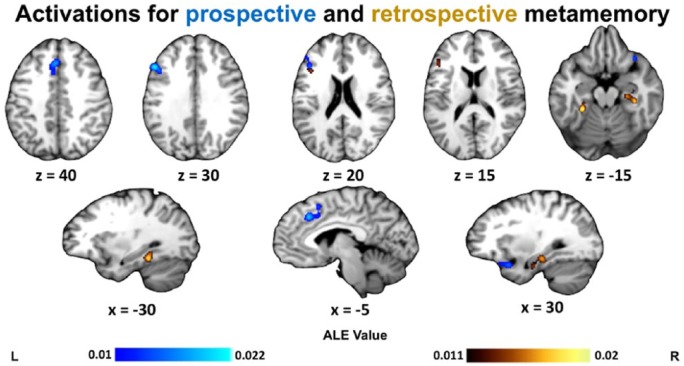
Within metamemory studies, we next examined possible differences in activation profile associated with prospective and retrospective metamemory judgements (Chua et al., 2009). Prospective judgements (such as JOLs or FOKs) included 14 studies with 232 foci, and retrospective judgements (such as recognition confidence) included 17 studies with 287 foci. The prospective analysis yielded three clusters: in posterior mPFC, left dorsolateral PFC, and right insula. The retrospective analysis revealed three clusters: in bilateral parahippocampal cortex and left inferior frontal gyrus (Table 5 and Figure 4).
Table 5.
ALE meta-analysis of prospective and retrospective metamemory-related activations (FWE cluster-level correction p < .05; cluster-defining threshold p < .001 uncorrected, 5000 permutations).
| Cluster | Peak coordinate (MNI) |
Volume (mm3) | Region | Maximum ALE value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Prospective | ||||||
| 1 | −2 | 28 | 36 | 2216 | L/R posterior medial frontal cortex | 0.0218 |
| 2 | −50 | 24 | 28 | 2056 | L dorsolateral prefrontal cortex | 0.0224 |
| 3 | 30 | 14 | −20 | 1040 | R insula/inferior frontal gyrus | 0.0152 |
| Retrospective | ||||||
| 1 | 34 | −28 | −16 | 1632 | R parahippocampal gyrus | 0.0185 |
| 2 | −28 | −38 | −14 | 1400 | L parahippocampal gyrus | 0.0203 |
| 3 | −42 | 22 | 18 | 784 | L inferior frontal gyrus | 0.0156 |
L: left; R: right; ALE: activation likelihood estimation.
Figure 4.
ALE results for contrasts of prospective metamemory (blue) and of retrospective metamemory (yellow). Clusters are displayed in MNI standard space. Multiple-comparisons correction was applied at the cluster level at a family-wise error-corrected threshold of p < .05, 5000 permutations, and a cluster-defining threshold of p < .001 uncorrected.
Comparing metacognition and ToM
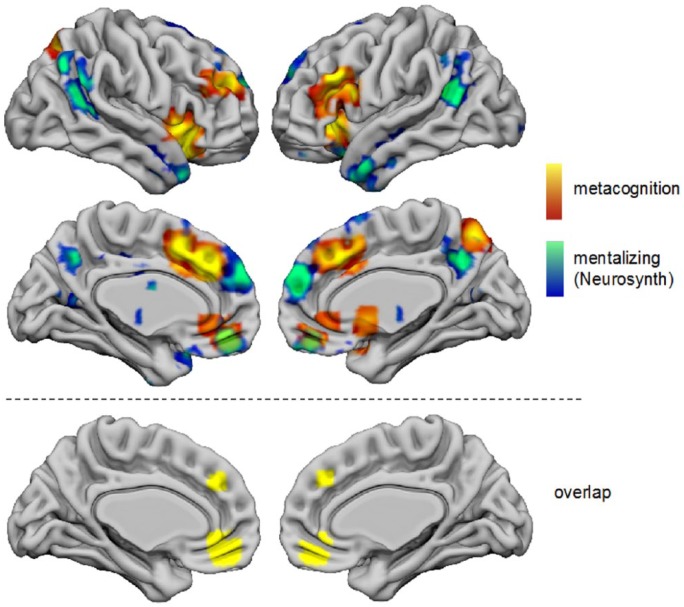
Finally, we examined a potential overlap between metacognition- and ToM-related activations by comparing our composite metacognition map (Figure 1) to a meta-analysis of ToM. ToM-related regions were obtained from the ‘reverse inference’ map for the term ‘mentalising’ in Neurosynth, which identifies regions that are preferentially associated with ToM over and above other terms in the database.
Figure 5 shows the two maps overlaid on the same cortical surface projection created using Surf Ice (https://www.nitrc.org/projects/surfice/). Activations for both metacognition and ToM were observed in mPFC and precuneus, with ToM activations tending to be anterior and ventral to metacognition-related activations. There was clear overlap between metacognition and ToM in vmPFC (cluster centre of mass (–3, 45, –12)) and a region of mid-dorsomedial PFC ((–4, 40, 34); Figure 5, bottom row). Unique activations for metacognition were observed in dorsolateral PFC, insula, and lateral parietal cortex; unique activations for ToM were observed in TPJ and temporal pole.
Figure 5.
ALE results for all studies on metacognition (from Figure 1, hot colours) as compared to the Neurosynth reverse inference map for the term ‘mentalising’ (cool colours). Clusters are displayed in a 3D rendering of MNI standard space.
Discussion
Deficits in metacognition – the ability to reflect on our cognitive processes – have clear and important consequences for functional capacity and quality of life, and are often found in psychiatric and neurological conditions (David et al., 2014; Koren et al., 2006). Metacognition has been considered a higher brain function that depends on the integrity of prefrontal and parietal association cortex (Shimamura, 2000) and that is particularly well-developed in humans compared to other animals (Metcalfe, 2008). However, the underlying neurocognitive architecture supporting metacognitive abilities remains poorly understood. By comparing the neural basis of metacognition across different judgement types (e.g. prospective versus retrospective judgements of performance) and tasks (e.g. decision-making and memory), we aimed to provide insight into the types of neurocognitive architecture (e.g. domain-general or domain-specific) that support human metacognition. In turn, we hope progress on this issue will aid in understanding the aetiology of metacognitive deficits.
Here, we present a first meta-analysis of the current neuroimaging literature on explicit metacognitive judgements of performance. We used quantitative ALE methods to synthesise findings from 47 structural or functional neuroimaging studies on metacognition, divided into categories based on domains (metamemory and metadecision), analysis type (judgement-related activity, parametric effect of confidence, and metacognitive sensitivity), and, for metamemory judgements, temporal focus (prospective and retrospective). We also compared our results on self-directed metacognition to those obtained in a previous meta-analysis of ToM, motivated by theoretical proposals that self-knowledge partly depends on co-opting machinery that originally evolved for mentalising about others (Carruthers, 2009, 2011; Fleming and Daw, 2017; Frith, 2012).
In a composite meta-analysis collapsing over both analysis type and domain, we found consistent involvement of a frontoparietal network. Previous reviews have highlighted the specific contribution of a network centred on medial/lateral aPFC in metacognition (Fleming and Dolan, 2014; Grimaldi et al., 2015; Metcalfe and Schwartz, 2015). We found evidence in line with this view, with metacognition-related activations in posterior mPFC, ventromedial PFC and bilateral aPFC/dorsolateral PFC. Notably, the lateral PFC activations were asymmetric: left lateral PFC was more posterior and corresponded closely to area 44d from the atlas of Neubert et al. (2014); the right lateral PFC cluster was more anterior, corresponding to Neubert et al.’s area 46. In addition to these prefrontal activations, we also observed the involvement of bilateral insula and dorsal precuneus. This is consistent with an emerging view that the parietal cortex, particularly precuneus, supports metacognition in concert with the PFC (Mccurdy et al., 2013; Simons et al., 2010). The insula, together with posterior mPFC has been implicated in error processing and error awareness (Bonini et al., 2014; Garavan et al., 2003; Ridderinkhof et al., 2004; Taylor et al., 2007; Ullsperger et al., 2010), and is a hub for interoception (Craig, 2009), thought to be a key modulator of, or input to, metacognitive appraisal (Allen et al., 2016; Stephan et al., 2016).
Subcomponents of metacognition
We next turn to the review results for each contrast type separately. Previous studies have drawn a distinction between activations tracking the requirement for a metacognitive judgement relative to a baseline or control condition and those parametrically tracking the level of the judgement (e.g. high vs low confidence). For judgement-related activity, we did not find any consistent regions across the studies, likely due to this analysis being underpowered, given the relatively fewer studies reporting results for this contrast. In contrast, regions parametrically tracking confidence level were widespread and highlight a similar network to that found in the composite analysis (posterior mPFC, bilateral insula, right dorsal precuneus, ventral striatum, left posterior dorsolateral PFC, and ventromedial PFC), suggesting parametric effects were a predominant driver of the overall pattern. Parametric effects of confidence in ventromedial PFC are consistent with recent findings that perigenual ACC tracks determinants of subjective confidence arising from multiple sources during perceptual decision-making (Bang and Fleming, 2018). We note, however, that parametric relationships with confidence in this meta-analysis may be due to a particular brain region tracking variables such as response time or stimulus difficulty that themselves covary with confidence, and we are unable to rule out the contribution of these covariates to these results.
A key aspect of metacognition is the extent to which judgements track objective performance, known as metacognitive sensitivity. Sensitivity is defined as the association between performance and confidence over multiple trials and is typically measured using individual-difference metrics such as area under the type 2 receiver operating characteristic curve (AUROC2) or meta-d’ (Fleming and Lau, 2014). Measures of metacognitive sensitivity are affected by task performance (Galvin et al., 2003; Maniscalco and Lau, 2012), making it important to control for differences in task performance either in the design of experiments (e.g. by using staircase procedures) or in analysis by computing metrics such as metacognitive efficiency (meta-d’/d’).
In the current meta-analysis, 9 of 11 studies reporting neural correlates of metacognitive sensitivity controlled for performance either in the design of the experiment or in data analysis. Unfortunately, this small sample was likely underpowered for the purposes of the current meta-analysis (Muller et al., 2018), and no significant clusters were observed after correction for multiple comparisons. However, at uncorrected thresholds, we observed involvement of a right aPFC region that was not observed in the parametric confidence meta-analysis (Figure S1). This pattern may indicate that the aPFC plays a role downstream of confidence formation – instead of monitoring performance, aPFC may contribute to updating a mapping between an internal feeling of confidence and the usage of confidence in communication or subsequent control of behaviour (Shekhar and Rahnev, 2018). However, further studies of the neural basis of metacognitive sensitivity (as opposed to confidence level per se) are required to test this hypothesis. The small number of studies reporting sensitivity analyses meant that we were also unable to establish potential domain-specific differences in the neural basis of metacognitive sensitivity, although recent studies have highlighted a specific contribution of precuneus to metamemory sensitivity (Baird et al., 2013, 2015; Mccurdy et al., 2013; Ye et al., 2018).
Comparing metamemory and metadecision
We observed common regions in separate analyses of metamemory and metadecision tasks, including insula, lateral PFC, and posterior mPFC, suggesting common inputs may drive judgements in both domains (Morales et al., 2018). This metamemory network is similar to that identified by White et al. (2014) in a meta-analysis of nine studies examining retrospective confidence judgements about memory. We also observed partially distinct networks engaged during metacognition of decision-making and memory tasks. Specific to the metamemory analysis were activations in left dorsolateral PFC and clusters in bilateral parahippocampal cortex, whereas specific to metadecision was the involvement of right anterior dorso lateral PFC.
Temporal focus of metamemory judgements
When separating metamemory judgements by temporal focus, retrospective metamemory activated bilateral parahippocampal cortex and left inferior frontal gyrus, whereas prospective metamemory activated posterior mPFC, left dorsolateral PFC, and right insula. Observing parahippocampal cortex activation for retrospective metamemory and PFC activation for prospective metamemory is consistent with elements of both direct access and inferential accounts of how metacognitive judgements about memory are formed (Metcalfe and Dunlosky, 2008). On one hand, fMRI activation and single-unit responses in the MTL have been linked not only to objective recognition performance (Kao et al., 2005) but also memory confidence (Rutishauser et al., 2015), and feelings of familiarity (Haskins et al., 2008; Henson et al., 2003; Montaldi et al., 2006), consistent with a first-order contribution of mnemonic representations to metacognitive judgement. In contrast, medial prefrontal activation covaries with JOLs independently of first-order performance (Kao et al., 2005) and PFC lesions impair JOL accuracy but not performance (Schnyer et al., 2004), potentially consistent with an inferential basis for prospective confidence.
Comparing metacognition and mentalising
An appealing model is that metacognition and ToM share a common computational basis that involves recursive inference about our own and others’ mental states. Neural processes supporting ToM are typically assessed by asking subjects to read stories that describe a character’s true or false beliefs while undergoing functional brain imaging (Saxe et al., 2006). These studies have led to the identification of a network encompassing dorsomedial PFC, TPJ, and precuneus as involved in ToM (Amodio and Frith, 2006; Frith and Frith, 1999; Molenberghs et al., 2016; Schurz et al., 2014). However, despite surface similarities in activation location (e.g. precuneus), up until recently, the overlap between large-scale brain networks involved in metacognition and ToM has remained unclear. A notable exception is a study by Valk et al. (2016), who analysed individual differences in cortical thickness and white matter anisotropy related to metacognitive sensitivity on perceptual and higher-order cognitive tasks. It was found that medial prefrontal regions, in which cortical thickness predicted metacognitive ability, overlapped with those from neuroimaging meta-analyses of mentalising.
Here, we assess overlap between our composite meta-analysis of metacognition-related activations and a meta-analysis of mentalising obtained from Neurosynth. While a similar midline network was engaged in both cases, there was in fact minimal overlap between the maps in posterior mPFC and precuneus; instead, metacognition engaged more dorsal and posterior regions. However, overlap was observed in ventromedial and anterior dorsomedial PFC. vmPFC has been specifically associated with self-reflective processing (D’Argembeau et al., 2007; Jenkins and Mitchell, 2011), and its role in ToM tasks is thought to support a simulation of what oneself would do in another’s situation (Jenkins et al., 2008). Intriguingly, in contrast, anterior dorsomedial PFC has been suggested to support second-order representations of mental states, irrespective of whether they originate from self or other (Nicolle et al., 2012; Yoshida et al., 2010). Unique activations for metacognition were observed in insula and lateral PFC, perhaps reflecting the specific contribution of interoception/error monitoring and the formation of confidence estimates, respectively, during self-directed judgements. ToM, in contrast, was uniquely associated with activations in TPJ and temporal pole, consistent with previous findings that these regions are biased towards other-referential processing (Saxe et al., 2006).
These data may tentatively speak to the difference between conceptual and non-conceptual forms of metacognition (Arango-Muñoz, 2011; Proust, 2013). It is plausible that a subset of the unique regions associated with metacognition here (e.g. posterior mPFC and insula) mediate non-conceptual, lower-level epistemic feelings of uncertainty. This perspective is consistent with these regions being found in our parametric confidence meta-analysis activations (Figure 2). In contrast, mPFC may support a conceptual second-order representation of one’s own mental states (conscious elaboration of epistemic feelings) and, in doing so, co-opt similar neural machinery to that engaged when reflecting on or inferring the mental states of others (Carruthers, 2009, 2011; Lombardo et al., 2010). A strong test of this hypothesis would be to compare neural correlates of explicit and implicit measures of self-directed metacognition (Logan and Crump, 2010) with activity engaged when mentalising about others. We would predict that only variation in explicit metacognitive judgements would share commonalities with ToM. However, it is also likely that differences in content between typical ToM and metacognition studies may drive the differences observed here (e.g. judging another person’s emotions or social intentions vs judging one’s own cognitive or decision processes). Further within-subject studies are needed with matched task domain/stimulus materials to draw strong conclusions about the relation between the neural substrates of self- and other-directed metacognition.
Implications of domain-specific differences in metacognition for neuropsychiatry
One implication of domain-specific neural correlates of metacognition is that damage or disorder affecting these regions may help explain the various types of introspective deficits observed in neuropsychiatry (David et al., 2014). The level of insight into one’s symptoms in schizophrenia have been linked to metacognitive ability over and above differences in executive function (Gilleen et al., 2016), and previous studies of lack of insight have highlighted similar regions to those identified here, including mPFC (van der Meer et al., 2013), insula (Spalletta et al., 2014), inferior frontal gyrus (Orfei et al., 2012), and dorsolateral PFC (Shad, 2004). Individuals with addictions, and those in remission from addiction, have been found to have deficits in metacognition which were predicted by loss of structural integrity in mPFC (Moeller et al., 2016). Furthermore, change in mPFC function has been found to predict the severity and prognosis of addictions (Moeller and Goldstein, 2014).
Neurodegenerative disorders have also been known to bring about progressive anosognosia, or symptom unawareness (García-Cordero et al., 2016). Specifically, Alzheimer’s disease has been associated with metamemory deficits independent of memory deficits, with various studies finding both better and worse insight relative to performance (McGlynn and Kaszniak, 1991; Moulin et al., 2003; Souchay, 2007). The parahippocampal cortex, found in our meta-analysis of retrospective metamemory, is known to be one of the earliest affected regions in the typical progression of Alzheimer’s, potentially consistent with behavioural observations of metamemory deficits (Cosentino, 2014; Didic et al., 2011).
Analysis limitations
Our study represents a first attempt to consolidate and synthesise findings in the neuroimaging literature on metacognitive judgements and is accompanied by several limitations. First, coordinate-based meta-analyses inevitably sacrifice experimental control to allow aggregating over studies. We were unable to balance the types of tasks (e.g. visual and semantic) used most frequently in different domains, and such imbalances may affect our results. For instance, metadecision studies are typically conducted using visual perceptual tasks, which may bias our results towards this modality. Related to this, most studies in our corpus only examined one particular domain. Because of this, and prominent differences between metamemory and metadecision tasks (i.e. stimulus type), it is not possible to estimate the extent to which differences between domains are related to differences in task. Inferences on parameteric confidence level–related activations are also limited by not incorporating the directionality (e.g. high >low confidence) of the contrast. Second, our analyses collapse across many different judgement types (e.g. FOKs, confidence ratings, and JOLs) that may affect our results if each judgement relies on different processes (Chua et al., 2009; Leonesio and Nelson, 1990; Metcalfe and Dunlosky, 2008). Notably, in metadecision, all judgements were retrospective, so we are unable to assess whether temporality may differentiate metacognitive judgements more generally, or only within metamemory judgements. Finally, all contrasts in our study were univariate, whereas domain-specific differences in confidence-related activation have recently been associated with multivariate patterns of activation in PFC (Morales et al., 2018).
Conclusion
Despite metacognition occupying a central role in human cognition, the relevant neurocognitive architecture has remained underdetermined, partly due to study-specific differences in both domain and type of metacognitive judgement under study. We used quantitative ALE methods to synthesise findings from 47 neuroimaging studies on metacognition, divided into categories based on the target of metacognitive evaluation (memory and decision-making), analysis type, and, for metamemory judgements, temporal focus (prospective and retrospective). We find engagement of mPFC and lateral PFC, precuneus, and insula in tracking the level of confidence in self-performance of both decision-making and memory tasks, suggesting domain-general contributions to metacognitive judgements. We find, however, preferential engagement of parahippocampal cortex in metamemory experiments and right anterior dorsolateral PFC in metadecision experiments. Finally, by comparing our results to comparable analyses of mentalising, we obtain evidence for common engagement of the ventromedial and anterior dorsomedial PFC in metacognition and mentalising, suggesting that these regions may support second-order representations for thinking about the thoughts of oneself and others.
Supplemental Material
Supplemental material, VaccaroFleming_SupplementaryMaterials for Thinking about thinking: A coordinate-based meta-analysis of neuroimaging studies of metacognitive judgements by Anthony G. Vaccaro and Stephen M. Fleming in Brain and Neuroscience Advances
Acknowledgments
The authors thank Marion Rouault for comments on an earlier draft of this manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The Wellcome Centre for Human Neuroimaging is supported by core funding from the Wellcome Trust (grant no. 203147/Z/16/Z). S.M.F. is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and Royal Society (grant no. 206648/Z/17/Z).
ORCID iD: Stephen M. Fleming  https://orcid.org/0000-0003-0233-4891
https://orcid.org/0000-0003-0233-4891
References
- Ais J, Zylberberg A, Barttfeld P, et al. (2016) Individual consistency in the accuracy and distribution of confidence judgments. Cognition 146: 377–386. [DOI] [PubMed] [Google Scholar]
- Allen M, Frank D, Schwarzkopf DS, et al. (2016) Unexpected arousal modulates the influence of sensory noise on confidence. Elife 5: e18103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. (2006) Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience 7(4): 268–277. [DOI] [PubMed] [Google Scholar]
- Arango-Muñoz S. (2011) Two levels of metacognition. Philosophia 39(1): 71–82. [Google Scholar]
- Baird B, Cieslak M, Smallwood J, et al. (2015) Regional white matter variation associated with domain-specific metacognitive accuracy. Journal of Cognitive Neuroscience 27(3): 440–452. [DOI] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Gorgolewski KJ, et al. (2013) Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. Journal of Neuroscience 33(42): 16657–16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang D, Fleming SM. (2018) Distinct encoding of decision confidence in human medial prefrontal cortex. Proceedings of the National Academy of Sciences 115(23): 6082–6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini F, Burle B, Liegeois-Chauvel C, et al. (2014) Action monitoring and medial frontal cortex: Leading role of supplementary motor area. Science 343(6173): 888–891. [DOI] [PubMed] [Google Scholar]
- Carruthers P. (2009) How we know our own minds: The relationship between mindreading and metacognition. Behavioral and Brain Sciences 32(2): 121–138. [DOI] [PubMed] [Google Scholar]
- Carruthers P. (2011) The Opacity of Mind: An Integrative Theory of Self-Knowledge. New York: Oxford University Press. [Google Scholar]
- Chiou K, Carlson RA, Arnett P, et al. (2011) Metacognitive monitoring in moderate and severe traumatic brain injury. Journal of the International Neuropsychological Society 17(4): 1–12. [DOI] [PubMed] [Google Scholar]
- Chua EF, Pergolizzi D, Weintraub RR. (2014) The cognitive neuroscience of metamemory monitoring: Understanding metamemory processes, subjective levels expressed, and metacognitive accuracy. In Fleming SM, Frith CD. (eds) The Cognitive Neuroscience of Metacognition. Berlin; Heidelberg: Springer, pp. 267–291. [Google Scholar]
- Chua EF, Schacter DL, Sperling RA. (2009) Neural correlates of metamemory: A comparison of feeling-of-knowing and retrospective confidence judgments. Journal of Cognitive Neuroscience 21(9): 1751–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S. (2014) Metacognition in Alzheimer’s disease. In Fleming SM, Frith CD. (eds) The Cognitive Neuroscience of Metacognition. Berlin; Heidelberg: Springer, pp. 389–407. [Google Scholar]
- Craig AD. (2009) How do you feel – Now? The anterior insula and human awareness. Nature Reviews Neuroscience 10(1): 59–70. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, et al. (2007) Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience 19(6): 935–944. [DOI] [PubMed] [Google Scholar]
- David AS, Bedford N, Wiffen B, et al. (2014) Failures of metacognition and lack of insight in neuropsychiatric disorders. In Fleming SM, Frith CD. (eds) The Cognitive Neuroscience of Metacognition. Berlin; Heidelberg: Springer, pp. 345–366. [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. (1994) Localization of a neural system for error detection and compensation. Psychological Science 5(5): 303–305. [Google Scholar]
- Didic M, Barbeau EJ, Felician O, et al. (2011) Which memory system is impaired first in Alzheimer’s disease? Journal of Alzheimer’s Disease 27(1): 11–22. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, et al. (2012) Activation likelihood estimation meta-analysis revisited. Neuroimage 59(3): 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, et al. (2009) Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping 30(9): 2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre N, Filevich E, Solovey G, et al. (2018) Behavioural, modeling, and electrophysiological evidence for supramodality in human metacognition. The Journal of Neuroscience 38(2): 263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LM, Arvaneh M, Dockree PM. (2017) Domain-specific and domain-general processes underlying metacognitive judgments. Consciousness and Cognition 49: 264–277. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Daw ND. (2017) Self-evaluation of decision-making: A general Bayesian framework for metacognitive computation. Psychological Review 124(1): 91–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Dolan RJ. (2014) The neural basis of metacognitive ability. In: Fleming SM, Frith CD. (eds) The Cognitive Neuroscience of Metacognition. Berlin; Heidelberg: Springer, pp. 245–266. [Google Scholar]
- Fleming SM, Frith CD. (2014) The Cognitive Neuroscience of Metacognition. Berlin; Heidelberg: Springer. [Google Scholar]
- Fleming SM, Lau HC. (2014) How to measure metacognition. Frontiers in Human Neuroscience 8: 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Ryu J, Golfinos JG, Blackmon KE. (2014) Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. Brain 137(10): 2811–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. (2012) The role of metacognition in human social interactions. Philosophical Transactions of the Royal Society B: Biological Sciences 367(1599): 2213–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. (1999) Interacting minds a biological basis. Science 286(5445): 1692–1695. [DOI] [PubMed] [Google Scholar]
- Galvin SJ, Podd JV, Drga V, et al. (2003) Type 2 tasks in the theory of signal detectability: Discrimination between correct and incorrect decisions. Psychonomic Bulletin & Review 10(4): 843–876. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross T, Kaufman J, et al. (2003) A midline dissociation between error-processing and response-conflict monitoring. Neuroimage 20(2): 1132–1139. [DOI] [PubMed] [Google Scholar]
- García-Cordero I, Sedeño L, Fuente LD, et al. (2016) Feeling, learning from and being aware of inner states: Interoceptive dimensions in neurodegeneration and stroke. Philosophical Transactions of the Royal Society B: Biological Sciences 371(1708): 20160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Manassei MF, Hamilton-Fletcher G, et al. (2016) Interoceptive dimensions across cardiac and respiratory axes. Philosophical Transactions of the Royal Society B: Biological Sciences 371(1708): 20160014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison J, Erdeniz B, Done J. (2013) Prediction error in reinforcement learning: A meta-analysis of neuroimaging studies. Neuroscience and Biobehavioral Reviews 37(7): 1297–1310. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, et al. (1993) A neural system for error detection and compensation. Psychological Science 4(6): 385–390. [Google Scholar]
- Gilleen J, David A, Greenwood K. (2016) Self-reflection and set-shifting mediate awareness in cognitively preserved schizophrenia patients. Cognitive Neuropsychiatry 21(3): 185–196. [DOI] [PubMed] [Google Scholar]
- Grimaldi P, Lau H, Basso MA. (2015) There are things that we know that we know, and there are things that we do not know we do not know: Confidence in decision-making. Neuroscience & Biobehavioural Reviews 55: 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins AL, Yonelinas AP, Quamme JR, et al. (2008) Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron 59(4): 554–560. [DOI] [PubMed] [Google Scholar]
- Hembacher E, Ghetti S. (2014) Don’t look at my answer. Psychological Science 25(9): 1768–1776. [DOI] [PubMed] [Google Scholar]
- Henson R, Cansino S, Herron J, et al. (2003) A familiarity signal in human anterior medial temporal cortex? Hippocampus 13(2): 301–304. [DOI] [PubMed] [Google Scholar]
- Hester R, Foxe JJ, Molholm S, et al. (2005) Neural mechanisms involved in error processing: A comparison of errors made with and without awareness. Neuroimage 27(3): 602–608. [DOI] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. (2009) Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology 34(11): 2450–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. (1989) Memory and metamemory: Comparisons between patients with frontal lobe lesions and amnesic patients. Psychobiology 17(1): 3–11. [Google Scholar]
- Jenkins AC, Mitchell JP. (2011) Medial prefrontal cortex subserves diverse forms of self-reflection. Social Neuroscience 6(3): 211–218. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Macrae CN, Mitchell JP. (2008) Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proceedings of the National Academy of Sciences of the United States of America 105(11): 4507–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, et al. (2012) FSL. NeuroImage 62(2): 782–790. [DOI] [PubMed] [Google Scholar]
- Kao Y, Davis ES, Gabrieli JD. (2005) Neural correlates of actual and predicted memory formation. Nature Neuroscience 8(12): 1776–1783. [DOI] [PubMed] [Google Scholar]
- Kelemen WL, Frost PJ, Weaver CA. (2000) Individual differences in metacognition: Evidence against a general metacognitive ability. Memory & Cognition 28(1): 92–107. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N. and Zariwala Mainen (2008) Neural correlates, computation and behavioural impact of decision confidence. Nature 455(7210): 227–231. [DOI] [PubMed] [Google Scholar]
- Kiani R, Shadlen MN. (2009) Representation of confidence associated with a decision by neurons in the parietal cortex. Science 324(5928): 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren D, Seidman LJ, Goldsmith M, Harvey PD. (2006) Real-world cognitive - and metacognitive - dysfunction in schizophrenia: a new approach for measuring (and remediating) more “right stuff”. Schizophrenia Bulletin 32(2): 310–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonesio RJ, Nelson TO. (1990) Do different metamemory judgments tap the same underlying aspects of memory? Journal of Experimental Psychology: Learning, Memory, and Cognition 16(3): 464–467. [DOI] [PubMed] [Google Scholar]
- Lockl K, Schneider W. (2007) Knowledge about the mind: Links between theory of mind and later metamemory. Child Development 78(1): 148–167. [DOI] [PubMed] [Google Scholar]
- Logan GD, Crump MJC. (2010) Cognitive illusions of authorship reveal hierarchical error detection in skilled typists. Science 330(6004): 683–686. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, et al. (2010) Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience 22(7): 1623–1635. [DOI] [PubMed] [Google Scholar]
- Mccurdy LY, Maniscalco B, Metcalfe J, et al. (2013) Anatomical coupling between distinct metacognitive systems for memory and visual perception. Journal of Neuroscience 33(5): 1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn SM, Kaszniak AW. (1991) When metacognition fails: Impaired awareness of deficit in Alzheimer’s disease. Journal of Cognitive Neuroscience 3(2): 183–187. [DOI] [PubMed] [Google Scholar]
- Maniscalco B, Lau H. (2012) A signal detection theoretic approach for estimating metacognitive sensitivity from confidence ratings. Consciousness and Cognition 21(1): 422–430. [DOI] [PubMed] [Google Scholar]
- Metcalfe J. (2008) Evolution of metacognition. In: Dunlosky J, Bjork R. (eds) Handbook of Metamemory and Memory. London: Psychology Press, pp. 27–46. [Google Scholar]
- Metcalfe J, Dunlosky J. (2008) Metamemory. In: Roediger HL III. (eds) Learning and Memory: A Comprehensive Reference. Oxford: Elsevier, pp. 349–362. [Google Scholar]
- Metcalfe J, Schwartz B. (2015) The ghost in the machine: Self-reflective consciousness and the neuroscience of metacognition. In: Dunlosky J, Tauber S. (eds) The Oxford Handbook of Metacognition. Oxford: Oxford University Press, pp. 407–424. [Google Scholar]
- Metcalfe J, Shimamura AP. (1996) Metacognition: Knowing about Knowing. Cambridge, MA: The MIT Press. [Google Scholar]
- Miyamoto K, Setsuie R, Osada T, et al. (2018) Reversible silencing of the frontopolar cortex selectively impairs metacognitive judgment on non-experience in primates. Neuron 97(4): 980–989 e6. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Goldstein RZ. (2014) Impaired self-awareness in human addiction: Deficient attribution of personal relevance. Trends in Cognitive Sciences 18(12): 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Fleming SM, Gan G, et al. (2016) Metacognitive impairment in active cocaine use disorder is associated with individual differences in brain structure. European Neuropsychopharmacology 26(4): 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Johnson H, Henry JD, et al. (2016) Understanding the minds of others: A neuroimaging meta-analysis. Neuroscience and Biobehavioral Reviews 65: 276–291. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, et al. (2006) The neural system that mediates familiarity memory. Hippocampus 16(5): 504–520. [DOI] [PubMed] [Google Scholar]
- Morales J, Lau H, Fleming SM. (2018) Domain-specific patterns of activity support metacognition in human prefrontal cortex. Journal of Neuroscience 38(14): 3534–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin CJ, James N, Perfect TJ, et al. (2003) Knowing what you cannot recognise: Further evidence for intact metacognition in Alzheimer’s disease. Aging, Neuropsychology, and Cognition 10(1): 74–82. [Google Scholar]
- Muller VI, Cieslik EC, Laird AR, et al. (2018) Ten simple rules for neuroimaging meta-analysis. Neuroscience and Biobehavioral Reviews 84: 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert F, Mars R, Thomas A, et al. (2014) Comparison of human ventral frontal cortex areas for cognitive control and language with areas in monkey frontal cortex. Neuron 81(3): 700–713. [DOI] [PubMed] [Google Scholar]
- Neubert F, Mars RB, Sallet J, et al. (2015) Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proceedings of the National Academy of Sciences of the United States of America 112(20): E2695–E2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle A, Klein-Flugge MC, Hunt LT, et al. (2012) An agent independent axis for executed and modeled choice in medial prefrontal cortex. Neuron 75(6): 1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfei MD, Piras F, Macci E, et al. (2012) The neuroanatomical correlates of cognitive insight in schizophrenia. Social Cognitive and Affective Neuroscience 8(4): 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannu JK, Kaszniak AW, Rapcsak SZ. (2005) Metamemory for faces following frontal lobe damage. Journal of the International Neuropsychological Society 11(6): 668–676. [DOI] [PubMed] [Google Scholar]
- Pollack C, Ashby NC. (2018) Where arithmetic and phonology meet: The meta-analytic convergence of arithmetic and phonological processing in the brain. Developmental Cognitive Neuroscience 30: 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust J. (2013) The Philosophy of Metacognition. Oxford: Oxford University Press. [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, et al. (2004) The role of the medial frontal cortex in cognitive control. Science 306(5695): 443–447. [DOI] [PubMed] [Google Scholar]
- Rouault M, McWilliams A, Allen MG, et al. (in press) Human metacognition across domains: Insights from individual differences and neuroimaging. Personality Neuroscience 1: E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Ye S, Koroma M, et al. (2015) Representation of retrieval confidence by single neurons in the human medial temporal lobe. Nature Neuroscience 18(7): 1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, et al. (2013) The organization of dorsal frontal cortex in humans and macaques. Journal of Neuroscience 33(30): 12255–12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, et al. (2006) Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive and Affective Neuroscience 1(3): 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, et al. (2012) Introspective minds: Using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS ONE 7(2): e30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. (2004) Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage 22(2): 941–947. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Verfaellie M, Alexander MP, et al. (2004) A role for right medial prefrontal cortex in accurate feeling-of-knowing judgments: Evidence from patients with lesions to frontal cortex. Neuropsychologia 42(7): 957–966. [DOI] [PubMed] [Google Scholar]
- Schraw G. (1996) The effect of generalized metacognitive knowledge on test performance and confidence judgments. The Journal of Experimental Education 65(2): 135–146. [Google Scholar]
- Schurz M, Radua J, Aichhorn M, et al. (2014) Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neuroscience and Biobehavioral Reviews 42: 9–34. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Diaz F. (2014) Quantifying human metacognition for the neurosciences. In: Fleming SM, Frith CD. (eds) The Cognitive Neuroscience of Metacognition. Berlin; Heidelberg: Springer, pp. 9–23. [Google Scholar]
- Shad M. (2004) Insight and prefrontal cortex in first-episode schizophrenia. Neuroimage 22(3): 1315–1320. [DOI] [PubMed] [Google Scholar]
- Shekhar M, Rahnev D. (2018) Distinguishing the roles of dorsolateral and anterior PFC in visual metacognition. Journal of Neuroscience 38(22): 5078–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP. (2000) Toward a cognitive neuroscience of metacognition. Consciousness and Cognition 9(2): 313–323. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. (1986) Memory and metamemory: A study of the feeling-of-knowing phenomenon in amnesic patients. Journal of Experimental Psychology: Learning, Memory, and Cognition 12(3): 452–460. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Mazuz YS, et al. (2010) Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cereb Cortex 20(2): 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski HM, Fias W, Ononye CB, et al. (2017) Are numbers grounded in a general magnitude processing system? A functional neuroimaging meta-analysis. Neuropsychologia 105: 50–69. [DOI] [PubMed] [Google Scholar]
- Song C, Kanai R, Fleming SM, et al. (2011) Relating inter-individual differences in metacognitive performance on different perceptual tasks. Consciousness and Cognition 20(4): 1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchay C. (2007) Metamemory in Alzheimer’s disease. Cortex 43(7): 987–1003. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Piras F, Piras F, et al. (2014) The structural neuroanatomy of metacognitive insight in schizophrenia and its psychopathological and neuropsychological correlates. Human Brain Mapping 35(9): 4729–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Manjaly ZM, Mathys CD, et al. (2016) Allostatic self-efficacy: A metacognitive theory of dyshomeostasis-induced fatigue and depression. Frontiers in Human Neuroscience 10: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. (2007) Neural systems for error monitoring. The Neuroscientist 13(2): 160–172. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, et al. (2012) Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping 33(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, et al. (2010) Conscious perception of errors and its relation to the anterior insula. Brain Structure & Function 214(5–6): 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk SL, Bernhardt BC, Böckler A, et al. (2016) Substrates of metacognition on perception and metacognition on higher-order cognition relate to different subsystems of the mentalizing network. Human Brain Mapping 37(10): 3388–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, de Vos AE, Stiekema AP, Pijnenborg GH, van Tol MJ, Nolen WA, David AS, Aleman A. (2013) Insight in schizophrenia: involvement of self-reflection networks? Schizophrenia Bulletin 39(6): 1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TP, Engen NH, Sørensen S, et al. (2014) Uncertainty and confidence from the triple-network perspective: Voxel-based meta-analyses. Brain and Cognition 85: 191–200. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, et al. (2011) Large-scale automated synthesis of human functional neuroimaging data. Nature Methods 8(8): 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Zou F, Lau H, Hu Y, Kwok SC. (2018) Causal evidence for mnemonic metacognition in human precuneus. Journal of Neuroscience 38(28): 6379–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida W, Seymour B, Friston KJ, et al. (2010) Neural mechanisms of belief inference during cooperative games. The Journal of Neuroscience 30(32): 10744–10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, VaccaroFleming_SupplementaryMaterials for Thinking about thinking: A coordinate-based meta-analysis of neuroimaging studies of metacognitive judgements by Anthony G. Vaccaro and Stephen M. Fleming in Brain and Neuroscience Advances