Abstract
Background:
The Gorham–Stout syndrome (GSS), also known as phantom bone disease, is a rare bone condition of unknown etiology. Involvement of the spine is described in <50 cases in the literature. Here, we report a case of thoracic spine fracture dislocation in a young female who was known to have GSS.
Case Description:
A 23-year-old female developed a left spontaneous hemothorax 10 years previously along with left ribs lytic lessions. Pleural and rib biopsies diagnosed angiomatosis and the clinical diagnosis of GSS was established. Following a minor trauma, she presented with a Frankel B deficit attributed to a T3-T4 fracture dislocation with severe spinal cord compression. The patient underwent halo traction with CT. Following reduction, decompression and C5 to T8 with instrumented fusion (posterior only), she neurologically improved to Frankel D, 2 years postoperatively.
Conclusion:
Although GSS is a rare condition in the spine, it may lead to gross instability and catastrophic vertebral fracture/dislocation with paraparesis. Acute spinal cord decompression with stabilization may be warranted to achieve neurological improvement.
Keywords: Gorham, Gorham–Stout, spinal surgery, spine deformity, spine fracture, spine surgery
INTRODUCTION
The Gorham–Stout syndrome (GSS), also known as phantom bone disease, is a rare bone condition of unknown etiology. Histologically, is a non-neoplastic syndrome involving endothelial vascular proliferation inside the bones leading to focal bone resorption, fibrosis substitution, and fracture. Although it can occur in any part of the skeleton, it is most common in shoulder, pelvic ring, and mandible.
Involvement of the spine with GSS is described in <50 cases in the literature. Here, we diagnosed a T3-T4 fracture dislocation in a young female with the prior well-established diagnosis of GSS.
CASE REPORT
A 23-year-old female, 10 years previously, developed a spontaneous left sided hemothorax. She was also found to have left rib lytic lessions.
Pleural and rib biopsies led to the diagnosis of GSS.
She received radiotherapy treatment focused on the left hemithorax. At age 18, she had a car accident and an upper thoracic kyphosis of 50 degrees without dislocation was diagnosed. Her neurological examen revealed a Frankel D score, with mild weakness and numbness on her left leg. She refused surgery at that time.
Eighteen months later, she presented with a paraparesis (Frankel B), due to a spontaneous (no trauma) T3-T4 fracture dislocation resulting in severe spinal cord compression [Figure 1].
Figure 1.
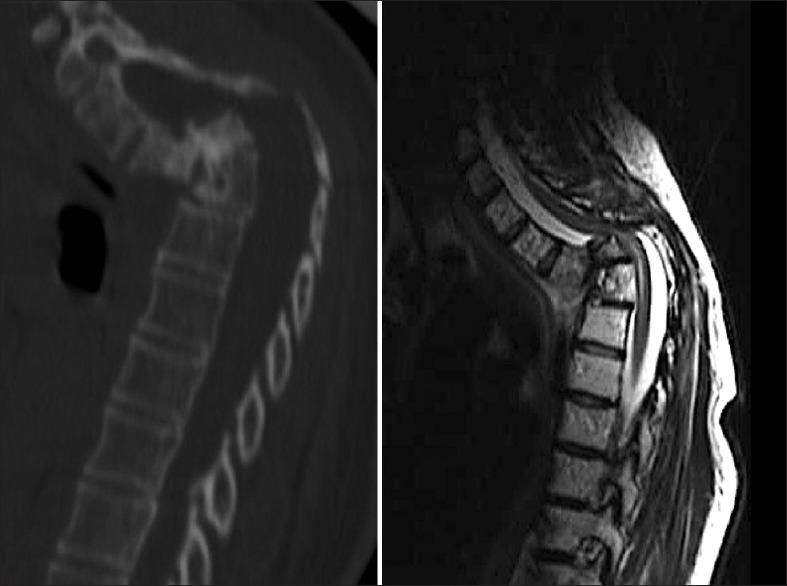
Preoperative CT and MRI where thoracic spine luxation can be observed. Note the severe spinal cord compression and the kyphotic deformity
With halo traction, she underwent reduction, decompression, and C5 to T8 instrumented fusion [Figures 2 and 3].
Figure 2.
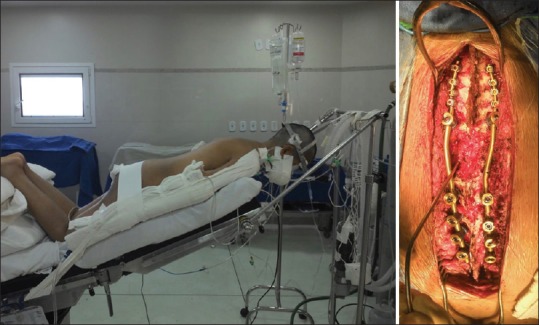
Intraoperative halo traction to get correction and the final construct obtained
Figure 3.
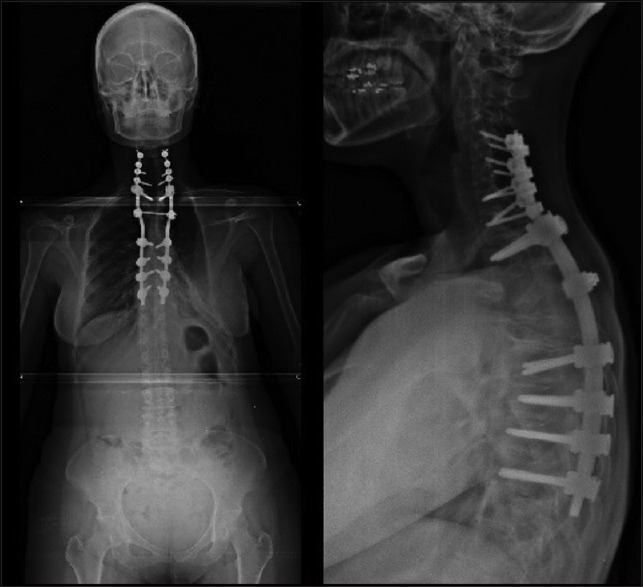
Postoperative X-rays with reduction and sagittal and coronal balance achieved
Postoperatively, the patient improved to Frankel D, ambulating 2 years after surgery with only mild dysfunction.
DISCUSSION
GSS is a rare, idiopathic condition. It was first described by Jackson et al. in 1838; they reported a young man with severe humerus osteolisis. In 1955, Gorham and Stout defined the histopathological criteria for GSS, based on clinical experience, and a literature review.[2,3,4,9]
Typical features of GSS include endothelial proliferation inside bone blood vessels that lead to fibrous tissue replacement of some of the bone matrix. Clinical symptoms depend on localization, but usually includes pain, sweating, and osteolysis on X-ray studies. This may lead to thoracic cage and rib cage instability and some cases leading to spontaneous chilotorax.[1,2]
Other problems may include respiratory insufficiency, with a survival rate <40%.[9]
The most frequent regions affected include the shoulder, pelvis, and mandible, whereas spine involvement is rare (e.g., 10%) with about 30 cases reported.[8,9]
Patients are typically under the age of 40, and this equally impacts males and females. X-ray, MRI, and CT findings typically demonstrate fracture, but only anatomopathology can confirm the diagnosis.[2]
Differential diagnosis include multicentric idiopatic osteolisis (ussually carpal and tarsal involvement), neurogenic osteolisis, Joseph-osteolisis, Shinz-osteolisis, Hajdu-osteolisis, Farber disease, and Winchester syndrome.[8,9]
Treatment typically involves surgery to stabilize the GSS resultant fractures. Other therapies have included radiotherapy and pharmacological treatments (e.g., anti-osteoclasis, alfa-2b interferón, zoledronic acid, etc.)[5,6,7]
When fusions are performed, prostheses should be used instead of bone grafts to avoid recurrent bony resorption.[9]
CONCLUSION
Although GSS is a rare condition in spine, it may lead to gross instability (e.g., acute fracture/dislocation) and catastrophic neurological deterioration. Early decompression and fusion may be warranted to promote neurological recovery.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Alexandre Jaccard, Email: jaccard.alexandre@gmail.com.
César Macedo, Email: medmacedo@gmail.com.
Gabriel Castro, Email: gbrcastro@gmail.com.
Alfredo Guiroy, Email: alfreguiroy@gmail.com.
REFERENCES
- 1.Aizawa T, Sato T, Kokubun S. Gorham disease of the spine: A case report and treatment strategies for this enigmatic bone disease. Tohoku J Exp Med. 2005;205:187–96. doi: 10.1620/tjem.205.187. [DOI] [PubMed] [Google Scholar]
- 2.Chong Ng L, Sell P. Gorham disease of the cervical spine-A case report and review of the literature. Spine (Phila Pa 1976) 2003;28:E355–8. doi: 10.1097/01.BRS.0000084557.38858.85. [DOI] [PubMed] [Google Scholar]
- 3.Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37:985–1004. [PubMed] [Google Scholar]
- 4.Gorham LW, Wright AW, Schultz HH, Maxon FC. Disappearing bones: A rare form of massive osteolysis. Am J Med. 1954;17:674–82. doi: 10.1016/0002-9343(54)90027-3. [DOI] [PubMed] [Google Scholar]
- 5.Heyd R, Micke O, Surholt C, Berger B, Martini C, Fuller J, et al. Radiation therapy for Gorham-Stout syndrome: Results of a national patterns of care study and literature review. Int J Radiat Oncol Biol Phys. 2011;81:e179–85. doi: 10.1016/j.ijrobp.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Kuriyama D, McElligot S, Glaser D, Thompson K. Treatment of Gorham-Stout disease with zoledronic acid and Interferon-a: A case report and literature review. J Pediatr Hematol Oncol. 2010;32:579–84. doi: 10.1097/MPH.0b013e3181edb464. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann G, Pfeil A, Bottcher J, Kaiser W, Fuller J, Hein G, et al. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham–Stout syndrome. Arch Orthop Trauma Surg. 2009;129:967–72. doi: 10.1007/s00402-008-0742-3. [DOI] [PubMed] [Google Scholar]
- 8.Maillot C, Cloche T, Le Huec JC. Thoracic osteotomy for Gorham-Stout disease of the spine: A case report and literature review. Eur Spine J. 2018;27:2285–90. doi: 10.1007/s00586-014-3613-3. [DOI] [PubMed] [Google Scholar]
- 9.Ruggieri P, Montalti M, Angelini A, Alberghini M, Mercuri M. Gorham-Stout disease: The experience of the Rizzoli Institute and review of the literature. Skeletal Radiol. 2011;40:1391–7. doi: 10.1007/s00256-010-1051-9. [DOI] [PubMed] [Google Scholar]


