Abstract
Background:
Germinal matrix-intraventricular hemorrhage (IVH) is the most common brain hemorrhage in preterm neonates. The importance of this hemorrhage lies in its severe complications. There is no definite treatment for IVH in neonates; therefore, the prevention of IVH should be considered. Some studies have shown that Vitamin E can probably decrease the risk of IVH and the other study has not shown its efficacy. The aim of this study is to evaluate the effects of Vitamin E on incidence and severity of IVH in preterm neonates.
Methods:
This study is a randomized clinical trial conducted on 76 neonates with gestational age of ≤30 weeks in the Isfahan University of Medical Science. The neonates were divided into two groups. The group one was administered with 10 units of Vitamin E for 3 days and the second group with placebo. In the 4th and 7th days after birth, brain sonography was conducted to evaluate IVH. The presence of sepsis, incidence of necrotizing enterocolitis, and hypotension were examined.
Results:
In this study, 76 neonates with the mean age of 28.49 ± 1.46 weeks participated. The incidence of hemorrhage in the 4th day was 26.3% in cases and 42.1% in controls with no significant difference (P = 0.3). The findings of the second sonography reported the incidence of IVH in 17.1% of cases and 36.8% of control group.
Conclusions:
Based on findings of the current study, Vitamin E use did not significantly decrease IVH in neonates. Further studies with larger sample size are needed.
Keywords: Intraventricular hemorrhage, preterm, Vitamin E
Introduction
Germinal matrix-intraventricular hemorrhage (IVH) is the most common brain hemorrhage in neonates, especially in preterm ones.[1] This hemorrhage can be associated with severe complications.[2] The incidence of IVH accounts for about 15%–25% of preterm neonates with birth weight of <1500 g.[3]
IVH in neonates can lead to posthemorrhagic ventricular dilation or periventricular hemorrhagic infarctions.[4]
The gold standard method for IVH diagnosis in neonates is sonography which is safe and accessible.[3]
There is no definitive treatment for IVH in neonates; therefore, this disease should be prevented as much as possible.[5] Methods of preventing IVH in neonates include prevention of preterm labor (PTL), natural birth delivery, administration of steroids before delivery, prevention of asphyxia, avoidance of rapid intravenous therapy, especially hypertonic fluids therapy and correction of acid-base state and coagulation.[1]
Vitamin E was evaluated in several studies as a mean to prevent IVH. The mechanism of any beneficial effect of Vitamin E may likely be related to the potent antioxidant properties of this vitamin. Presumably, Vitamin E operates as a free radical scavenger to protect matrix capillary endothelial cells from injury. Some studies have shown that Vitamin E can probably decrease IVH in neonates,[6] whereas other studies have given no significant role to Vitamin E.[7] In general, approach to Vitamin E use for IVH prevention is controversial.
A potential role for Vitamin E in neonates and lack of sufficient data on its benefit deserves this study to evaluate the effects of Vitamin E on incidence and severity of IVH in preterm neonates.
Methods
This study is a blinded randomized clinical-trial conducted on 76 neonates hospitalized in Al-Zahra Hospital (affiliated to Isfahan University of Medical Sciences) since June 2015.
This study was approved based on IRCT2015042521910N2 code from Iranian Registry of Clinical Trials.
An informed consent was obtained from patients parents.
Seventy-six neonates with 30-week gestational age or less were enrolled in this study. The inclusion criteria were prematurity with gestational age equal or <30 weeks, lack of any malformations and central nervous system disorder, one course of corticosteroids prescription for mother, no presence of chorioamnionitis, no need for mechanical ventilation, arterial oxygen saturation ≥90%, normal blood pressure, 5-min Apgar scores of ≥7, no presence of sepsis, abdominal distension, bile secretion, and IVH.
The neonates who needed sodium bicarbonate or resuscitation or mechanical ventilation in the first 3 days after birth were excluded from the study. Besides that, abdominal distension and bile secretion during the study were considered other exclusion criteria. The consort diagram of studied population is shown in Figure 1.
Figure 1.
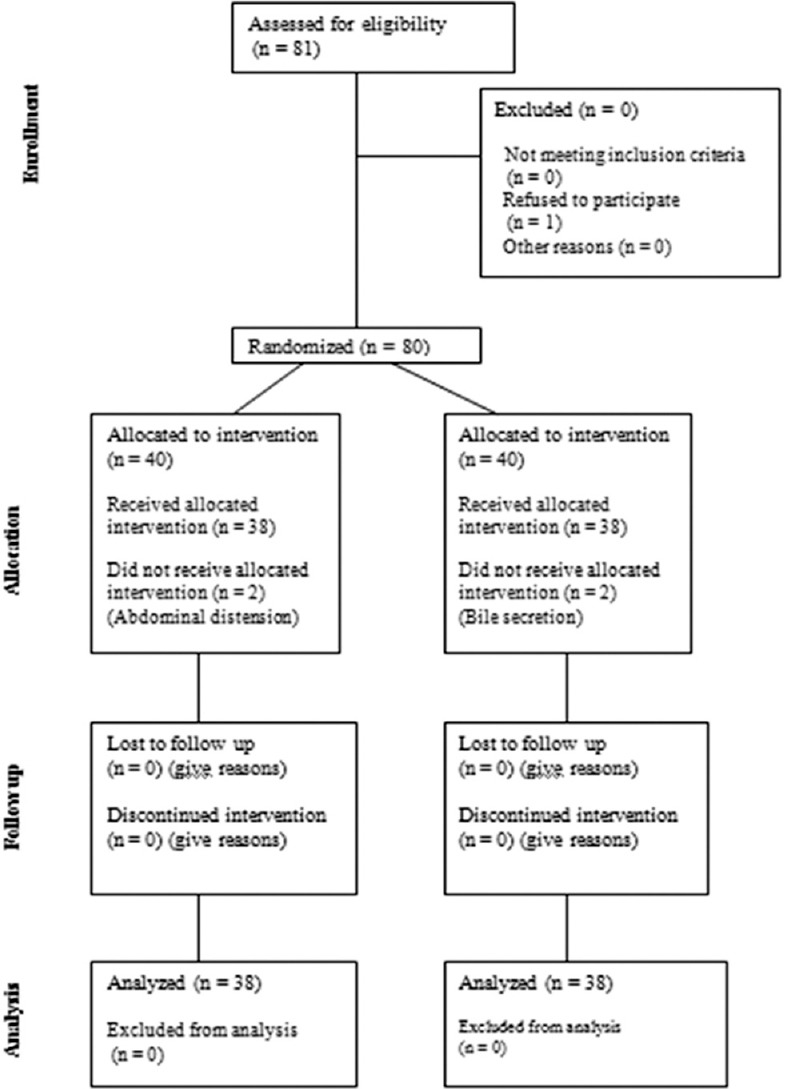
Consort diagram of studied population
The neonates were randomly divided into two groups matched for age and gender. Immediately after birth, all neonates underwent brain sonography to determine if they had IVH. For the cases group (consist of 38 newborns), 10 units of Vitamin E (1 cc of Vitamin E per a drop) were administered orally through orogastric tube for 3 days. In the control group, (consist of 38 newborns) 1cc distilled water was administered through the orogastric tube as placebo. In the 4th and 7th day after birth, brain sonography was conducted again to evaluate IVH in neonates. If hemorrhage was detected in sonographic findings, the severity of hemorrhage was evaluated. Hemorrhage Grades I and II were determined as mild hemorrhage and Grades III and IV as severe.
In this study, the presence of sepsis was evaluated during the study and defined in three groups including no sepsis, clinical sepsis, and positive blood culture. The incidence of necrotizing enterocolitis (NEC) was evaluated by clinical sign and abdominal X-ray and defined in three groups including no NEC, suspected NEC, and proven NEC. The blood pressure of neonates measured each 6 h in neonatal intensive care unit and in case of hypotension presence, it was reported. The cause of prematurity in neonates was evaluated by searching in patient medical records.
Data collection and analysis
All data on the neonates including gestational age, gender, weight, having hemorrhage or not, hemorrhage severity, blood pressure, and other complications were recorded in checklists. The descriptive data were reported by mean ± standard deviation. Comparison of the data was made by independent t-test and paired t-test. The Research Council and Ethics Committee of the School of Medicine approved the study protocol.
Results
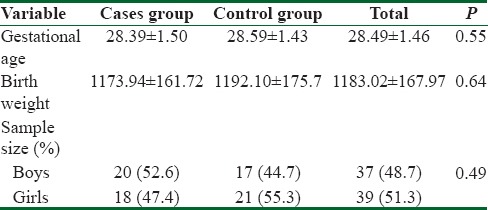
In this clinical trial, 76 neonates were participated (38 neonates in each group). Thirty-seven (48.7%) neonates were male and the rest (51.3%) were female. The mean age of neonates was 28.49 ± 1.46 weeks ranged from 24.3 to 30 weeks. The birth weight of neonates ranged 660–1400 g with mean weight 1183.02 ± 167.97 g. The demographic characteristics of the neonates in the two groups are shown in Table 1.
Table 1.
Demographic characteristics of two groups
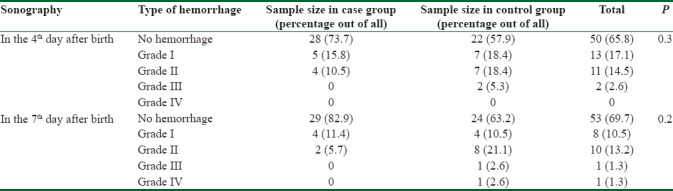
Brain sonography in 4th day after birth showed that in 50 (65.8%) neonates, there was no hemorrhage, 13 (17.1%) had Grade I hemorrhage, 11 (14.5%) Grade II hemorrhage, and only 2 (2.6%) Grade III hemorrhage. The incidence of hemorrhage was lower in the case group than in the controls (26.3% vs. 42.1%) with no significant difference. Brain sonography was repeated in the 7th day after birth to detect IVH in the neonates. There was no IVH in 53 (69.7%) neonates, 8 (10.5%) had hemorrhage Grade I, 10 (13.2%) Grade II, 1 (1.3%) Grade III, and only one (1.3%) Grade IV. The incidence of IVH was lower in the case group yet insignificantly (17.1% vs. 36.8%). All data about the incidence of IVH in 4th and 7th days of birth in the two groups are shown in Table 2.
Table 2.
Incidence and severity of intraventricular hemorrhage in two groups of neonates
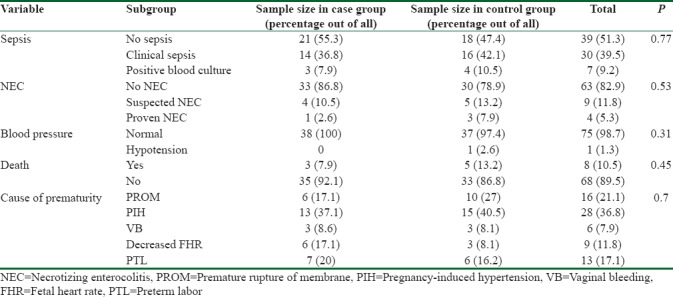
Clinical sepsis was seen in 30 (39.5%) neonates, 7 (9.2%) neonates had positive blood cultures that were indicative of sepsis, and 39 (51.3%) neonates had no signs/symptoms of sepsis. NEC was detected in 13 neonates (17.1%), 9 (11.8%), of whom were suspected for NEC, and 4 (5.3%) had definitely diagnosed NEC. Blood pressure measurement showed that 75 (98.7%) of neonates had normal blood pressure and hypotension was reported only in one (1.3%) neonate in the control group and all patients in the case group had normal blood pressure.
In this study, eight premature neonates died during the study. Mortality was insignificantly higher in the control group.
The causes of preterm labor were as following: premature rupture of membrane (21.1%), pregnancy-induced hypertension (36.8%), vaginal bleeding (7.9%), decreased fetal heart rate (11.8%), and PTL (17.1%).
The abovementioned characteristics are shown in Table 3.
Table 3.
Other clinical characteristics of the two groups of neonates
Discussion
Controversial opinions about Vitamin E administration for IVH prevention in premature neonates was the question that made us conduct the current study.
The mean gestational age in the two groups was approximately equal with no significant difference, suggesting that the case and control groups were matched for age. The difference in birth weight between the two groups was not statistically significant, showing that the two groups were matched for birth weight. In general, age and weight as possible confounding variables, were not significantly different between cases and controls.
Brain sonography was done in the 4th day after birth to detect IVH. The incidence of IVH was higher in the control group administered with placebo, suggesting that Vitamin E had protective effects on IVH in newborns, but the decrease was not statistically significant. Sonography was repeated on the 7th day after birth. The findings were similar to those of the first sonography and the incidence of IVH was insignificantly higher in control group. In case group, no newborn had Grade III IVH in first sonography whereas 2 newborns in control group experienced Grade III IVH although there was no significant difference between groups. One newborn of control group experienced Grade IV of IVH in the second sonography. Our study was not accompanied with significant decrease in incidence of IVH between two groups, but it was shown that IVH severity decreased in case group though it was not statistically significant.
These findings are consistent with other findings reported by other studies. The study of Chiswick et al. on 44 very low birthweight (≤1500 g) newborns showed that intramuscular injection of 20 mg/kg Vitamin E in the first 12 h after birth did not significantly decrease the incidence of IVH but decreased incidence of severe IVH significantly.[7]
Another case–control study on 134 newborns with <1500 g birth weight showed that intramuscular injection of Vitamin E in first, second, 4th and 6th days after birth decreased the incidence of IVH significantly.[6]
A review article about the effect of Vitamin E on IVH reported that using Vitamin E in the 1st day after birth could prevent incidence of IVH but it had no remarkable effect on IVH severity.[8]
A meta-analysis presented that Vitamin E administration was associated with less incidence of IVH but as use of doses >3.5 mg/dl was accompanied with higher rate of sepsis, it is not recommended to use Vitamin E routinely.[9]
McCrea and Ment have reported that Vitamin E consumption in premature neonates as an antioxidant has decreased rate of IVH but have not affected rate of mortality and outcomes.[3]
IVH occurs due to hypoxic-ischemic reperfusion injury of the germinal matrix, and Vitamin E is an antioxidant which protects endothelial cell membrane against oxidative injuries and decreases hemorrhage.[7] Vitamin E has been demonstrated to be a free rapid scavenger which can decrease the incidence of IVH in neonates.[10]
Sepsis was evaluated in neonates by clinical assessment and blood culture. Positive blood culture and clinical sepsis were more prevalent in the control group, yet the difference was not considerable. It suggests that Vitamin E can decrease risk of sepsis in newborns.
A systematic review reported that using Vitamin E in neonates could increase risk of sepsis, especially in neonates with very low birth weight and if over 30 IU/kg/day of Vitamin E has been used.[11] Another study on 50 neonates who received intramuscular Vitamin E showed that Vitamin E has no effect on sepsis.[12]
In this study, NEC was less prevalent in cases suggesting that Vitamin E might help decrease the risk for NEC yet this decrease in risk was not statistically significant. A systematic review on the effects of Vitamin E showed that this antioxidant had no effects on NEC.[7] A study on 914 premature neonates showed that using Vitamin E could increase the risk for both sepsis and NEC. It has been reported that pharmacological level of Vitamin E in serum is accompanied with less ability of oxygen-dependent intracellular killing which can lead to decreased resistance to infection in preterm newborns. Intravenous administration of Vitamin E can increase serum level of Vitamin E more than oral use, thus monitoring of serum Vitamin E during supplemental Vitamin E administration for preterm infants is recommended.[13] Mortality in our study was higher in the control group yet insignificantly. In a study on 149 newborns with lower than 1000 g birth weight showed that using intramuscular Vitamin E can decrease mortality rate.[12] However, a study reported that Vitamin E had no effect on mortality rate in infants.[11] The cause of death due to Vitamin E administration may be due to complications such as sepsis, NEC, and IVH which can lead to increased mortality of neonates.
Limitations
One of the limitations of this study was related to the sample size which was too small to be generalizable to whole community. Another limitation was lack of measuring Vitamin E level before starting the program, because some neonates might have appropriate levels of Vitamin E and it was more advisable to prescribe Vitamin E in neonates with lower levels of Vitamin E. To evaluate the effects of Vitamin E on incidence of IVH, mortality and morbidity in neonates, further studies are needed to investigate the factors that potentially affect the results.
Conclusions
It should be mentioned that Vitamin E administration in preterm neonates was not significantly accompanied with IVH reduction but rate of IVH occurrence was less in neonates underwent Vitamin E therapy. In addition, although findings of the current study were not significant mortality, sepsis rate and NEC incidence were less in cases in comparison to controls.
Financial support and sponsorship
This study was financially supported by Isfahan University of medical sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The current paper was conducted with the support of research deputy of Isfahan University of Medical Sciences. Kind supports of respective people are highly acknowledged.
References
- 1.Linder N, Haskin O, Levit O, Klinger G, Prince T, Naor N, et al. Risk factors for intraventricular hemorrhage in very low birth weight premature infants: A retrospective case-control study. Pediatrics. 2003;111:e590–5. doi: 10.1542/peds.111.5.e590. [DOI] [PubMed] [Google Scholar]
- 2.Kadri H, Mawla AA, Kazah J. The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv Syst. 2006;22:1086–90. doi: 10.1007/s00381-006-0050-6. [DOI] [PubMed] [Google Scholar]
- 3.McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol. 2008;35:777–92, vii. doi: 10.1016/j.clp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwer A, Groenendaal F, van Haastert IL, Rademaker K, Hanlo P, de Vries L, et al. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr. 2008;152:648–54. doi: 10.1016/j.jpeds.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Ballabh P. Intraventricular hemorrhage in premature infants: Mechanism of disease. Pediatr Res. 2010;67:1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speer ME, Blifeld C, Rudolph AJ, Chadda P, Holbein ME, Hittner HM, et al. Intraventricular hemorrhage and Vitamin E in the very low-birth-weight infant: Evidence for efficacy of early intramuscular Vitamin E administration. Pediatrics. 1984;74:1107–12. [PubMed] [Google Scholar]
- 7.Chiswick ML, Johnson M, Woodhall C, Gowland M, Davies J, Toner N, et al. Protective effect of Vitamin E (DL-alpha-tocopherol) against intraventricular haemorrhage in premature babies. Br Med J (Clin Res Ed) 1983;287:81–4. doi: 10.1136/bmj.287.6385.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Protocol SB. RPA Newborn Care Guidelines [Google Scholar]
- 9.Kuperman AA, Kenet G, Papadakis E, Brenner B. Intraventricular hemorrhage in preterm infants: Coagulation perspectives. Semin Thromb Hemost. 2011;37:730–6. doi: 10.1055/s-0031-1297163. [DOI] [PubMed] [Google Scholar]
- 10.Sinha S, Davies J, Toner N, Bogle S, Chiswick M. Vitamin E supplementation reduces frequency of periventricular haemorrhage in very preterm babies. Lancet. 1987;1:466–71. doi: 10.1016/s0140-6736(87)92087-3. [DOI] [PubMed] [Google Scholar]
- 11.Chiswick M, Gladman G, Sinha S, Toner N, Davies J. Vitamin E supplementation and periventricular hemorrhage in the newborn. Am J Clin Nutr. 1991;53:370S–2S. doi: 10.1093/ajcn/53.1.370S. [DOI] [PubMed] [Google Scholar]
- 12.Fish WH, Cohen M, Franzek D, Williams JM, Lemons JA. Effect of intramuscular Vitamin E on mortality and intracranial hemorrhage in neonates of 1000 grams or less. Pediatrics. 1990;85:578–84. [PubMed] [Google Scholar]
- 13.Johnson L, Bowen FW, Jr, Abbasi S, Herrmann N, Weston M, Sacks L. Relationship of prolonged pharmacologic serum levels of Vitamin E to incidence of sepsis and necrotizing enterocolitis in infants with birth weight 1,500 grams or less. Pediatrics. 1985;75:619–38. [PubMed] [Google Scholar]


