Abstract
Background
Bacterial infection and inflammation of the testis impairs fertility, yet an understanding of inflammatory responses of the testis is incomplete. We are interested in identifying gene pathways involved in the detection and clearance of infectious microbes in the male reproductive tract. In previous studies in our lab focused on hypoxia-responsive genes of the testis, preliminary experiments suggested that genes classically categorized as hypoxia genes are also activated during antimicrobial responses. The purpose of this study was to identify hypoxia and inflammatory gene pathways that contribute to antimicrobial protection of the testis and to consider possible cross-talk and interactions between these pathways. Inflammation was induced in Sprague-Dawley rats using P. aeruginosa or E. coli lipopolysaccharide (LPS). Levels of hypoxia-inducible factor-1 (HIF-1α) protein and nuclear factor kappa B (NF-κB) were measured, and hypoxia and inflammatory gene expression patterns in testis were analyzed by gene expression profiling using real-time quantitative PCR arrays.
Results
In LPS-treated rats, HIF-1α protein increased with no change in Hif-1α mRNA. Western Blot analysis also demonstrated no change in NF-κB and inhibitory NFKB alpha (IκBα) protein levels following LPS treatment. Five hypoxia pathway genes (Angptl4, Egr1, Ier3, Pai1, and Glut1), and 11 inflammatory pathway genes (Ccl12, Cc13, Cd14, Cxcl10, Icam1, Il10, Il1b, Il6, Nfkbia, Tlr2, Tnf) up-regulated after 3 h of inflammation. Angptl4, Ccl12, Cc13, Cd14, Egr1, Nfkbia, Tlr2, and Tnf remained elevated at 6 h. Six genes were up-regulated at 6 h only (Bhlhe40, C3, Jak2, Nlrp3, Slc11a1, Tlr1). One gene (Tlr5) was down-regulated after 3 h and no genes at 6 h. Electrophoretic mobility shift assay results suggest a decrease in NF-κB binding activity following LPS treatment.
Conclusions
Testicular HIF-1α is up-regulated following LPS-induced inflammation. In contrast to other tissues, in which HIF-1α is up-regulated through transcriptional activation via NF-κB, we conclude that HIF-1α in the testis is not up-regulated through an increase in Hif-1α mRNA or through NF-κB-dependent mechanisms. Hypoxia pathway genes and genes involved in Toll-like receptor (TLR) and cytokine-mediated signaling comprise major functional categories of up-regulated genes, demonstrating that both hypoxia and classic inflammatory pathways are involved in inflammatory responses of the testis.
Electronic supplementary material
The online version of this article (10.1186/s12610-018-0079-x) contains supplementary material, which is available to authorized users.
Keywords: Hypoxia, Orchitis, Pathogens, Lipopolysaccharide, Inflammation
Résumé
Contexte
L’infection et l’inflammation bactériennes du testicule altèrent la fertilité; cependant la compréhension des réponses inflammatoires du testicule est. encore incomplète. Nous nous sommes intéressés à l’identification des voies des gènes impliqués dans la détection et l’élimination des microbes infectieux dans l’appareil reproductif masculin. Dans de précédentes études menées dans notre laboratoire, et centrées sur des gènes sensibles à l’hypoxie, les expérimentations préliminaires suggéraient que les gènes classiquement catégorisés comme gènes de l’hypoxie étaient aussi activés au cours des réponses antimicrobiennes. Le but de la présente étude était d’identifier les voies des gènes qui contribuaient à la protection antimicrobienne du testicule et d’examiner de potentiels intermodulations et interactions entre ces voies.
L’inflammation a été induite chez des rats Sprague-Dawley en utilisant des lypopolysaccharides (LPS) de P. aeruginosaet d’E. coli. Les taux de protéine du facteur-1 inductible par l’hypoxie (HIF1- α) et du facteur nucléaire kappa B (NF- kB) ont été mesurés; les profils d’expression des gènes de l’hypoxie et de l’inflammation dans le testicule ont été analysés par profilage de l’expression génique par PCR quantitative en temps réel.
Résultats
Chez les rats traités par LPS, la protéine HIF-1 α a augmenté sans modification de Hif-1αmRNA. L’analyse par Western Blot a aussi montré l’absence de modifications des taux de NF-kB et de la protéine inhibitrice NFKB alpha (IkB α) après traitement. Cinq gènes de la voie hypoxie (Angptl4, Egr1, Ier3, Pai1,et Glut1), et 11 gènes de la voie inflammatoire (Ccl12, Cc13, Cd14, Cxcl10, Icam1, Il10, Il1b, Il6, Egr1, Nfkbia, Tlr2, et Tnf) ont été régulés à la hausse après 3 heures d’inflammation. Angptl4, Ccl12, Cc13, Cd14, Egr1, Nfkbia, Tlr2, et Tnfsont restés élevés à 6 heures. Six gènes n’ont ont été régulés à la hausse qu’à 6 heures (Bhlhe40, C3, Jak2, Nlrp3, Slc11a1, Tlr1). Un gène (Tlr5) a été régulé à la baisse après 3 heures et aucun gène à 6 heures. Les résultats du test de décalage de la mobilité électrophorétique suggèrent une baisse de l’activité de liaison de NF- kB après traitement par LPS.
Conclusions
HIF-1α testiculaire est. régulé à la hausse après inflammation induite par LPS. Contrairement à d’autres tissus, dans lesquels HIF-1α est. régulé à la hausse par activation transcriptionnelle via NF- kB, nous concluons que HIF-1α dans le testicule n’est. pas régulé à la hausse par une augmentation de Hif-1 αmRNA ou par des mécanismes NF-kB-dépendants. Les gènes de la voie hypoxie et les gènes impliqués dans le récepteur Toll-like (TLR) et dans la signalisation médiée par les cytokines comprennent des catégories fonctionnelles majeures de gènes régulés à la hausse, ce qui démontre qu’à la fois les voies de l’hypoxie et les voies classiques de l’inflammation sont impliquées dans les réponses inflammatoires du testicule.
Mots-clés: Hypoxie, Orchite, Pathogènes, Lypopolysaccharides, Inflammation
Background
Infection and resulting inflammation of male reproductive organs impairs fertility by decreasing sperm mobility through the tract and reducing androgen production among other effects on spermatozoa and on the testis and epididymis [1–3]. Roughly half of all infertility cases are caused by male infertility and over 30% of male infertility is idiopathic, yet the relevance of infection and inflammation in male infertility, although widely studied clinically, is still subject to debate [4, 5]. Research to better understand the effects of pathogens on the male reproductive tract and cellular and molecular mechanisms involved in the detection and response to infection of male reproductive organs is essential.
In vivo and in vitro models involving the administration of lipopolysaccharide (LPS), a major pathogenic component of the cell wall of Gram negative bacteria, is a common experimental approach to evaluate inflammatory responses [6, 7]. Pathogen-specific recognition sensors, such as Toll-like receptors (TLR), have been identified in the testis and implicated in the physiology of Leydig, Sertoli and spermatogenic cells [8, 9]. It is well-known that across species a number of antimicrobial peptides are produced by the testis including Spag11, members of the defensin family, Eppin and others [8, 10–17].
Effects of uropathogenic bacteria and LPS in the testis include inhibition of steroidogenesis and reduced androgen production [18, 19], decreased intracellular cAMP and reduced sperm motility [20], induction of proinflammatory cytokines and activation of antimicrobial pathways [10], and epigenetic regulation of antimicrobial gene expression [21]. However, relatively little is known about interactions between regulatory pathways and mechanisms involved in the response to inflammation of the testis.
In other tissues, cross-talk between hypoxia regulatory pathways and classic inflammatory pathways has been demonstrated [22, 23]. This led us to hypothesize that both pathways contribute significantly to inflammatory responses of the testis. We were intrigued by this hypothesis in part because of our prior work on testis hypoxia and the transcription factor hypoxia-inducible factor-1 (HIF-1). Recognized as the master regulator of hypoxia, HIF-1 is known to activate transcription of over 100 genes crucial for cellular responses to hypoxia [24–26]. Increasingly, HIF-1 activation has been implicated in a range of inflammatory responses [27]. For example, HIF-1 is induced by proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin beta (IL-1ß) [28], and by LPS under normoxic conditions [29].
HIF-1α is expressed by Leydig cells in the normoxic adult rat testis and is unregulated by hypoxia [30]. We have also demonstrated that myeloid leukemia cell differentiation 1 (Mcl-1) is a target gene for testicular HIF-1 with potentially important roles in antiapoptotic protection of Leydig cells [31]. Understanding mechanisms of HIF-1 activation under normoxic, hypoxic, and other physiological conditions, such as inflammation, in the testis is of significant interest to investigators interested in the roles of HIF-1 on Leydig cell physiology.
The present study was initiated to (1) investigate whether HIF-1 is affected by LPS-induced inflammation of the testis as an initial step to determine if co-activation of hypoxia and inflammatory pathways occurs in the testis, (2) determine if other hypoxia pathway genes are activated by inflammation, and (3) to explore potential cross-talk between expression of hypoxia and inflammatory pathway genes.
Methods
Animals
Adult male, retired-breeder Sprague-Dawley rats (475–750 g, 9–12 months, Charles River Laboratories (Stone Ridge, NY) were housed individually on a 12:12-h light/dark cycle and controlled temperature with free access to food and water.
Administration of Lipopolysaccharides
P. aeruginosa LPS (Type 10, L7018; Sigma-Aldrich, St. Louis, Missouri) and E. coli 055:B5 LPS (L2880; Sigma-Aldrich) were selected because both are from known pathogens of the urogenital tract and cause tissue-specific inflammatory responses. A dosage of 5.0 mg/kg body weight was chosen to maximize activation of inflammatory responses. Others have clearly demonstrated LPS doses that generate inflammatory responses in vivo (1–5 mg/kg body weight) and in vitro (0.1–1.0 mg/ml), models [10, 16, 18, 19, 32]. LPS was reconstituted in sterile 1× phosphate buffered saline (PBS; 10 mM sodium phosphate, 150 mM sodium chloride, pH 7.8) and administered via intraperitoneal injection. Sham controls were injected with sterile PBS. After 1, 3, 6, and 12 h of treatment (n = 5–7/time point), animals were euthanized via CO2 gas. Testes and control tissues were excised then frozen in liquid nitrogen and stored at − 80 °C.
Serum testosterone assays
Blood was collected via cardiac puncture at the time of sacrifice, clotted at room temperature for 30 min, centrifuged at 13,000 rpm for 10 min at 4 °C then sera was removed and stored at − 80 °C prior to testosterone measurements. Testosterone radioimmunoassays were carried out using a Packard 1900TR Liquid Scintillation Analyzer (Canberra, Meriden, CT). Internal controls included a no testosterone control and a positive control sample of 100 ng/ml testosterone.
Protein extraction
Frozen tissue samples were homogenized on ice in three volumes of lysis buffer (10 mM Tris-HCL [pH 7.5], 1.5 mM MgCl2, 1 mM dithiothreitol, 1 mM Na3VO4 containing protease inhibitor cocktail; P8340; Sigma-Aldrich). The homogenate was maintained on ice for 10 min then centrifuged for 5 min at 3500 rpm at 4 °C. Supernatant was removed for cytoplasmic proteins. Nuclei were resuspended in three volumes of 0.42 M KCl, 20 mM Tris-HCL (pH 7.5), 1.5 mM MgCl2, 20% glycerol, mixed for 30 min at 150 rpm at 4 °C, and centrifuged at 13,500 rpm for 30 min at 4 °C to isolate nuclear proteins. Protein extracts were stored at − 80 °C and protein concentrations determined by the Bradford assay (Bio-Rad, Hercules, CA).
Immunoblot analysis
Proteins were separated by denaturing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) through 7.5% PAGEr® Gold Precast Gels (Lonza, Rockland, MD) in 1× Tris-glycine SDS buffer (0.25 mM Tris, 192 mM glycine, and 0.1% (w/v) SDS [pH 8.3]). COS-7 simian virus 40-transformed kidney cell nuclear extract (Active Motif, Carlsbad, CA) was included as a positive control for detecting HIF-1α. RAW 264.7 cell extract (Santa Cruz Biotechnology, Santa Cruz, CA) was included as positive control for NF-κB and IκBα. Proteins were electroblotted onto Trans-Blot nitrocellulose (Bio-Rad), blocked with 1× Western wash (50 mM Tris, 30 mM NaCl, 0.001% Tween 20 [pH 7.6]) containing 5% nonfat dry milk (NFDM) for 30 min at room temperature, then incubated in primary antibody overnight at 4 °C in 1× Western wash, 5% NFDM.
Primary antibody dilutions were as follows: 0.5 μg/mL HIF-1α mouse monoclonal antibody (AF1935; R&D Systems, Minneapolis, MN), 0.5 μg/mL NF-κB rabbit polyclonal antibody (ADI-KAP-TF112, Enzo Life Sciences, Farmingdale, NY), 0.5 μg/mL IκB rabbit polyclonal antibody (9242; Cell Signaling, Danvers, MA). Actin was detected as a loading control for protein quantification using a 1:2000 dilution of rabbit polyclonal antibody (A2066, Sigma-Aldrich). Blots were incubated in 1× Western wash, 5% NFDM containing appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (1:20,000 dilution), washed in 1× Western wash, developed by enhanced chemiluminescence using either SuperSignal® West Pico substrate or SuperSignal® West Femto substrate (Pierce, Rockford, IL), and analyzed with a ChemiDoc™ XRS+ Molecular Imager with Image Lab™ Software (Bio-Rad).
Gene expression profile analysis by real-time quantitative PCR (qPCR)
Total RNA was isolated from frozen tissue using TRIzol reagent (Invitrogen, Grand Island, NY). Gene expression profiles for hypoxia pathway genes and inflammatory pathway genes were analyzed using Rat Hypoxia Signaling Pathway and Rat Innate and Adaptive Immune Response Pathway RT2 Profiler™ PCR Arrays (Qiagen, Frederick, MD). One microgram of genomic-DNA-free total RNA was reverse transcribed using the RT2 First Strand cDNA Kit (330,401, Qiagen). Real-time qPCR assays were carried out with SYBR Green dye and amplified for 40 cycles in a Stratagene Mx3500P® thermocycler. Each array contained 84 genes involved in the hypoxia or innate and adaptive immune response pathways. Listings of all genes queried and accession numbers are available from the Qiagen website.
Positive controls and housekeeping genes were included to normalize for differences in sample loading, genomic DNA contamination, and amplification efficiency. PCR controls included no RT and no template negative controls. Relative quantification of all target genes was calculated by first normalizing comparative cycle threshold (Ct) values of target genes to ß-actin. Normalized values were used to calculate target gene expression in treatment groups compared to shams. Ct values of controls were ~ 8–10 cycles beyond RT test samples indicating that no contaminating DNA was present. Genes that displayed an average (n = 4–7) minimum of three-fold change (up- or down-regulation) were considered statistically significant (p < 0.05) by analysis of variance (ANOVA).
In silico analysis
Computer-based (in silico) methods were utilized for the analysis of candidate genes involved in inflammatory responses of the testis. Methods included literature searches using PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and bioinformatics databases such as UniProt (https://www.uniprot.org) for gene expression and protein distribution data. These electronic resources were used to determine if there was existing data available about these genes, such as expression and regulation data, cell-type-specific expression in the testis, and protein expression data from other researchers. Information about each gene of interest was gathered to propose pathway maps. The Database for Annotation, Visualization, and Integrated Discovery (DAVID) Bioinformatics Resources 6.7 available through the National Institute of Allergy and Infectious Diseases (NIAID) was utilized for functional annotation and pathway map analysis (https://david.ncifcrf.gov).
Electrophoretic mobility shift assays
Electrophoretic Mobility Shift Assays (EMSA) were performed using the Thermo Scientific LightShift® Chemiluminescent EMSA kit according to manufacturer’s instructions (Thermo Scientific, Rockford, IL). Terminal deoxynucleotidyl transferase (TdT) was used to catalyze biotin labeling of oligonucleotides (Biotin 3′ End DNA Labeling Kit, Pierce). Binding reactions were carried out in 20 μl volumes with protein extracts incubated with 10× binding buffer, 1 μg/μl poly (dI•dC), 50% glycerol, 1% NP-40, 1 M KCl, 100 mM MgCl2, and 200 mM EDTA and biotin-labeled oligonucleotide for 20 min at room temperature. Epstein-Barr Nuclear Antigen (EBNA) and biotin-labeled double-stranded target DNA were included as positive controls. Unlabeled EBNA oligonucleotides were used in negative control and competition experiments. NF-κB double-stranded oligonucleotide with a consensus binding site for NF-κB /c-Rel homodimeric and heterodimeric complexes (5’-AGTTGAGGGGACTTTCCCAGGC -3′; sc-2505, Santa Cruz Biotechnology) which is similar to the sequence present in the upstream region of the HIF-1 gene and mutant oligonucleotides (sc-2511) with a C-G substitution in the consensus site were used for EMSA.
EMSA reactions were separated through 6% polyacrylamide TBE PAGEr® Gold gels (Lonza) electrophoresed in 0.5× tris-borate-EDTA buffer (AccuGENE® 10× TBE Buffer, Lonza), transferred onto nylon (Biodyne® B, Thermo Scientific) and membranes cross-linked at 120 mJ/cm2 for 60 s using a UV-light cross-linker. Blots were blocked, incubated with a 1:300 dilution of stabilized streptavidin-HRP conjugate for 15 min at room temperature, then reacted with luminal/enhancer solution. Digital images were captured with a ChemiDoc™ XRS+ quantitative analysis performed using Image Lab™ Software.
Results
Serum testosterone levels decrease following LPS treatment
A rat model of orchitis, or testicular inflammation, was established via administration of P. aeruginosa or E. coli LPS at a dosage of 5 mg/kg bw. It is well-established that one effect of LPS on the testis is diminished testosterone output and reductions in plasma testosterone levels [19]. To determine if 1 h, 3 h, and 6 h P. aeruginosa and 6 h E. coli LPS treatment caused acute inflammation of the testis and the expected reduction in circulating testosterone, serum testosterone levels were measured via radioimmunoassay. No decrease in serum testosterone levels was observed at 1 h P. aeruginosa LPS treatment (Fig. 1). Statistically significant decreases in serum testosterone levels were observed at 3 h and 6 h P. aeruginosa and 6 h E. coli LPS treatment as compared to sham (P < 0.05; Fig. 1), demonstrating the expected and well-reported physiological effect of LPS inflammation on the testis under our experimental conditions.
Fig. 1.
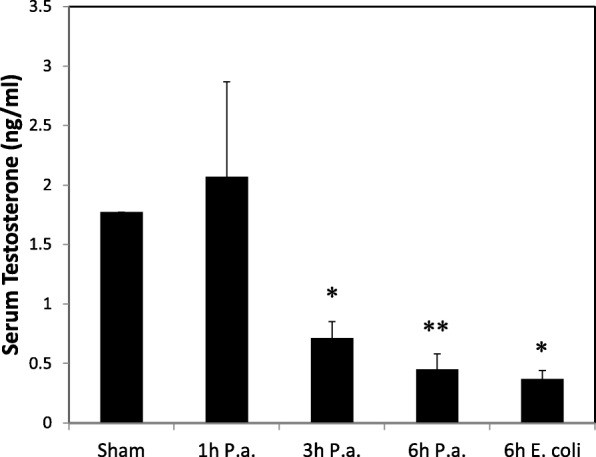
Serum testosterone levels decrease following LPS-induced inflammation. *Indicates statistically significant difference as compared to sham (ANOVA, P < 0.05; n = 3–5); **Indicates P < 0.001. P.a., Pseudomonas aeruginosa
Increased expression of Il-6 has been reported in the testis following LPS-induced inflammation at both 3 and 9-h time points [21]. As mentioned later in the manuscript, we detected elevated mRNA expression for Il-6 mRNA in 3 h LPS treatment groups providing further evidence for inflammation of the testis under these experimental conditions (see Table 2).
Table 2.
Changes in expression of genes involved in innate and adaptive immune responses after 3 and 6 h of LPS-induced inflammation
| Gene Name | Fold Change | Gene Functions | |
|---|---|---|---|
| 3 h | 6 h | ||
| Complement component 3 (C3) Rn.11378, NM_016994 |
No change | 7.72 (p = 0.002) |
Activation of the complement system. |
| Chemokine (C-C motif) ligand 12 (Cc112); Rn.137780, NM_001105822 | 8.52 (p = 0.000) |
26.15 (p = 0.035) |
Angiogenesis; cellular response to fibroblast growth factor stimulus; participates in chemokine and cytokine mediated and cytokine signaling pathways. |
| Chemokine (C-C motif) ligand 3 (Cc13) Rn.10139, NM_013025 | 10.62 (p = 0.000) |
14.63 (p = 0.043) |
Monokine with inflammatory and chemokinetic properties. |
| CD 14 molecule (Cd14) Rn.42942, NM_021744 |
7.17 (p = 0.002) |
6.67 (p = 0.002) |
Accessory protein for TLR4. |
| Chemokine (C-X-C motif) ligand 10 (Cxc110); Rn.10584, NM_139089 | 24.23 (p = 0.000) |
NSS | Proinflammatory cytokine; may participate in T-cell effector function and development. Inhibits expression of StAR D1. |
| Intercellular adhesion molecule 1 (1cam1) Rn. 12, NM_012967 | 25.26 (p = 0.000) |
NSS | Up-regulated by TLR2, TLR4, TLR5, and TLR6; ligand for leukocyte adhesion protein. |
| Interleukin 10 (1110) Rn.9868, NM_012854 |
4.43 (p = 0.021) |
No change | Inhibits cytokine synthesis. |
| Interleukin 1 beta (111b) Rn.9869, NM_031512 |
11.32 (p = 0.000) |
14.18 (p = 0.053) |
Endogenous pyrogen, proinflammatory cytokine. |
| Interleukin 6 (116) Rn.9873, NM_012589 |
69.61 (p = 0.000) |
NSS | Disrupts integrity of blood-testis-barrier by altering steady state levels of membrane proteins. |
| Janus kinase 2 (Jak2) Rn.18909, NM_031514 |
No change | 3.77 (p = 0.032) |
Non-receptor tyrosine kinase known to be involved in innate and adaptive immunity signaling. |
| Nuclear factor of kappa light polypeptide gene (Nfkbia) Rn.12550, NM_001105720 |
4.84 (p = 0.000) |
3.99 (p = 0.018) |
Inhibitor of NF-ĸB; binds NF-ĸB and retains it in the cytoplasm. |
| NLF family, pyrin domain containing 3 (Nlrp3) Rn.214177, XM_220513 |
No change | 8.27 (p = 0.001) |
No functional assignments in Uniprot. |
| Solute carrier family 11 (proton-coupled divalent; Slc11a1) Rn.105919, NM_001031658 |
No change | 3.79 (p = 0.000) |
Macrophage protein associated with resistance or susceptibility to intracellular pathogens. |
| Toll-like receptor 1 (Tlr1) Rn. 107,212, NM_001172120 |
No change | 3.36 (p = 0.018) |
Stimulated by bacterial lipoproteins. |
| Toll-like receptor 2 (Tlr2) Rn. 46,387, NM_198769 |
6.35 (p = 0.000) |
8.70 (p = 0.027) |
Increase expression of inflammatory cytokines IL-α, IL-6, and interferon-α, and β |
| Toll-like receptor 5 (Tlr5) Rn. 198,962, NM_001145828 |
−3.37 (p = 0.052) |
No change | Increase expression of inflammatory cytokines IL-α, IL-6, and interferon-α, and β |
| Tumor necrosis factor (TNF superfamily, member 2; Tnf) Rn.2275,NM_012675 |
8.78 (p = 0.000) |
4.58 (p = 0.029) |
Chemokine-mediated signaling, induces germ cell apoptosis in autoimmune orchitis. |
Genes were reported that showed more than a three-fold change following LPS-induced inflammation with p < 0.05. NSS, not statistically significant, greater than three-fold change observed with p > 0.05
HIF-1α protein levels increase following LPS-induced inflammation
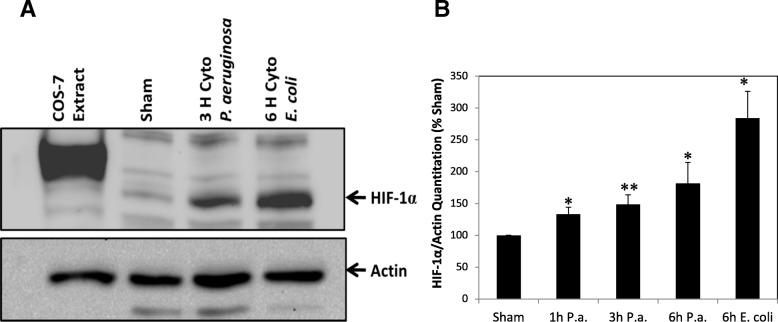
To determine if testis inflammation results in the activation of hypoxia pathways through HIF-1, HIF-1α protein levels in testis from sham and treatment groups were detected by Western blot analysis (Fig. 2a). Statistically significant increases in HIF-1α protein were observed at 1 h, 3 h and 6 h P. aeruginosa LPS treatment as compared to sham and following 6 h E. coli LPS treatment (Fig. 2b). In an initial pilot study to determine time course and LPS dosage, 1 h and 3 h E. coli LPS treatment experiment was performed but no apparent changes in HIF-1α protein were detected (data not shown), therefore it appears that P. aeruginosa LPS produces a more acute phase response in elevating HIF-1 than E. coli LPS.
Fig. 2.
HIF-1α protein levels increase following LPS-induced inflammation in rat testis. a Results of HIF-1α immunoblot analysis of testicular cytoplasmic proteins isolated from sham and LPS treated animals. COS-7 cell line protein extract was included as a positive control and ß-actin was included as an internal control. HIF-1α was detected at ~ 120 kDa. Shown is a 6 h sham as an example but time-matched shams (1, 3 and 6 h) were used for all experiments and for quantitation. Representative time points and results are shown in panel (a). b Histogram shows relative HIF-1α protein levels normalized to ß-actin and as compared to sham for all time points and treatment samples analyzed. *Indicates statistically significant difference as compared to sham (ANOVA, P < 0.05; n = 4–7). **Indicates P < 0.001
NF-κB and IκB protein levels following LPS-induced inflammation
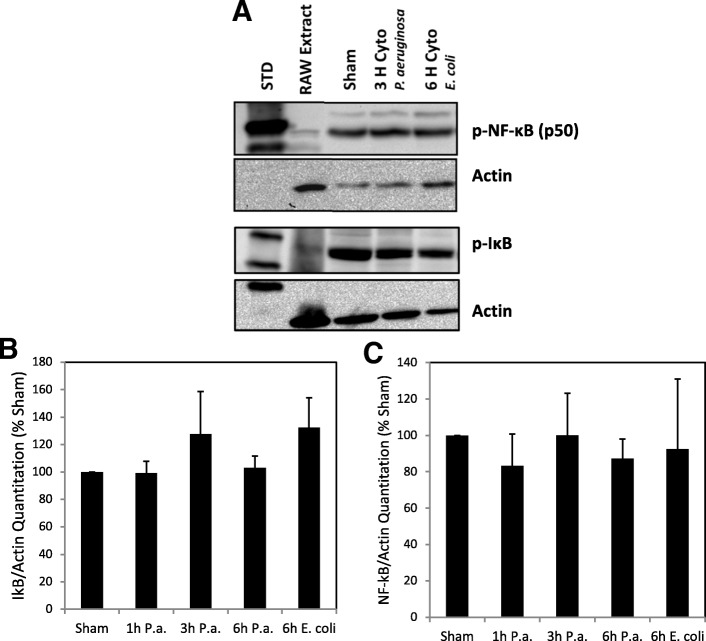
NF-κB is a major activator of inflammatory pathways and has been shown to transcriptionally regulate HIF-1 under conditions of inflammation in other cell types [33, 34]. In an effort to identify the mechanism(s) responsible for HIF-1α protein accumulation following LPS-induced inflammation of the testis, phospho-NF-κB and phospho-IκB protein levels in testicular cytoplasmic protein extracts were measured via Western blot analysis. No differences in phospho-NF-κB and phospho-IκB protein levels were observed in the testis following LPS-induced inflammation at all time points as compared to sham (P < 0.05; Figs. 3a-c).
Fig. 3.
Phospho-NF-κB and IκB protein levels do not change following LPS-induced inflammation in rat testis. a Results of phospho-NF-κB and phospho-IκB immunoblot analysis of testicular cytoplasmic proteins isolated from sham and LPS treated animals. RAW cell line protein extract was included as a positive control and ß-actin was included as an internal control. Representative time points and results are shown in panel (a). Histograms show relative phospho-IκB (b) and phospho-NF-κB (c) protein levels normalized to ß-actin and as compared to sham for all time points and treatment samples analyzed. No statistically significant differences in phospho-IκB and phospho-NF-κB protein levels were observed between sham and LPS treated groups (ANOVA, P < 0.05; n = 3–5). STD, protein molecular weight standards
NF-κB DNA binding activity decreases following LPS-induced inflammation
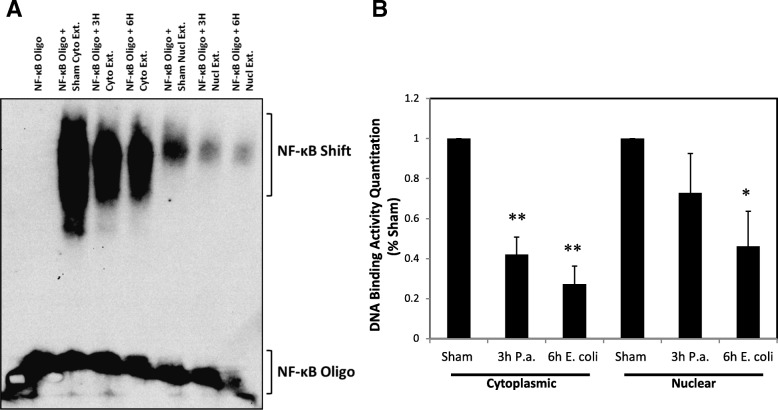
EMSAs were performed to evaluate the DNA binding activity of NF-κB. An oligonucleotide with a consensus binding site for NF-κB homo- and heterodimeric complexes, similar in sequence to a binding site upstream of the HIF-1 gene, was used for EMSA with both testicular cytoplasmic and nuclear protein extracts (Fig. 4a). These time points were chosen to determine if there was any difference in inflammatory response caused by the different treatments. A decrease in binding activity was observed in the cytoplasmic extracts of both 3 h P. aeruginosa and 6 h E. coli LPS treatment and nuclear extracts of 6 h E. coli LPS treatment as compared to sham (P < 0.05; Fig. 4b).
Fig. 4.
Electrophoretic mobility shift assay (EMSA) for NF-κB binding to the HIF-1α gene. a NF-κB binding to a consensus sequence from the HIF-1α gene in both cytoplasmic and nuclear protein extracts decreased following LPS treatment. b Relative DNA binding activity as compared to sham. *Indicates statistically significant difference as compared to sham (ANOVA, n = 3–5; P < 0.05). **Indicates P < 0.001
Gene expression profiling of hypoxia pathway genes
That HIF-1 increased following LPS-induced inflammation suggests roles for hypoxia regulated genes in inflammatory responses of the testis. Gene expression profiling arrays were used to identify hypoxia pathway genes that are regulated following LPS administration. Also, to determine if regulation of HIF-1α protein accumulation following LPS-induced inflammation of the testis occurs at the transcriptional level, Hif-1α mRNA levels were measured in these arrays. No statistical significant difference in Hif-1α mRNA levels was observed at any time point following LPS-induced inflammation in the testis by the arrays or by independent RT-PCR assays for Hif-1α (data not shown), suggesting that HIF-1 protein up-regulation is occurring at the post-transcriptional level. Stabilization and degradation of HIF-1 protein is very well characterized as the primary mechanism controlling HIF-1 protein levels [26].
Pathway array results demonstrated that 5 genes in the hypoxia pathway, Angptl4, Egr1, Ier3, Pai1, and Slc2a1 (Glut1), were up-regulated after 3 h of LPS-induced inflammation and expression and showed more than a three-fold change (p < 0.05; Table 1). Following 6 h of LPS-induced inflammation, 3 of these genes (Angptl4, Bhlhe40, and Egr1), showed statistically significant up-regulation (Table 1). No genes were down-regulated following LPS administration. In silico analysis of LPS-stimulated genes indicates that these transcripts are predominantly expressed in supporting cells of the testis (Sertoli, myoid, and Leydig cells) and not in developing germ cells. Egr1, Fos, Ier3, and Pai1 are known target genes for the transcription factor NF-κB. None of the up-regulated genes are known HIF-1 targets.
Table 1.
Up-regulation of hypoxia pathway genes after 3 and 6 h of LPS-induced inflammation
| Gene Name Unigene, GenBank | Fold Change | |
|---|---|---|
| 3 h (n = 7) | 6 h (n = 6) | |
| Angiopoietin-like 4 (Angptl4)Rn.119611, NM_199115 | 20.38 (p = 0.007) | 14.12 (p = 0.005) |
| Basic helix-loop-helix family, member (Bhlhe40) Rn.81055, NM_053328 |
No change | 3.31 (p = 0.000) |
| Early growth response 1 (Egr1) Rn.9096, NM_012551 |
29.16 (p = 0.006) |
6.67 (p = 0.046) |
| Immediate early response 3 (Ier3) Rn.23638, NM_212505 |
9.85 (p = 0.001) |
No change |
| Plasminogen activator inhibitor type 1 (Pai1) Rn.29367, NM_012620 |
15.05 (p = 0.023) |
No change |
| Solute carrier family 2 (facilitated glucose transporter), member 1 (Slc2a1, Glut1) Rn.3205, NM_138827 |
3.41 (p = 0.002) |
No change |
Only genes that showed at least a three-fold increase in expression with p < 0.05 were considered statistically significant
Gene expression profiling of innate and adaptive immune response pathway genes
Because recent research has demonstrated cross-talk between hypoxia pathways and inflammatory pathways, we chose to also evaluate expression profiles for innate and adaptive immune response genes. At 3 h following P. aeruginosa LPS treatment, 11 genes showed a statistically significant up-regulation (Cc112, Cc13, Cd14, Cxc110, Icam1, Il10, Il1b, Il6, Nfkbia, Tlr2, and Tnf) and one gene was down-regulated (Tlr5; Table 2). Twelve genes were up-regulated at 6 h (C3, Cc112, Cc13, Cd14, Il1b, Jak2, Nfkbia, Nlrp3, Slc11a1, Tlr1, Tlr2, and Tnf). Seven genes remained elevated at both 3 and 6 h (Cc112, Cc13, Cd14, Il1b, Nfkbia, Tlr2, and Tnf).
Pathway analysis of inflammatory genes affected by LPS-induced inflammation
DAVID Bioinformatics Resources 6.7 was utilized for functional annotation and pathway map analysis. This allowed for identification of multiple genes involved in specific signaling pathways. Of the inflammatory genes affected by LPS, 9 genes are part of the TLR signaling pathway (Additional file 1: Figure S1). With the exception of Tlr5, which was down-regulated 3 h following LPS-induced inflammation, genes circled in red were up-regulated at 3 and/or 6 h following LPS administration (Additional file 1: Figure S1).
Discussion
This study was designed to begin to understand effects of E. coli and P. aeruginosa LPS-induced inflammation in activating HIF-1 and hypoxia pathway gene responses in the testis. Regulation of HIF-1 during inflammation has been studied in vivo and in vitro and reviewed extensively [23]. One hypothesis is that HIF-1 is up-regulated in microenvironments of inflamed tissues characterized by low levels of oxygen and glucose and high levels of inflammatory cytokines, reactive oxygen species (ROS), and nitrogen species, to protect cells against secondary inflammatory damage [32].
Steroidogenesis and spermatogenesis are inhibited by infection and inflammation of the testis [18]. For example, in mice using relatively high doses of LPS, Leydig cell steroidogenesis is inhibited via reduced synthesis of the cholesterol transport protein steroidogenic acute regulatory protein and steroidogenic enzymes [19]. To validate inflammation in our model system, serum testosterone levels were measured and decreases in serum testosterone after the 3 h and 6 h P. aeruginosa and 6 h E. coli LPS treatments confirmed LPS-induced inflammation and compromised testicular functions.
Previously we have shown that HIF-1 is highly expressed by normoxic Leydig cells and that, unlike other tissues, testicular HIF-1 expression is not induced by hypoxia [35]. This may be because physiologic oxygen tension of the testis is already hypoxic. Thus, studying the activation of a hypoxic response during inflammation in what may be an already hypoxic environment is of particular interest. In vitro studies have established the activation of hypoxia genes by LPS in multiple systems including pulmonary artery smooth muscle cells, macrophages, monocytes, and glioblastomas [33, 34, 36–38]. The increase in testicular HIF-1 protein we detected following LPS-induced inflammation indicates a role for HIF-1 and Leydig cells in the activation of (or protection against) an inflammatory response.
The accumulation of testicular HIF-1α protein following inflammation suggests crosstalk between inflammatory responses and hypoxic responses in the testis. Before determining the effects of HIF-1 up-regulation following LPS-induced inflammation, we chose to make an initial effort to identify potential pathways responsible for the crosstalk between inflammatory and hypoxic responses and up-regulation of HIF-1α. Studies suggest this crosstalk occurs via transcriptional regulation of HIF-1α by NF-κB, a key transcription factor responsible for mediating inflammatory responses [34, 37]. A functional NF-κB binding site in the HIF-1α promoter has been demonstrated [36] and LPS induces HIF-1 activation in human monocytes via NF-κB [33].
This led us to consider that NF-κB may be an important transcriptional activator of HIF-1α following LPS-induced inflammation in our model. EMSA experiments to measure NF-κB binding to the HIF-1α promoter revealed that binding activity decreased following LPS-induced inflammation. Nfkb1 mRNA and protein expression did not increase and our pathway array results also demonstrated increased expression of Nfkbia mRNA. Taken together these results suggest that NF-κB is probably not a transcriptional activator of HIF-1α in the testis in vivo.
We also demonstrated that testicular HIF-1α is not up-regulated through an increase in Hif-1α mRNA. It is likely then that post-translational regulation of HIF-1α protein levels (via ubiquitination and protein stability) as occurs under hypoxic conditions, and not transcriptional regulation of HIF-1α is the likely mechanism for up-regulating HIF-1α protein levels in the testis during inflammation.
One of the functional clusters of inflammatory genes up-regulated in our study is the TLRs. In our work expression of Tlr2 was shown to be elevated at both 3 and 6-h time points but Tlr4 mRNA was not. Although others have demonstrated elevated expression of TLR-4 following LPS challenge of the testis [10]. But the absence of significant change in expression of Tlr4 has been reported before. For example, Winnall et al. reported a significantly higher expression of TLR2 in relation to expression of the LPS receptor TLR4 and its co-receptor protein CD14 [7]. Sertoli cells have been shown to respond to bacteria through both TLR2 and TLR4 [39]. TLR4-dependent activation of HIF-1 in the lung has been demonstrated [40]. Our studies showed elevated expression of CD14. In addition, TLR stimulation elevates the synthesis of proinflammatory cytokines such as IL-1β and TNF (both of which showed elevated mRNA expression in our study) so perhaps TLR-dependent pathways and not NF-κB is the predominant activation pathway for HIF-1 in the testis. Recently we have also demonstrated that expression of a number of microRNAs in the testis are downregulated by LPS including miRNAs involved in regulating the NF-κB pathway [41].
Testicular macrophages are important for modulating inflammatory responses [1]. In testicular macrophages challenged with LPS, it has been shown that the NF-κB signaling pathway is blocked due to a lack of IκBα ubiquitination and degradation [42]. Rather LPS stimulates testicular macrophages to activate MAPK, AP-1, CREB and other pathways that increase production of proinflammatory cytokines such as IL-6 and TNF. Both IL-6 and TNF are known to have inhibitory roles on steroidogenesis of Leydig cells [1, 43] and so the elevated mRNA expression of these genes observed in our studies was expected. In this study up-regulated expression of mRNAs for proinflammatory cytokines such as IL-1 and IL-6, TNF underscores the importance of cytokine-mediated signaling pathways that are thought to be important for inflammatory responses of the testis, as demonstrated by other investigators [1].
Finally, the up-regulation of HIF-1α protein may also be caused by several physiologic changes. For example, a decrease in oxygen tension caused by inflammatory activity occurring in the testis may result in a hypoxic environment leading to HIF-1 activation. Similarly ROS released during inflammatory responses can also stabilize and elevate HIF-1α protein levels [23].
Results from hypoxia pathway arrays demonstrated that six hypoxic pathway genes (Angptl4, Bhlhe40, Egr1, Ier3, Pai1, and Glut1) are up-regulated 3 or 6 h following LPS-induced inflammation. Among 84 hypoxic genes, no genes appear to be down-regulated in response to LPS. Those that did show a significant increase do not appear to belong to any particular functional cluster. Due to the variety of functions exhibited by these genes, we do not want to speculate about the roles these genes may plan in inflammatory responses in the testis. These genes are the subject of future studies.
Although we did not determine the cell-type specific location of the genes analyzed in our study, in silico analysis of hypoxia pathways genes revealed that only Bhlhe40 is known to be expressed in spermatogenic cells. Angptl4, Egr1, Ier3, and Pai1 are expressed in myoid cells. Pai1 is expressed in germ cells. Egr1, Ier3, Pai1, and Slc2a1 are expressed in Sertoli cells, and Egr1 is expressed in Leydig cells. These results suggest that supporting cells, and not germ cells, are primarily involved in inflammatory responses of the testis.
In addition, the literature includes few studies on hypoxic gene expression in the testis in response to LPS. Bourdon et al. detected Pai1 expression in cultured Sertoli cells following LPS treatment [44]. Further experimental evidence is necessary to elucidate the specific mechanisms and functions of these genes, and will provide a greater understanding of why some appear to be early response genes while others may be late responders. These studies could also provide an explanation for why certain genes such as Angptl4 and Egr1 show a sustained response, with elevated expression at both 3 and 6-h time points.
While the up-regulation of HIF-1α suggests a role for the HIF-1 pathway in inflammatory responses of the testis, further studies are needed to determine possible HIF-1 target genes. HIF-1 was initially thought to play a significant role in the regulation of these hypoxic genes, but only Glut1 is a known target gene for HIF-1. However, Egr1, Ier3, and Pai1 are known target genes of NF-κβ. Also, although HIF-1 is known to activate over 100 target genes, not all targets were built into the array; therefore, we cannot exclude the possibility that there may be other HIF-1 target genes involved in inflammation.
Conclusions
Regardless of the underlying reasons for HIF-1 activation, we conclude that the mechanism of HIF-1α protein accumulation following LPS-induced inflammation is not at the transcriptional level and likely not mediated through NF-κB. Further studies will be carried out to identify target genes regulated by HIF-1. This study provides a baseline analysis of hypoxia and inflammatory pathway gene expression changes following LPS-induced inflammation of the testis that will be a useful resource for researchers in the future. The range of genes affected and their potential functions, reflects the complexity of the inflammatory response of the testis and underscores that both hypoxic and inflammatory gene expression pathways are involved.
Additional file
Figure S1. Toll-like receptor signaling pathway including innate and adaptive immune response genes up-regulated or down-regulated by LPS-induced inflammation. (DOCX 359 kb)
Acknowledgements
We thank Dr. Renshan Ge and Chantal Sottas at the Population Council of the Center for Biomedical Research for performing serum testosterone radioimmunoassays. Results from this study were presented at the 34th annual meeting of the American Society for Reproductive Immunology (Long Beach, NY, June 2014), the 39th annual meeting of the American Society of Andrology (Atlanta, GA, April 2014), the 112th Annual Meeting of the American Society for Microbiology (San Francisco, CA, June 2012) and the 37th Annual Meeting of the American Society of Andrology (Tucson, AZ, April 2012).
Funding
Supported by NIH grant R15-HD046451 to M. Palladino, the Independent College Fund of New Jersey-Merck Undergraduate Science Endeavors Program to support G. Fasano, and the Bristol-Myers Squibb Charitable Giving Grant to the Monmouth University Summer Research Program to support D. Patel.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its Additional files].
Abbreviations
- ANOVA
analysis of variance
- cAMP
cyclic AMP
- cDNA
complementary DNA
- Ct
cycle threshold
- DAVID
Database for Annotation, Visualization, and Integrated Discovery
- DNA
deoxyribonucleic acid
- EBNA
Epstein-Barr Nuclear Antigen
- EBNA
Epstein-Barr Nuclear Antigen
- EDTA
ethylene-Diamine-Tetra-Acetic acid
- EMSA
Electrophoretic Mobility Shift Assays
- HIF-1α
hypoxia-inducible factor-1 alpha
- HRP
horseradish peroxidase
- IL-1ß
interleukin beta
- IκBα
inhibitory NFKB alpha
- LPS
lipopolysaccharide
- Mcl-1
myeloid leukemia cell differentiation 1
- NFDM
nonfat dry milk
- NIAID
National Institute of Allergy and Infectious Diseases
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- qPCR
real-Time Quantitative PCR
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- RT
reverse transcriptase
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TdT
terminal deoxynucleotidyl transferase
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor alpha
Authors’ contributions
MAP designed the study, participated in its experimental design, data analysis, and drafting and final writing and submission of the manuscript. GAF carried out hypoxia and inflammatory pathway array experiments and drafting of the manuscript. DP carried out western blot experiments and drafting of the manuscript. CD and ML carried out EMSA experiments and drafting of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Animal research protocols were approved by the Monmouth University IACUC and conform to guidelines established in The Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Note: Co-authors GF, DP, CD and ML were students at Monmouth University when the work was carried out. All have graduated and are currently in graduate school, medical school or in positions in industry
Contributor Information
Michael A. Palladino, Phone: (732) 571-7550, Email: mpalladi@monmouth.edu
Genevieve A. Fasano, Email: genevieve.allegra.fasano@drexel.edu
Dharm Patel, Email: dharm16@gmail.com.
Christine Dugan, Email: cdugan07@gmail.com.
Marie London, Email: marie.london01@gmail.com.
References
- 1.Hedger Mark P. Knobil and Neill's Physiology of Reproduction. 2015. The Immunophysiology of Male Reproduction; pp. 805–892. [Google Scholar]
- 2.Azenabor A, Ekun AO, Akinloye O. Impact of inflammation on male reproductive tract. J Reprod Infertil. 2015;16:123–129. [PMC free article] [PubMed] [Google Scholar]
- 3.Bachir BG, Jarvi K. Infectious, inflammatory, and immunologic conditions resulting in male infertility. Urol Clin North Am. 2014;41:67–81. doi: 10.1016/j.ucl.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Ochsendorf FR. What are the consequences of sexually transmitted infections on male reproduction? Handbook of Andrology. Lawrence: Allen Press, Inc; 2009.
- 5.Hamada A, Esteves SC, Agrawal A. The role of contemporary andrology in unraveling the mystery of unexplained male infertility. Open Reprod Sci J. 2011;3:27–41. doi: 10.2174/1874255601103010027. [DOI] [Google Scholar]
- 6.Hedger M, Klug J, Fröhlich S, Müller R, Meinhardt A. Regulatory cytokine expression and interstitial fluid formation in the normal and inflamed rat testis are under leydig cell control. J Androl. 2005;26:379–386. doi: 10.2164/jandrol.04149. [DOI] [PubMed] [Google Scholar]
- 7.Winnall WR, Muir JA, Hedger MP. Differential responses of epithelial Sertoli cells of the rat testis to toll-like receptor 2 and 4 ligands: implications for studies of testicular inflammation using bacterial lipopolysaccharides. Innate Immun. 2011;17:123–136. doi: 10.1177/1753425909354764. [DOI] [PubMed] [Google Scholar]
- 8.Palladino M, Johnson T, Gupta R, Chapman J, Ojha P. Members of the toll-like receptor family of innate immunity pattern-recognition receptors are abundant in the male rat reproductive tract. Biol Reprod. 2007;76:958–964. doi: 10.1095/biolreprod.106.059410. [DOI] [PubMed] [Google Scholar]
- 9.Girling JE, Hedger MP. Toll-like receptors in the gonads and reproductive tract: emerging roles in reproductive physiology and pathology. Immunol Cell Biol. 2007;85:481–489. doi: 10.1038/sj.icb.7100086. [DOI] [PubMed] [Google Scholar]
- 10.Biswas B, Yenugu S. Antimicrobial responses in the male reproductive tract of lipopolysaccharide challenged rats. Am J Reprod Immunol. 2011;65:557–568. doi: 10.1111/j.1600-0897.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 11.Palladino MA, Mallonga TA, Mishra MS. Messenger RNA (mRNA) expression for the antimicrobial peptides beta-defensin-1 and beta-defensin-2 in the male rat reproductive tract: beta-defensin-1 mRNA in initial segment and caput epididymidis is regulated by androgens and not bacterial lipopolysaccharides. Biol Reprod. 2003;68:509–515. doi: 10.1095/biolreprod.102.008953. [DOI] [PubMed] [Google Scholar]
- 12.Biswas B, Narmadha G, Choudhary M, French FS, Hall SH, Yenugu S. Identification of toll-like receptors in the rat (Rattus norvegicus): messenger RNA expression in the male reproductive tract under conditions of androgen variation. Am J Reprod Immunol. 2009;62:243–252. doi: 10.1111/j.1600-0897.2009.00732.x. [DOI] [PubMed] [Google Scholar]
- 13.Com E, Bourgeon F, Evrard B, Ganz T, Colleu D, Jégou B, et al. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod. 2003;68:95–104. doi: 10.1095/biolreprod.102.005389. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Widgren EE, Sivashanmugam P, O’Rand MG, Richardson RT. Association of eppin with semenogelin on human spermatozoa. Biol Reprod. 2005;72:1064–1070. doi: 10.1095/biolreprod.104.036483. [DOI] [PubMed] [Google Scholar]
- 15.Yenugu S, Chintalgattu V, Wingard CJ, Radhakrishnan Y, French FS, Hall SH. Identification, cloning and functional characterization of novel beta-defensins in the rat (Rattus norvegicus) Reprod Biol Endocrinol. 2006;4:7. doi: 10.1186/1477-7827-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues A, Queiróz DB, Honda L, Silva EJ, Hall SH, Avellar MC. Activation of toll-like receptor 4 (TLR4) by in vivo and in vitro exposure of rat epididymis to lipopolysaccharide from Escherichia Coli. Biol Reprod. 2008;79:1135–1147. doi: 10.1095/biolreprod.108.069930. [DOI] [PubMed] [Google Scholar]
- 17.Hall SH, Hamil KG, French FS. Host defense proteins of the male reproductive tract. J Androl. 2002;23:585–597. [PubMed] [Google Scholar]
- 18.O’Bryan MK, Schlatt S, Phillips DJ, De Kretser DM, Hedger MP. Bacterial lipopolysaccharide-induced inflammation compromises testicular function at multiple levels in vivo. Endocrinology. 2000;141:238–246. doi: 10.1210/endo.141.1.7240. [DOI] [PubMed] [Google Scholar]
- 19.Allen J, Diemer T, Janus P, Hales K, Hales B. Bacterial endotoxin lipopolysaccharide and reactive oxygen species inhibit leydig cell steroidogenesis via perturbation of mitochondria. Endocrine. 2004;25:265–275. doi: 10.1385/ENDO:25:3:265. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Zhang D, He Y, Ding Z, Mao F, Luo T, et al. Lipopolysaccharide compromises human sperm function by reducing intracellular cAMP. Tohoku J Exp Med. 2016;238:105–112. doi: 10.1620/tjem.238.105. [DOI] [PubMed] [Google Scholar]
- 21.Biswas B, Yenugu S. Lipopolysaccharide induces epididymal and testicular antimicrobial gene expression in vitro: insights into the epigenetic regulation of sperm-associated antigen 11e gene. Immunogenetics. 2013;65:239–253. doi: 10.1007/s00251-012-0674-5. [DOI] [PubMed] [Google Scholar]
- 22.Bartels K, Grenz A, Eltzschig HK. Hypoxia and inflammation are two sides of the same coin. Proc Natl Acad Sci U S A. 2013;110:18351–18352. doi: 10.1073/pnas.1318345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devraj Gayatri, Beerlage Christiane, Brüne Bernhard, Kempf Volkhard A.J. Hypoxia and HIF-1 activation in bacterial infections. Microbes and Infection. 2017;19(3):144–156. doi: 10.1016/j.micinf.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008;33:526–534. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Brocato J, Chervona Y, Costa M. Molecular responses to hypoxia-inducible factor 1α and beyond. Mol Pharmacol. 2014;85:651–657. doi: 10.1124/mol.113.089623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dehne N, Brüne B. HIF-1 in the inflammatory microenvironment. Exp Cell Res. 2009;315:1791–1797. doi: 10.1016/j.yexcr.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Tewari R, Choudhury SR, Ghosh S, Mehta VS, Sen E. Involvement of TNFα-induced TLR4-NF-κB and TLR4-HIF-1α feed-forward loops in the regulation of inflammatory responses in glioma. J Mol Med. 2012;90:67–80. doi: 10.1007/s00109-011-0807-6. [DOI] [PubMed] [Google Scholar]
- 29.Imtiyaz HZ, Simon MC. Hypoxia-inducible factors as essential regulators of inflammation. Curr Top Microbiol Immunol. 2010;345:105–120. doi: 10.1007/82_2010_74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell JD, Elshtein R, Forest DJ, Palladino MA. Stimulation of hypoxia-inducible Factor-1 alpha (HIF-1α) protein in the adult rat testis following ischemic injury occurs without an increase in HIF-1α messenger RNA expression. Biol Reprod. 2002;67:995–1002. doi: 10.1095/biolreprod.101.002576. [DOI] [PubMed] [Google Scholar]
- 31.Palladino MA, Shah A, Tyson R, Horvath J, Dugan C, Karpodinis M. Myeloid cell leukemia-1 (Mc1–1) is a candidate target gene of hypoxia-inducible factor-1 (HIF-1) in the testis. Reprod Biol Endocrinol. 2012;10:104. doi: 10.1186/1477-7827-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frede S, Berchner-Pfannschmidt U, Fandrey J. Regulation of hypoxia-inducible factors during inflammation. Meth Enzymol. 2007;435:405–419. doi: 10.1016/S0076-6879(07)35021-0. [DOI] [PubMed] [Google Scholar]
- 33.Frede S, Stockmann C, Freitag P, Fandrey J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem J. 2006;396:517–527. doi: 10.1042/BJ20051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorlach A, Bonello S. The cross-talk between NF-κB and HIF-1: further evidence for a significant liaison. J Biochem. 2008;412:e17. doi: 10.1042/BJ20080920. [DOI] [PubMed] [Google Scholar]
- 35.Palladino MA, Pirlamarla PR, McNamara J, Sottas CM, Korah N, Hardy MP, et al. Normoxic expression of hypoxia-inducible factor 1 in rat Leydig cells in vivo and in vitro. J Androl. 2011;32:307–323. doi: 10.2164/jandrol.110.011494. [DOI] [PubMed] [Google Scholar]
- 36.Bonello S, Zähringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, et al. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 37.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, et al. NF-|[kgr]|B links innate immunity to the hypoxic response through transcriptional regulation of HIF-1|[agr]|. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blouin CC, Pagé EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1α. Blood. 2004;103:1124–1130. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 39.Hedger MP. Toll-like receptors and signalling in spermatogenesis and testicular responses to inflammation—a perspective. J Reprod Immunol. 2011;88:130–141. doi: 10.1016/j.jri.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Z, Zhu X, Chen J, Yang S, Sun R, Yang G. The interaction between toll-like receptor 4 signaling pathway and hypoxia-inducible factor 1α in lung ischemia-reperfusion injury. J Surg Res. 2014;188:290–297. doi: 10.1016/j.jss.2013.11.1086. [DOI] [PubMed] [Google Scholar]
- 41.Parker MI, Palladino MA. MicroRNAs downregulated following immune activation of rat testis. Am J Repro Immunol. 2017;00:e12673. doi: 10.1111/aji.12673. [DOI] [PubMed] [Google Scholar]
- 42.Bhushan S, Tchatalbachev S, Lu Y, Fröhlich S, Fijak M, Vijayan V, et al. Differential activation of inflammatory pathways in testicular macrophages provides a rationale for their subdued inflammatory capacity. J Immunol. 2015;194:5455–5464. doi: 10.4049/jimmunol.1401132. [DOI] [PubMed] [Google Scholar]
- 43.Hales DB. Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol. 2002;57:3–18. doi: 10.1016/S0165-0378(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 44.Bourdon V, Defamie N, Fenichel P, Pointis G. Regulation of tissue-type plasminogen activator and its inhibitor (PAI-1) by lipopolysaccharide-induced phagocytosis in a Sertoli cell line. Exp Cell Res. 1999;247:367–372. doi: 10.1006/excr.1998.4369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Toll-like receptor signaling pathway including innate and adaptive immune response genes up-regulated or down-regulated by LPS-induced inflammation. (DOCX 359 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its Additional files].