Abstract
Background:
Published literature on intracerebral haemorrhage (ICH) from the Indian subcontinent is very scarce. The study aims to assess the prognostic factors influencing outcome and validating the ICH score which is widely used to prognosticate the disease in this financially constraint population. Prognosticating the outcome at the time of admission is important to customize treatment in a cost-effective manner.
Materials and Methods:
We conducted a prospective study of all Spontaneous ICH patients admitted from February 2015 to May 2016. Data pertaining to patient demographics, clinical findings, biochemical parameters and cranial computed tomography (CT) findings were recorded. mRS (modified Rankin score) was used to assess outcome at discharge and at three month follow up.
Results:
A total of 215 patients with hypertensive haemorrhage were analysed. The mean age of our cohort was 57.64 years and volume of bleed was 24.5ml. 73% pf patients with GCS<8, 46% with Intraventricular extension and 57% with hematoma volume >30 were died at the end of 3 months. Twenty eight patients succumbed during hospitalization while 38 died after their discharge. Mortality rates were 5%,16%, 33%, 54% and 93% for ICH Scores of 0, 1, 2, 3 and 4. The rICH score after modifying the age parameter in the ICH score to 70 years had mortality rates of 6%,15%,25%,51%,75% and 100%.
Conclusion:
ICH Score failed to accurately predict mortality in our cohort. ICH is predominately seen at a younger age group in our country and hence have better outcomes in comparison to the west. We propose a minor modification in the ICH score by reducing the age criteria by 10 years to prognosticate the disease better in our population.
Keywords: Hypertensive bleed, intracerebral hemorrhage, intracerebral hemorrhage score, stroke
INTRODUCTION
Intracerebral hemorrhage (ICH) is the second most common cause of stroke and accounts for 7.5%–30% of all strokes.[1,2] The incidence of ICH is increasing over the years,[3] and ICH remains the most dreadful among stroke subtypes with a 30-day mortality of 40%–50%[4] and half of these deaths occurring in the first 2 days.[5,6] Only 12%–39% of symptomatic ICH (SICH) patients achieve functional independence at the end of 6 months.[7] Incidence of hemorrhagic stroke in India is higher in comparison to the western population,[8] and the population at risk is younger compared to the western developed world. Considering the poor long-term outcome for SICH patients, an effective prognosticating scale is important to optimize the management plan for careful use of available resources, especially in developing countries like India.[9] The ICH score remains one of the most widely used scoring systems[10] in prognosticating the disease at the time of admission but has its own limitations. In developing countries like India which exhibit a different demographic profile, the ICH score is not an ideal tool for prognostication. The purpose of this study was to validate the ICH score for the Indian population and suggest modifications for better prediction of mortality and morbidity.
MATERIALS AND METHODS
This study was conducted at Kasturba Medical College Hospital, a premier tertiary care referral hospital of the Manipal University located in Manipal, Udupi district of Karnataka, India, catering to the local population of nearly one million from February 2016 to May 2016. All patients with a computed tomography (CT) evidence of spontaneous ICH above the age of 18 years were included in this study. Hematoma volume was calculated by the (axbxc)/2 method on plain CT scans, and hematoma expansion was defined as any increase in volume in the follow-up scan or at patient deterioration. Patients with coagulation abnormalities, aneurysmal hematomas, and vascular malformations were excluded from the study. The study group thus comprised 215 patients with hypertensive spontaneous ICH in a period of 15 months. Outcome was measured using the modified Rankin Scale (mRS). Statistical analysis was done using SPSS version 23 (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp).
RESULTS
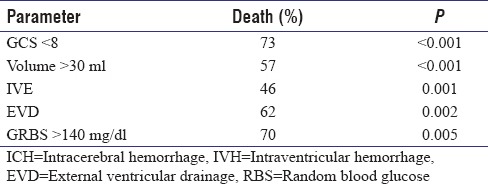
The mean age of patients in this study was 57.64 years with a range of 24–89 years, and 73.5% of patients in this study were males. A history of hypertension was available in only half (50%) of our patients [Table 1]. The mean blood glucose values on admission in the entire cohort were 160.25 mg/dl (42–407 mg/dl). The distribution of the 186 (86.5%) patients with supratentorial hematoma was as follows: basal ganglia (110), thalamus (36), and lobar (31). Of the 29 patients with infratentorial hematomas, 20 had cerebellar bleeds and 9 had brain stem hematomas [Table 2]. Intraventricular extension of the hematoma was seen in 80 (37.2%) in which 37 (46%) succumbed to the illness at the end of 3 months (P = 0.001). Hydrocephalus developed in 68 patients (31.6%). An external ventricular drain was placed in 21 of these patients of whom 13 patients (62%) succumbed (P = 0.002).
Table 1.
Results of 215 patients with hypertensive intracranial hemorrhage (n=215)
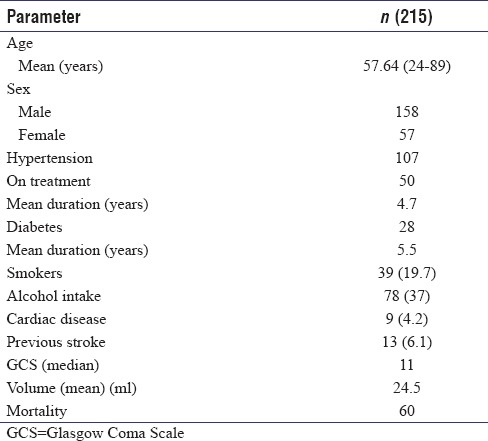
Table 2.
Radiological characteristics
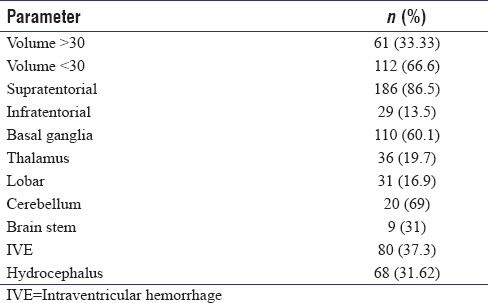
The mean hematoma volume was 24.50 ml with standard deviation (SD) of 17.98 and range of 5–110 ml. We dichotomized this cohort of supratentorial bleed into two groups of volume <30 ml and >30 ml with 122 and 61 cases, respectively. Mann–Whitney U test showed statistical significance in hematoma volume and mortality with a mean rank of 140.66 ml for dead patients and 90.37 for alive patients with P ≤ 0.001. Receiver operating characteristic (ROC) curve plotted for supratentorial hematoma volume in dead patients showed a 77.6% area under the curve with P < 0.001. The mean volume of infratentorial hematomas was 10.1 ml (2 ml to 20 ml) with a SD of 5.75 ml. Infratentorial hematoma volume had no correlation with mortality (P = 0.78). Thirteen patients had hematoma expansion documented by CT scans. This, however, did not influence the outcome (P = 1.227).
Surgery was offered to all patients with a supratentorial clot volume >30 ml, cerebellar clot volume >3 cm, age <70 years, Glasgow Coma Scale (GCS) >8, and presentation within 48 h of ictus. A total of 37 patients in this study (17.2%) underwent surgical evacuation of hematoma. Surgery involved craniotomy and evacuation of the hemorrhage through a corticectomy placed in the middle frontal gyrus in front of the coronal suture. Cerebellar hematomas were drained following a suboccipital craniectomy. The mean volume of the supratentorial clots was 42.29 ± 16.48 ml. Surgical mortality at discharge was 6/37 (16.2%) and at 3 months was 16/37 (43%). Wilcoxon signed-rank test showed no significance in the change in mRS for the surgical group (P = 0.175). While 14 patients had an improvement in mRS at 3 months, 9 patients had a deterioration of mRS and 11 patients remained the same. Cross-tabulation of surgery versus mortality also failed to show any improvement in mortality rates following surgery.
A total of 69 patients (32.15%) in this cohort succumbed to the illness. While 28 patients (13%) died in the hospital, 38 patients (17.7%) died within 3 months of ictus and 3 patients (1.4%) died within 6 months of ictus. Of the surviving patients, 65 (30.2%) had an mRS ≤3 on discharge. At the end of 3 months, many patients had improved and 109 patients had an mRS <3 at the end of 3 months. Similarly, of the 122 patients with mRS of 4 and 5 on nearly 98 improved and at the end of 3 months, only 24 patients were left with an mRS of >4. Wilcoxon signed-rank test showed a statistical significance in the improvement of mRS at 3 months (P < 0.001).
ICH score was computed for all patients at admission. Forty-one patients were categorized as score 0, 80 as score 1, 45 as score 2, 35 as score 3, and 14 as score 4. Mortality rates were then combined to validate the ICH score. Mortality rates were 5%, 16%, 33%, 54%, and 93% in comparison to 0%, 13%, 26%, 72%, 94%, and 100% for scores of 0, 1, 2, 3, 4, and 5.
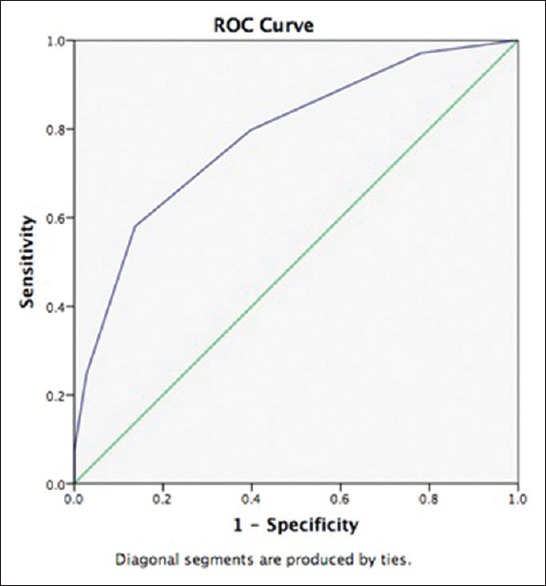
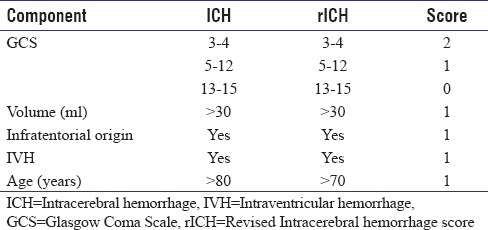
After adjusting the age factor in the original ICH score, 34 patients had a score of 0, 68 with score of 1, 53 with score of 2, 39 with score of 3, 16 with score of 4, and 5 with score of 5. Mortality rates with the new score were 6%, 15%, 25%, 51%, 75%, and 100%, respectively [Table 3]. ROC curve for the new modified SICH score was 78.4% with P ≤ 0.001 [Figure 1].
Table 3.
Comparing morality data with the original and our intracerebral hemorrhage scores
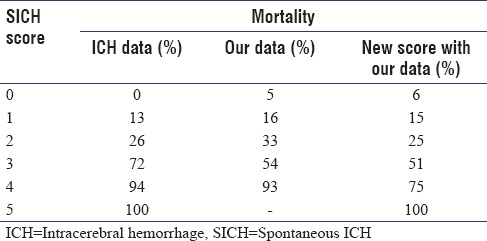
Figure 1.
Receiver operating characteristic curve for new intracerebral hemorrhage score
DISCUSSION
The original ICH score designed by Hemphill et al. in 2001 is one of the most widely used scoring systems which has been validated by multiple external studies.[11,12,13,14,15,16,17,18] The ICH score, however, fails to account for prebleed cognitive impairment. Few other drawbacks of the ICH score are its poor ability to predict functional outcome and failure to incorporate the NIHSS score which is a better predictor of outcome than GCS. The ICH score has evolved with the development of the modified ICH[15] score and mICH score A and B.[19] Both these scores are more complicated and difficult to apply in an emergency setting. Moreover, they offer only minimal or no advantage over the original ICH score in predicting mortality at 30 days. Other scores to predict functional outcome such as the ICH-grading scale score,[9] FUNC score,[20] the new ICH score,[15] the Essen score,[21] simplified ICH score,[22] and the mICH score[23] too have not become popular among neurologists.
The ICH score has been validated in North American, European, and Asian population but is yet to be validated in an Indian context.[11,16,17,24] The pattern and demography of SICH in India is different from the western world. The incidence of ICH is predominantly in the younger age group in comparison to the western population where the mean age was 70–79 years.[10,11,25] Most of the available studies from India report mean ages of 50–58 years.[26,27,28,29] Hypertensive ICH in India occurs 15–20 years earlier than their counterparts in the west. This can be attributed to the poor detection of hypertension in this younger population and use of irregular medications. Only 7 patients out of 215 (3.25%) were above the age of 80 in this group. The 2016 WHO report states that the average life expectancy of an Indian at birth is 68.3 years, and at 60 years, it is 17.9. Thus, most of our population fail to live beyond the age of 80 which is one of the cutoff criteria for the existing ICH scoring system. This was evident from our observation that none of the patients in this study group had a SICH score of 5.
We had 45 patients in the 70–79 years' age group and only 7 patients above the age of 80. Replacing the cutoff criteria of 80 years with 70 years, we observed that patients below 70 years had significantly lesser mortality rates (29%) compared to those above 70 years (42%). As the number of patients in the 71–80-year category was few, the mortality rate remained unchanged by reducing the cutoff age from 80 to 70 years. ROC curve for age >60 was only 41%, while for >70 years, it was 61%. Thus, we reduced the age criteria of the ICH score from 80 years to 70 years to accommodate for this differential occurrence of disease in the fourth-fifth decade.
With the new score, mortality rates in score 3 and 4 were ≈20% lesser than the original ICH data [Table 3]. We attribute this to better survival of younger patients and improvement of ICU and rehabilitation facilities in the 15 years that have passed since the development of ICH score. Validating the score helps us give better prognosis to patients with SICH score of 3 and 4, where aggressive treatment can help them survive and lead a meaningful life at the end of 1 year. We also calculated mortality based on 3-month mortality data instead of the 30-day endpoint, as extremely sick patients with mRS 5, when discharged succumb to the illness due to lack of airway care and infections within the first 3 months. Patients, who survive the first 3 months, improve thereafter and make good neurological recovery over time. Patients with mRS >3 had poor outcome mostly progressing to moribund conditions or death in this study, while patients with mRS of 1–3 make good functional recovery. Good prognosis can be offered to patients with SICH score of <3 in view of maximum mortality rates of 51%, with the new SICH score [Table 4].
Table 4.
The original intracerebral hemorrhage score with our proposed modification (rICH)
As described in earlier series, a GCS score of <8, clot volume of >30 ml, intraventricular extension of hematoma, an admission blood glucose of >140 mg/dl on admission, occurrence of hydrocephalus, and requirement of an external ventricular drainage were all significant risk factors to predict mortality in this study group [Table 5]. Modifying the other parameters of the ICH score such as GCS, volume of hematoma, infratentorial origin of hematoma, and intraventricular extension did not seem to change the outcome prognostication.
Table 5.
Significant factors affecting mortality in this study
This new scoring system is essentially aimed at optimizing and rationalizing medical facilities in a resource-constrained society like India. SICH patients in the 70–80-year group would now be assigned one extra point (unlike the existing scoring system) which would upgrade their overall SICH score, thereby worsening their prognosis. This would help medical caregiver and the family to optimize and tailor their resources knowing the ultimate prognosis. Indirectly, this would result in preferential allocation of resources to those with younger age and lower SICH score who constitute the majority of our patients.
CONCLUSION
The demographics of SICH in the Indian subcontinent are different from that observed in the western population. The ICH score has not been validated for an Indian population and in its current form doesn't ideally prognosticate outcome. We believe that a minor modification in the age component of the ICH score would suit the younger onset of disease in our country.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–44. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: Results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28:491–9. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Suri MF, Nasar A, Kirmani JF, Ezzeddine MA, Divani AA, et al. Changes in cost and outcome among US patients with stroke hospitalized in 1990 to 1991 and those hospitalized in 2000 to 2001. Stroke. 2007;38:2180–4. doi: 10.1161/STROKEAHA.106.467506. [DOI] [PubMed] [Google Scholar]
- 4.Fogelholm R, Murros K, Rissanen A, Avikainen S. Long term survival after primary intracerebral haemorrhage: A retrospective population based study. J Neurol Neurosurg Psychiatry. 2005;76:1534–8. doi: 10.1136/jnnp.2004.055145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broderick JP, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 1993;78:188–91. doi: 10.3171/jns.1993.78.2.0188. [DOI] [PubMed] [Google Scholar]
- 6.Hemphill JC, 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A Guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–60. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 7.Daverat P, Castel JP, Dartigues JF, Orgogozo JM. Death and functional outcome after spontaneous intracerebral hemorrhage. A prospective study of 166 cases using multivariate analysis. Stroke. 1991;22:1–6. doi: 10.1161/01.str.22.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Jiang B, Wang WZ, Chen H, Hong Z, Yang QD, Wu SP, et al. Incidence and trends of stroke and its subtypes in China: Results from three large cities. Stroke. 2006;37:63–8. doi: 10.1161/01.STR.0000194955.34820.78. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Sandoval JL, Chiquete E, Romero-Vargas S, Padilla-Martínez JJ, González-Cornejo S. Grading scale for prediction of outcome in primary intracerebral hemorrhages. Stroke. 2007;38:1641–4. doi: 10.1161/STROKEAHA.106.478222. [DOI] [PubMed] [Google Scholar]
- 10.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–7. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 11.Clarke JL, Johnston SC, Farrant M, Bernstein R, Tong D, Hemphill JC, 3rd, et al. External validation of the ICH score. Neurocrit Care. 2004;1:53–60. doi: 10.1385/NCC:1:1:53. [DOI] [PubMed] [Google Scholar]
- 12.van Asch CJ, Velthuis BK, Greving JP, van Laar PJ, Rinkel GJ, Algra A, et al. External validation of the secondary intracerebral hemorrhage score in the Netherlands. Stroke. 2013;44:2904–6. doi: 10.1161/STROKEAHA.113.002386. [DOI] [PubMed] [Google Scholar]
- 13.Hemphill JC, 3rd, Farrant M, Neill TA., Jr Prospective validation of the ICH score for 12-month functional outcome. Neurology. 2009;73:1088–94. doi: 10.1212/WNL.0b013e3181b8b332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer DM, Begtrup K, Grotta JC. Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Is the ICH score a valid predictor of mortality in intracerebral hemorrhage? J Am Assoc Nurse Pract. 2015;27:351–5. doi: 10.1002/2327-6924.12198. [DOI] [PubMed] [Google Scholar]
- 15.Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke. 2003;34:1717–22. doi: 10.1161/01.STR.0000078657.22835.B9. [DOI] [PubMed] [Google Scholar]
- 16.Godoy DA, Boccio A. ICH score in a rural village in the republic of Argentina. Stroke. 2003;34:e150–1. doi: 10.1161/01.STR.0000089493.23505.CA. [DOI] [PubMed] [Google Scholar]
- 17.Jamora RD, Kishi-Generao EM, Jr, Bitanga ES, Gan RN, Apaga NE, San Jose MC, et al. The ICH score: Predicting mortality and functional outcome in an Asian population. Stroke. 2003;34:6–7. doi: 10.1161/01.str.0000047847.18178.d3. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Lu J, Wang C, Wang Y, Li H, Zhao X, et al. Prognostic value of ICH score and ICH-GS score in Chinese intracerebral hemorrhage patients: Analysis from the China National Stroke Registry (CNSR) PLoS One. 2013;8:e77421. doi: 10.1371/journal.pone.0077421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godoy DA, Piñero G, Di Napoli M. Predicting mortality in spontaneous intracerebral hemorrhage: Can modification to original score improve the prediction? Stroke. 2006;37:1038–44. doi: 10.1161/01.STR.0000206441.79646.49. [DOI] [PubMed] [Google Scholar]
- 20.Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: The FUNC score. Stroke. 2008;39:2304–9. doi: 10.1161/STROKEAHA.107.512202. [DOI] [PubMed] [Google Scholar]
- 21.Weimar C, Benemann J, Diener HC. German Stroke Study Collaboration. Development and validation of the Essen Intracerebral Haemorrhage Score. J Neurol Neurosurg Psychiatry. 2006;77:601–5. doi: 10.1136/jnnp.2005.081117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang YC, Chen YM, Peng SK, Peng SY. Risk stratification for predicting 30-day mortality of intracerebral hemorrhage. Int J Qual Health Care. 2009;21:441–7. doi: 10.1093/intqhc/mzp041. [DOI] [PubMed] [Google Scholar]
- 23.Cho DY, Chen CC, Lee WY, Lee HC, Ho LH. A new modified intracerebral hemorrhage score for treatment decisions in basal ganglia hemorrhage – A randomized trial. Crit Care Med. 2008;36:2151–6. doi: 10.1097/CCM.0b013e318173fc99. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes H, Gregson BA, Siddique MS, Mendelow AD. Testing the ICH score. Stroke. 2002;33:1455–6. doi: 10.1161/01.str.0000018666.74574.9b. [DOI] [PubMed] [Google Scholar]
- 25.Masotti L, Lorenzini G, Di Napoli M, Godoy DA. Prognostic ability of four clinical grading scores in spontaneous intracerebral hemorrhage. Acta Neurol Belg. 2017;117:325–7. doi: 10.1007/s13760-016-0609-2. [DOI] [PubMed] [Google Scholar]
- 26.Namani G, Rampure DM, MM Clinical profile and mortality in patients presenting with intra-cerebral hemorrhage in a tertiary care centre. Sch J Appl Med Sci. 2014;2:3005–6. [Google Scholar]
- 27.Bhatia R, Singh H, Singh S, Padma MV, Prasad K, Tripathi M, et al. A prospective study of in-hospital mortality and discharge outcome in spontaneous intracerebral hemorrhage. Neurol India. 2013;61:244–8. doi: 10.4103/0028-3886.115062. [DOI] [PubMed] [Google Scholar]
- 28.Suthar NN, Patel KL, Saparia C, Parikh AP. Study of clinical and radiological profile and outcome in patients of intracranial hemorrhage. Ann Afr Med. 2016;15:69–77. doi: 10.4103/1596-3519.176259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayan SK, Sivaprasad P, Sushma S, Sahoo RK, Dutta TK. Etiology and outcome determinants of intracerebral hemorrhage in a South Indian population, A hospital-based study. Ann Indian Acad Neurol. 2012;15:263–6. doi: 10.4103/0972-2327.104333. [DOI] [PMC free article] [PubMed] [Google Scholar]


