Abstract
Objectives:
Many community-based and hospital-based studies across the world have yielded contradictory results regarding association of positive Toxocara canis serology and epilepsy. The present study was planned to analyze disease burden of epilepsy in rural community of North India and its association with exposure to T. canis in this part of the world.
Methods:
A door-to-door screening survey was carried out in the rural community using a validated questionnaire for epilepsy by trained field workers, which was finally confirmed by trained neurologists. The risk factors for epilepsy and for predisposing infections were also enquired. The results were compared with an equal number of age- and sex-matched healthy controls enrolled from the same community. Serologic evaluation was carried out to detect antibodies against T. canis.
Results:
A total of 41,973 persons from the rural community in 49 villages were enrolled in the study. Two hundred and eleven persons were confirmed to be suffering from active epilepsy, resulting in a crude prevalence of 5 per 1000 population. More than 50% of people with epilepsy were in the second or third decade of life. The prevalence of antibodies to T. canis was similar in people with epilepsy (13.7%; 29 of 211 individuals) and controls (9.95%; 21 of 211 individuals). Of the 151 persons with epilepsy, who underwent CT scan, 34 people (22.3%) had evidence of inflammatory granuloma, thereby confirming high incidence of this infestation in rural Northern India.
Significance:
Our study does not support the association between epilepsy and exposure to T. canis in rural Northern Indian population.
Keywords: Case–control study, epilepsy, neurocysticercosis, Toxocara canis
INTRODUCTION
A parasitic zoonosis, human toxocariasis results from ingestion (contaminated fomites, food, water, or soil etc.) of embryonated eggs of Toxocara Canis or catis, residing in lumen of small intestine of dogs or cats, their definitive hosts. Once eggs are ingested, the larva hatch and pass though intestine wall to reach blood circulation and their favorite tissues, namely liver, lungs, and central nervous system (CNS) including eyes. Once in these tissues, larva can induce severe local reactions which produce symptoms and signs associated with toxocariasis. The larvae can locate in the CNS leading to a variety of neurological disorders. In fact, several case reports and small series have reported neurological disorders such as meningitis, encephalitis, meningoencephalitis, myelitis, and optic neuritis to be associated with migrating T. Canis (Dog roundworm) larvae. Infection with T. canis, though cosmopolitan, is more common in rural populations of tropical countries as their hot and humid climate favors survival of eggs and poor oral hygienic practices common in rural areas of resource-poor countries predispose to infection with Toxocara.[1,2,3,4,5,6]
Epilepsy, a common neurological disorder affecting more than 70 million people worldwide, is more common in developing nations mainly due to the presence of infections which are endemic to these regions.[6,7] For several decades, researchers have focused on putative association between T. canis and epilepsy. However, a clear-cut relation between the two has not emerged till date. Though several studies[8,9,10] reported a positive association between Toxocara seropositivity and epilepsy, other studies[11,12,13] have yielded contradictory results. A recent meta-analysis of 7 studies included 4 studies with strong positive relationship, 1 with equivocal results, and 2 studies with negative association.[6] Although this meta-analysis supported positive association of Toxocara seropositivity with epilepsy, it also emphasized on need for future studies to clarify association between the two. The knowledge of contribution of toxocariasis to epilepsy is of paramount importance as it will help in institution of better preventive and therapeutic measures against this infection at the community level. The present study was planned to look at the disease burden of epilepsy and to study causal relationship between Toxocara seropositivity and epilepsy in a rural population in Northern India.
METHODS
The current study was a prospective, community-based, case–control observational study carried out from October 1, 2010 to September 30, 2013, in a rural population in Northern India.
Study design and sample size
The study was based on a door–to-door screening survey using a validated questionnaire for epilepsy followed by comprehensive assessments by two trained neurologists with experience in field of epilepsy. Based on the previous epidemiological surveys in India on Toxocara seroprevalence of close to 20%,[14,15] it was estimated that we needed to recruit at least 240 individuals (120 cases and 120 controls) to detect an odds ratio (OR) of 2.0 with 80% power at a 2-sided level of significance of 5%. However, in the present survey, we screened a population of about 42,000 people in 49 villages of Boothgarh block and stopped further screening because of time and financial constraints. Moreover, we had already enrolled more than desired individuals (211 cases and 211 controls against the planned 120 cases and controls each).
Community survey and recording of data
The chosen community belonged to Boothgarh block [Figure 1/Supplementary Table S1 (774.4KB, tif) ] in Mohali district of state of Punjab (India). This community was chosen due to its proximity to the institute where the study was undertaken. Necessary permissions were obtained from concerned health regulatory authorities before starting the study. We surveyed 49 villages of this block [listed in Supplementary Table, S1 (774.4KB, tif) ], details of which were obtained from Department of health and family welfare, Punjab, India. With the help of doctors, nurses and auxiliary nursing midwives posted in dispensaries, subsidiary, and primary health centers of Boothgarh block, rapport was built with village heads, and a door-to-door survey was conducted. To collect data, a validated screening questionnaire for detection of epilepsy was used which was adapted from previous epidemiological studies.[16,17] Three field workers were employed for this survey and educated about symptoms and signs of epilepsy. They were formally trained for 2 months by expert from Community Medicine (JST) for data collection in community and trained in the epilepsy clinics by trained Neurologists (MM, PSK and GS) for identifying epileptic patients. The questionnaire on epilepsy was applied to patients attending the neurology clinic of the institute in presence of the investigators. Once the field workers were fully trained and aware of the disease, they were sent to the rural areas to conduct the survey. The workers went to individual houses and collected information from the family head and other members as per pro forma. In case some persons were not available, revisits were made at appropriate times. The socioeconomic status of all the people was determined using modified Uday Pareek Scale.[18]
Figure 1.
Map of Boothgarh block villages
Field visit by neurologists
Once the people with suspected epilepsy were identified by field workers, two qualified neurologists (MM, PSK) with experience in epilepsy went to field and examined the shortlisted individuals. Epilepsy was defined as a condition characterized by recurrent (≥2) epileptic seizures, unprovoked by any immediate identified cause (ILAE, 1993).[19] Multiple seizures occurring in a 24-h period were considered a single event. Active epilepsy was defined as a person with epilepsy who has had at least one epileptic seizure in previous 5 years, regardless of treatment.[19]
All the shortlisted individuals underwent a detailed history and examination, after which they were labeled as suffering from epilepsy. History of alcohol and drug abuse was obtained in all individuals and seizures related to their overdosage or withdrawal were not considered epileptic.
Workup of patients
Five milliliter of blood was collected from all the patients and immediately shifted to laboratory for centrifugation in an ice box. The serum was stored at −80°C till further use. The people with epilepsy who consented for further workup were then called to our institute for evaluation regarding cause of epilepsy. Majority of patients (151 out of 211; 71.6%) underwent computed tomography (CT) scan of brain on high resolution 256 slice Philips CT scanner in the Department of Radiodiagnosis and Imaging under supervision of one of the investigator (NK). These patients also underwent electroencephalography (EEG) examination on Digital EEG (NIC Vue Viasys Healthcare Inc., Version 2.9.1, Database version 27, USA) in the Department of Neurology of the institute.
Controls
To compare the data, 211 age- and sex-matched controls were randomly selected from the same community. Written informed consent was obtained from all the patients and controls before inclusion in the study. The study was approved by Institutional Ethics Committee.
Detection of antitoxocara antibodies
Antibodies against T. canis were assayed using commercially available enzyme-linked immunosorbent assay (ELISA) (in vitro Diagnostic Research Carlshad, USA). The results were read using an ELISA reader at 450/650-620 nm. Absorbance reading of >0.3 OD units was considered positive. The sera of patients detected positive by ELISA method were further analyzed by using an immunoblot assay (Toxocara Immunoblot IgG, LD Bio Diagnostics, Lyon, France) as per the methodology provided with the kit, which is considered highly specific for Toxocariasis.
Statistical analysis
Descriptive statistics was used to analyze the data. Pearson's Chi-square test/Fisher's exact test was used for categorical data and working out the association between variables. To find out exact influence of various variables, multiple logistic regression analysis was used. P < 0.05 was considered significant. SPSS IBM SPSS Statistics for Windows, (Version 21.0, Armonk, NY: IBM Corp) was used for statistical analysis.
RESULTS
Three hundred and fifty individuals were screened positive by the field workers from the rural population comprising of 41,973 people. Of these, 104 individuals were excluded from the study (39 were cases of inactive epilepsy, 37 were related to alcohol and drug abuse, 15 were nonepileptic, 7 had single seizure, and 6 had only febrile seizures). The current study finally recruited 211 people with active epilepsy, thereby resulting in a crude prevalence of 5/1000 population. The results were compared with 211 age- and sex-matched healthy controls from the same community.
Demographic profile
The mean age was 25.6 ± 16.6 (range: 2–85 years) and 25.6 ± 15.9 (range: 4–82 years) in study and control groups, respectively. There were 109 (51.7%) men in the study group and 101 (47.9%) men in the control group. Both the groups were well matched with respect to age and sex. Most patients in the current study were in 2nd decade of life (n = 61; 28.9%) followed by 3rd decade (n = 46; 21.8%) and 4th decade, respectively (n = 33; 15.6%). Thus, epilepsy affected people in prime of their life.
Type of seizures
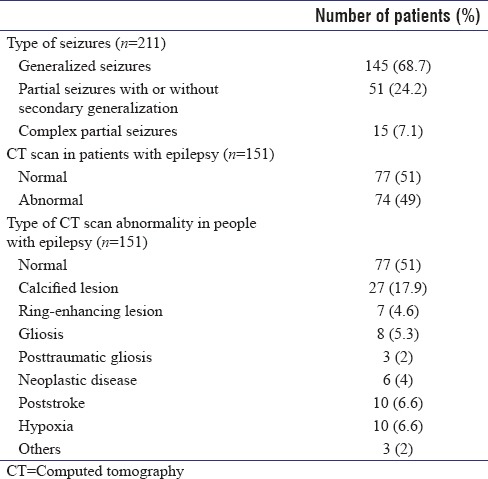
In the current study, we also determined type of seizures in people with epilepsy. Majority of patients have generalized tonic–clonic seizures (n = 145; 68.7%) followed by partial seizures with or without secondary generalization (n = 51; 24.2%) and complex partial seizures (n = 15; 7.1%) [Table 1].
Table 1.
Types of seizures and underlying etiology in people with epilepsy (n=211)
Etiology of epilepsy
In the current study, 151 out of 211 (71.6%) patients consented for workup and underwent CT scan of brain. CT scan of brain was abnormal in 74 (49%) patients, with inflammatory granuloma being the most common abnormality seen in 34 (22.5%) of patients ([calcified lesion: 27/151 (17.9%); ring-enhancing lesion suggestive of neurocysticercosis: 7/151 (4.6%)]). Majority of these patients with inflammatory granulomas (23 out of 34; 69%) were in the age group of 11–30 years. All the patients also underwent EEG to better delineate the epilepsy syndrome. Finally, based on clinical and investigational data, a consensus was obtained regarding the etiology of epilepsy. The type of seizures and CT brain findings are summarized in [Table 1].
Comparison of various socioeconomic factors among patients with and without epilepsy
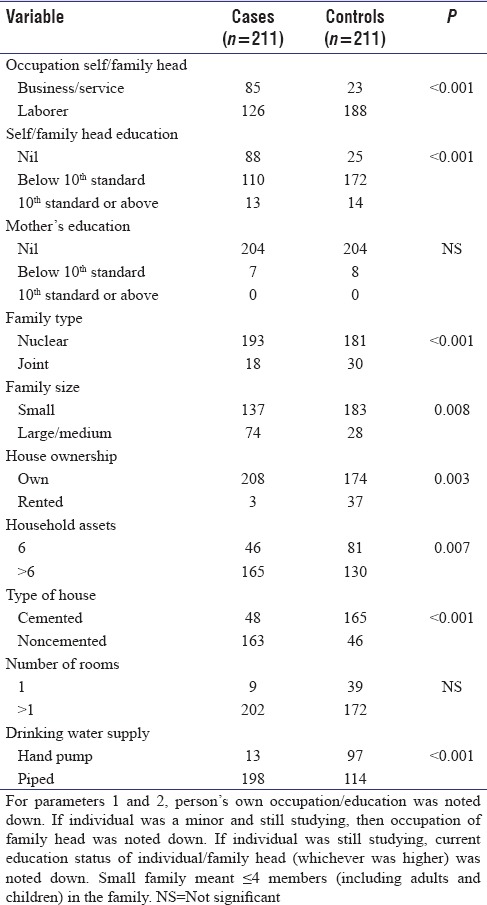
We compared influence of various socioeconomic and demographic factors such as occupation, education, family type, and size, on occurrence of epilepsy in cases and controls [Table 2].
Table 2.
Comparison of various socioeconomic variables among patients with and without epilepsy (multivariate logistic regression analysis)
Exposure to Toxocara Canis as well evaluation of risk factors predisposing to toxocariasis in people with epilepsy
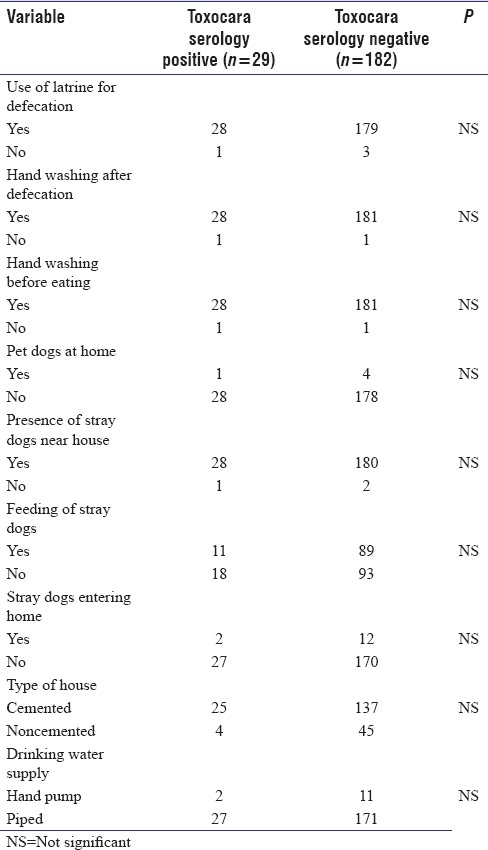
In the current study, Toxocara serology was positive in 31/211 patients with ELISA and was finally confirmed in 29/211 (13.7%) patients with epilepsy by western blot assay. In the control population, 23/211 individuals were positive for toxocara serology by ELISA, which was confirmed in 21/211 (9.95%) controls by western blot. There was no significant association between positive Toxocara serology and presence of epilepsy in rural North Indian population [Table 3]. We also analyzed influence of various epidemiological risk factors which are known to predispose to toxocariasis in people with epilepsy [Table 4].
Table 3.
Relationship between positive Toxocara canis serology with epilepsy
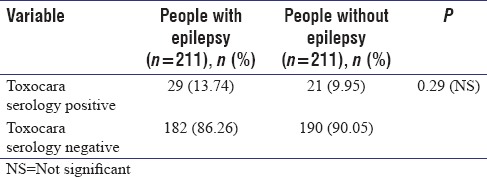
Table 4.
Comparison of factors known to predispose to Toxocara canis infection in toxocara serology-positive people with epilepsy and toxocara serology-negative people with epilepsy (multivariate logistic regression analysis)
DISCUSSION
In the current study, we aimed to determine prevalence of epilepsy and its relationship to T. canis infection in rural North Indian population. The crude prevalence of epilepsy in current study was 5/1000 population with approximately half the patients in 2nd and 3rd decade of life (107 out of 211 patients, 50.71%). Similar prevalence of active epilepsy (5.8 per 1000 population) was reported in a study carried out in rural pig farming community of North India.[20] However, the prevalence of epilepsy in the current study was lower as compared to two previous studies done in Northern India (7.2 and 7.67/1000, respectively).[12,21] Another study from rural population of Uttrakhand, North India reported a crude prevalence of 10/1000 individuals.[22] The reported prevalence of epilepsy world over is as low as 3.3/1000 population in developed countries of Europe[23] compared to 12.5/1000 in resource-poor countries like Zambia.[24] The prevalence of active epilepsy across 5 centers in a recent study in Sub-Saharan Africa varied from 7.8 to 10.3 per 1000 population.[10] The possible reason for lower prevalence of epilepsy in the current study may be related to the differences in socioeconomic and educational status as well as better access to healthcare facilities in our study population.
In the current study, >50% of patients with epilepsy were in 2nd and 3rd decade of life. Similar observation has been made in a recently published meta-analysis, where peak prevalence rates were reported in the second and third decades.[25] A Chinese study also reported the prevalence of epilepsy to increase till 2nd and 3rd decade followed by a fall in later years.[26] Of the 107 patients of epilepsy in this age group of second and third decade, 23 patients (21.5%) had evidence of inflammatory granuloma on imaging. While higher prevalence of epilepsy in this age group may be related to premature mortality in people with epilepsy, more exposure to CNS infections and infestations in this age group may also be contributing to higher epilepsy prevalence.
In the current study, inflammatory granuloma (Neurocysticercosis, NCC) accounted for 22.5% of all cases of epilepsy. A slum-based study reported evidence of neurocysticercosis in 17% of patients with active epilepsy from another region in Northern India. A still higher incidence of NCC has been reported in other community-based prevalence studies from other regions of India. In the study from Vellore, South India, 34% of patients with active epilepsy were confirmed to have NCC by CT scan and enzymelinked immunotransfer blot (EITB)[27] and another study from Dehradun, North India, 35% patients of epilepsy had NCC based only on CT scan.[22] These evidences suggest an urgent need for preventive programs aimed at curtailing this infection through public education measures, more so in resource-poor nations.
Regarding various socioeconomic factors which can influence prevalence of epilepsy, we found a significant positive association between factors which predict a better socioeconomic status (availability of toilet in household, higher educational status of self/family head, own house, small family size, cemented house, and more household assets) and prevalence of epilepsy. The overall low prevalence of active epilepsy in the present study could be a possible explanation for this observation.
In the present study, no association could be found between T. canis seropositivity and epilepsy. In one study, 231 people with epilepsy were evaluated for the presence of antibodies against T. canis, and results were compared with 201 healthy controls. While T. canis antibodies were positive in 38% of patients, corresponding figure was 6.6% for controls, yielding as OR of 2.85 for occurrence of epilepsy in people who were positive for T. Canis antibodies. On subgroup analysis, it was found that there was a significant higher risk (OR - 3.9) of partial seizures compared to generalized seizures (OR - 1.74) in patients who were T. Canis seropositive.[28] In another study, 191 people with epilepsy were evaluated for the presence of antibodies against T. canis and results were compared with 191 healthy controls. T. canis antibodies were positive in 59.7% of patients and 50.8% of controls. There was a significantly higher risk of epilepsy (OR - 2.13; 95% confidence interval: 1.18–3.83) in patients who were positive for T. canis antibodies.[29] Other studies[9,10] have also reported a positive association between presence of T. canis antibodies and epilepsy. On the other hand, several researchers have reported lack of such an association.[11,12,13] This may be due to different methodologies adopted by different authors as well as to differences in population characteristics, risk factor profile for epilepsy, and degree of endemicity for T. canis. In addition, though some studies do suggest a modest association of T. canis seropositivity with epilepsy in certain geographic areas, its causal relationship still needs to be determined. The neuropathological and structural basis of presumed cerebral infestation to epilepsy still remains elusive.
In the current study, we also tried to determine the influence of various factors, which are reported to be associated with T. canis seropositivity such as use of latrine for defecation, hand washing practices, and pet dog/stray dog presence. However, we could not find any factor predisposing to T. canis seropositivity in our study. Our results were similar to another study with respect to type of housing,[12] with respect to defecation in open space,[9,29] and with respect to dog ownership and contact with dogs.[28] Our results contrasted from a study in children[30] who reported high Toxocara seropositivity in homes with gardens underlining exposure to clay as a risk factor for toxocariasis. Our results also contrasted from some studies, who reported positive association of Toxocara seropositivity with contact of dogs and lack of safe water supply, respectively.[12,15] A significant association with Toxocara seropositivity (P < 0.05) and presence of puppies at home was reported by Schantz et al.[31] The main reason for these differences may be the good socioeconomic status and different genetic predisposition of study population and the different methodologies used by different authors.
The main strength of our study was a homogeneous population, large sample size, and well-conducted prospective study wherein majority of patients underwent good evaluation for underlying cause. The main limitation of our study was that we could not carry out imaging of brain in all people with epilepsy. We also could not do EITB assays for Taenia solium infestation in patients with epilepsy to confirm the diagnosis of definitive NCC due to financial constraints.
Furthermore, we focused only on people with new-onset epilepsy and ignored patients with single unprovoked seizures which could have been included in the study. To conclude, our study demonstrated lack of association T. canis seropositivity and epilepsy in rural Northern Indian population. Future studies including both people with epilepsy and single unprovoked seizures may help to understand this association in a better way.
Financial support and sponsorship
The study was funded by Indian Council of Medical Research, New Delhi.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The research project was supported by Indian Council of Medical Research, New Delhi vide Project no. 5/4–5/22/Neuro/2010-NCD-I. We acknowledge the support by Ms. Supriya for data entry, coordination of the CT scans and EEG's of patients; Mr. Ashok Kumar for statistical analysis; Mr. Sandeep Sharma for blood sample processing.
REFERENCES
- 1.Gould IM, Newell S, Green SH, George RH. Toxocariasis and eosinophilic meningitis. Br Med J (Clin Res Ed) 1985;291:1239–40. doi: 10.1136/bmj.291.6504.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rüttinger P, Hadidi H. MRI in cerebral toxocaral disease. J Neurol Neurosurg Psychiatry. 1991;54:361–2. doi: 10.1136/jnnp.54.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ota S, Komiyama A, Johkura K, Hasegawa O, Kondo K. Eosinophilic meningo-encephalo-myelitis due to Toxocara canis. Rinsho Shinkeigaku. 1994;34:1148–52. [PubMed] [Google Scholar]
- 4.Wang C, Huang CY, Chan PH, Preston P, Chau PY. Transverse myelitis associated with larva migrans: Finding of larva in cerebrospinal fluid. Lancet. 1983;1:423. doi: 10.1016/s0140-6736(83)91544-1. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi E, Akao N, Fujita K. Evidence for the involvement of the optic nerve as a migration route for larvae in ocular toxocariasis of Mongolian gerbils. J Helminthol. 2003;77:311–5. doi: 10.1079/joh2003186. [DOI] [PubMed] [Google Scholar]
- 6.Quattrocchi G, Nicoletti A, Marin B, Bruno E, Druet-Cabanac M, Preux PM, et al. Toxocariasis and epilepsy: Systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1775. doi: 10.1371/journal.pntd.0001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amudhan S, Gururaj G, Satishchandra P. Epilepsy in India I: Epidemiology and public health. Ann Indian Acad Neurol. 2015;18:263–77. doi: 10.4103/0972-2327.160093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Critchley EM, Vakil SD, Hutchinson DN, Taylor P. Toxoplasma, toxocara, and epilepsy. Epilepsia. 1982;23:315–21. doi: 10.1111/j.1528-1157.1982.tb06197.x. [DOI] [PubMed] [Google Scholar]
- 9.Nicoletti A, Bartoloni A, Reggio A, Bartalesi F, Roselli M, Sofia V, et al. Epilepsy, cysticercosis, and toxocariasis: A population-based case-control study in rural Bolivia. Neurology. 2002;58:1256–61. doi: 10.1212/wnl.58.8.1256. [DOI] [PubMed] [Google Scholar]
- 10.Ngugi AK, Bottomley C, Kleinschmidt I, Wagner RG, Kakooza-Mwesige A, Ae-Ngibise K, et al. Prevalence of active convulsive epilepsy in Sub-Saharan Africa and associated risk factors: Cross-sectional and case-control studies. Lancet Neurol. 2013;12:253–63. doi: 10.1016/S1474-4422(13)70003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akyol A, Bicerol B, Ertug S, Ertabaklar H, Kiylioglu N. Epilepsy and seropositivity rates of Toxocara canis and Toxoplasma gondii. Seizure. 2007;16:233–7. doi: 10.1016/j.seizure.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Singh G, Bawa J, Chinna D, Chaudhary A, Saggar K, Modi M, et al. Association between epilepsy and cysticercosis and toxocariasis: A population-based case-control study in a slum in India. Epilepsia. 2012;53:2203–8. doi: 10.1111/epi.12005. [DOI] [PubMed] [Google Scholar]
- 13.Eraky MA, Abdel-Hady S, Abdallah KF. Seropositivity of Toxoplasma gondii and Toxocara spp. In children with cryptogenic epilepsy, Benha, Egypt. Korean J Parasitol. 2016;54:335–8. doi: 10.3347/kjp.2016.54.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malla N, Aggarwal AK, Mahajan RC. A serological study of human toxocariasis in North India. Natl Med J India. 2002;15:145–7. [PubMed] [Google Scholar]
- 15.Dar ZA, Tanveer S, Yattoo GN, Sofi BA, Wani SA, Dar PA, et al. Seroprevalance of toxocariasis in children in Kashmir, J and K State, India. Iranian J Parasitol. 2008;3:45–50. [Google Scholar]
- 16.Wang WZ, Wu JZ, Wang DS, Dai XY, Yang B, Wang TP, et al. The prevalence and treatment gap in epilepsy in China: An ILAE/IBE/WHO study. Neurology. 2003;60:1544–5. doi: 10.1212/01.wnl.0000059867.35547.de. [DOI] [PubMed] [Google Scholar]
- 17.Gourie-Devi M, Gururaj G, Satishchandra P, Subbakrishna DK. Prevalence of neurological disorders in Bangalore, India: A community-based study with a comparison between urban and rural areas. Neuroepidemiology. 2004;23:261–8. doi: 10.1159/000080090. [DOI] [PubMed] [Google Scholar]
- 18.Pareek U, Trivedi G. Manual of Socio-Economic Status Scale. Delhi, India: Manasyan Publishers; 1979. [Google Scholar]
- 19.Guidelines for epidemiologic studies on epilepsy. Commission on epidemiology and prognosis, international league against epilepsy. Epilepsia. 1993;34:592–6. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 20.Prasad KN, Prasad A, Gupta RK, Nath K, Pradhan S, Tripathi M, et al. Neurocysticercosis in patients with active epilepsy from the pig farming community of Lucknow district, North India. Trans R Soc Trop Med Hyg. 2009;103:144–50. doi: 10.1016/j.trstmh.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Hara HS, Gupta A, Singh M, Raj R, Singh H, Pawar G, et al. Epilepsy in Punjab (India): A Population-based epidemiologic study. Neuroepidemiology. 2015;45:273–81. doi: 10.1159/000438509. [DOI] [PubMed] [Google Scholar]
- 22.Goel D, Dhanai JS, Agarwal A, Mehlotra V, Saxena V. Neurocysticercosis and its impact on crude prevalence rate of epilepsy in an Indian community. Neurol India. 2011;59:37–40. doi: 10.4103/0028-3886.76855. [DOI] [PubMed] [Google Scholar]
- 23.Rocca WA, Savettieri G, Anderson DW, Meneghini F, Grigoletto F, Morgante L, et al. Door-to-door prevalence survey of epilepsy in three Sicilian municipalities. Neuroepidemiology. 2001;20:237–41. doi: 10.1159/000054796. [DOI] [PubMed] [Google Scholar]
- 24.Birbeck GL, Kalichi EM. Epilepsy prevalence in rural Zambia: A door-to-door survey. Trop Med Int Health. 2004;9:92–5. doi: 10.1046/j.1365-3156.2003.01149.x. [DOI] [PubMed] [Google Scholar]
- 25.Gourie-Devi M. Epidemiology of neurological disorders in India: Review of background, prevalence and incidence of epilepsy, stroke, Parkinson's disease and tremors. Neurol India. 2014;62:588–98. doi: 10.4103/0028-3886.149365. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Wu J, Dai X, Ma G, Yang B, Wang T, et al. Global campaign against epilepsy: Assessment of a demonstration project in rural China. Bull World Health Organ. 2008;86:964–9. doi: 10.2471/BLT.07.047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajshekhar V, Raghava MV, Prabhakaran V, Oommen A, Muliyil J. Active epilepsy as an index of burden of neurocysticercosis in Vellore district, India. Neurology. 2006;67:2135–9. doi: 10.1212/01.wnl.0000249113.11824.64. [DOI] [PubMed] [Google Scholar]
- 28.Nicoletti A, Sofia V, Mantella A, Vitale G, Contrafatto D, Sorbello V, et al. Epilepsy and toxocariasis: A case-control study in Italy. Epilepsia. 2008;49:594–9. doi: 10.1111/j.1528-1167.2007.01432.x. [DOI] [PubMed] [Google Scholar]
- 29.Nicoletti A, Bartoloni A, Sofia V, Mantella A, Nsengiyumva G, Frescaline G, et al. Epilepsy and toxocariasis: A case-control study in Burundi. Epilepsia. 2007;48:894–9. doi: 10.1111/j.1528-1167.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 30.Arpino C, Gattinara GC, Piergili D, Curatolo P. Toxocara infection and epilepsy in children: A case-control study. Epilepsia. 1990;31:33–6. doi: 10.1111/j.1528-1157.1990.tb05356.x. [DOI] [PubMed] [Google Scholar]
- 31.Schantz PM, Weis PE, Pollard ZF, White MC. Risk factors for toxocaral ocular larva migrans: A case-control study. Am J Public Health. 1980;70:1269–72. doi: 10.2105/ajph.70.12.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.