Abstract
Diagnostic and prognostic biomarkers of traumatic brain injury (TBI) are actively being pursued; potential candidates include glial fibrillary acid protein (GFAP), S100 calcium-binding protein B (S100B), and ubiquitin C-terminal hydrolase L1 (UCHL1), two of which the United States Food and Drug Administration (FDA) recently approved for marketing of blood tests for adult concussion. The relationship between biomarker-encoding genes and TBI outcomes remains unknown. This pilot study explores variation in 18 single nucleotide polymorphisms (SNPs) in biomarker-encoding genes as predictors of neurological outcome in a population of adults with severe TBI. Participants (n = 305) were assessed using the Glasgow Outcome Scale (GOS) at 3, 6, 12, and 24 months post-injury. Multivariate logistical regression was used to calculate the odds ratio (OR) and determine the odds of having a lower score on the GOS ( = 1–2 vs. 3–5) based on variant allele presence, while controlling for confounders. Possession of the variant allele of one S100B SNP (rs1051169) was associated with higher scores on the GOS at 3 months (OR = 0.39; p = 0.04), 6 months (OR = 0.34; p = 0.02), 12 months (OR = 0.32; p = 0.02), and 24 months (OR = 0.30; p = 0.02) post-severe TBI. The relationship among these polymorphisms, protein levels, and biomarker utility, merits examination. These findings represent a novel contribution to the evidence that can inform future studies aimed at enhancing interpretation of biomarker data, identifying novel biomarkers, and ultimately harnessing this information to improve clinical outcomes and personalize care.
Keywords: : biomarkers, genes, genotype, neurological outcomes, TBI
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability worldwide, affecting individuals of all ages.1 Many TBI survivors experience deficits long after injury, especially following severe TBI (sTBI),2 defined as an admission Glasgow Coma Scale (GCS) score of ≤8. There are no Food and Drug Administration (FDA)-approved therapies for TBI, and efforts to further investigate treatment modalities and individual differences to improve TBI outcomes are ongoing. A critical part of this effort includes identification of biomarkers that can serve as indicators of normal biological processes, pathogenic processes, or responses to an exposure/intervention in TBI patients.3 Identification and validation of blood-based biomarkers that have diagnostic and prognostic utility for the TBI population are especially of interest.4–6 Three promising biomarker candidates7 include: glial fibrillary acid protein (GFAP), S100 calcium-binding protein β (S100β), and ubiquitin C-terminal hydrolase-L1 (UCH-L1). GFAP is an intermediate filament protein implicated in the structure of many central nervous system cell types. The structural importance of GFAP makes it an excellent indicator of damage; it is one of the most well-studied biomarkers in the context of brain trauma.8–12 S100B is a cytoplasmic and nuclear protein that is found in a multiplicity of cell types and is involved in the regulation of basic cellular processes including differentiation and progression through the cell cycle, and has shown utility in European trauma centers;13–15 UCH-L1 is a deubiquitinating enzyme that is highly expressed in neurons; UCH-L1 levels in blood have been associated with poor outcomes after stroke,16 subconcussive hits,17 and TBI.9,18,19 Recently, the FDA granted Banyan Biomarker, Inc. permission to market the Brain Trauma Indicator as a blood test for the evaluation of mild TBI, which includes GFAP and UCH-L1.
Considering the promise of dynamic protein levels for TBI diagnosis and prognosis, it may prove clinically useful to identify stable biomarkers (e.g., single nucleotide polymorphisms (SNPs) in DNA) that impact biomarker protein expression and/or directly impact the biological and clinical responses to TBI. Such genetic variation may prove to be a strong independent predictor of outcomes or a confounder of biomarker utility for certain individuals. To date, the impact of genetic polymorphisms for the genes encoding these candidate biomarker proteins remains unknown, but SNPs in other genes have been found to independently predict outcomes after TBI,20 adding to the rationale for this study. This study examines 18 SNPs in genes encoding three putative protein biomarkers for TBI (GFAP, S100B, and UCH-L1); the SNPs chosen for analysis in the present study have been previously found to show variation at the population level. None of the 18 SNPs examined in this study have been previously explored in the context of TBI. For 12 of the SNPs (rs12222, rs2289679, rs11651396, rs3760379, rs3785891, rs2239574, rs2839365, rs34722617, rs10517002, rs10517003, rs16852986, and rs17528160), no published literature is available. The remaining six SNPs have been previously studied in another context, specifically: schizophrenia (rs1051169),21–23 dementia (rs4861387),24 Parkinson's disease (rs1051169),25 an isolated population from the island of Kosrae (rs17629022),26 or healthy controls (rs2839357, rs9984765, and rs881827).21,27 For those six SNPs that have been previously reported on, associations with neurological disease (e.g., schizophrenia; dementia)21–24 and circulating biomarkers were found.27 The abovementioned evidence, lack of published data for any SNP in TBI, and the known link between other SNPs and TBI recovery from our past studies, led to the hypothesis that variation in these 18 SNPs may be associated with outcome variability after TBI. The present pilot study represents an initial exploration of the association between neurological outcomes at 3, 6, 12 and 24 months after TBI and 18 SNPs in genes encoding GFAP, S100B, and UCH-L1.
Methods
Sample
Participants were recruited from a neurointensive care unit at a level 1 trauma center between 2000 and 2012 and co-enrolled under approved institutional review board (IRB) protocols (University of Pittsburgh IRB# 971212 and 001004). Prior to data collection, consent was obtained from the authorized legal representative of each sTBI participant. If, during the course of the study, the participant's status improved to the extent that (s)he had capacity, informed consent for continued participation was obtained.
Participants in the present study enrolled individuals based on the following inclusion criteria: (1) diagnosed with an sTBI (GCS ≤8), (2) 18–80 years old, and (3) presence of a ventriculostomy catheter. Exclusion criteria were: (1) penetrating TBI, (2) cardiac and/or respiratory arrest, and/or (3) any pre-existing neurological deficit that could confound scoring on the chosen measures (e.g., GCS, Glasgow Outcome Scale [GOS]), such as an intellectual disability, stroke, or dementia.
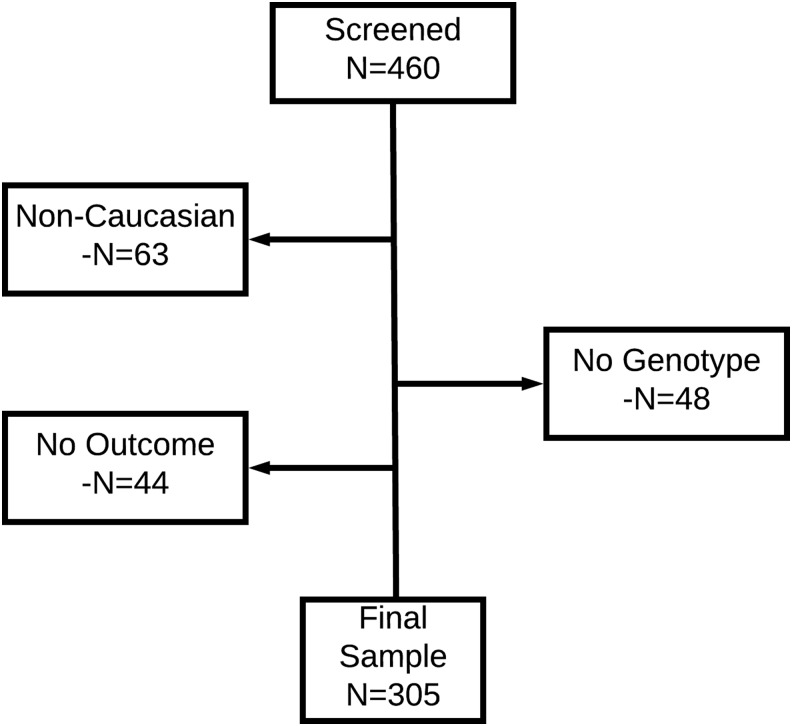
A total of 460 adults with sTBI were enrolled. Additional criteria for this genetic-based study approach were added, including the decision to limit the sample to Caucasians because of the low enrollment of black, Hispanic, and other non-Caucasian participants (n = 63), combined with the possible confounding effects of known allelic frequency differences based on ancestry. To address the goals of the study, individuals without genotypic data for at least one of the 18 SNPs of interest (n = 48) and those without valid GOS data for at least one post-injury time point (n = 44) were also eliminated. The final sample (n = 305) consisted of adult, Caucasian, sTBI patients with some genotypic and phenotypic data (Fig. 1).
FIG. 1.
Study sample-consort diagram.
Biospecimen collection, processing, and analysis
Biospecimens used for genotyping were either cerebrospinal fluid (CSF) or blood, both collected under the same IRB-approved protocol. CSF was collected from an indwelling ventriculostomy catheter and blood was collected from either an intravenous or an intra-arterial catheter. DNA was extracted from CSF using the Qiamp Midi Kit (QIAGEN Inc., Valencia, CA) and from blood by centrifugation, removal of buffy coat, and extraction of the DNA from buffy coat using a salting out procedure, as previously described.28 A total of 18 SNPs representing three candidate biomarker genes (GFAP, S100B, and UCHL1) were genotyped using the iPLEX MassARRAY multiplex assay platform (Sequenom Inc., San Diego, CA). Genotype quality control included independently double calling genotypes, inclusion of blind duplicates, and excluding SNPs not meeting a minimum 85% call rate from analyses.
Measures
Upon patients' admission, initial GCS score assessed by a neurosurgeon after resuscitation was confirmed for inclusion. GOS was assessed and collected by a trained neuropsychological technician at 3, 6, 12, and 24 months post-injury. To ensure that groups were sufficiently large and balanced for analysis, GOS scores were dichotomized as 1–2 versus 3–5, with lower scores corresponding to profound injury (i.e., death or a vegetative state). Whenever possible, GOS scoring was completed face to face, but when this was not possible, phone interviews were performed. Personnel involved in data collection were blinded to participant genotype.
Statistical analysis
SPSS version 24 software (IBM, Chicago, IL) was used for all statistical analyses. Variables were screened and summary statistics (e.g., mean, standard deviation, range) were generated for all continuous variables; frequencies were generated for categorical variables. Univariate associations between interval-level variables (age, Injury Severity Score [ISS] and GOS scores at 3, 6, 12, and 24 months were assessed with logistical regression. Univariate associations among categorical variables (e.g., sex, GCS) were assessed using χ2 analysis and, when appropriate, Fisher's exact test. Multivariate logistical regression controlling for age and sex, as well as admission GCS, was used to explore the relationship between variant allele presence and neurological outcomes (i.e., GOS = 1–2 vs. 3–5) assessed at 3, 6, 12, and 24 months. A priori criteria for statistical significance was set at p < 0.05. Because of the descriptive nature of this pilot study, and the primary goal of identifying possible confounders in biomarker studies, corrections for multiple comparisons were not performed. The rationale was that correcting for multiple comparisons would have increased the chance of type II errors, which would potentially make subsequent regression models too lenient.
Results
Sample demographics
Details for each SNP examined in this study are provided in Table 1. Highlighted summary information includes: related biomarker, protein function, wild-type (WT) versus variant allele, and SNP location. Data for each SNP are also reported in Table 1, including genotypic frequencies, the percent of the sample genotyped, and minor (i.e., variant) allele frequency (MAF); the MAF in a Caucasian population from the Single Nucleotide Polymorphism database (dbSNP).
Table 1.
Summary Information for the 18 Single Nucleotide Polymorphisms Examined in This Study
| SNP | Biomarker | Known function(s) of the candidate biomarker protein | Alleles WT/ variant (1000 genomes) | Homozygous wild type n = | Homozygous variant n = | Heterozygous n = | Genotyped n = (% of sample) | Study MAF | dbSNP MAF | Location of SNP |
|---|---|---|---|---|---|---|---|---|---|---|
| rs12222 | GFAP | Glial fibrillary acid protein (GFAP) is a type of intermediate filament critical to nerve structure and support. Following TBI, astroglial cells increase GFAP production. In February 2018, the FDA authorized marketing of blood tests for GFAP as a prognostic marker of adult concussion. | T/C | 172 | 26 | 92 | 290 (95.1%) | 0.248 | 0.244 | Downstream |
| rs17629022 | T/C | 180 | 16 | 93 | 289 (94.8%) | 0.216 | 0.184 | Intron | ||
| rs2289679 | G/C | 162 | 24 | 80 | 266 (87.2%) | 0.241 | 0.240 | Intron | ||
| rs11651396 | G/A | 140 | 121 | 1 | 262 (85.9%) | 0.464 | 0.244 | Intron | ||
| rs3760379 | C/T | 102 | 58 | 119 | 279 (91.5%) | 0.421 | 0.339 | Upstream | ||
| rs3785891 | C/T | 134 | 36 | 112 | 282 (92.5%) | 0.326 | 0.260 | Intron | ||
| rs2239574 | S100B | S100 calcium binding protein B (S100B) is involved in regulating a variety of cellular processes, including the cell cycle and differentiation. | C/T | 146 | 23 | 115 | 284 (93.1%) | 0.283 | 0.259 | Intron |
| rs2839357 | A/G | 232 | 7 | 42 | 281 (92.1%) | 0.100 | 0.133 | Intron | ||
| rs2839365 | G/C | 105 | 41 | 127 | 273 (89.5%) | 0.383 | 0.400 | Upstream | ||
| rs1051169 | G/C | 28 | 129 | 129 | 286 (93.8%) | 0.677 | 0.433 | Exon | ||
| rs9984765 | T/C | 168 | 13 | 103 | 284 (93.1%) | 0.227 | 0.283 | Intron | ||
| rs34722617 | A/T | 146 | 26 | 114 | 286 (89.5%) | 0.290 | 0.263 | Intron | ||
| rs881827 | C/T | 141 | 32 | 111 | 284 (93.1%) | 0.308 | 0.283 | Intron | ||
| rs10517002 | UCHL1 | Ubiquitin C-terminal hydrolase L1 (UCHL1) is an enzyme abundant in nerve cells. UCHL1 is hypothesized to be implicated in degrading unneeded or damaged cells, such as those that would arise as part of TBI pathology. In February 2018, the FDA authorized marketing of blood tests for UCHL1 as a prognostic marker of adult concussion. | A/C | 105 | 56 | 114 | 275 (90.2%) | 0.411 | 0.377 | Intron |
| rs10517003 | A/G | 168 | 24 | 91 | 283 (92.8%) | 0.246 | 0.103 | Intron | ||
| rs16852986 | T/C | 161 | 25 | 93 | 279 (91.5%) | 0.256 | 0.164 | Downstream | ||
| rs17528160 | G/A | 168 | 21 | 95 | 284 (93.1%) | 0.241 | 0.103 | Intron | ||
| rs4861387 | G/A | 156 | 26 | 91 | 273 (89.5%) | 0.262 | 0.173 | Intron |
Summary of SNPs in the gene(s) encoding each relevant biomarker, including: wild-type and variant alleles, frequencies of each genotype in the sample, the percent of the sample genotyped, sample minor allele frequency, published minor allele frequency, and location of the SNP with the gene.
TBI, traumatic brain injury; FDA, United States Food and Drug Administration; WT, wild type; MAF, minor (i.e., variant) allele frequency; SNP, single nucleotide polymorphism.
On admission, the sample population was primarily male (80.7%), Caucasian, with an average age of 39.1 years (SD = 16.4, range = 18–76 years). The most common cause of injury was automobile accidents (n = 138; 45.2%), followed by falls/jumps (n = 57; 18.7%). Other common causes of injury were transit related, including injuries related to: motorcycles (n = 47; 15.4%), all-terrain vehicles (ATV) (n = 17; 5.6%), bicycles (n = 8; 2.7%), trucks (n = 8; 2.6%), industrial/farming vehicles (n = 4; 1.3%), buses (n = 2; 0.7%), and trains (n = 1; 0.3%). The remaining injury mechanisms included assaults (n = 6; 2.6%), being hit by an object (n = 3; 1.0%), or other/unknown causes (n = 6; 2.6%). The ISS was available for 302 of the participants, and ranged from 8 to 75 with an average of 33.72 (SD 10.9). Only two patients had a score of 75, suggesting unsurvivable injuries. ISS was not significantly associated with outcomes at 3, 6, 12, or 24 month GOS time points (p = 0.962, p = 0.397, p = 0.218, p = 0.189; data not shown). All participants had sTBI (GCS ≤8); two dichotomized groups were formed (GCS = 3–5 vs. 6–8) and the majority (62.3%) of participants had an admission GCS score within the higher category, suggesting better functionality on admission. GOS score at all time points (3, 6, 12, and 24 months) was univariately associated with age and admission GCS score (all p's < 0.0005). Select baseline sample demographics are reported in Table 2.
Table 2.
Demographic Characteristics of the Sample (Age, Sex, Admission GCS) at Baseline and by Dichotomized GOS Group (1–2 vs. 3–5) at 3, 6, 12, and 24 Months after Severe TBI, as Assessed Using Logistical Regression (Age) or χ2 Analysis (Sex and GCS)
| 3 month GOS | 6 month GOS | 12 month GOS | 24 month GOS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Sample | 1–2 | 3–5 | Test and p | 1–2 | 3–5 | Test and p | 1–2 | 3–5 | Test and p | 1–2 | 3–5 | Test and p |
| Age (Years) | |||||||||||||
| n (total) | n = 305 | n = 108 | n = 177 | OR = 0.948 (95% CI = 0.932–0.964) p < 0.0005*** | n = 107 | n = 177 | OR = 0.943 (95% CI = 0.927–0.960) p < 0.0005*** | n = 109 | n = 167 | OR = 0.941 (95% CI = 0.925–0.958) p < 0.0005*** | n = 108 | n = 134 | OR = 0.939 (95% CI = 0.922–0.957) p < 0.0005*** |
| Mean | 39.1 | 48.1 | 34.7 | 48.8 | 34.1 | 48.9 | 33.7 | 49.3 | 33.7 | ||||
| (SD) | (16.4) | (17.0) | (14.0) | (16.6) | (14.1) | (16.8) | (13.7) | (16.8) | (13.7) | ||||
| Range | 18–76 | 18–76 | 18–74 | 18–76 | 18–74 | 18–76 | 18–74 | 18–76 | 18–74 | ||||
| Sex | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (total) | n = 305 | n = 108 | n = 177 | χ2 = 0.141 p = 0.710 | n = 107 | n = 177 | χ2 = 0.077 p = 0.781 | n = 109 | n = 167 | χ2 = 0.001 p = 0.977 | n = 108 | n = 134 | χ2 = 0.176 p = 0.675 |
| n Male | n = 246 | n = 88 | n = 141 | n = 85 | n = 143 | n = 86 | n = 132 | n = 87 | n = 105 | ||||
| (%) | (80.7%) | (81.5%) | (79.7%) | (79.4%) | (80.8%) | (78.9%) | (79.0%) | (80.6%) | (78.4%) | ||||
| n Female | n = 59 | n = 20 | n = 36 | n = 22 | n = 34 | n = 23 | n = 35 | n = 21 | n = 29 | ||||
| (%) | (19.3%) | (18.5%) | (20.3%) | (20.6%) | (19.2%) | (21.1%) | (21.0%) | (19.4%) | (21.6%) | ||||
| Admission GCS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (total) | n = 305 | n = 108 | n = 177 | χ2 = 34.972 p < 0.0005*** | n = 107 | n = 177 | χ2 = 32.869 p < 0.0005*** | n = 109 | n = 167 | χ2 = 24.896 p < 0.0005*** | n = 108 | n = 134 | χ2 = 18.624 p < 0.0005*** |
| n GCS 3–5 | n = 115 | n = 64 | n = 43 | n = 63 | n = 44 | n = 62 | n = 45 | n = 62 | n = 40 | ||||
| (%) | (37.7%) | (59.3%) | (24.3%) | (58.9%) | (24.9%) | (56.9%) | (26.9%) | (57.4%) | (29.9%) | ||||
| n GCS 6–8 | n = 190 | n = 44 | n = 134 | n = 44 | n = 133 | n = 47 | n = 122 | n = 46 | n = 94 | ||||
| (%) | (62.3%) | (40.7%) | (75.7%) | (41.1%) | (75.1%) | (43.1%) | (73.1%) | (42.6%) | (70.1%) | ||||
Age was significantly associated with GOS outcomes at all time points, with the “dead or in a vegetative state group” having an older mean age than the “mild, moderate, and severe disability” group. Similarly, initial GCS was significantly associated with outcomes (GOS) at all time points with the “dead or in a vegetative state group” group being more likely to have a lower GCS (3,4,5 vs. 6,7,8) on admission. Sex was not significantly associated with GOS at any time point.
GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Scale; TBI, traumatic brain injury; OR, odds ratio; CI, confidence interval.
Variant allele presence and GOS
Possessing the variant allele of either of two SNPs in S100B (rs1051169 or rs9984765) was significantly associated with GOS score after controlling for age, sex, and admission GCS (Table 3). One SNP held its significance across the 2 year follow-up period; specifically, the variant C allele of rs1051169 was significantly associated with reduced odds of being in the poorer GOS outcome group (i.e., dead or in a vegetative state) at 3 months (odds ratio [OR] = 0.39; p = 0.04), 6 months (OR = 0.34; p = 0.02), 12 months (OR = 0.32; p = 0.02), and 24 months (OR = 0.30; p = 0.02) post-TBI. Conversely, for rs9984765, variant C allele presence was associated with poorer outcomes at 6 months post-injury; specifically, the odds of having a lower 6 month GOS score (1–2 vs. 3–5) was significantly higher (OR = 1.89; p = 0.04) when the variant allele was present. There were no significant relationships among the other S100B SNPs examined or any of the SNPs in either GFAP or UCH-L1 and GOS score at any time point.
Table 3.
Association of Variant Allele Presence with 3, 6, 12, and 24 Month Glasgow Outcome Scale (GOS) (after Controlling for Age, Sex, and Admission Glasgow Coma Score [GCS])
| 3 month GOS | 6 month GOS | 12 month GOS | 24 month GOS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variant allele presence | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p |
| rs1051169 C Present | 0.39 | 0.16–0.96 | 0.04* | 0.34 | 0.13–0.87 | 0.02* | 0.32 | 0.13–0.83 | 0.02* | 0.30 | 0.11–0.82 | 0.02* |
| rs9984765 C Present | 1.31 | 0.73–2.36 | 0.36 | 1.89 | 1.03–3.47 | 0.04* | 1.43 | 0.78–2.62 | 0.24 | 1.44 | 0.76–2.72 | 0.26 |
| rs12222 C Present | 0.66 | 0.36–1.21 | 0.18 | 0.60 | 0.32–1.11 | 0.11 | 0.78 | 0.42–1.44 | 0.43 | 0.73 | 0.38–1.39 | 0.33 |
| rs17629022 C Present | 0.77 | 0.42–1.42 | 0.41 | 0.72 | 0.39–1.34 | 0.30 | 0.70 | 0.38–1.31 | 0.26 | 0.82 | 0.43–1.58 | 0.56 |
| rs2289679 C Present | 0.60 | 0.31–1.15 | 0.13 | 0.55 | 0.28–1.06 | 0.07 | 0.66 | 0.34–1.27 | 0.21 | 0.65 | 0.33–1.29 | 0.22 |
| rs11651396 A Present | 0.72 | 0.38–1.35 | 0.30 | 0.70 | 0.37–1.32 | 0.26 | 0.84 | 0.44–1.59 | 0.59 | 0.79 | 0.40–1.54 | 0.49 |
| rs3760379 T Present | 1.18 | 0.63–2.19 | 0.61 | 1.37 | 0.73–2.59 | 0.33 | 1.16 | 0.62–2.18 | 0.65 | 1.25 | 0.63–2.44 | 0.51 |
| rs3785891 T Present | 0.75 | 0.41–1.36 | 0.34 | 0.74 | 0.40–1.36 | 0.33 | 0.99 | 0.53–1.85 | 0.98 | 1.04 | 0.55–1.99 | 0.90 |
| rs2239574 T Present | 0.81 | 0.45–1.46 | 0.49 | 0.73 | 0.40–1.33 | 0.31 | 0.95 | 0.52–1.72 | 0.86 | 0.92 | 0.49–1.72 | 0.79 |
| rs2839357 G Present | 1.43 | 0.67–3.02 | 0.36 | 1.76 | 0.81–3.79 | 0.15 | 1.41 | 0.65–3.03 | 0.38 | 1.79 | 0.79–4.04 | 0.16 |
| rs2839365 C Present | 0.63 | 0.34–1.16 | 0.14 | 0.57 | 0.31–1.05 | 0.07 | 0.68 | 0.37–1.26 | 0.23 | 0.66 | 0.35–1.26 | 0.21 |
| rs34722617 T Present | 0.78 | 0.43–1.40 | 0.40 | 0.67 | 0.37–1.23 | 0.20 | 0.85 | 0.47–1.54 | 0.59 | 0.79 | 0.42–1.49 | 0.47 |
| rs881827 T Present | 0.71 | 0.39–1.29 | 0.26 | 0.65 | 0.35–1.19 | 0.16 | 0.63 | 0.34–1.16 | 0.14 | 0.68 | 0.36–1.29 | 0.24 |
| rs10517002 C Present | 1.03 | 0.55–1.94 | 0.92 | 0.97 | 0.52–1.83 | 0.93 | 1.13 | 0.60–2.15 | 0.70 | 0.98 | 0.50–1.91 | 0.95 |
| rs10517003 G Present | 0.66 | 0.36–1.21 | 0.18 | 0.76 | 0.42–1.40 | 0.39 | 0.88 | 0.48–1.63 | 0.69 | 1.01 | 0.53–1.91 | 0.99 |
| rs16852986 C Present | 0.70 | 0.38–1.30 | 0.26 | 0.75 | 0.40–1.39 | 0.35 | 0.89 | 0.47–1.66 | 0.70 | 1.04 | 0.55–2.00 | 0.90 |
| rs17528160 A Present | 0.67 | 0.37–1.23 | 0.20 | 0.81 | 0.44–1.49 | 0.49 | 0.91 | 0.49–1.68 | 0.76 | 1.02 | 0.54–1.93 | 0.96 |
| rs4861387 A Present | 0.75 | 0.41–1.39 | 0.36 | 0.87 | 0.47–1.62 | 0.66 | 1.06 | 0.56–1.99 | 0.87 | 1.31 | 0.68–2.54 | 0.42 |
Among the group of individuals who possessed the variant C allele of rs1051169, the odds of having a lower GOS (1–2 vs. 3–5) was significantly lower at 3 months (odds ratio [OR] = 0.39; p = 0.04), 6 months (OR = 0.34; p = 0.02), 12 months (OR = 0.32; p = 0.02), and 24 months post-severe traumatic brain injury (TBI); (OR = 0.30; p = 0.02). Among the group of individuals possessing the variant C allele of rs9984765, the odds of having a lower 6 month GOS (1–2 vs. 3–5) was significantly higher (OR = 1.89; p = 0.04).
CI, confidence interval. *p < 0.05.
Discussion
Novel contribution to the evidence
The final sample in this study (n = 305) consisted of adult Caucasians with sTBI. The rationale for including only Caucasian participants was twofold. First, public databases (e.g., dbSNP) report that allelic frequency differences exist across ancestral groups for genes of interest in this study, which could confound the results. Second, low racial and ethnic diversity at the parent study's recruitment site resulted in a subsample of non-Caucasian participants that was too small to statistically control for or stratify by race; moreover, the abovementioned allelic frequency differences negated collapsing all non-Caucasian races into one subgroup.
This is the first study to report associations between genetic polymorphisms related to candidate blood-based TBI biomarkers and global neurological outcome measures assessed longitudinally after sTBI. The variant allele presence on each of two SNPs in S100B (rs1051169 and rs9984765) was found to significantly predict outcome, one of which significantly predicted GOS scores at all time points examined (3, 6, 12, and 24 months). Specifically, the variant allele of rs1051169 was associated with more favorable outcomes on the GOS (i.e., a lower odds of being dead or vegetative) at all four post-injury time points examined; therefore, rs1051169 is a strong candidate for further evaluation. Interestingly, in other contexts such as Alzheimer's21 and schizophrenia,29 variation in rs1051169 was associated with prefrontal functioning and spatial reasoning tasks; together, these findings suggest that further examination of the effects of genetic variation at this locus on TBI recovery may be warranted.
S100B SNP (rs9984765) was associated with poorer outcome on the GOS (i.e., a higher odds of being dead or in a vegetative state) at the 6 month data collection point. Although this is the first report of either SNP in the context of TBI, both rs1051169 and to a lesser extent, rs9984765 have been characterized in other samples. One study examined publicly available gene expression data from postmortem samples,30 and found a relationship between rs9984765 and increased expression of S100B within the frontal cortex.27 Moreover, a haplotype containing rs9984765 along with rs11542311, rs2839356, and rs881827 was also associated with increased S100B mRNA in the postmortem frontal cortex samples.27 Notably, one of these SNPs (rs881827) was examined in the present study, but no significant associations were detected. The significant relationships reported in this study between SNPs related to biomarkers and neurological outcomes at 3, 6, 12, and 24 months after injury merit further exploration. Replication of these findings is necessary as are efforts to understand the mechanism behind replicated findings. It is possible, although untested, that genetic variation directly or indirectly affects transcription and translation of these critical biomarker proteins, thereby affecting outcomes.
Limitations of this study
A limitation of this pilot study surrounds the generalizability of the sample, which excluded pediatric cases and was predominately composed of male Caucasians from one geographic area. It is possible that genotypic differences that affect biomarker levels vary by ancestral background and affect the utility of some biomarkers for certain racial/ethnic groups. Likewise, all individuals had an sTBI, and the relationships should be confirmed in mild and moderate TBI. Overall, the small sample size in the present study and lack of control for multiple comparisons means that false positives are possible. Replication of these findings in more diverse samples with better control for potential confounders will be critical to improving generalizability and will facilitate subsequent translation efforts.
Another limitation surrounds inclusion of only GOS as an outcome measure. Dichotomizing GOS served to balance the groups so that sample size was sufficient for analyses. Larger studies may analyze GOS as an ordinal variable and incorporate additional outcome measures that provide more details about recovery. A related limitation is that no statistical correction for multiple comparisons was applied in this pilot study.
Notably, other biomarkers have been empirically tested and have shown promise,31–33 but SNPs related to these proteins were not included in this study. Likewise, other SNPs in GFAP, S100B, and UCH-L1 exist but were not included in this study, representing a possible future direction. Often, a single protein biomarker does not have the predictive power that could be achieved with a panel of two or more biomarkers,34 as has been reported previously.9 Likewise, a predictive model with multiple SNPs may prove beneficial. Multi-marker panels have been proposed to improve the quality of biomarker evidence. Models with predictive biomarkers and/or relevant genetic information should also consider traditional predictor variables (e.g., pupil reactivity) to enhance predictability.19 In this study, limited outcome data were available, and it is likely that inclusion of additional predictors would be informative. Future studies are needed to verify and reproduce the finding that these allele variants are robustly associated with outcome and to explore downstream implications (e.g., effects on protein biomarker expression).
Conclusion
This is the first study to examine the association between polymorphisms in genes encoding candidate TBI biomarkers and long-term outcomes of TBI. Genetic variation at two loci was associated with GOS scores. One SNP (rs1051169) was significantly associated with favorable outcome (i.e., a lower odds of death or being in a vegetative state) at all time points (3, 6, 12, and 24 months). Limitations in the study sample including low representation of women and a lack of non-Caucasians and children, restricting the generalizability of these findings. Future studies should directly explore the effects of genotypic variation on levels of the protein biomarkers of interest and additional outcome measures. Better predictive models including demographic, genomic, proteomic, and clinical variables are needed for improved diagnosis and prognosis in the context of TBI.
Acknowledgments
Funds in support of this study were provided by the National Institute of Nursing Research (R00NR013176, F31NR014957, R01NR013342, and T32NR009759).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hyder A.A., Wunderlich C.A., Puvanachandra P., Gururaj G., and Kobusingye O.C. (2007). The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 22, 341–353 [PubMed] [Google Scholar]
- 2.Whiteneck G.G., Cuthbert J.P., Corrigan J.D., and Bogner J.A. (2016). Prevalence of self-reported lifetime history of traumatic brain injury and associated disability: a statewide population-based survey. J. Head Trauma Rehabil. 31, E55–62 [DOI] [PubMed] [Google Scholar]
- 3.NIH-FDA Joint Biomarker Team (2017). Biomarker Guidances and Reference Materials. Food and Drug Administration, Silver Spring, MD and National Institutes of Health, Bethesda, MD [Google Scholar]
- 4.Mondello S., and Hayes R.L. (2015). Biomarkers. Handb. Clin. Neurol. 127, 245–265 [DOI] [PubMed] [Google Scholar]
- 5.Sharma R., and Laskowitz D.T. (2012). Biomarkers in traumatic brain injury. Curr. Neurol. Neurosci. Rep. 12, 560–569 [DOI] [PubMed] [Google Scholar]
- 6.Maas A.I.R., Lingsma H.F., and Roozenbeek B. (2015). Predicting outcome after traumatic brain injury. Handb. Clin. Neurol. 128, 455–474 [DOI] [PubMed] [Google Scholar]
- 7.Gao J., and Zheng Z. (2015). Development of prognostic models for patients with traumatic brain injury: a systematic review. Int. J. Clin. Exp. Med. 8, 19,881–19,885 [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J.Y., Lee C.Y., Kim H.R., Lee C.-H., Kim H.W., and Kim J.H. (2015). A role of serum-based neuronal and glial markers as potential predictors for distinguishing severity and related outcomes in traumatic brain injury. J. Korean Neurosurg. Soc. 58, 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz-Arrastia R., Wang K.K.W., Papa L., Sorani M.D., Yue J.K., Puccio A.M., McMahon P.J., Inoue T., Yuh E.L., Lingsma H.F., Maas A.I.R., Valadka A.B., Okonkwo D.O., Manley G.T., and TRACK-TBI Investigators. (2014). Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 31, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokobori S., Hosein K., Burks S., Sharma I., Gajavelli S., and Bullock R. (2013). Biomarkers for the clinical differential diagnosis in traumatic brain injury–a systematic review. CNS Neurosci. Ther. 19, 556–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okonkwo D., Yue J., Puccio A., Panczykowski D., Inoue T., McMahon P., Sorani M., Yuh E., Lingsma H., Maas A., Valadka A., Manley G., and Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Investigators. (2013). GFAP-BDP as an acute diagnostic marker in traumatic brain injury: results from the prospective transforming research and clinical knowledge in traumatic brain injury study. J. Neurotrauma 30, 1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahon P., Panczykowski D., Yue J., Puccio A., Inoue T., Sorani M., Lingsma H., Maas A., Valadka A., Yuh E., Mukherjee P., Manley G., Okonkwo D., and Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Investigators. (2015). Measurement of the GFAP-BDP biomarker for the detection of traumatic brain injury compared to CT and MRI. J. Neurotrauma 32, 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinelis V.G., Sorokina E.G., Semenova J.B., Karaseva O. V, Mescheryakov S. V, Chernisheva T.A., Arsenieva E.N., and Roshal L.M. (2015). Biomarkers in children with traumatic brain injury [in Russian]. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 115, 66–72 [DOI] [PubMed] [Google Scholar]
- 14.Goyal A., Failla M.D., Niyonkuru C., Amin K., Fabio A., Berger R.P., and Wagner A.K. (2013). S100b as a prognostic biomarker in outcome prediction for patients with severe traumatic brain injury. J. Neurotrauma 30, 946–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thelin E.P., Johannesson L., Nelson D., and Bellander B.-M. (2013). S100B is an important outcome predictor in traumatic brain injury. J. Neurotrauma 30, 519–528 [DOI] [PubMed] [Google Scholar]
- 16.Kiiski H., Tenhunen J., Ala-Peijari M., Huhtala H., Hämäläinen M., Långsjö J., Moilanen E., Narkilahti S., Öhman J., and Peltola J. (2016). Increased plasma UCH-L1 after aneurysmal subarachnoid hemorrhage is associated with unfavorable neurological outcome. J. Neurol. Sci. 361, 144–149 [DOI] [PubMed] [Google Scholar]
- 17.Puvenna V., Brennan C., Shaw G., Yang C., Marchi N., Bazarian J.J., Merchant-Borna K., and Janigro D. (2014). Significance of ubiquitin carboxy-terminal hydrolase L1 elevations in athletes after sub-concussive head hits. PLoS One 9, e96296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger R.P., Hayes R.L., Richichi R., Beers S.R., and Wang K.K.W. (2012). Serum concentrations of ubiquitin C-terminal hydrolase-L1 and αII-spectrin breakdown product 145 kDa correlate with outcome after pediatric TBI. J. Neurotrauma 29, 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takala R.S.K., Posti J.P., Runtti H., Newcombe V.F., Outtrim J., Katila A.J., Frantzén J., Ala-Seppälä H., Kyllönen A., Maanpää H.-R., Tallus J., Hossain M.I., Coles J.P., Hutchinson P., van Gils M., Menon D.K., and Tenovuo O. (2016). Glial fibrillary acidic protein and ubiquitin c-terminal hydrolase-l1 as outcome predictors in traumatic brain injury. World Neurosurg. 87, 8–20 [DOI] [PubMed] [Google Scholar]
- 20.Osier N.D., Bales J.W., Pugh B., Shin S., Wyrobek J., Puccio A.M., Okonkwo D.O., Ren D., Alexander S., Conley Y.P., and Dixon C.E. (2017). Variation in PPP3CC genotype is associated with long-term recovery after severe brain injury. J. Neurotrauma 34, 86–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhai J., Cheng L., Dong J., Shen Q., Zhang Q., Chen M., Gao L., Chen X., Wang K., Deng X., Xu Z., Ji F., Liu C., Li J., Dong Q., and Chen C. (2012). S100B gene polymorphisms predict prefrontal spatial function in both schizophrenia patients and healthy individuals. Schizophr. Res. 134, 89–94 [DOI] [PubMed] [Google Scholar]
- 22.Zhai J., Zhang Q., Cheng L., Chen M., Wang K., Liu Y., Deng X., Chen X., Shen Q., Xu Z., Ji F., Liu C., Dong Q., Chen C., and Li J. (2011). Risk variants in the S100B gene, associated with elevated S100B levels, are also associated with visuospatial disability of schizophrenia. Behav. Brain Res. 217, 363–368 [DOI] [PubMed] [Google Scholar]
- 23.Liu J., Shi Y., Tang J., Guo T., Li X., Yang Y., Chen Q., Zhao X., He G., Feng G., Gu N., Zhu S., Liu H., and He L. (2005). SNPs and haplotypes in the S100B gene reveal association with schizophrenia. Biochem. Biophys. Res. Commun. 328, 335–41 [DOI] [PubMed] [Google Scholar]
- 24.Shibata N., Motoi Y., Tomiyama H., Ohnuma T., Kuerban B., Tomson K., Komatsu M., Hattori N., and Arai H. (2012). Lack of genetic association of the UCHL1 gene with Alzheimer's disease and Parkinson's disease with dementia. Dement. Geriatr. Cogn. Disord. 33, 250–254 [DOI] [PubMed] [Google Scholar]
- 25.Guo Y., Yang H., Deng X., Song Z., Yang Z., Xiong W., Yuan L., Xu H., Deng S., and Deng H. (2013). Genetic analysis of the S100B gene in Chinese patients with Parkinson disease. Neurosci. Lett. 555, 134–136 [DOI] [PubMed] [Google Scholar]
- 26.Kenny E.E., Kim M., Gusev A., Lowe J.K., Salit J., Smith J.G., Kovvali S., Kang H.M., Newton-Cheh C., Daly M.J., Stoffel M., Altshuler D.M., Friedman J.M., Eskin E., Breslow J.L., and Pe'er I. (2011). Increased power of mixed models facilitates association mapping of 10 loci for metabolic traits in an isolated population. Hum. Mol. Genet. 20, 827–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hohoff C., Ponath G., Freitag C.M., Kästner F., Krakowitzky P., Domschke K., Koelkebeck K., Kipp F., von Eiff C., Deckert J., and Rothermundt M. (2010). Risk variants in the S100B gene predict elevated S100B serum concentrations in healthy individuals. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 153B, 291–7 [DOI] [PubMed] [Google Scholar]
- 28.Miller S.A., Dykes D.D., and Polesky H.F. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16, 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhai J., Zhang Q., Cheng L., Chen M., Wang K., Liu Y., Deng X., Chen X., Shen Q., Xu Z., Ji F., Liu C., Dong Q., Chen C., and Li J. (2011). Risk variants in the S100B gene, associated with elevated S100B levels, are also associated with visuospatial disability of schizophrenia. Behav. Brain Res. 217, 363–368 [DOI] [PubMed] [Google Scholar]
- 30.Myers A.J., Gibbs J.R., Webster J.A., Rohrer K., Zhao A., Marlowe L., Kaleem M., Leung D., Bryden L., Nath P., Zismann V.L., Joshipura K., Huentelman M.J., Hu-Lince D., Coon K.D., Craig D.W., Pearson J. V, Holmans P., Heward C.B., Reiman E.M., Stephan D., and Hardy J. (2007). A survey of genetic human cortical gene expression. Nat. Genet. 39, 1494–9 [DOI] [PubMed] [Google Scholar]
- 31.Mondello S., Buki A., Italiano D., and Jeromin A. (2013). α-Synuclein in CSF of patients with severe traumatic brain injury. Neurology 80, 1662–1668 [DOI] [PubMed] [Google Scholar]
- 32.Zhang B., and Zhao J. (2015). Red blood cell distribution width as a prognostic biomarker for mortality in traumatic brain injury. Int. J. Clin. Exp. Med. 8, 19172–5 [PMC free article] [PubMed] [Google Scholar]
- 33.Korley F.K., Diaz-Arrastia R., Wu A.H.B., Yue J.K., Manley G.T., Sair H.I., Van Eyk J., Everett A.D., TRACK-TBI investigators, Okonkwo D.O., Valadka A.B., Gordon W.A., Maas A.I.R., Mukherjee P., Yuh E.L., Lingsma H.F., Puccio A.M., and Schnyer D.M. (2016). Circulating brain-derived neurotrophic factor has diagnostic and prognostic value in traumatic brain injury. J. Neurotrauma 33, 215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Battista A.P., Buonora J.E., Rhind S.G., Hutchison M.G., Baker A.J., Rizoli S.B., Diaz-Arrastia R., and Mueller G.P. (2015). Blood biomarkers in moderate-to-severe traumatic brain injury: potential utility of a multi-marker approach in characterizing outcome. Front. Neurol. 6, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]