Abstract
Purpose:
To investigate the implications of a cancer diagnosis on medication adherence for pre-existing comorbid conditions, we explored statin adherence patterns prior to and following a new diagnosis of breast, colorectal or prostate cancer among a multi-ethnic cohort.
Methods:
We identified adults enrolled at Kaiser Permanente Northern California who were prevalent statin medication users, newly diagnosed with breast, colorectal or prostate cancer between 2000–2012. Statin adherence was measured using the proportion of days covered (PDC) during the two years pre-cancer diagnosis and the two years post-cancer diagnosis. Adherence patterns were assessed using generalized estimating equations, for all cancers combined and stratified by cancer type and race/ethnicity, adjusted for demographic, clinical, and tumor characteristics.
Results:
Among 10,177 cancer patients, statin adherence decreased from pre- to postcancer diagnosis (Adjusted Odds Ratio (ORadj):0.91, 95% Confidence Interval (95% CI):0.88–0.94). Statin adherence decreased from pre- to post-cancer diagnosis among breast (ORadj:0.94, 95% CI:0.90–0.99) and colorectal (ORadj:0.79, 95% CI:0.74–0.85) cancer patients. No difference in adherence was observed among prostate cancer patients (ORadj:1.01, 95% CI:0.97–1.05). Prior to cancer diagnosis, adherence to statins was generally higher among non-Hispanic whites and multi-race patients than other groups. However, statin adherence after diagnosis decreased only among these two populations (ORadj:0.85, 95% CI:0.85–0.92 and ORadj:0.86, 95% CI:0.76–0.97), respectively.
Conclusions:
We found substantial variation in statin medication adherence following diagnosis by cancer type and race/ethnicity among a large cohort of prevalent statin users in an integrated health care setting.
Implications for Cancer Survivors:
Improving our understanding of comorbidity management and polypharmacy across diverse cancer patient populations is warranted to develop tailored interventions that improve medication adherence and reduce disparities in health outcomes.
Keywords: Adherence, race/ethnicity, breast cancer, colorectal cancer, prostate cancer, comorbidities
INTRODUCTION
There are over 15.5 million cancer survivors in United States (U.S.), with nearly 1.7 million new cancers expected to be diagnosed in 2018 [1]. Individuals with cancer have disproportionately higher burdens of comorbid chronic conditions compared to individuals without a cancer history [2, 3]. In particular, hypertension, diabetes and lipid disorders (conditions treated with statins) represent the most common comorbid chronic conditions among individuals diagnosed with cancer [2–4]. For individuals with pre-existing chronic conditions, a new cancer diagnosis can lead to tremendous challenges, including the coordination of care and providers as well as the management of multiple medications for comorbid conditions alongside cancer treatment [5, 6]. In such scenarios, treatment focus may shift away from day to day chronic disease management toward life-saving or life-prolonging strategies related to the cancer [7].
Despite the complexity of these interactions, few studies have evaluated how a new cancer diagnosis affects medication adherence for preexisting comorbid conditions [8–12]. Additionally, none of these studies reported findings on racial/ethnic variation in adherence, even though racial/ethnic differences in medication adherence are well-documented in the literature [13–17]. The objective of our study was to describe the patterns of medication adherence in the 2-year period prior to and 2-year period following a new breast, colorectal or prostate cancer diagnosis, among a large multi-ethnic cohort of prevalent statin users in an integrated health care setting. The prevalence of statin medication use [2–4], clinical effectiveness of statins in reducing cardiovascular disease events and mortality [18–22], and evidence of non-adherence or non-persistence with statin medication [22–28] present a unique opportunity in which to assess medication management among individuals diagnosed with cancer. We hypothesized that: (1) statin adherence would decrease following a new cancer diagnosis and (2) statin adherence among individuals diagnosed with cancer would vary by race/ethnicity.
METHODS
Data Source and Study Sample
This retrospective cohort study was conducted at Kaiser Permanente Northern California (KPNC), a multi-specialty health care delivery system that serves over 3.4 million current members. The KPNC Tumor Registry (KPTR) was used to identify adults 18 years and older newly diagnosed with a primary breast, colorectal or prostate cancer between January 1, 2000 and May 30, 2012 (see Supplemental Figure 1 for Study Participant Flow Diagram). The KPTR reports to the California Cancer Registry and the Surveillance, Epidemiology and End Results (SEER) program of registries and has data of similar completeness and accuracy [29]. Individuals with a previous cancer diagnosis, a subsequent cancer diagnosis within 24 months of incident cancer diagnosis, and/or with distant metastasis at diagnosis were excluded (n=6,984).
The study cohort consisted of prevalent users of statin medication. Statin use was ascertained from the KPNC electronic pharmacy database, which records information on all prescription medications dispensed at KPNC pharmacies and includes information on drug name, dose and quantity, days supply and dispensing dates for all fills. Over 90% of KPNC members have a pharmacy benefit. Study participants had to be KPNC members with continuous enrollment and prescription drug coverage ≥36 months prior to their incident cancer diagnosis and ≥24 months following cancer, ≥2 prescription for a statin medication in the 24–36 months prior to incident cancer diagnosis, and be alive ≥3 years following their incident cancer. The latter was to minimize the potential impact of palliative and end of life care on estimates of adherence. We excluded individuals with only 1 prescription refill in the 24–36 months prior to incident cancer diagnosis (n=168) and those with a prescription for a combination of statin + other agent (n=1,346). Our final cohort was comprised of 10,177 patients.
Measures of medication adherence
Medication adherence was measured as the proportion of days covered (PDC), as a recommended metric by the Pharmacy Quality Alliance [30], which is based on the quantity of medication dispensed and number of days supply from each filled prescription. The PDC was calculated as the total number of days for which the drug was available during four distinct study intervals for each participant: 12–24 months prior to incident cancer diagnosis (Year –2); 0–11 months prior to incident cancer diagnosis (Year –1); month of incident cancer to 11 month following incident cancer diagnosis (Year +1); and 12–24 months following incident cancer diagnosis (Year +2). For individuals taking more than one statin drug during a given interval, we calculated a single summary measure by combining the number of days in the interval with any statin availability. For descriptive analyses, the adherence status of each patient was categorized as follows: adherent (PDC≥0.80); partially-adherent (PDC=0.20–0.79) and non-adherent (PDC<0.20) [27, 31, 32].
Patient Characteristics
We obtained patient demographic and clinical data on patients from the electronic medical record, including age, sex, race/ethnicity and Charlson Comorbidity Index. Information on tumor characteristics was obtained from the KPTR, including primary tumor site, stage at diagnosis and year of diagnosis.
Statistical Analysis
Descriptive statistics were used to assess patients’ sociodemographic and tumor characteristics at baseline. To evaluate trends in statin medication adherence, we estimated mean PDC and proportion of patients by adherence status, for all cancers combined and stratified by cancer site, during each study interval. Next, we tested the study hypotheses by assessing change in adherence over the study period. The dependent variable was the proportion of days covered by statin medication each year. We evaluated change in adherence status in the two-year period prior to versus the two-year period following cancer diagnosis. Generalized estimating equations (GEE) were used to fit marginal models with a logit link function for the correlated longitudinal data [33]. Marginal multivariable regression models for adherence over time were adjusted for age at diagnosis, year of diagnosis, cancer site, stage at diagnosis, Charlson Comorbidity Index, sex, and race/ethnicity. Differences in the effect of time by cancer site were estimated by using an interaction term for time by cancer site. Differences in the effect of time by race/ethnicity were estimated by using an interaction term for time by race/ethnicity.
A sensitivity analysis was conducted to evaluate change in adherence status in the one-year period prior to versus the one-year period following cancer diagnosis, by cancer site (Supplemental Table 1). All statistical analyses were performed using SAS 9.3, where GEE models were fit using PROC GENMOD.
RESULTS
Population Characteristics
Baseline characteristics of the 10,177 patients are shown in Table 1. Most patients were older (age ≥65) at cancer diagnosis (68%), male (61%), non-Hispanic white (64%), and had a Charlson Comorbidity Score of ≥1 (60%). Patients diagnosed with prostate cancer represented the largest proportion of participants (52%), followed by those diagnosed with breast (31%) and colorectal cancer (17%). The majority of patients were diagnosed with localized disease (82%) and between the years 2005 to 2012 (72%).
Table 1.
Characteristics of Study Population
| Characteristic | Patients (n = 10,177) | |
|---|---|---|
| n | % | |
| Age at Cancer Diagnosis, years | ||
| <55 | 589 | 5.8 |
| 55–64 | 2,688 | 26.4 |
| 65–74 | 4,277 | 42.0 |
| 75+ | 2,623 | 25.8 |
| Sex | ||
| Female | 3,980 | 39.1 |
| Male | 6,197 | 60.9 |
| Race/Ethnicity | ||
| Asian, non-Hispanic | 1,074 | 10.6 |
| African American, non-Hispanic | 937 | 9.2 |
| Hispanic | 1,054 | 10.4 |
| White, non-Hispanic | 6,497 | 63.8 |
| Native American/Alaskan Native | 33 | 0.3 |
| Multi-race | 577 | 5.7 |
| Unknown | 5 | 0.1 |
| Charlson Comorbidity Index Score | ||
| 0 | 4,105 | 40.3 |
| 1 | 2,765 | 27.2 |
| 2 | 1,683 | 16.5 |
| 3+ | 1,624 | 16.0 |
| Cancer Site | ||
| Breast | 3,153 | 31.0 |
| Colorectal | 1,762 | 17.3 |
| Prostate | 5,262 | 51.7 |
| Stage at Diagnosis | ||
| Localized | 8,372 | 82.3 |
| Regional | 1,805 | 17.7 |
| Year of Cancer Diagnosis | ||
| 2000–2004 | 1,856 | 18.2 |
| 2005–2008 | 4,010 | 39.4 |
| 2009–2012 | 4,311 | 42.4 |
Notes: Percentages may not equal 100 due to rounding. Abbreviation: n=number of participants
Statin Use and Adherence Status Prior to Cancer Diagnosis
The mean PDC and adherence status at each study interval, overall and by cancer site, are reported in Table 2. Among all cancers, the mean PDC for statin use in the two years prior to cancer diagnosis (i.e., Year –2 and Year –1) was 0.80 and 0.79, respectively. The majority of all patients were adherent to their statin medication in the two years prior to cancer diagnosis. During Year –2 approximately 69.7% of all patients were adherent, 24.4% were partially-adherent and 6.0% were non-adherent. Breast cancer patients had the lowest pre-cancer diagnosis adherence, with 67.1% adherent in both Year –2 and Year –1. Similar proportions of patients adherent to their statin medications in Year –2 and Year –1 were observed for colorectal cancer (AdherentYear– 2=70.8% and AdherentYear–1=69.7%) and prostate cancer patients (AdherentYear– 2=70.8% and AdherentYear–1=70.9%).
Table 2.
Unadjusted Mean, Standard Deviation and Interquartile Range of Proportion of Days Covered (PDC) by a Statin and Adherence Status of Users over Each Interval, Overall and by Cancer Site
| Adherence Status | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PDC | PDC ≥0.80 | PDC 0.20–0.79 | PDC<0.20 | |||||||
| All cancers | n | Mean | SD | IQR | n | % | n | % | n | % |
| Year–2 | 10,177 | 0.8 | 0.28 | 0.73–1.00 | 7,089 | 69.7 | 2,478 | 24.4 | 610 | 6 |
| Year –1 | 10,177 | 0.8 | 0.29 | 0.72–1.00 | 7,073 | 69.5 | 2,361 | 23.2 | 743 | 7.3 |
| Year +1 | 10,177 | 0.79 | 0.29 | 0.72–1.00 | 7,060 | 69.4 | 2,280 | 22.4 | 837 | 8.2 |
| Year +2 | 10,177 | 0.79 | 0.3 | 0.73–1.00 | 7,135 | 70.1 | 2,155 | 21.2 | 887 | 8.7 |
| Breast cancer | ||||||||||
| Year –2 | 3,153 | 0.79 | 0.29 | 0.68–1.00 | 2,115 | 67.1 | 825 | 26.2 | 213 | 6.8 |
| Year –1 | 3,153 | 0.78 | 0.29 | 0.68–1.00 | 2,114 | 67.1 | 789 | 25 | 250 | 7.9 |
| Year +1 | 3,153 | 0.77 | 0.31 | 0.68–0.99 | 2,131 | 67.6 | 726 | 23 | 296 | 9.4 |
| Year +2 | 3,153 | 0.78 | 0.31 | 0.69–1.00 | 2,128 | 67.5 | 724 | 23 | 301 | 9.6 |
| Colorectal cancer | ||||||||||
| Year –2 | 1,762 | 0.81 | 0.27 | 0.74–1.00 | 1,247 | 70.8 | 414 | 23.5 | 101 | 5.7 |
| Year –1 | 1,762 | 0.8 | 0.28 | 0.73–1.00 | 1,228 | 69.7 | 417 | 23.7 | 117 | 6.6 |
| Year +1 | 1,762 | 0.76 | 0.31 | 0.68–0.97 | 1,146 | 65 | 445 | 25.3 | 171 | 9.7 |
| Year +2 | 1,762 | 0.78 | 0.31 | 0.71–1.00 | 1,202 | 68.2 | 378 | 21.5 | 182 | 10.3 |
| Prostate cancer | ||||||||||
| Year –2 | 5,262 | 0.81 | 0.27 | 0.74–1.00 | 3,727 | 70.8 | 1,239 | 23.6 | 296 | 5.6 |
| Year –1 | 5,262 | 0.81 | 0.28 | 0.74–1.00 | 3,731 | 70.9 | 1,155 | 22 | 376 | 7.2 |
| Year +1 | 5,262 | 0.81 | 0.28 | 0.76–1.00 | 3,783 | 71.9 | 1,109 | 21.1 | 370 | 7 |
| Year +2 | 5,262 | 0.81 | 0.29 | 0.76–1.00 | 3,805 | 72.3 | 1,053 | 20 | 404 | 7.7 |
Notes: Estimates are presented for each study interval: Year –2 is the 12–24 months prior to cancer diagnosis; Year –1 is the 0–11 months prior to cancer diagnosis; Year +1 is the 0–11 months following cancer diagnosis; Year +2 is the 12–24 months following cancer diagnosis. Adherent levels were defined as: adherent (PDC≥0.80); partial-adherent (PDC=0.20–0.79) and non-adherent (PDC<0.20). The proportion of patients across adherence levels, in any year, may not add to 100% due to rounding. Abbreviations: n=number of participants evaluated at each study interval; PDC=proportion of days covered; SD=standard deviation; IQR=interquartile range.
Change in Statin Use and Adherence Status following Cancer Diagnosis Among all patients, the mean PDC in the two years following cancer diagnosis appeared similar to that during the pre-cancer diagnosis period in descriptive analyses (Table 2). The mean PDC among breast cancer patients was 0.80 in both Year +1 and Year +2, while for colorectal cancer patients it was PDCYear+1=0.78 and PDCYear+2=0.80 and for prostate cancer patients was PDCYear+1=0.84 and PDCYear+2=0.83.
The proportion of all patients who were adherent to their statin medications was stable in two years following cancer diagnosis (Table 2). Among patients with breast cancer, the proportion who were adherent following cancer remained relatively unchanged (67.1% in Years −1 and −2, 67.6% in Year +1 and 67.5% in Year +2). The greatest change in adherence following cancer diagnosis was observed among patients with colon/rectal cancer, as the proportion who were adherent decreased from 69.5% in Year −1 to 65% in Year +1. The proportion of patients with prostate cancer who were adherent in Year +1 (71.9%) and Year +2 (72.3%).
The results from GEE regression analyses are presented in Table 3. Among all patients, the odds of medication adherence decreased slightly following cancer diagnosis (adjusted OR (ORadj)=0.91, 95% CI:0.88–0.94, p<0.001). Similarly, in cancer-specific analyses, there was a slight decrease in medication adherence following diagnosis among patients with breast cancer (ORadj =0.94, 95% CI:0.90–0.99, p=0.011) and colon/rectal cancer (ORadj =0.79, 95% CI:0.74–0.85, p<0.001). Among patients with prostate cancer, no statistically significant changes in medication adherence were observed over time. Results from the sensitivity analysis, assessing change in statin adherence from 1-year prior (Year −1) to 1-year following (Year +1) cancer diagnosis, revealed consistent patterns of adherence over time, by cancer site (Supplemental Table 1).
Table 3.
Adjusted Change in Proportion of Days Covered (PDC) from Pre-Cancer Diagnosis Period to Post-Cancer Diagnosis Period, by Cancer Site and Race/Ethnicity
| Odds Ratio [95% CI] | p-value | ||
|---|---|---|---|
| By Cancer Type | |||
| All cancers (n=10,177) | |||
| Pre-cancer | 1.0 (Referent) | <.0001 | |
| Post-cancer | 0.91 [0.88, 0.94] | ||
| Breast cancer (n=3,153) | |||
| Pre-cancer | 1.0 (Referent) | 0.011 | |
| Post-cancer | 0.94 [0.90, 0.99] | ||
| Colorectal cancer (n=1,762) | |||
| Pre-cancer | 1.0 (Referent) | <.0001 | |
| Post-cancer | 0.79 [0.74, 0.85] | ||
| Prostate cancer (n=5,262) | |||
| Pre-cancer | 1.0 (Referent) | 0.657 | |
| Post-cancer | 1.01 [0.97, 1.05] | ||
| By Race/Ethnicity | |||
| White, NH (n=6,497) | |||
| Pre-cancer | 1.0 (Referent) | <0.001 | |
| Post-cancer | 0.85 [0.85, 0.92] | ||
| Asian/Pacific Islander, NH (n=1,074) | |||
| Pre-cancer | 1.0 (Referent) | 0.176 | |
| Post-cancer | 0.94 [0.86, 1.03] | ||
| African American, NH (n=937) | |||
| Pre-cancer | 1.0 (Referent) | 0.368 | |
| Post-cancer | 0.96 [0.89, 1.05] | ||
| Hispanic (n=1,054) | |||
| Pre-cancer | 1.0 (Referent) | 0.596 | |
| Post-cancer | 0.98 [0.90, 1.06] | ||
| Multi-race (n=577) | |||
| Pre-cancer | 1.0 (Referent) | 0.015 | |
| Post-cancer | 0.86 [0.76, 0.97] | ||
| Native American/Alaskan Native (n=33) | |||
| Pre-cancer | 1.0 (Referent) | 0.998 | |
| Post-cancer | 1.00 [0.68, 1.46] | ||
Notes: Estimates based on generalized estimating equations (GEE) using PROC GENMOD for binomial proportion with logit link function. Overall adherence is defined as proportion of days covered using a multivariable generalized linear model adjusted for cancer site, race-ethnicity, age at diagnosis, year of diagnosis, stage at diagnosis, Charlson Comorbidity Index, time (as one-year periods), sex (for colorectal cancer only), and cancer site-time interaction. Pre-cancer (post-cancer) diagnosis period includes the two-year period prior to (after) cancer. Multi-race patients are those that self-reported more than one race category.
Overall Adherence Status by Race/Ethnicity
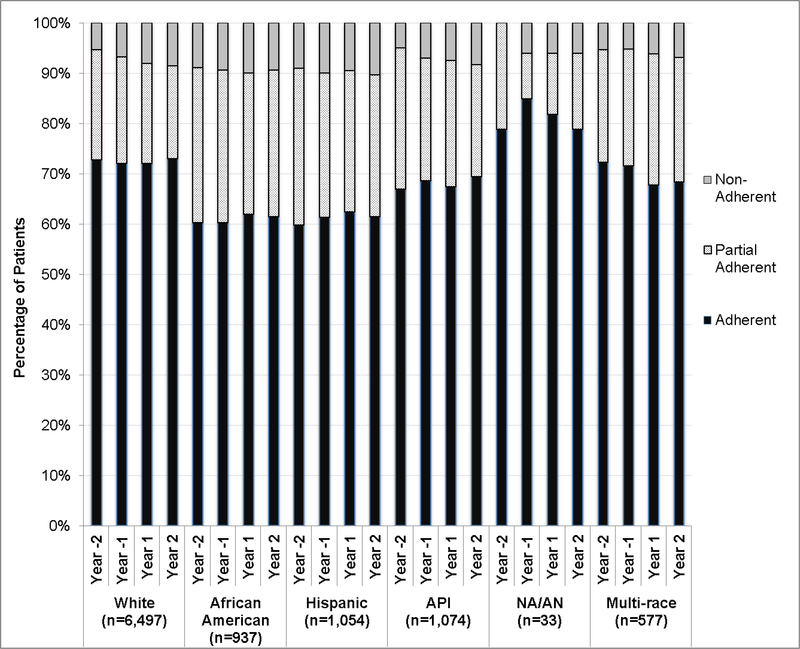
Adherence to statin medication by patient race/ethnicity is presented in Figure 1. A lower proportion of Asian/Pacific Islander, African American and Hispanic patients (all cancers) were adherent to their statin medication in the periods prior to and following their cancer diagnosis than non-Hispanic white patients. Adherence to statin medication was highest among Native-American/Alaskan Native patients, over the entire study period, although numbers in this subgroup were very small (n=33).
Figure 1. Unadjusted Percentage of Patients by Adherence Status (Adherent, Partially Adherent and Non-Adherent) at Each Time Interval, by Race/Ethnicity.
Notes: Year –2 is the 12–24 months prior to cancer diagnosis; Year –1 is the 0–11 months prior to cancer diagnosis; Year +1 is the 0–11 months following cancer diagnosis; Year +2 is the 12–24 months following cancer diagnosis. Adherent defined as having PDC≥80% in the time interval, Partially Adherent defined as having PDC=20%−79% in the time interval. Non-Adherent defined as PDC<20% in the time interval. Abbreviations: API=Asian or Pacific Islander; NA/AN=Native American or Alaska Native. Estimates of adherence for NA/AN based on a sample of n=33. Multi-race patients are those that self-reported more than one race category.
There was a significant decrease in medication adherence following cancer diagnosis among non-Hispanic white (ORadj=0.89, 95% CI:0.85–0.92) and multi-race patients (ORadj=0.86, 95% CI:0.76-, 0.97] (Table 3). Adherence to statin medication among Asian American and African American patients decreased following cancer diagnosis, although these differences were not statistically significant. No changes in statin adherence following diagnosis were observed among Hispanic and Native American/Alaskan Native patients. These differences by race/ethnicity were not statistically significant (p=0.134).
DISCUSSION
Our study of a large, retrospective cohort of 10,177 patients in an integrated health care system provides valuable information on the interaction and complexity of co-managing cancer and pre-existing disease. Consistent with our hypothesis, among all cancer patients, we found that adherence to statin medication was slightly lower during the two-year period following a new cancer diagnosis as compared to the two-year period preceding diagnosis; although, adherence patterns varied by cancer site. Furthermore, changes in statin adherence were statistically significantly different among non-Hispanic white and multi-race cancer patients only. Population-based prevalence estimates indicating that 30–32% of older (ages 66 and older) breast and prostate cancer patients and 41% of older colorectal cancer patients have at least one comorbidity underscore the importance of our findings for understanding the potential implications for treatment and health outcomes associated with the co-existence of cancer and chronic disease.
Our results support the few prior studies of medication adherence for treatment of chronic conditions among individuals newly diagnosed with cancer [8–12]. Calip et al. [9, 10] found significant declines in adherence to statins (mean medication possession ratio (MPR) ranging from 0.78 to 0.69) and diabetes medications (mean MPR ranging from 0.86 to 0.52) between one year prior to and three years following cancer diagnosis among women newly diagnosed with breast cancer. Stuart et al. found declines in adherence, between the 6 months prior to and 6 months after a new cancer diagnosis, to statins (absolute change in PDC of −2.1), oral hypoglycemic agents (absolute change in PDC of −2.3) and renin-angiotensin-aldosterone system inhibitors (absolute change in PDC of −4.5), among Medicare beneficiaries with diabetes who lived at least 1 year following diagnosis [8]. Similarly, Yang and colleagues [11] reported a decline in medication adherence for hypertension (from 89.6% to 74.0%), hyperlipidemia (adherence defined as MPR ≥0.80, from 83.2% to 57.1%), diabetes (from 79.9% to 53.1%), hyperlipidemia (from 83.2% to 57.1%), gastroesophageal reflux disease (from 79.8% to 55.0%), and thyroid disease(from 89.7% to 69.1%), between the 1 year prior to and 1.5 years following a new diagnosis of early stage breast cancer. A study by Chou et al. [12], assessing changes in anti-depressant adherence among women diagnosed with breast cancer diagnosis and a non-cancer control group, found that a breast cancer diagnosis was not significantly associated with changes in the PDC.
Collectively, findings from this growing body of research suggest that challenges in optimal care management for patients with comorbidities who are newly diagnosed with cancer may vary by type of cancer and the type of medication used to treat a pre-existing condition. The complexity of care needs and the required coordination of care for these patients may be a primary factor, since it may necessitate a shift in the attention, of providers and patients, away from chronic disease management to focus on the cancer treatment [34]. In addition to the number of medications that a patient may need to take due to multimorbidity, the overall illness and symptom burdens of multiple medications may influence adherence [7]. Prior research has shown reduced medication adherence associated with increasing number of concurrent prescription drugs taken, among patients with comorbidity who are diagnosed with cancer [11, 35, 36]. Such patterns of non-adherence are often the result of increased likelihood of medication interference and adverse reactions related to the multiple medications for the pre-existing comorbidity and cancer treatment [37]. With nearly one-third of cancer patients exposed to potential drug-drug interactions [38, 39], which could potentially lead to increased toxicity or reduced therapy effectiveness [40–42], it is likely that appropriate reductions in statin use for certain patients could have contributed to the observed variation in statin adherence following diagnosis between cancer sites in our study. However, with limited evidence on risk of therapeutic complications among cancer patients with comorbidity, and most clinical trials excluding patients with substantial comorbidity, more research is needed to better understand the extent to which treatment-related toxicities and effectiveness influence medication management among this population [7, 34, 43].
Treatment plans may serve as a tool that can be leveraged by providers and health care systems to document recommended medication use, provider roles, cost of treatment, and follow-up care to help patients as they manage their multi-morbidities [44]. Gatwood and Bailey suggest that these treatment plans may also help in treatment discussions between patients and providers, as they consider the challenges experiences with taking multiple medications, including the risk of outcomes associated with nonadherence and the costs associated with multiple treatments [44]. Further, as patients progress along the cancer care continuum, the information from the treatment plans can be revised and potentially used to develop survivorship care plans, which have been shown to improve survivors’ ability to identify the appropriate provider for specific follow-up care and may lead to improved health outcomes [45].
Cost-related medication non-adherence has also been shown to be of significant concern among individuals diagnosed with cancer [46–49]. Two population-based studies [46, 49] found that compared to individuals without a cancer history, cancer survivors were significantly more likely to report changes in their prescription medication use for financial reasons. Findings from Zheng et al. [46] show that recently diagnosed (within 2 years of diagnosis) cancer survivors were significantly more likely than non-cancer controls to report a change in prescription medication use for financial reasons; furthermore, the percentage of cancer survivors reporting changes in medication use due to financial reasons increased as the number of comorbidities increased [46]. Consequently, the growing cost burden for patients with comorbidity who are diagnosed with cancer may also have significant implications on adherence to both non-cancer and cancer medications. Future studies that explore the association between medication costs and adherence, of both non-cancer and cancer drugs, among patients with comorbidity who are diagnosed cancer are needed to better understand the potential negative effects of multimorbidity in this unique patient population.
Of noteworthy is our finding of racial/ethnic variation in overall adherence to statin medications, across the study period, despite finding no significant differences by race/ethnicity in the changes in statin adherence prior to and following cancer diagnosis. Lower adherence to statins and other prescription medications among racial/ethnic minority groups, compared to white patients, is well-documented in the literature [15, 17, 50–54]. However, few studies have assessed the association between race/ethnicity and medication adherence within the context of a cancer diagnosis. Calip et al. [55] found no racial/ethnic differences in adherence to antihypertensive, diabetes or statin medications in the second year following cancer treatment, among breast cancer survivors. While these findings provide a better understanding of medication adherence following treatment of cancer among women of different race/ethnicity, it is imperative that future studies investigate whether patterns of medication adherence in the time during cancer diagnosis and treatment differ by race/ethnicity and lead to disparities in health outcomes.
Our study has certain limitations that deserve consideration. Our study was conducted among cancer patients enrolled in an integrated health plan. Therefore, our findings are most generalizable to similar populations. However, there were several advantages to this population as well, including the stability of the population over time, access to both prescribing and dispensing data, and our ability to track patients across providers. Use of pharmacy data on medication dispensings does not guarantee that patients took the medication as directed, potentially leading to overestimation of adherence. However, pharmacy records are generally considered to be more accurate than self-reported medication use. Although we were not able to assess the specific reasons for decreased statin adherence following cancer diagnosis in our current data, future studies that collect such information from both patients and providers are warranted. Findings on Native-American/Alaskan Native patients are based on a small sample (n=33) and, thus, we did not have the statistical power to detect meaningful differences. Nevertheless, Last, our findings are based on a cohort of prevalent users, and may not be applicable to new user populations; since, prevalent users may exhibit different health risks and health behaviors associated with statin use and adherence than individuals newly initiating statin medication [56].
Importantly, our study was comprised of a large cohort with comprehensive, long-term data on relevant clinical, pharmacy and tumor characteristics. Additionally, the inclusion criteria and measurement time points employed in this study enabled us to evaluate long-term patterns of statin use both prior to and following cancer diagnosis among a stable cohort of patients with continuous drug coverage benefits. Thus, while differences in study design, population and measurements make direct comparisons with other studies of statin medication adherence difficult, our estimates provide a valid assessment of statin adherence patterns among individuals with long-term use of medication for a comorbid condition who are newly diagnosed with cancer.
CONCLUSION
Despite the recognized challenges and hardship that are brought upon by a cancer diagnosis, there is a large gap in evidence regarding the added complexity that individuals with pre-existing chronic conditions who are diagnosed with cancer encounter. Understanding the extent to which the observed variation in statin adherence by cancer type is driven by providers, through strategic reductions in medication, or by patients, in response to mounting side effects, is imperative. Future research efforts are warranted that examine medical care patterns and behaviors among individuals with existing chronic conditions who are diagnosed with cancer across disease profiles, health care settings and sociodemographically diverse populations.
Supplementary Material
Acknowledgments
Funding: This work was supported by PHS grant from the National Cancer Institute (R01 CA098838, Habel, PI; R01AG032249, Adams, PI; Cancer Research Network: U24 CA17152, Kushi, PI).
This work was supported by PHS grant from the National Cancer Institute (R01 CA098838, Habel, PI; R01AG032249, Adams, PI; Cancer Research Network: U24 CA17152, Kushi, PI). Parts of this study were presented at the 8th American Association for Cancer Research Conference on The Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved. The study sponsor had no role in study design; collection, analysis, or interpretation of data; writing of the report; or the decision to submit the report.
Potential Conflicts of Interest: MPB has received research grants from AstraZeneca and LAH has received research grants from Genentech, for projects outside of this work.
Footnotes
This study was a secondary analysis of existing dataset with no PHI and, therefore, did not meet the definition of human subjects research
COMPLIANCE WITH ETHICAL STANDARDS
Ethical approval: This study was a secondary analysis of existing dataset with no PHI and, therefore, did not meet the definition of human subjects research. For this type of study formal consent is not required.
DATA AVAILABILITY
The datasets generated during and/or analyzed during the current study are not publicly available due HIPAA. Inquiries about the data can be sent to Dr. Habel.
REFERENCES
- 1.American Cancer Society, Cancer Facts & Figures 2018. 2018, American Cancer Society: Atlanta, GA. [Google Scholar]
- 2.Edwards BK, et al. , Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer, 2014. 120(9): p. 1290–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith AW, et al. , Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev, 2008. 29(4): p. 41–56. [PMC free article] [PubMed] [Google Scholar]
- 4.Deckx L, et al. , Chronic Diseases among Older Cancer Survivors. J Cancer Epidemiol, 2012. 2012: p. 206414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilmer SN, et al. , A drug burden index to define the functional burden of medications in older people. Arch Intern Med, 2007. 167(8): p. 781–7. [DOI] [PubMed] [Google Scholar]
- 6.Cashman J, Wright J, and Ring A, The treatment of co-morbidities in older patients with metastatic cancer. Support Care Cancer, 2010. 18(5): p. 651–5. [DOI] [PubMed] [Google Scholar]
- 7.Ritchie CS, Kvale E, and Fisch MJ, Multimorbidity: an issue of growing importance for oncologists. J Oncol Pract, 2011. 7(6): p. 371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuart BC, Davidoff AJ, and Erten MZ, Changes in Medication Management After a Diagnosis of Cancer Among Medicare Beneficiaries With Diabetes. J Oncol Pract, 2015. 11(6): p. 429–34. [DOI] [PubMed] [Google Scholar]
- 9.Calip GS, Boudreau DM, and Loggers ET, Changes in adherence to statins and subsequent lipid profiles during and following breast cancer treatment. Breast Cancer Res Treat, 2013. 138(1): p. 225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calip GS, et al. , Adherence to oral diabetes medications and glycemic control during and following breast cancer treatment. Pharmacoepidemiol Drug Saf, 2014. 24(1): p. 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, et al. , Nonadherence to Oral Medications for Chronic Conditions in Breast Cancer Survivors. J Oncol Pract, 2016. 12(8): p. e800–9. [DOI] [PubMed] [Google Scholar]
- 12.Chou YT, et al. , Assessing disruptions in adherence to antidepressant treatments after breast cancer diagnosis. Pharmacoepidemiol Drug Saf, 2017. 26(6): p. 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gellad WF, Haas JS, and Safran DG, Race/ethnicity and nonadherence to prescription medications among seniors: results of a national study. J Gen Intern Med, 2007. 22(11): p. 1572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poon I, et al. , Racial/ethnic disparities in medication use among veterans with hypertension and dementia: a national cohort study. Ann Pharmacother, 2009. 43(2): p. 185–93. [DOI] [PubMed] [Google Scholar]
- 15.Adams AS, et al. , Medication adherence and racial differences in A1C control. Diabetes Care, 2008. 31(5): p. 916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y and Baik SH, Race/Ethnicity, disability, and medication adherence among medicare beneficiaries with heart failure. J Gen Intern Med, 2014. 29(4): p. 602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams AS, et al. , Health system factors and antihypertensive adherence in a racially and ethnically diverse cohort of new users. JAMA Intern Med, 2013. 173(1): p. 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heart Protection Study Collaborative, G., MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet, 2002. 360(9326): p. 7–22. [DOI] [PubMed] [Google Scholar]
- 19.Taylor F, et al. , Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev, 2013(1): p. CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou R, et al. , Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA, 2016. 316(19): p. 2008–2024. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen TR, et al. , High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA, 2005. 294(19): p. 2437–45. [DOI] [PubMed] [Google Scholar]
- 22.Shepherd J, et al. , Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet, 2002. 360(9346): p. 1623–30. [DOI] [PubMed] [Google Scholar]
- 23.Bates TR, Connaughton VM, and Watts GF, Non-adherence to statin therapy: a major challenge for preventive cardiology. Expert Opin Pharmacother, 2009. 10(18): p. 2973–85. [DOI] [PubMed] [Google Scholar]
- 24.Turin A, Pandit J, and Stone NJ, Statins and Nonadherence: Should We RELATE Better? J Cardiovasc Pharmacol Ther, 2015. 20(5): p. 447–56. [DOI] [PubMed] [Google Scholar]
- 25.Lemstra M and Blackburn D, Nonadherence to statin therapy: discontinuation after a single fill. Can J Cardiol, 2012. 28(5): p. 567–73. [DOI] [PubMed] [Google Scholar]
- 26.Lemstra M, et al. , Proportion and risk indicators of nonadherence to statin therapy: a meta-analysis. Can J Cardiol, 2012. 28(5): p. 574–80. [DOI] [PubMed] [Google Scholar]
- 27.Benner JS, et al. , Long-term persistence in use of statin therapy in elderly patients. JAMA, 2002. 288(4): p. 455–61. [DOI] [PubMed] [Google Scholar]
- 28.Vinker S, et al. , Adherence with statins over 8 years in a usual care setting. Am J Manag Care, 2008. 14(6): p. 388–92. [PubMed] [Google Scholar]
- 29.Oehrli MD and Quesenberry CP, Northern California Cancer Registry: 2015 Annual Report on Trends, Incidence, and Outcomes. 2015, Kaiser Permanente: Oakland, CA. [Google Scholar]
- 30.Nau DP Proportion of days covered (PDC) as the preferred method of measuring medication adherence. 02/01/2016]; Available from: www.pqaalliance.org/images/uploads/files/PQA%20PDC%20vs%20%20MPR.pdf
- 31.Nichol MB, et al. , Transition probabilities and predictors of adherence in a California Medicaid population using antihypertensive and lipid-lowering medications. Value Health, 2009. 12(4): p. 544–50. [DOI] [PubMed] [Google Scholar]
- 32.Slejko JF, et al. , Adherence to Statins in Primary Prevention: Yearly Adherence Changes and Outcomes. J Manag Care Pharm, 2014. 20(1): p. 51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzmaurice GM, Laird NM, and Ware JH, eds. Applied Longitudinal Analysis. Second ed. 2011, John Wiley and Sons: New York, New York. [Google Scholar]
- 34.Sarfati D, Koczwara B, and Jackson C, The impact of comorbidity on cancer and its treatment. CA Cancer J Clin, 2016. 66(4): p. 337–50. [DOI] [PubMed] [Google Scholar]
- 35.Shin DW, et al. , Antihypertensive medication adherence in cancer survivors and its affecting factors: results of a Korean population-based study. Support Care Cancer, 2010. 19(2): p. 211–20. [DOI] [PubMed] [Google Scholar]
- 36.Tam-McDevitt J, Polypharmacy, aging, and cancer. Oncology (Williston Park), 2008. 22(9): p. 1052–5, discussion 1055, 1058, 1060. [PubMed] [Google Scholar]
- 37.daCosta DiBonaventura M, et al. , Patient preferences and treatment adherence among women diagnosed with metastatic breast cancer. Am Health Drug Benefits, 2014. 7(7): p. 386–96. [PMC free article] [PubMed] [Google Scholar]
- 38.Riechelmann RP, et al. , Potential drug interactions and duplicate prescriptions among cancer patients. J Natl Cancer Inst, 2007. 99(8): p. 592–600. [DOI] [PubMed] [Google Scholar]
- 39.Riechelmann RP, et al. , Potential drug interactions in cancer patients receiving supportive care exclusively. J Pain Symptom Manage, 2008. 35(5): p. 535–43. [DOI] [PubMed] [Google Scholar]
- 40.Riechelmann RP and Del Giglio A, Drug interactions in oncology: how common are they? Ann Oncol, 2009. 20(12): p. 1907–12. [DOI] [PubMed] [Google Scholar]
- 41.Beijnen JH and Schellens JH, Drug interactions in oncology. Lancet Oncol, 2004. 5(8): p. 489–96. [DOI] [PubMed] [Google Scholar]
- 42.Blower P, et al. , Drug-drug interactions in oncology: why are they important and can they be minimized? Crit Rev Oncol Hematol, 2005. 55(2): p. 117–42. [DOI] [PubMed] [Google Scholar]
- 43.LeBlanc TW, et al. , Polypharmacy in patients with advanced cancer and the role of medication discontinuation. Lancet Oncol, 2015. 16(7): p. e333–41. [DOI] [PubMed] [Google Scholar]
- 44.Gatwood J and Bailey JE, Improving medication adherence in hypercholesterolemia: challenges and solutions. Vasc Health Risk Manag, 2014. 10: p. 615–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobsen PB, et al. , Systematic Review of the Impact of Cancer Survivorship Care Plans on Health Outcomes and Health Care Delivery. J Clin Oncol, 2018. 36(20): p. 2088–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Z, et al. , Do cancer survivors change their prescription drug use for financial reasons? Findings from a nationally representative sample in the United States. Cancer, 2017. 123(8): p. 1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darkow T, et al. , Treatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics, 2007. 25(6): p. 481–96. [DOI] [PubMed] [Google Scholar]
- 48.Dusetzina SB, et al. , Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol, 2014. 32(4): p. 306–11. [DOI] [PubMed] [Google Scholar]
- 49.Kaul S, et al. , Cost-related medication nonadherence among adolescent and young adult cancer survivors. Cancer, 2017. 123(14): p. 2726–2734. [DOI] [PubMed] [Google Scholar]
- 50.Adams AS, et al. , Changes in use of lipid-lowering medications among black and white dual enrollees with diabetes transitioning from Medicaid to Medicare Part D drug coverage. Med Care, 2014. 52(8): p. 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mann DM, et al. , Predictors of nonadherence to statins: a systematic review and meta-analysis. Ann Pharmacother, 2010. 44(9): p. 1410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y, et al. , Predictors of medication nonadherence among patients with diabetes in Medicare Part D programs: a retrospective cohort study. Clin Ther, 2009. 31(10): p. 2178–88; discussion 2150–1. [DOI] [PubMed] [Google Scholar]
- 53.Rolnick SJ, et al. , Patient characteristics associated with medication adherence. Clin Med Res, 2013. 11(2): p. 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ritchey M, et al. , Vital Signs: Disparities in Antihypertensive Medication Nonadherence Among Medicare Part D Beneficiaries - United States, 2014. MMWR Morb Mortal Wkly Rep, 2016. 65(36): p. 967–76. [DOI] [PubMed] [Google Scholar]
- 55.Calip GS, Elmore JG, and Boudreau DM, Characteristics associated with nonadherence to medications for hypertension, diabetes, and dyslipidemia among breast cancer survivors. Breast Cancer Res Treat, 2017. 161(1): p. 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Danaei G, Tavakkoli M, and Hernan MA, Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a metaanalysis of statins. Am J Epidemiol, 2012. 175(4): p. 250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.