Abstract
Chronic wounds are a significant medical and economic problem worldwide. Individuals over the age of 65 are particularly vulnerable to pressure ulcers and impaired wound healing. With this demographic growing rapidly there is a need for effective treatments. We have previously shown that defective hypoxia signaling through destabilization of the master hypoxia-inducible factor 1α (HIF-1α) underlies impairments in both aging and diabetic wound healing. To stabilize HIF-1α, we developed a transdermal delivery system of the FDA-approved small molecule deferoxamine (DFO) and found that transdermal DFO could both prevent and treat ulcers in diabetic mice. Here, we show that transdermal DFO can similarly prevent pressure ulcers and normalize aged wound healing. Enhanced wound healing by DFO is brought about by stabilization of HIF-1α and improvements in neovascularization. Transdermal DFO can be rapidly translated into the clinic and may represent a new approach to prevent and treat pressure ulcers in aged patients.
Keywords: Deferoxamine, Wound Healing, Aging, Pressure Ulcers, Skin Repair, Hypoxia Signaling
INTRODUCTION:
There are currently 46.2 million people in the United States over 65 years old. By 2060, this number is expected to rise to 98 million1. Chronic wounds including venous leg ulcers, diabetic foot ulcers, arterial insufficiency and pressure ulcers disproportionately affect these elderly individuals and lead to substantial morbidity, mortality and expense for the healthcare system2. While several therapies for wound healing exist, they are only moderately effective in preventing and treating impaired wound healing in aged patients2,3. Thus, there is an urgent clinical need to understand wound healing in human aging so that effective therapies to treat wounds can be developed for this population segment4.
We have previously identified the critical molecular and cellular pathways responsible for normal wound healing that are impaired by advanced age5–8. Specifically, we have identified that destabilization of HIF-1α impairs neovascularization, local fibroblast function and distal progenitor cell recruitment, causing deficiencies in aged wound healing9–13. To enhance hypoxia signaling during impaired wound healing, we previously tested two FDA-approved drugs, dimethyloxalylglycine (DMOG) and deferoxamine (DFO) by topical application of these drugs in solution form on diabetic and aged mice with wounds13. DFO solution was found to improve wound healing in both diabetic and aged mice13. Furthermore, systemic delivery of DFO by intraperitoneal (IP) injections was found to improve survival of ischemic tissue in aged mice by enhancing new blood vessel formation5.
While our findings repeatedly demonstrated that DFO in solution improved wound healing, there was a need to develop a reliable and consistent delivery system that could be used clinically to deliver the drug through both intact and injured skin of patients with chronic wounds. A DFO transdermal drug delivery system (DFO-TDDS) was developed, which when applied topically, released the drug in a sustained manner through the impermeable stratum corneum into the dermis11. We first tested DFO-TDDS by application on ulcers in diabetic mice. DFO-TDDS both prevented and healed ulcers in diabetic mice11.
Here, we verify the effectiveness of DFO-TDDS in accelerating wound healing in aged mice. We first demonstrate that systemic injection of DFO can prevent and heal pressure ulcers in aged mice. Next, we test the effects of DFO-TDDS on wound healing in aged mice and demonstrate its effectiveness in stabilizing HIF-1α, improving neovascularization in aged wounds and restoring healing in aged mice to near normal.
METHODS
Animals
C57Bl/6 mice were acquired from the National Institute on Aging (NIA, Bethesda, MD). Young mice were 3 months of age, while aged mice were over 21 months of age. All mice were housed in the Stanford University Veterinary Service Center in accordance with NIH and institution-approved animal care guidelines. All procedures were approved by the Stanford Administrative Panel on Laboratory Animal Care.
Pressure ulcer model
Pressure ulcers were induced on the dorsum of the mice as previously described11. An ischemia-reperfusion cycle was performed to create each pressure ulcer. Ischemia induction was stimulated with the application of two ceramic magnets for a period of six hours, followed by a six-hour reperfusion period initiated by removal of the magnets. Each mouse had two separate ulcers formed on their back. Mice (n=4) at least 21 months of age were randomized into two groups where they were injected with either phosphate buffered saline (PBS) or DFO. Injections started the day before ulcer formation, and continued every other day until wound closure. All wounds were covered with an occlusive dressing (Tegaderm; 3M).
Histology
After the mice were euthanized, wounds were harvested with a 2-mm rim of unwounded skin. Frozen tissue samples for CD31 and terminal deoxynucleotidyl transferase dUTP Nick-End Labeling (TUNEL) immunohistochemistry were prepared by immediate OCT embedding (Sakura Finetek USA, Inc.). Trichrome staining was performed on sections of healed wounds that were either treated with DFO-TDDS or left untreated.
Formulation of DFO-TDDS
Transdermal DFO patches were formulated as previously described11. Briefly, DFO is encapsulated within nonionic surfactants to generate reverse-micelles that allows for permeation through the stratum corneum. The reverse-micelles are dispersed throughout a degradable slow-release matrix which allows for continuous and efficient application of DFO into the dermis.
Excisional wound healing and DFO-TDDS treatment
The excisional wound model was used as previously described14. Briefly, 6-mm wounds were generated on the dorsa with the use of a biopsy punch. The wounds were stented open with silicone rings that were sutured to the mice with 6–0 sutures. Mice at least 21 months of age were randomized into two groups whereby one group received DFO-TDDS topically every day until closure and the other served as an untreated control. A third group of untreated young mice was also used as control.
Western blotting
For Western blot analysis, protein was separated on a 4–12% polyacrylamide gel and then transferred to a nitrocellulose membrane. Anti- HIF-1α (ab179483 1:1000, Abcam, Cambridge, UK) and anti-β-actin (ab8227, Abcam, Cambridge, UK) were used as primary antibodies, while an HRP-conjugated secondary antibody was used at a 1:10,000 dilution (Abcam, Inc., Cambridge, UK) and detected using an ECL Plus Western Blotting Detection Kit (GE Healthcare, Chicago, IL).
Enzyme-linked immunosorbent assay (ELISA)
Protein was isolated from harvested wounds via homogenization of the tissue in RIPA buffer combined with protease inhibitor. Vascular endothelial growth factor (VEGF) levels were measured with a VEGF ELISA kit (R&D Systems, Minneapolis, MN), following the manufacturer’s protocol.
Statistical analysis
SPSS 12 software was used to perform univariate Student t-tests and multivariate ANOVA for wound healing analysis. Power analysis was accomplished using G- Power software (Parkville, Australia).
RESULTS
Systemic delivery of DFO prevents and enhances healing of pressure ulcers
We first tested the effectiveness of DFO in preventing and treating pressure ulcers in aged mice. Two pressure ulcers were created on the dorsum of aged mice (Figure 1A). One group of mice was treated with systemic DFO, while the other group was injected with PBS and served as the control. Aged mice treated with systemic DFO demonstrated a decreased incidence of ulcer formation and lesser grade ulceration (Figure 1B-C). Next, we compared new blood vessel formation in the two groups by performing immunohistochemistry for CD31 on histological sections of the pressure ulcers. We found a statistically significant two-fold increase in neovascularization in the pressure ulcers treated with DFO (*p<0.05) (Figure 1D). We also analyzed the histological sections for apoptosis by TUNEL assay. Treatment with DFO brought about a statistically significant two-fold decrease in apoptosis (*p<0.05) (Figure 1E). Our findings suggest that DFO provides an efficient means of preventing and treating pressure ulcers in debilitated elderly patients.
Figure 1. Systemic delivery of DFO enhances healing of pressure ulcers.
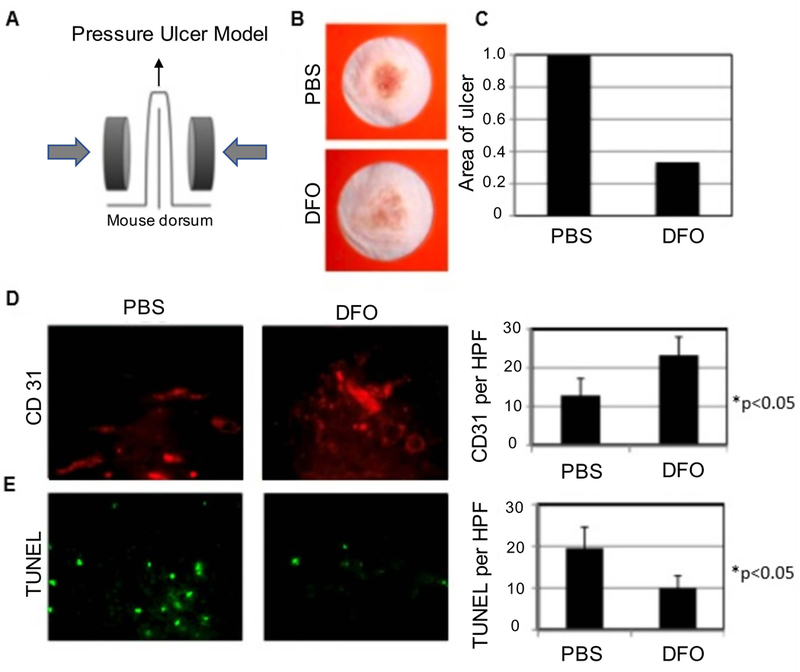
(A) Graphic depicting method by which pressure ulcers were created on mice. (B) Photographs of ulcer wounds treated with PBS and DFO respectively. (C) Comparison of wound area between control and DFO treatment groups. (D) Immunofluorescence staining for CD31 (red) comparing DFO treatment with control and quantification of CD31 positive pixels per high power field (HPF). (E) Immunofluorescence staining for TUNEL (green) comparing DFO treatment with control and quantification of TUNEL positive pixels per HPF.
Transdermal DFO enhances healing of wounds in aged mice
We next wanted to verify if DFO-TDDS could enhance wound healing in aged mice. Wound closure of the DFO-TDDS treated group was compared to wound closure in both the untreated young (3 months) and aged (21 month) control groups (Figure 2A). Wounds in untreated aged mice closed significantly slower than those in the untreated young mice, consistent with previous findings7. Aged mice treated with DFO-TDDS showed significantly accelerated healing over the untreated aged control group (Figure 2B). Following euthanasia of the mice, skin samples were isolated from the healed wounds and used for molecular analysis. CD31 immunohistochemistry staining displayed similar neovascularization between the young untreated mice and the aged DFO-TDDS treated mice. The number of vessels per high power field (HPF) in both of these groups was found to be significantly higher (*p<0.05) than the aged untreated control following statistical analysis (Figure 2C). Next, protein was extracted from the skin samples and analyzed by ELISA. The DFO-TDDS treated group displayed significantly higher levels of VEGF (in ng/mL) compared to the untreated aged controls (*p<0.05) (Figure 2D). We also performed Western blot analysis on the wound lysates. DFO-TDDS treated mice displayed higher expression of HIF-1α compared to the untreated aged group (*p<0.05) (Figure 2E).
Figure 2. DFO-TDDS enhances healing of excisional wounds in aged mice.

(A) Excisional wounds in aged mice treated with DFO-TDDS compared with those in young untreated and aged untreated control groups. (B) Wound healing curves as a function of time showing the DFO-TDDS treated group displaying similar time to wound closure as the young control. (C) Immunofluorescence staining for CD31 (red) comparing untreated young and aged mice with DFO-TDDS treated aged mice and quantification of CD31 immunofluorescence staining. The aged DFO-TDDS treated group displayed a significantly higher measure of blood vessel growth similar to the young untreated group. (D) Quantification of VEGF concentration from ELISA assay. The DFO-TDDS treated group displayed significantly higher concentration of VEGF compared to their untreated counterparts. (E) Western blot analysis of HIF-1α protein on post-operative day 5. DFO-TDDS treated mice show an increase in HIF-1α expression compared to their untreated aged counterparts.
Histological sections of healed wounds in both aged untreated and aged DFO-TDDS groups were subjected to trichrome staining. The dermal thickness was significantly higher in DFO-TDDS treated mice (*p<0.05) and DFO-TDDS treated wounds displayed re-appearance of epidermal appendages, indicating enhanced wound remodeling (Figure 3A-B). Histological sections of healed wounds were also subjected to TUNEL staining to label for DNA fragmentation and dying cells. There was significantly higher cell death (*p<0.05) in untreated wounds compared to DFO-TDDS treated wounds (Figure 3C-D). Thus, we demonstrate that DFO-TDDS significantly accelerates wound healing in aged mice through increased HIF-1α expression and VEGF production. The healed wounds following DFO-TDDS treatment exhibit a significantly thicker dermis and lesser cell death.
Figure 3. DFO-TDDS enhances dermal thickness and wound remodeling in aged mice.

(A) Histological sections of healed wounds treated with DFO-TDDS and untreated wound samples were subjected to trichrome staining. Scale bar: 500um (B) Dermal thickness was calculated from the stained sections and represented in arbitrary units (a.u). (C) Histological sections of healed wounds treated with DFO-TDDS and untreated wound samples were subjected to TUNEL staining to identify dead cells (red) and counterstained with DAPI (blue). (D) The fluorescence intensity of TUNEL stain was quantified. Scale bar: 10um.
DISCUSSION
Advanced age is a known risk factor for pressure ulcer formation. Elderly bed-ridden patients are at significant risk for the development of pressure ulcers and have a prolonged recovery period due to impairments in neovascularization. Recurrence rates are high, and in many cases, lead to amputations and mortality. Repetitive cycles of ischemia and reperfusion characterize the pathophysiology of pressure ulcers, resulting in increased reactive oxygen species (ROS). These elevated levels of ROS induce oxidative tissue damage, partially through an iron-driven hydroxyl radical formation pathway6,7. Various strategies for stabilizing and increasing HIF-1α have been employed to improve new blood vessel formation and wound healing, including HIF-1α gene transfection and delivery of stem and progenitor cells with increased HIF-1α activity15,16. We have exogenously applied the iron chelator DFO, which stabilizes HIF-1α and protects cells from free-radical injury.
DFO is a small molecule FDA-approved iron-chelator approved for treating hemochromatosis in diseases such as thalassemia and sickle cell disease17. We have previously shown that systemic delivery of DFO can improve new blood vessel formation by stabilizing HIF-1α and enhancing the survival of ischemic flaps in aged mice. We have also shown that DFO solution dripped on wounds can enhance healing13. However, pressure ulcers in humans begin with an intact epidermis and to prevent them requires delivery through intact skin. It is very difficult to consistently deliver DFO, a hydrophilic small molecule through the hydrophobic epidermis, which limits the efficacy of simple topical administration. Any proposed topical delivery of DFO such as an ointment or cream needs to overcome these biochemical constraints to be effective as a prophylactic therapeutic. After looking at a variety of delivery strategies, we developed DFO-TDDS, where the DFO is enclosed within nanoscale reverse micelles that can penetrate the hydrophobic epidermis and release the small molecule drug specifically into the dermis. In vitro characterization of DFO-TDDS using a Franz cell set-up showed uniformity of drug release into the human dermis11. DFO-TDDS was found to both prevent and treat ulcers in diabetic mice11.
Similar to humans, aged mice demonstrate impaired wound regeneration, characterized by greater tissue necrosis and lesser blood vessel formation7. Here, we first confirmed that systemic DFO delivery through IP injections enhanced healing of aged pressure ulcers. We were unable to use the DFO-TDDS on these mice because it physically interfered with the application of the magnets which are essential to the pressure ulcer model. We will need to do these studies in a more human-like, porcine model. We then topically applied DFO-TDDS every day on wounds in aged mice, as would be eventually performed in aged patients, and compared the healing of the treated group to young and aged untreated counterparts. We found that DFO significantly increased wound regeneration in aged mice by increasing neovascularization and VEGF expression, while also decreasing apoptosis. The TDDS optimized the drug delivery process, offering an effective alternative to IP injection. DFO-TDDS can be rapidly scaled and optimized for treating both pressure ulcers and chronic wounds in aged patients.
ACKNOWLEDGEMENTS
The authors would like to thank Yujin Park for her assistance with tissue processing. Funding for this research was provided by the Hagey Family Endowed Fund in Stem Cell Research and Regenerative Medicine, the National Institute on Aging (R01-AG025016), the National Institutes of Health (R01- DK074095-13) and the Oak Foundation. Melanie Rodrigues was supported by the 3M and Wound Healing Foundation Fellowship.
REFERENCES
- 1.Statistics. Accessed 3.2.2016. Administration for Community Living US Department of Health and Human Services. [Google Scholar]
- 2.Gould L et al. Chronic wound repair and healing in older adults: current status and future research. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 23, 1–13, 10.1111/wrr.12245 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Premnath Shenoy AH Elderyly patients’ participation in clinical trials. Perspect Clin Res 6, 184–189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJ et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2197–2223, 10.1016/S0140-6736(12)61689-4 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Chang EI et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation 116, 2818–2829, 10.1161/CIRCULATIONAHA.107.715847 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara T et al. Age-associated intracellular superoxide dismutase deficiency potentiates dermal fibroblast dysfunction during wound healing. Exp Dermatol, 10.1111/exd.13404 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Fujiwara T et al. Extracellular superoxide dismutase deficiency impairs wound healing in advanced age by reducing neovascularization and fibroblast function. Exp Dermatol 25, 206–211, 10.1111/exd.12909 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rennert RC et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res Ther 5, 79, 10.1186/scrt468 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thangarajah H et al. HIF-1alpha dysfunction in diabetes. Cell Cycle 9, 75–79, 10.4161/cc.9.1.10371 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Thangarajah H et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci U S A 106, 13505–13510, 10.1073/pnas.0906670106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duscher D et al. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proc Natl Acad Sci U S A 112, 94–99, 10.1073/pnas.1413445112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duscher D et al. Fibroblast-Specific Deletion of Hypoxia Inducible Factor-1 Critically Impairs Murine Cutaneous Neovascularization and Wound Healing. Plast Reconstr Surg 136, 1004–1013, 10.1097/PRS.0000000000001699 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duscher D et al. Comparison of the Hydroxylase Inhibitor Dimethyloxalylglycine and the Iron Chelator Deferoxamine in Diabetic and Aged Wound Healing. Plast Reconstr Surg 139, 695e–706e, 10.1097/PRS.0000000000003072 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galiano RD, Michaels J. t., Dobryansky M, Levine JP & Gurtner GC Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen 12, 485–492, 10.1111/j.1067-1927.2004.12404.x (2004). [DOI] [PubMed] [Google Scholar]
- 15.Du J et al. Combination of HIF-1alpha gene transfection and HIF-1-activated bone marrow-derived angiogenic cell infusion improves burn wound healing in aged mice. Gene Ther 20, 1070–1076, 10.1038/gt.2013.32 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Zhang X et al. Impaired angiogenesis and mobilization of circulating angiogenic cells in HIF-1alpha heterozygous-null mice after burn wounding. Wound Repair Regen 18, 193–201, 10.1111/j.1524-475X.2010.00570.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minniti CP & Kato GJ Critical Reviews: How we treat sickle cell patients with leg ulcers. Am J Hematol 91, 22–30, 10.1002/ajh.24134 (2016). [DOI] [PubMed] [Google Scholar]


