Abstract
Netrins and semaphorins, members of the neuronal guidance cue family, exhibit a rich biology with significant roles that extend beyond chemotactic guidance of the axons to build the neuronal patterns of the body. Screening of adult tissues and specific cellular subsets have illuminated that these proteins are also abundantly expressed under both steady state and pathological scenarios. This observation suggests that, in addition to their role in the development of the axonal tree, these proteins possess additional novel functions in adult physiopathology. Notably, a series of striking evidence has emerged in the literature describing their roles as potent regulators of both innate and adaptive immunity, providing extra dimension to our knowledge of neuronal guidance cues. In this review, we summarize the key complex roles of netrins and semaphorins outside the central nervous system (CNS) with focus on their immunomodulatory functions that impact pathophysiological conditions.
Keywords: neuronal guidance cues, inflammation, innate immunity, acquired immunity, macrophage, chemotaxis
1. Introduction
During the development of the CNS, axons are the major source of neuronal guidance cues, which are comprised of the large family members: netrins, semaphorins, ephrins and slits [1]. Utilizing repulsion and attraction forces in combination with paracrine and autocrine effects, these cues guide the protruding axons appropriately to integrate communication between the tissues and their environment. It has only been during the last two decades that research has uncovered a unique biology of these proteins in complex adult physiopathological states unassociated with their original neuronal guidance cue functions. In this review, we will focus on the novel roles of netrins and semaphorins outside the CNS as described by the mounting literature investigating their functions in various inflammatory illnesses. Most notably, the pleiotropic functions executed by these cues influence organogenesis, direct cell migration and mediate cell-cell adhesion in numerous organs including the lung, pancreas, mammary gland, vasculature and muscle [2–7]. Because the cells comprising the immune system employ similar mechanisms to perform their functions, it was not surprising when recent studies uncovered the roles of netrins and semaphorins in guiding the immune response. However, a major knowledge gap persisted as these cues were thought to operate only during development. By taking advantage of advanced technologies, such as genome-wide screening, several research groups have established that immune subsets express both netrins and semaphorins. The subsequent challenge was to uncover the mechanism by which netrins and semaphorins were regulated, as well as what roles they played in these cellular subsets. In adults, it is often factors present locally within the tissue microenvironment that dictates their expression, which can be transcriptionally and/or translationally regulated [3, 8–10]. Initially, the identification of the roles of netrins and semaphorins were restricted as some members of the family were embryonincally lethal when completely deleted in mice models. However, the generation of the lox/Cre system has allowed for a more precise indication of each neuronal cue in specific cellular subsets both under steady biological states or under experimental or clinically diseased scenarios. More recently, their role as potential clinical biomarkers has also been actively investigated. This can be attributed to the fact that some netrins and semaphorins are secreted or truncated and that their circulating levels correlated with disease symptoms and severity. In this review, we will attempt to address some of the major immonumodulatory functions of netrins and semaphorins as they are described in the literature.
1.1 Netrin family
Only since the early nineties has research been aimed at the neuronal guidance cue family. The first identified member, uncoordinated-6 (UNC-6) was found while studying the neuronal development of nematode C. elegans [11]. Since this discovery, Netrin-1, 3, 4, G1, G2 and GPI-linked netrins have been discovered to be genetically conserved from invertebrates to mammals. Netrins encompass a family of diffusible proteins known to possess bona fide chemoattractant and chemo repellent guiding cue properties that direct axons and migrating cells during the development of the nervous system. Netrins are intuitively named after the Sanskrit word “netr” which means “one who guides”. Additional informative studies have shown that netrins influence key cellular and molecular events including chemotaxis and adhesion under pathological situations [2, 4, 5] as summarized in Table 1.
Table 1.
Variation of the expression and roles of netrins & semaphorins in inflammatory –related pathologies.
Recapitulative listing of studies that describe the impact of modulating levels of netrins and semaphorins in inflammatory diseases.
| Diseases | Neuronal Cue | Function | Reference |
|---|---|---|---|
|
| |||
| Atherosclerosis | ↑ Netrin-1/UNC5b | -Prevented macrophage foam cell emigration out of arterial wall | [52, 54] |
| ↑ Sema3E | -Regulated macrophage polarization and migration | [53, 55] | |
| Netrin-1/Sema3A | -Regulated monocyte adhesion to endothelium | [62] | |
|
| |||
| Obesity | ↑ Netrin-1/UNC5b | -Trapped adipose tissue macrophages in the white adipose tissue | [57] |
| ↑ Sema3E/PlexinD1 | -Reported to have pro-migratory functions in ATM; acts as chemoattractant for immune cells and regulates macrophage migration | [58] | |
|
| |||
| Cardiac Failure | Netrin-1/UNC5b | -Increased inflammatory status and accumulation of neutrophils/macrophages | [56, 59] |
| ↑Sema4D | -Plasma soluble form found in cohort of heart failure patients | [48] | |
|
| |||
| Kidney Injury | ↑ Sema3A ↑ Netrin-1 |
- Decreased injury when genetically deleted; excess disrupts glomerular filtration barrier; increased in urine following liver transplant | [49] |
|
| |||
| Rheumatoid Arthritis | ↑ Sema4A/PlexinB1 | - Promoted migration of synovial fibroblasts; found in serum and synovial fluid | [41] |
| ↑ Sema7a | - Increased Th1/Th17 cytokine secretion & T-bet upregulation in T cells | [42] | |
| ↑ Netrin-1 | -Increased in macrophages in inflamed areas of bone resorption; associated with osteoclast differentiation | [69, 71] | |
Netrin-1, a 604-amino-acid protein structurally similar to laminin, is the most widely studied secreted member of this neuronal guidance cue family. It is composed of an N-terminal laminin repeat, three cysteine-rich EGF modules and a positively charged C terminal domain. Netrin G ligands (NGLs) NGL-1 and NGL-2 bind to GPI-linked netrins, but possess a structure and function that differs from the extracellular receptor netrins [12]. Several plasma-membrane receptors that bind netrins have been described to mediate intracellular responses as covered in the detailed review by Cirulli et al [13]. These single-pass type I transmembrane proteins are members of the immunoglobulin (Ig) superfamily and include deleted in colorectal cancer (DCC), neogenin, down syndrome cell adhesion molecule (DSCAM), and UNC-5 homologue family UNC5A-D in mammals. DCC is comprised of the immunoglobulin superfamily of cell adhesion proteins and mediates intracellular signaling through the activation of tyrosine kinases, Src and focal adhesion kinase (FAK), to attract protruding axons [13]. Neogenin, which is expressed at the cell surface, is composed of fibronectin type III domains, immunoglobulin, transmembrane and a C terminal domain, and shares structural homology with DCC [14]. UNC5B members encode proteins termed dependence receptors as they induce apoptosis in the absence of the ligand and are responsible for mediating the repellent effects of Netrin-1 [9, 15, 16]. DSCAM can cooperate with DCC to mediate Netrin-1-dependent axonal movement in vertebrates [17].
The embryonic lethality of loss-of-function of Netrin-1 and Unc5b mice have limited the studies aimed at investigating their roles in adult physiopathology. Recently, the generation of floxed mice [18] has allowed researchers to bypass this issue and expand novel roles for these cues at the cellular and tissue-specific level which will be further developed below.
1.2 Semaphorin family
Semaphorins were discovered in the mid-nineties, shortly after the netrin family and were found to have similar axonal guidance characteristics [19] [20]. Through its Greek etymology, Semaphorin means “sign-bearer”. This large family displays a high structural and functional degree of conservation across species throughout evolution from unicellular viruses to mammals. Currently, over 20 semaphorins have been reported and are divided into eight major classes based on their structural and phylogenetic homology. An updated structural overview of each member can be appreciated in a recent review [21].
Semaphorin ligands are secreted or membrane-associated glycoproteins that share a common ~500 amino acid region called the sema domain. The sema domain, which has only been partly characterized by crystallography, confers specificity for receptor-ligand interactions that determine the underlying signaling pathways engendered [22]. Classes 1 and 2 are present only in invertebrates while class 5 members (Sema5A, Sema5B) are found in both vertebrates and invertebrates. Members of classes 3 (Sema3A–G), 4 (Sema4A–D, Sema4F–G), 6 (SemaA–D), and 7(Sema7A) are vertebrate only. Class V is termed so as it is only encoded by viruses. The presence of intrinsic anchored protein sequences in classes 1, 4, 5, and 6 makes them membrane bound. Classes 2 and 3 are synthesized as secreted proteins, while class 7 ligands are typically membrane-bound, but can also undergo proteolytic cleavage [23] to act as soluble ligands.
The predominant semaphorin receptors are classified in two groups: the plexins and neuropilins. The plexin family is comprised of large conserved transmembrane proteins [24] that were initially known to facilitate cell adhesion but were later found to have additional more complex roles [25] as summarized in Table 1 and Figure 1 & 2 below. The eleven members of the plexin family are divided into four classes (PlexinA–D). Each has an extracellular N-terminal sema domain responsible for binding to semaphorins and possess intracellular signal transduction capacities [26]. Semaphorins often require dimerization in order to bind to plexin receptors; failure to dimerize compromises downstream signaling without preventing binding [27, 28]. The highly conserved cytoplasmic region of plexins contains a crucial guanosine triphosphate (GTPase)-activating protein (GAP) domain that is segmented by a RHO-GTPase-binding domain (RBD). Following complex helix-loop conformational changes, the GAP domain triggers signaling events through activation of small GTPases, RAP, and a catalytic “arginine finger” to integrate semaphorin-plexin responses such as changes in cell morphology, cell adhesion, cell proliferation [29] and cell polarity [30]. Interestingly, the GAP domain of plexins can also exert catalytic inactivation by converting GTP to GDP which contributes to decreased neuronal collapse. Some plexins, such as those of the B-type, can physically interact with other neighboring receptors such as tyrosine kinases (RTK) to mediate their transactivation.
Figure 1. Involvement of Netrins and Semaphorins in major innate immune responses.
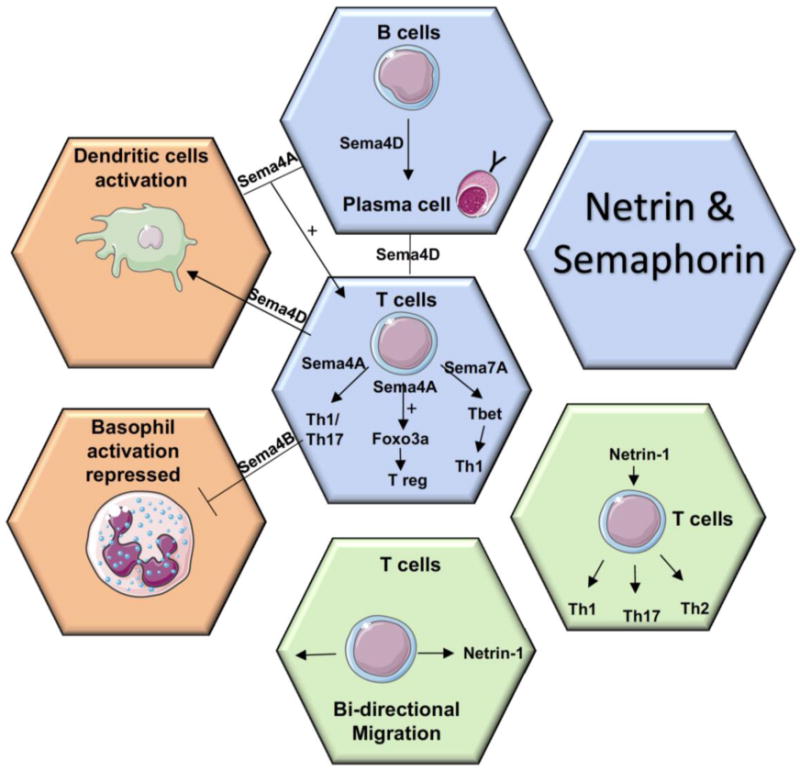
In the innate immune system, Sema4A expressed by dendritic cells (DC) and B cells is involved in the activation of T cells, induced Th1/Th17 responses as well as promoted Treg stability by inducing Foxo3a. T cells-derived Sema4D activated the humoral response of B cells and DC. In contrast, Sema4B repressed basophil activation and Th2 differentiation. Sema7A promoted inflammation by inducing the differentiation of Th1 cells. Recombinant Netrin-1 induced the expression of Th1/Th17/Th2 cytokines and regulated the bidirectional migration of T cells.
Figure 2. Diverse mechanisms regulating Netrins & Semaphorins expression in macrophages.
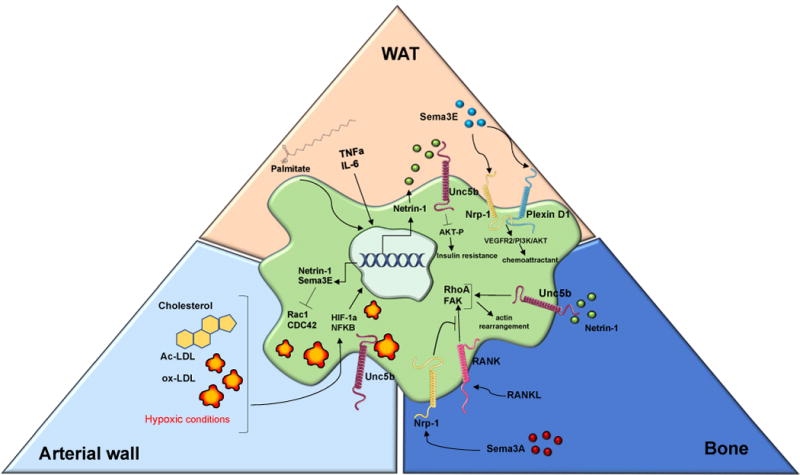
In macrophage-foam cells in the arterial wall, accumulation of cholesterol, acetylated low-density lipoprotein (Ac-LDL), oxidized low-density lipoportein (ox-LDL) and hypoxic conditions induced the expression of the transcription factors nuclear factor kappa-light-chain-enhancer of activated B cells (NFKB) and hypoxia inducible factor 1 α (HIF-1 α) to regulate the expression of Netrin-1 and Sema3E. Rac1 and CDC42 are inhibited, critical pathways for actin cytoskeleton organization. In the white adipose tissue (WAT), circulating levels of Sema3E bound to its receptors PlexinD1 and neuropilin-1 (Nrp-1) to activate VEGFR2/PI3K/AKT pathways leading to and IL-6 chemoattraction of adipose tissue macrophage (ATM). Free fatty acid palmitate, TNF-α and IL-6 tempered the expression of netrin-1 and its receptor Unc5b and inhibited insulin-induced AKT phosphorylation thereby contributing to insulin resistance. In bone marrow differentiated osteoclast, Sema3A/Nrp-1 complex repressed osteoclast differentiation by inhibiting receptor activator nuclear factor-kβ ligand (RANKL) and its downstream targets. Netrin-1 promoted osteoclast differentiation by activating Ras homolog gene family member (RhoA) and focal adhesion kinase (FAK).
Nrp1 and Nrp2, members of the neuropilin family, commonly act as cell surface co-receptors for secreted semaphorin ligands due to their very short intracellular C-terminal domain that contains a PDZ-binding motif [31]. Neuropilins are conserved in all vertebrates and harbor two coagulation factor homology domains, two complement binding domains and a MAM (meprin, A-5 protein, and receptor protein-tyrosine phosphatase mu) domain in their extracellular section [32]. Recently, vascular endothelial growth factor family (VEGF) members have been described to bind to neuropilins to form receptor complexes with semaphorins. Through these interactions, they can regulate key events in angiogenesis [33]. With the exception of Semaphorin 3E, class 3 semaphorins must interact with a neuropilin co-receptor in order to transduce a signal to the plexin receptor. However, most semaphorins require only specific plexin binding for signal transduction [34, 35].
2- The role of netrins and semaphorins in adaptive immunity
2.1 Netrins and lymphoid cells
Thus far, the ability of netrins to direct the adaptive immune response was primarily studied in T lymphocytes as shown in figure 1. Since the motility of CD4+ T cells is critical for their function, Boneschansker et al utilized an elegant microfluidic device in which, unlike unidirectional traditional migration assays, could characterize both negatively and positively directed migration patterns [36]. The effects of Netrin-1 on CD4+ T cells was proven to be complex. Netrin-1 induced the bidirectional locomotion of CD4+T cells both towards and away from concentration gradients of Netrin-1. This could be attributed to the varying levels of Netrin-1 receptors expressed by the cells. To test this hypothesis, the authors used a profiling approach to map the receptors of Netrin-1 expressed by CD4+ T cells. The interaction of Netrin-1 with neogenin seemed important in mediating the bidirectional migration response in vitro and in vivo using humanized SCID mice exposed to recombinant Netrin-1. Interestingly, we found a similar biphasic migration pattern of macrophages to different concentrations of Netrin-1 [52]. The mechanisms responsible for these puzzling findings are yet to be fully defined. Although we can imagine various theories depicting scenarios that operate in multicellular tissues, it will be important for future studies to place emphasis on elucidating the cellular source of Netrin-1 by utilizing technologies such a single-cell RNA sequencing. Because Netrin-1 harbors a laminin-like domain, we can also predict its likeliness to bind to extracellular matrix components exhibiting additional haptotactic potentials. Substrate-bound Netrin-1 could then impact its bioavailability-by either diluting its circulating concentration or creating complex chemoattractant gradient webs within the tissue. In addition, the migration force exerted by various chemokines will undoubtedly impact the outcome of the net migratory effect in vivo. It is also reasonable to suspect that the effects of Netrin-1 that dominate in this context are its role in regulating the resolution of inflammation. As such, the effects of Netrin-1 in T lymphocytes might be interpreted as a role in directing the egress of T lymphocytes either out of tissues or to direct them to lymph nodes.
Furthermore, Netrin-1 has been shown to repress the expression of a panel of both pro and anti-inflammatory cytokines released by Th1/Th2/Th17 CD4 subsets [15]. The mechanisms responsible for these in vitro observations are unclear as the expression of the receptors is poorly characterized. Similarly, the signaling pathways through which Netrin-1 could exert such actions on T cells remain to be defined. Further studies using Netrin-1 deletion in specific adaptive immune subsets will certainly provide novel mechanistic insights necessary to the field.
2.2- Semaphorins and lymphoid cells
Pioneer studies led by Kikutani & al, have uncovered the immuno-modulatory functions of class 4 semaphorins [37, 38]. They found that Sema4A, acting through novel receptor Tim-2, was expressed by B cells and dendritic cells and induced the activation and differentiation of T cells into antigen-specific subsets [39]. Consistent with these findings, the binding of Sema4A to neuropilin-1 expressed by regulatory T cells (Treg) inhibited AKT phosphorylation via phosphatase activation thereby increasing the nuclear localization of the protein forkhead box O 3 (Foxo3a), which dampened inflammation by fostering the survival and stability of Treg [40]. In contrast, the activation of the Sema4A/PlexinB1 axis [41], promoted inflammation in the setting of rheumatoid arthritis similarly to Sema7A which induced the upregulation of the transcription factor, T-bet, in T-cells in this disease [42]. These controversial inflammatory roles are yet to be explained mechanistically but are most probably due to the complex microenvironmental settings amenable for the transcriptional programing of T cells in the tissues. Notably, although the receptor for Sema4B has not yet been described, in order to regulate the IgE-mediated humoral memory response, basophil activation was repressed by Sema4B through cell-cell interactions with Th2 cells [43] (Figure 1). These findings were exciting as the junctions where T cells and other activated immune cells communicate mimick the neurological synapse where important neuronal information is exchanged. Intuitively, one could have hypothesized that neurological cues could direct the fate of T cell/antigen presenting cells based on their initially described function. This novel set of information has facilitated our understanding of the innate immune regulation by providing important instructive clues on how the semaphorins can impact T cell polarization but has also opened the doors for new avenues of research. Notably, future research endeavors fueled by inspiration from the well established role of neuronal cues in the CNS could help uncover unsolved issues in inflammatory deseases.
In an alternative study, a soluble activated form of transmembrane Sema4D was detected in supernatants of lymphocytes, which enhanced CD-40-mediated B-cell response suggesting a role for unbound semaphorins, besides membrane-anchored isoforms, in guiding the immune response [44]. The genetic manipulation of Sema4D gene to generate a truncated form of the protein validated these findings. In addition to regulating the B cell response by mitigating CD72 inhibitory signals allowing downstream BCR signaling to proceed [38, 45, 46], these mice manifested improved T-cell responses [47]. Consistent with these observations, serum levels of soluble Sema4D positively correlated with increased CD3+, CD4+ and CD8+ cells in a clinical cohort of patients suffering from heart failure [48]. These sets of data were compelling as they further evidenced the similarity between the highly patterned and circulating signaling mediators in both the nervous and immune systems. Similarly to cytokines, truncated semaphorins could act in a paracrine mode and signal to distant target cells expressing their cognate receptors. It will be useful to elucidate whether other members of the neuronal guidance cues function similarly to cytokines and hopefully, in the near future, we will describe a novel nomenclature for the “neurokine” family.
3- The role of netrins and semaphorins in innate immunity
3.1 Netrins/semaphorins and tissue macrophages
Based on the rationale that analogous patterning mechanisms operate to establish the vascular network and the CNS and the fact that tissue macrophages are central contributors to the physiopathology of diseases affecting the blood vessels, several studies questioned the role of netrins and semaphorins in this context. Notably, a distinguished feature of arterial macrophages is their ability to rapidly respond to chemoattractive and/or chemorepulsive signals to direct their locomotion to their site of action. Since the dichotomous role of Netrin-1 in embryonic patterning is well established, our group hypothesized that Netrin-1 could direct arterial inflammation by regulating macrophage accumulation characterized by their recruitment and egress in atherosclerosis. This severe arterial complication is a chronic inflammatory disease of the arterial wall dominated by the accumulation of cholesterol-engorged macrophages (foam cells) in the arterial lesion [49] [50] [51]. Athero-prone lipids, such as low-density lipoproteins (LDL) that concentrate in the arterial wall transcriptionally regulate the expression of Netrin-1 and its receptor Unc5b [52] as well as Sema3E [53] in macrophage foam cells partly via the activation of the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NFKB) and hypoxia-inducible factor 1 alpha subunit (HIF-1α) [54]. These findings were consistent with studies which demonstrated that in the lung and colon, where ambient oxygen levels were compromised, Netrin-1 mRNA was shown to be upregulated in epithelial cells in a HIF-1α-dependent manner [55] (Figure 2). Notably, in cardiac ischemia/reperfusion injury, Netrin-1 regulated the inflammatory status of tissue macrophages [56] suggesting that, in addition to directing their migration, Netrin-1 could modulate the polarization of macrophages which can impact the repair process. While numerous studies had focused on the mechanisms that regulate the recruitment of circulating monocytes into the diseased vascular beds, we provocatively demonstrated that Netrin-1/Unc5b protein-receptor complex prevented the emigration of macrophages out of the arterial wall following their accumulation by inhibiting CCL2-mediated migration through the induction of FAK and Rac1 signaling cascades. We confirmed these effects in a second model of chronic inflammation — obesity — where the accumulation of adipose tissue macrophages (ATM) in crown like structures within the white adipose tissue (WAT) impairs glucose metabolism. In this model, we and others demonstrated that the increased expression of Netrin-1 [57] and Sema3E/plexinD1 [58] by ATM seeded in obese WAT compared to lean fat depots, fostered chronic inflammation and contributed to insulin resistance. A free-fatty acid, palmitate, induced the expression of Netrin-1 indirectly by promoting the expression of pro-inflammatory cytokines such as TNFα and IL-6 (Figure 2) in bone marrow derived macrophages. Likewise, reduced IL-6 was observed in Netrin-1-receptor deficient Unc5b+/− mice which attenuated inflammation and reduced myocardial infarct size [59]. These studies were key to the field as they uncovered that the egress of macrophages was a regulated process and identified Netrin-1 as a potential candidate to mobilise tissue macrophages. The non-motile chemo-static macrophages within the damaged tissue mayhem could then foster chronic inflammation by compromising the resolution of inflammation [52]. Similar to its role in obese ATM, Sema3E has also been shown to regulate CCL19 and CCL2-mediated macrophage migration in vitro. These chemokines contributed to direct macrophage efflux from atherosclerotic plaques to neighboring draining lymph nodes [53, 60, 61]. These findings further emphasized the role of netrins and semaphorins in orchestrating the emigration of cells from tissues. Interestingly, while studying their expression in the endothelial lining of the aorta, we found that Netrin-1 expression prevented monocyte binding to the endothelial cell in addition to impeding leukocyte migration to proatherogenic chemokines [62]. Since the accumulation of monocytes within the lesions rely on chemotaxis mechanisms, we can imagine that the differential expression of Netrin-1 and Sema3E and their receptors could intercept in this context. So far, no data is available on the effect of Netrin-1 on circulating Ly6Chi and Ly6Clo monocytes subpopulations, which have been reported to be key contributors of atherosclerosis development [63, 64].
Of note, atherosclerotic plaques have been shown to commonly develop in areas characterized by turbulent blood flow [65]. Interestingly, shear stress at the level of endothelial cells regulated the expression of Netrin-1 and Sema3A. Notably, we demonstrated that Netrin-1 and Sema3A were downregulated in the inner aortic curvature prone to atherosclerosis development versus the outer aortic curvature, which is protected against lesion formation [62]. One can also speculate that the regulation of netrins/semaphorin signaling induced by disturbed shear stress might orchestrate inflammatory cells infiltration and activation mode. Nevertheless, the overall mechanisms by which netrins regulate vascular inflammation appear to be complex and incomplete as endothelial-derived Netrin-1 seemed to repell pro-atherogenic leukocytes while the expression of Netrin-1 by macrophages seeded in advanced lesions promoted inflammation associated with disease progression. Since the activation of the endothelium intervenes in early stages of atherosclerosis while the accumulation of foamy macrophages in the vessel wall contribute to disease progression characterized by vulnerable lesions prone to rupture, future studies performed in a timed manner that allow for the identification of the exact cellular souce of Netrin-1 would better help to characterize its multifaceted contribution to either the initiation or progression of the disease. Currently, these knowledge gaps have hampered the development of viable therapeutic strategies aimed at targeting Netrin-1 to reduce the burden of atherosclerosis.
3.2 Netrins and semaphorins and bone-derived macrophages
The two hundred thirteen bones constituting the adult human skeletal tree are architecturally programmed to provide structural support to the body. Given the crucial dynamic patterning role of netrins and semaphorins, several members of these families have been uncovered to play distinct roles in regulating bone homeostasis. Bone mass determinants, important for the maintenance of equilibrium between osteoclasts and osteoblasts, remained unclear until the critical role of Sema3A was discovered as an important factor for bone remodeling. Interestingly, the crosstalk between neuronal derived Sema3A and bone homeostasis has been unraveled using conditional osteoblast-deficient mice or mice lacking Sema3A in neurons. Sensory innervations expressed Sema3A, which could regulate bone remodeling by indirectly acting on osteoblasts to promote bone formation [66]. This study was key in demonstrating the additional regulatory roles of neuronal derived cues to regulate bone homeostasis. Mechanistic studies have uncovered that the interaction of Sema3A with neuropilin-1 triggered intracellular signaling events that inhibited the receptor activator nuclear factor-kb ligand (RANKL), a key osteoclast differentiation marker, while also inhibiting immunoreceptor tyrosine-based activation motif (ITAM) and the expression of cytoskeletal regulator, Ras homolog gene family member (RhoA) pathways. As such, this interaction exerted a critical dual action on both bone cell types by suppressing osteoclast-resorption and promoting osteoblastic bone formation simultaneously [67]. The same group demonstrated the deleterious effect of Sema4D ligation to PlexinB1, which instead activates RhoA and thus inhibits bone formation by osteoclast [68]. Interestingly, Netrin-1 was also shown to intercept in the RhoA pathway in macrophages within inflamed areas of bone resorption. Via its Unc5b receptor, Netrin-1 acted as an autocrine and paracrine factor necessary for the differentiation of osteoclasts, which promoted bone resorption. Recombinant Netrin-1 promoted the differentiation of osteoclast from both mouse and human precursors by inducing the expression of RhoA and FAK therefore modifying actin filaments and impacting cellular shape [69] (Figure 2). The activation of Unc5b induced a signaling cascade via LARG (regulator of Rho-GEF) and RGMa (repulsive guidance molecule) to activate RhoA which induced critical cytoskeletal rearrangements necessary for osteoclast differentiation [69]. In accordance, bone mineral density and volume were increased in chimera mice reconstituted with bone marrow-deficient Netrin-1 compared to WT reconstituted chimeras [70]. These findings point to the possible therapeutic role of targeting Netrin-1 in overactive osteoclast diseases. As such, antibody-mediated blockade of Netrin-1 and Unc5b, but not DCC, reduced paw inflammation, bone erosion and osteoclast numbers in a mouse model of inflammatory arthritis [71].
4. Conclusions and perspectives
Altogether, these emerging findings on netrins and semaphorins outside the CNS revealed the complexity of the mechanisms pertaining to their function and further emphasize the importance of investigation in these new directions. Aided by the recent exquisite tissue-specific and inducible knockouts to overcome the embryonic lethal phenotype of genetic deletion of some netrins and semaphorins, the literature uncovering exciting mechanistic insights in the interplay between the neuronal cues and the immune cell subsets has been expanding. Because of the important roles semaphorins and netrins play in the pathogenesis of inflammatory diseases, they have become an important drug target for therapeutic benefits. Notably, currently there are limited therapies to curb the burden of illnesses such as atherosclerosis, in which Netrin-1 has been shown to play a complex causative role. Of note, several clinical trials aiming to target specific netrin and semaphorin candidates in various pathological settings have been registered and are underway. Presently, a group in France from the Centre Léon Bérard has designed a monocolonal antibody to target Netrin-1 in advanced/metastaic tumor patients. It will be beneficial to address whether this antibody can confer athero-prediction and/or protection in a clinical trial. The results may shed the light on novel treatment that will not only allow us to predict disease outcomes in healthy individuals presenting high cardiovascular risk but also target to stabilize or prevent the rupture of more advanced lesions in patients with diagnosed plaques. A combination of targets may also be proven appropriate as several neuronal cues have been shown to be detrimental in these chronic inflammatory complications.
Given the pivotal role of netrins and semaphorins in inflammatory-related diseases, one can also speculate that circulating levels of Sema3A and Netrin-1 could have prognostic value for patients. Interestingly, Netrin-1 and Sema3A levels were elevated in patients with acute kidney injury following liver transplantation [72] suggesting that netrins and semaphorins could be used as diagnostic biomarkers and thus optimize healthcare management for patient populations with defective innate or adaptive immune responses. Notably, a study is registered to measure urine Netrin-1 levels as a prognostic marker to assess the severity of kidney damage in patients with lower pole/renal pelvis stones and is aiming to optimize current treatment options. However, albeit these translational advances, the strategy utilized for the specific cellular targeting of these cues remains challenging and requires further research in drug engineering and delivery. Recent attempts with small molecule drugs have been unsuccessful due to their difficulty interacting with the semaphorin-plexin complex. Matsanuga et al. identified an alternative allosteric site between the Plexin B1 and Semaphorin 4D complex that provided deeper insight into the morphology of these proteins [73]. To circumvent this, nanoparticle-mediated blockade approaches of these neuronal targets that confer cellular specificity should be considered. Notably, nanomedicine without toxic side-effects has shown promise in dampening inflammation and protecting against atherosclerotic lesions development in mice models of the disease [74].
Highlights.
Neuronal guidance cues netrins and semaphorins exhibit novel immunomodulatory roles.
They direct the innate and adaptive immune response in physiopathology.
Strategies that target netrins and semaphorins may show promising therapeutic potential to modulate inflammatory cell-related effects in pathophysiological setting.
Acknowledgments
This work was supported in part by the National Heart and Lung Blood Institute of the National Institute of Health (R00 HL125667 to BR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing financial interests.
Contributor Information
Jordyn Feinstein, Division of Vascular Surgery, Department of Surgery, New York University School of Medicine, 530 First Avenue, New York, NY 10016, USA.
Bhama Ramkhelawon, Department of Cell Biology, New York University School ofMedicine, 530 First Avenue, New York, NY 10016, USA.
References
- 1.Hinck L. The versatile roles of “axon guidance” cues in tissue morphogenesis. Dev Cell. 2004;7(6):783–93. doi: 10.1016/j.devcel.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Lejmi E, et al. Netrin-4 inhibits angiogenesis via binding to neogenin and recruitment of Unc5B. Proc Natl Acad Sci U S A. 2008;105(34):12491–6. doi: 10.1073/pnas.0804008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, et al. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr Biol. 2004;14(10):897–905. doi: 10.1016/j.cub.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu X, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432(7014):179–86. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 5.Yebra M, et al. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev Cell. 2003;5(5):695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan K, et al. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell. 2003;4(3):371–82. doi: 10.1016/s1534-5807(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 7.Kang JS, et al. Netrins and neogenin promote myotube formation. J Cell Biol. 2004;167(3):493–504. doi: 10.1083/jcb.200405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranganathan P, et al. UNC5B receptor deletion exacerbates tissue injury in response to AKI. J Am Soc Nephrol. 2014;25(2):239–49. doi: 10.1681/ASN.2013040418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, et al. Netrin-1 overexpression protects kidney from ischemia reperfusion injury by suppressing apoptosis. Am J Pathol. 2009;175(3):1010–8. doi: 10.2353/ajpath.2009.090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ly NP, et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102(41):14729–34. doi: 10.1073/pnas.0506233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii N, et al. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 1992;9(5):873–81. doi: 10.1016/0896-6273(92)90240-e. [DOI] [PubMed] [Google Scholar]
- 12.Rajasekharan S, Kennedy TE. The netrin protein family. Genome Biol. 2009;10(9):239. doi: 10.1186/gb-2009-10-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol. 2007;8(4):296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- 14.Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138(11):2153–69. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- 15.Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol. 2010;185(6):3750–8. doi: 10.4049/jimmunol.1000435. [DOI] [PubMed] [Google Scholar]
- 16.Ranganathan P, et al. Semaphorin 3A inactivation suppresses ischemia-reperfusion-induced inflammation and acute kidney injury. Am J Physiol Renal Physiol. 2014;307(2):F183–94. doi: 10.1152/ajprenal.00177.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ly A, et al. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133(7):1241–54. doi: 10.1016/j.cell.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bin JM, et al. Complete Loss of Netrin-1 Results in Embryonic Lethality and Severe Axon Guidance Defects without Increased Neural Cell Death. Cell Rep. 2015;12(7):1099–106. doi: 10.1016/j.celrep.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 19.Luo Y, et al. A family of molecules related to collapsin in the embryonic chick nervous system. Neuron. 1995;14(6):1131–40. doi: 10.1016/0896-6273(95)90261-9. [DOI] [PubMed] [Google Scholar]
- 20.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75(2):217–27. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 21.Worzfeld T, Offermanns S. Semaphorins and plexins as therapeutic targets. Nat Rev Drug Discov. 2014;13(8):603–21. doi: 10.1038/nrd4337. [DOI] [PubMed] [Google Scholar]
- 22.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90(4):739–51. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 23.Siebold C, Jones EY. Structural insights into semaphorins and their receptors. Semin Cell Dev Biol. 2013;24(3):139–45. doi: 10.1016/j.semcdb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Artigiani S, Comoglio PM, Tamagnone L. Plexins, semaphorins, and scatter factor receptors: a common root for cell guidance signals? IUBMB Life. 1999;48(5):477–82. doi: 10.1080/713803563. [DOI] [PubMed] [Google Scholar]
- 25.Ohta K, et al. Plexin: a novel neuronal cell surface molecule that mediates cell adhesion via a homophilic binding mechanism in the presence of calcium ions. Neuron. 1995;14(6):1189–99. doi: 10.1016/0896-6273(95)90266-x. [DOI] [PubMed] [Google Scholar]
- 26.Antipenko A, et al. Structure of the semaphorin-3A receptor binding module. Neuron. 2003;39(4):589–98. doi: 10.1016/s0896-6273(03)00502-6. [DOI] [PubMed] [Google Scholar]
- 27.Nogi T, et al. Structural basis for semaphorin signalling through the plexin receptor. Nature. 2010;467(7319):1123–7. doi: 10.1038/nature09473. [DOI] [PubMed] [Google Scholar]
- 28.Janssen BJ, et al. Structural basis of semaphorin-plexin signalling. Nature. 2010;467(7319):1118–22. doi: 10.1038/nature09468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, et al. Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci Signal. 2012;5(207):ra6. doi: 10.1126/scisignal.2002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gloerich M, Bos JL. Regulating Rap small G-proteins in time and space. Trends Cell Biol. 2011;21(10):615–23. doi: 10.1016/j.tcb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Takagi S, et al. The A5 antigen, a candidate for the neuronal recognition molecule, has homologies to complement components and coagulation factors. Neuron. 1991;7(2):295–307. doi: 10.1016/0896-6273(91)90268-5. [DOI] [PubMed] [Google Scholar]
- 32.Parker MW, et al. Function of members of the neuropilin family as essential pleiotropic cell surface receptors. Biochemistry. 2012;51(47):9437–46. doi: 10.1021/bi3012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo HF, Vander Kooi CW. Neuropilin Functions as an Essential Cell Surface Receptor. J Biol Chem. 2015;290(49):29120–6. doi: 10.1074/jbc.R115.687327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu C, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5(1):45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chauvet S, et al. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56(5):807–22. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boneschansker L, et al. Netrin-1 Augments Chemokinesis in CD4+ T Cells In Vitro and Elicits a Proinflammatory Response In Vivo. J Immunol. 2016;197(4):1389–98. doi: 10.4049/jimmunol.1502432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi W, et al. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000;13(5):633–42. doi: 10.1016/s1074-7613(00)00063-7. [DOI] [PubMed] [Google Scholar]
- 38.Kumanogoh A, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13(5):621–31. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 39.Kumanogoh A, et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002;419(6907):629–33. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- 40.Delgoffe GM, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501(7466):252–6. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, et al. Expression of Semaphorin 4A and its potential role in rheumatoid arthritis. Arthritis Res Ther. 2015;17:227. doi: 10.1186/s13075-015-0734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie J, Wang H. Semaphorin 7A as a potential immune regulator and promising therapeutic target in rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):10. doi: 10.1186/s13075-016-1217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakagawa Y, et al. Identification of semaphorin 4B as a negative regulator of basophil-mediated immune responses. J Immunol. 2011;186(5):2881–8. doi: 10.4049/jimmunol.1003485. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, et al. Functional soluble CD100/Sema4D released from activated lymphocytes: possible role in normal and pathologic immune responses. Blood. 2001;97(11):3498–504. doi: 10.1182/blood.v97.11.3498. [DOI] [PubMed] [Google Scholar]
- 45.Adachi T, et al. The B cell surface protein CD72 recruits the tyrosine phosphatase SHP-1 upon tyrosine phosphorylation. J Immunol. 1998;160(10):4662–5. [PubMed] [Google Scholar]
- 46.Kumanogoh A, et al. Requirement for CD100-CD72 interactions in fine-tuning of B-cell antigen receptor signaling and homeostatic maintenance of the B-cell compartment. Int Immunol. 2005;17(10):1277–82. doi: 10.1093/intimm/dxh307. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe C, et al. Enhanced immune responses in transgenic mice expressing a truncated form of the lymphocyte semaphorin CD100. J Immunol. 2001;167(8):4321–8. doi: 10.4049/jimmunol.167.8.4321. [DOI] [PubMed] [Google Scholar]
- 48.Lu Q, et al. Increased levels of plasma soluble Sema4D in patients with heart failure. PLoS One. 2013;8(5):e64265. doi: 10.1371/journal.pone.0064265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138(5 Pt 2):S419–20. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 50.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10(1):36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–55. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Gils JM, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13(2):136–43. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wanschel A, et al. Neuroimmune guidance cue Semaphorin 3E is expressed in atherosclerotic plaques and regulates macrophage retention. Arterioscler Thromb Vasc Biol. 2013;33(5):886–93. doi: 10.1161/ATVBAHA.112.300941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramkhelawon B, et al. Hypoxia induces netrin-1 and Unc5b in atherosclerotic plaques: mechanism for macrophage retention and survival. Arterioscler Thromb Vasc Biol. 2013;33(6):1180–8. doi: 10.1161/ATVBAHA.112.301008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenberger P, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10(2):195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 56.Mao X, et al. Netrin-1 attenuates cardiac ischemia reperfusion injury and generates alternatively activated macrophages. Inflammation. 2014;37(2):573–80. doi: 10.1007/s10753-013-9771-3. [DOI] [PubMed] [Google Scholar]
- 57.Ramkhelawon B, et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med. 2014;20(4):377–84. doi: 10.1038/nm.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimizu I, et al. Semaphorin3E-induced inflammation contributes to insulin resistance in dietary obesity. Cell Metab. 2013;18(4):491–504. doi: 10.1016/j.cmet.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Kohler D, et al. The uncoordinated-5 homolog B (UNC5B) receptor increases myocardial ischemia-reperfusion injury. PLoS One. 2013;8(7):e69477. doi: 10.1371/journal.pone.0069477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi YI, et al. PlexinD1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity. 2008;29(6):888–98. doi: 10.1016/j.immuni.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jimenez F, et al. CCR2 plays a critical role in dendritic cell maturation: possible role of CCL2 and NF-kappa B. J Immunol. 2010;184(10):5571–81. doi: 10.4049/jimmunol.0803494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Gils JM, et al. Endothelial expression of guidance cues in vessel wall homeostasis dysregulation under proatherosclerotic conditions. Arterioscler Thromb Vasc Biol. 2013;33(5):911–9. doi: 10.1161/ATVBAHA.112.301155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.An G, et al. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117(25):3227–37. doi: 10.1161/CIRCULATIONAHA.108.771048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swirski FK, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117(1):195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gimbrone MA, Jr, et al. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–9. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–40. [DOI] [PubMed] [Google Scholar]
- 66.Fukuda T, et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497(7450):490–3. doi: 10.1038/nature12115. [DOI] [PubMed] [Google Scholar]
- 67.Hayashi M, et al. Osteoprotection by semaphorin 3A. Nature. 2012;485(7396):69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- 68.Negishi-Koga T, et al. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17(11):1473–80. doi: 10.1038/nm.2489. [DOI] [PubMed] [Google Scholar]
- 69.Mediero A, et al. Netrin-1 is highly expressed and required in inflammatory infiltrates in wear particle-induced osteolysis. Ann Rheum Dis. 2016;75(9):1706–13. doi: 10.1136/annrheumdis-2015-207593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mediero A, et al. Netrin-1 is a critical autocrine/paracrine factor for osteoclast differentiation. J Bone Miner Res. 2015;30(5):837–54. doi: 10.1002/jbmr.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mediero A, et al. Ticagrelor regulates osteoblast and osteoclast function and promotes bone formation in vivo via an adenosine-dependent mechanism. Faseb j. 2016;30(11):3887–3900. doi: 10.1096/fj.201600616R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewandowska L, et al. Netrin-1 and semaphorin 3A predict the development of acute kidney injury in liver transplant patients. PLoS One. 2014;9(10):e107898. doi: 10.1371/journal.pone.0107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsunaga Y, et al. Allosteric Inhibition of a Semaphorin 4D Receptor Plexin B1 by a High-Affinity Macrocyclic Peptide. Cell Chem Biol. 2016;23(11):1341–1350. doi: 10.1016/j.chembiol.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 74.Mulder WJ, et al. Imaging and nanomedicine in inflammatory atherosclerosis. Sci Transl Med. 2014;6(239):239sr1. doi: 10.1126/scitranslmed.3005101. [DOI] [PMC free article] [PubMed] [Google Scholar]


