Abstract
Mesenchymal stem cells (MSCs) are an attractive cell type for cartilage repair that can undergo chondrogenesis in a variety of three-dimensional (3D) scaffolds. Hyaluronic acid (HA) hydrogels provide a biologically relevant interface for cell encapsulation. While previous studies have shown that MSC-laden HA constructs can mature in vitro to match native mechanical properties using cells from animal sources, clinical application will depend on the successful translation of these findings to human cells. Though numerous studies have investigated chondrogenesis of human MSC (hMSC)-laden constructs, their functional outcomes were quite inferior to those using animal sources, and donor-specific responses to 3D HA hydrogels have not been fully investigated. To that end, hMSCs were derived from seven donors, and their ability to undergo chondrogenesis in pellet culture and HA hydrogels was evaluated. Given the initial observation of overt cell aggregation and/or gel contraction for some donors, the impact of variation in cell and HA macromer concentration on functional outcomes during chondrogenesis was evaluated using one young/healthy donor. The findings show marked differences in functional chondrogenesis of hMSCs in 3D HA hydrogels based on donor. Increasing cell density resulted in increased mechanical properties, but also promoted construct contraction. Increasing the macromer density generally stabilized construct dimensions and increased extracellular matrix production, but limited the distribution of formed matrix at the center of the construct and reduced mechanical properties. Collectively, these findings suggest that the use of hMSCs may require tuning of cell density and gel mechanics on a donor-by-donor basis to provide for the most robust tissue formation for clinical application.
Keywords: : human mesenchymal stem cells, hyaluronic acid hydrogel, chondrogenesis, cartilage tissue engineering, donor variation, cell and macromer density
Introduction
Cartilage tissue engineering aims to develop functional constructs to replace damaged or degenerated tissues.1 Although chondrocytes have been utilized as a primary source for cartilage repair, their availability and quality is limited, especially when derived from human arthritic donors.2–4 Given this, mesenchymal stem cells (MSCs) have arisen as an attractive cell type for cartilage tissue engineering, as they are multipotent and easy to obtain and expand. MSCs can also undergo chondrogenesis in a variety of three-dimensional (3D) platforms.5–8 To date, many studies have focused on improving MSC chondrogenesis to recapitulate the native properties of cartilage in biologic replacements.9–11 Although there has been considerable progress toward optimizing functional properties of MSC-laden constructs, successful recapitulation of the native functional properties has only been achieved by MSCs derived from animal sources.12,13
From a clinical perspective, successful translation of findings from animal sources to human MSCs (hMSCs) is critical for effective repair, preferably those derived from an autologous source. However, some have noted species-to-species variation in chondrogenic potential. For instance, in contrast to bovine MSCs, hMSCs encapsulated with hydrogels lacking adhesion moieties showed a marked loss in viability with time, demonstrating that chondrogenesis of hMSCs in hydrogels may depend on the 3D microenvironment.14 Even in humans, there are different responses on the effect of age and disease status in hMSC chondrogenesis. Murphy et al. reported that disease (i.e., osteoarthritis) or age status affected the chondrogenic capacity of hMSCs.15
Conversely, Scharstuhl et al. showed that the chondrogenic potential of hMSCs was independent of age or disease status.16 Further, several studies have demonstrated donor-to-donor variability in chondrogenic,17 osteogenic,18,19 or endothelial20 differentiation of hMSCs in monolayer or pellet culture. However, as the microenvironment (i.e., extracellular matrix) influences chondrogenesis of MSCs,14,21 donor-dependent chondrogenic differentiation of hMSCs in 3D hydrogels needs to be verified and optimized to enhance matrix accumulation and mechanical properties.
A decade ago, Burdick et al., developed photocrosslinkable hyaluronic acid (HA) hydrogel networks for cartilage tissue engineering applications.22 HA is a nonsulfated glycosaminoglycan (GAG), and it is one of the major components of cartilage extracellular matrix.23 Studies have shown that HA provides a native-like microenvironment for MSCs (i.e., through the cell adhesion receptor CD44) and can enhance functional chondrogenesis compared to other synthetic hydrogels such as polyethylene glycol (PEG).23–26 The authors have likewise shown that bovine MSCs encapsulated in HA of varying macromer density achieve different levels of mechanical properties and biochemical content.27 While higher macromer densities (2% and 5%) started out with superior properties, after 42 days of culture, low macromer (1%) HA constructs matured to the greatest extent.
Building from these studies, and to elucidate better the consistency of construct maturation using different hMSC donors in HA hydrogels, the first objective was to assess the donor-dependent response of hMSC-laden HA hydrogel constructs and the effects of age and disease status on functional chondrogenesis. Based on the marked contraction observed in some donors, a subsequent study sought to optimize MSC chondrogenesis by manipulating cell and macromer density. The findings demonstrate marked donor-to-donor variability by hMSCs in response to their microenvironment and variation in their functional chondrogenesis in these 3D HA hydrogels. The studies further show that functional chondrogenesis of hMSCs can be improved by modulation of cell and gel density, suggesting that donor-specific manipulation of HA hydrogel might be required for the generation of functional constructs for patient-specific cartilage repair.
Materials and Methods
Isolation and expansion of hMSCs
Eight hMSC donors (age range 18–62 years) were used for this study (seven donors for the first study, and one donor for the second study), and each donor was identified by age/sex code (Table 1). These donors were obtained from a commercial vendor (21M and 22F; Lonza, Walkersville, MD), bone-marrow aspirates of patients undergoing treatment for osteochondritis dissecans (OCD; 18M), or from patients undergoing total hip/knee replacement (42F, 46F, 57M, 61F, and 62M). All locally sourced human materials were obtained with patient consent and under the auspices of an approved Institutional Review Board protocol at the University of Pennsylvania. Bone-marrow aspirates from patient donors or frozen cells (∼750,000 cells/vial) from a commercial vendor were plated on a separate tissue culture dish (round; Ø15 cm; 4–5 million cells at 80–90% confluency in 7–10 days; 08-772-24; Thermo Fisher Scientific, Waltham, MA), isolated, and re-plated to a large square plate (500 cm2; 9–10 million cells at 80–90% confluency in 7–10 days; 07-200-599; Thermo Fisher Scientific). MSCs were expanded to passage 3–4 in basal media (BM) to obtain sufficient cell numbers for 3D hydrogel or pellet culture. hMSCs from individual donors that failed to adhere, form colonies, or proliferate during the cell expansion were excluded from this study. Based on colony formation, proliferation, and retention of initial cell morphology during the expansion period in a timely manner, a total of eight hMSC donors were used for the study. BM consisted of high-glucose Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (Gibco, Grand Island, NY) and 1% penicillin/streptomycin/Fungizone (PSF; Gibco).
Table 1.
Details of Human Mesenchymal Stem-Cell Donors and Sourcing
| Donor | ||
|---|---|---|
| Study | Age/sex | Source |
| 1 | 18M | Patient (OCD) |
| 22F | Lonza | |
| 42F | Patient (OA) | |
| 46F | Patient (OA) | |
| 57M | Patient (OA) | |
| 61F | Patient (OA) | |
| 62M | Patient (OA) | |
| 2 | 21M | Lonza |
Each donor was identified by an age/sex code. Of eight donors, seven donors were used for the first study, and one donor was used for the second study.
F, female; M, male; OA, patient undergoing joint replacement surgery as a consequence of hip or knee osteoarthritis; OCD, patient with osteochondritis dissecans.
Methacrylated HA synthesis
Synthesis of methacrylated HA (MeHA) was as previously described.22,27 Briefly, methacrylic anhydride (Sigma–Aldrich, St. Louis, MO) was reacted with 1 wt% HA (65 kDa MW; Lifecore, Chaska, MN) dissolved in deionized water on ice while continuously maintaining pH at 8.0 via dropwise addition of 5 N sodium hydroxide (Sigma–Aldrich) for 6 h. After the reaction was completed, the macromer solution was purified via dialysis (MW cutoff 6–8k) against distilled water for 1 week, with changes to fresh water twice daily. The MeHA solution was then lyophilized, and the degree of modification was assessed by nuclear magnetic resonance spectroscopy. Next, lyophilized MeHA was dissolved in phosphate-buffered saline with 0.05% w/v of the photoinitiator I2959 (2-methyl-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone; Ciba-Geigy, Tarrytown, NY) to enable UV-mediated photo-polymerization.
MSC encapsulation
Evaluation of donor-to-donor variability
To investigate chondrogenic capacity of hMSCs among different donors, seven hMSC donors (18M, 22F, 42F, 46F, 57M, 61F, and 62M) were used. Cells were encapsulated in 1% MeHA hydrogels or formed into pellets. To culture in the pellet format, 250,000 hMSCs from each donor were placed in a 96-well round-bottom polypropylene plate (Nunc, Naperville, IL) and centrifuged at 300 g for 5 min. These cell pellets were aggregated over the first 24 h and were cultured in 200 μL/pellet of a chemically defined media (CM) supplemented with 10 ng/mL TGF-β3 (CM+; R&D Systems, Minneapolis, MN) for 2 and 4 weeks to induce chondrogenesis. Media were changed twice weekly. CM consisted of high-glucose DMEM with 1 × PSF; 0.1 μM dexamethasone, 50 μg/mL ascorbate 2-phosphate, 40 μg/mL l-proline, 100 μg/mL sodium pyruvate, 6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 ng/mL selenious acid (ITS), 1.25 mg/mL bovine serum albumin, and 5.35 μg/mL linoleic acid. For 3D MeHA hydrogel culture, hMSCs were suspended in 1% MeHA solution at 20 million cells/mL (∼1 million cells/construct), and hMSC-laden MeHA suspensions were then cast in a gel casting device (spacers of 2.25 mm) and photo-polymerized by UV exposure using a 365 nm Blak-Ray® UV lamp (UVL-56; UVP, San Gabriel, CA) for 10 min.27 Cylindrical constructs (Ø4 × 2.25 mm) were cored from the gel slab and cultured in CM+ (1 mL/construct) for 4 and 8 weeks to provide sufficient time to induce optimal maturation in mechanical properties and biochemical content, with media changed twice weekly.
Optimization of cell and macromer density
Given the donor-dependent response of hMSCs in 3D culture in terms of matrix accumulation and volumetric changes, next cell and gel concentrations were modulated to optimize functional chondrogenesis and construct geometry. To do this, hMSCs were cast in MeHA hydrogels at different cell (20 and 60 million cell/mL) and macromer (1%, 1.5%, and 2% MeHA) densities and cultured in CM+ for 8 weeks using one new young donor (21M; Lonza). During media changes, cell–matrix interaction and volumetric changes were observed closely, and macroscopic images were captured at 2 and 4 weeks. Media were changed three times a week, and this experiment was repeated twice.
Mechanical testing
Unconfined compression testing was performed to determine compressive equilibrium (EY) and dynamic (|G*|) moduli of constructs, as in Mauck et al.28 Compressive modulus was determined via stress relaxation applied at 0.05%/s to 10% strain, following a creep deformation to 0.02 N applied over 5 min to seat the sample. After gels reached equilibrium in the stress relaxation test, a 1% strain amplitude sinusoidal deformation was applied at 1.0 Hz.29 Before mechanical testing, the diameter and height of the constructs were independently measured. The volume was calculated and is reported relative to the initial volume as the % change. Then, the top and bottom surfaces of the constructs were carefully trimmed using a freezing stage microtome to ensure an even contact surface. Equilibrium modulus was calculated from the stress relaxation test and sample geometry, while the dynamic modulus was estimated from the dynamic compression test and sample geometry, as outlined in Soltz and Ateshian.30
Biochemical analysis
After mechanical testing, construct wet weights (WW) were measured, followed by papain digestion at 60°C for 16 h. Sulfated GAG content was assessed using the 1,9-dimethylmethylene blue assay.31 Collagen content was measured via reaction with chloramine T and diaminobenzaldehyde.32 Collagen content was extrapolated from hydroxyproline (OHP) content using a 1:7.14 ratio of OHP:collagen.33 Given the variations in contractility and chondrogenic capacity of hMSC donors, it was not feasible to obtain a reliable WW from both constructs and pellets. Thus, to evaluate matrix production in the two distinct contexts (e.g., pellet and hydrogel), GAG and collagen content were normalized to the starting cell number per construct (1 million and 250,000 cells for MeHA hydrogel and pellet, respectively) for the first study. For the second study, biochemical contents were normalized per construct (μg/construct) or %WW due to the varying cell and macromer density.
Histological analysis and cell viability
Constructs were fixed in 4% paraformaldehyde for 24 h and embedded in paraffin. Sections (8 μm) were deparaffinized in Citrisolv, followed by rehydration in a graded series of ethanol/water, and stained with Alcian Blue (pH 1.0), Safranin-O (1%), and Picrosirius Red (0.1% w/v in saturated picric acid) for proteoglycans (PG) and collagens, respectively. Immunohistochemistry was performed to visualize type I collagen, type II collagen, and chondroitin sulfate (CS). Primary antibodies for type I collagen (MAB3391; Millipore, Billerica, MA), type II collagen (II-II6B3; DSHB, Iowa City, IA), and CS (C8035; Sigma–Aldrich) were used for immunostaining, as in Kim et al.34 On separate constructs, cell viability was evaluated via LIVE/DEAD staining, as per the manufacturer's instructions (Molecular Probes, Eugene, OR).
Statistical analysis
Statistical analysis was performed using SYSTAT v10.2 (SYSTAT Software, Inc., San Jose, CA). Significance was determined by one-way analysis of variance with Tukey's post hoc testing (p < 0.05).
Results
Donor-to-donor variation in hMSC chondrogenesis
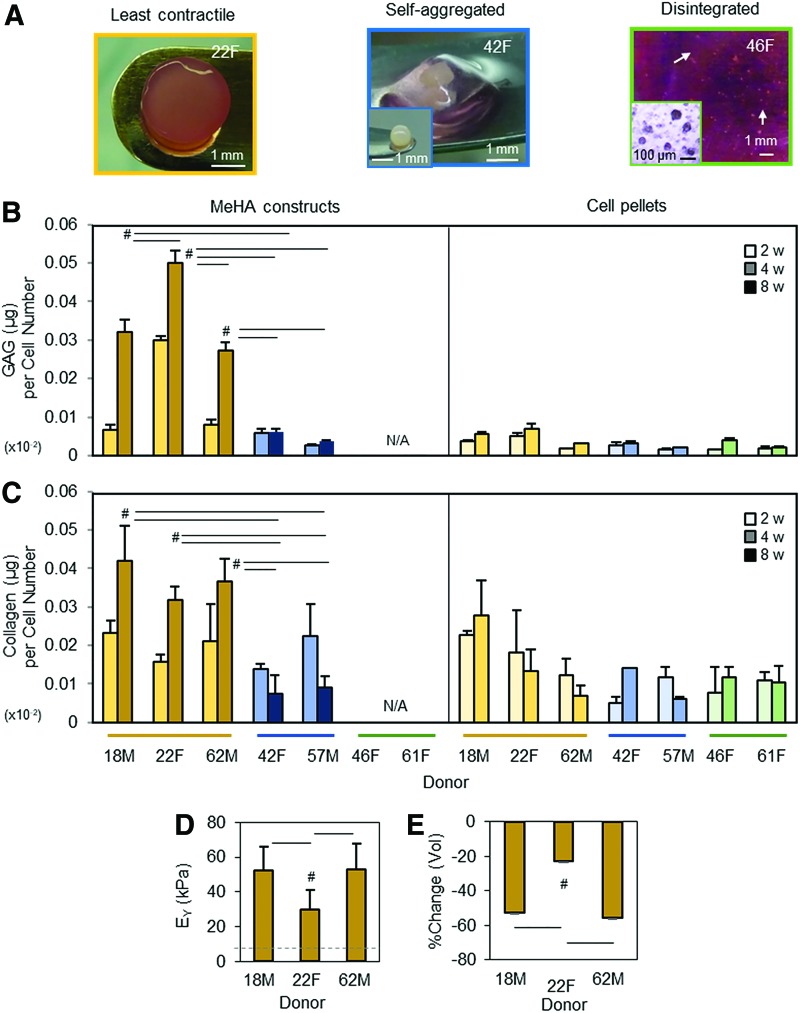
All donor cells were viable but responded differently when encapsulated in 1% MeHA hydrogels (Fig. 1 and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). Consistent with previous findings,27 all constructs contracted to some extent over the first few days of culture in CM+. Of the seven donors, three donors (18M, 22F, and 62M) were least contractile and maintained their cylindrical shape in 1% MeHA construct (left/yellow; the least contractile group; Fig. 1A). Two donors (42F and 57M) markedly self-aggregated without interaction with the surrounding hydrogels, gradually separated from hydrogels, and eventually formed a pellet-like mass (middle/blue; the self-aggregated group). The remaining two donors (46F and 61F) failed to maintain their structural integrity and disintegrated into small aggregated particles (right/green; the disintegrated group) within a few days in CM+, precluding further analysis. The biochemical content of these hMSC donors within 1% MeHA hydrogels as well as in donor-matched pellet format are shown in Figure 1B and C. GAG content in 1% MeHA hydrogels formed from the least contractile donors (18M, 22F, and 62M; yellow bars) increased with time (4 and 8 weeks) and was greater than the other two groups. In pellets from these same donors, the content was similar to the other two groups (2 and 4 weeks). The overall content in the pellets was lower compared to that in constructs (Fig. 1B). Notably, the GAG content of constructs from the donors with severe contraction (42F and 57M; blue bars) was similar to those in pellets from the same donors, as well as those with the remaining two donors (46F and 61F; green bars) formed in pellets. Donor-matched collagen production between 1% MeHA hydrogels and pellet culture was similar when compared to the content at 4 weeks, but the least contractile group (yellow bars) was slightly greater than the other donor groups (blue or green bars; Fig. 1C). Due to the self-aggregation or disintegration, only three donors (18M, 22F, and 62M; the least contractile group; yellow bars) were testable for mechanical properties. The constructs seeded with the least contractile donors yielded functional properties, reaching equilibrium moduli ranging from 30 to 53 kPa by 8 weeks (Fig. 1D). While these three donors retained a cylindrical shape, significant volumetric changes (% change) were observed, with decreases in the order of around 22–55% (p < 0.05; Fig. 1E).
FIG. 1.
Donor-dependent response of hMSCs in 1% MeHA hydrogels and pellets during chondrogenic differentiation. (A) hMSC donors laden in 1% MeHA hydrogels (20 million cells/mL) were cultured in CM+. Of the seven donors, three donors (18M, 22F, and 62M) maintained their cylindrical morphology with little contraction (left). Two other donors (42F and 57M) aggregated and separated from the hydrogels (middle; inset = a cell aggregate in 1% MeHA hydrogels after 1 week in CM+; scale bar = 1 mm). The remaining two donors (46F and 61F) failed to maintain their structural integrity and degraded (arrows) the MeHA hydrogels within 1 week (right; inset = disintegrated cells and/or small aggregates; scale bar = 100 μm). (B) Two young/healthy donors and one aged/arthritic donor (18M, 22F, and 62M) that demonstrated the least contraction had the greatest GAG and collagen content at 8 weeks (left panels) compared to two aged/arthritic donors (42F and 57M) that markedly self-aggregated. The remaining two aged/arthritic donors (46F and 61F) that failed to maintain a cylindrical geometry disintegrated within a few days and were unable to be assessed for biochemical properties. In pellet cultures, GAG production from those same donors was low overall compared to those in hydrogels, and GAG content in pellets was similar at 2 and 4 weeks (right panel). (C) Collagen production was slightly greater than GAG production, but the levels of collagen production were also similar between the donors, except for one young/healthy (18M) donor at 4 weeks (right panel). GAG and collagen contents were normalized to their respective starting cell number (construct = 106; pellet = 0.25 × 106) due to the variations in response (n = 3–4/group). (D and E) Due to the self-aggregation or disintegration, constructs from only three donors (yellow bars; 18M, 22F, and 62M) were assessed for mechanical properties at 8 weeks. (D) Equilibrium modulus (EY; kPa) of constructs from the 22F donor was 44% (30 kPa; #p < 0.05) lower than the 18M (52 kPa) and 62M (53 kPa) donors. (E) Constructs seeded with cells from donor 22F were less contractile (−22%; #p < 0.05) based on changes in volume (% change) compared to those from donors 18M (−52%) and 62M (−55%; light bars = 2w, medium bars = 4w, dark bars = 8w; n = 3–4/group; #p < 0.05). CM, chemically defined media; GAG, glycoaminoglaycan; hMSC, human mesenchymal stem cell; MeHA, methacrylated hyaluronic acid. Color images available online at www.liebertpub.com/tea
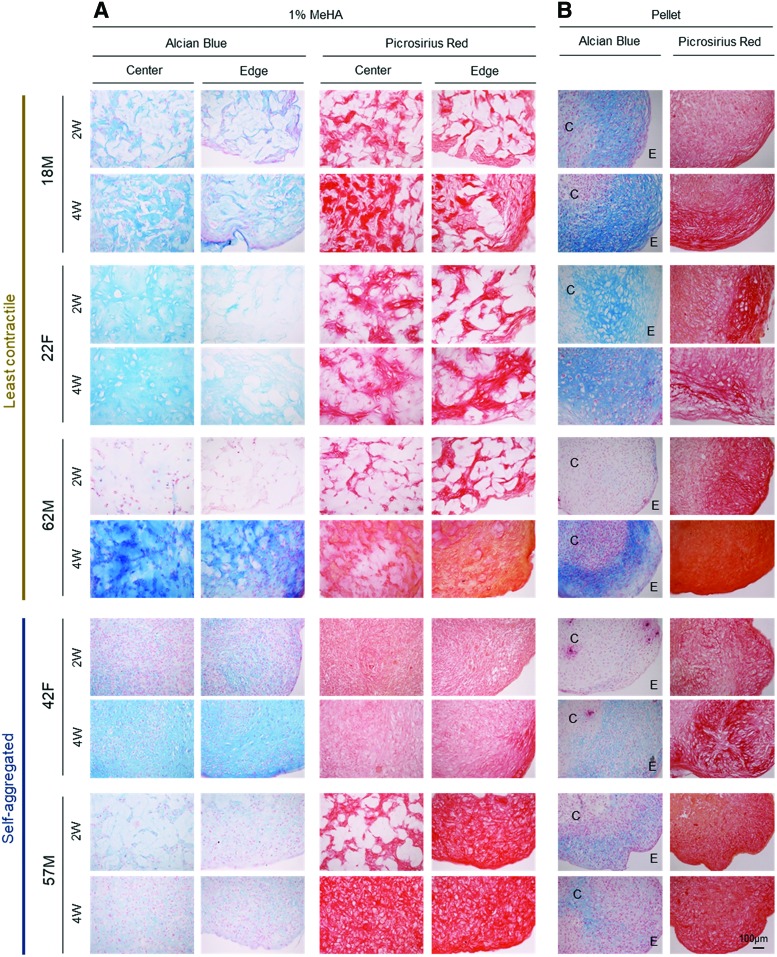
Histological evaluation for those donors with the least contraction (18M, 22F, and 62M) showed that a dense matrix of PG and collagen accumulated by week 2 in the two young donors (18M and 22F), with even distribution of the matrix (by week 4) in the one aged donor (62F) for both 1% MeHA hydrogels and pellet culture (Fig. 2A and Supplementary Figs. S2A and S3). Conversely, constructs with severe contraction (42F and 57M) showed weak staining for PG, even after aggregation at the center, and the staining intensity was similar to that of donor-matched pellets. Interestingly, constructs and pellets from all donors showed positive collagen staining, regardless of cell contractility (Fig. 2B and Supplementary Fig. S2B).
FIG. 2.
Histological assessment of hMSC-laden 1% MeHA constructs and pellets. Alcian blue and Picrosirius red staining of hMSC-laden 1% MeHA constructs (A) and pellets (B) at 2 and 4 weeks. Constructs seeded with MSC donors maintained a cylindrical morphology with least contraction (18M, 22F, and 62M) are shown in the top panel, and those markedly contracted (42F and 57M) are shown in the bottom panel (C = center; E = edge of pellet; scale bar = 100 μm). Color images available online at www.liebertpub.com/tea
Optimization of cell and macromer density impacts functional properties and biochemical contents
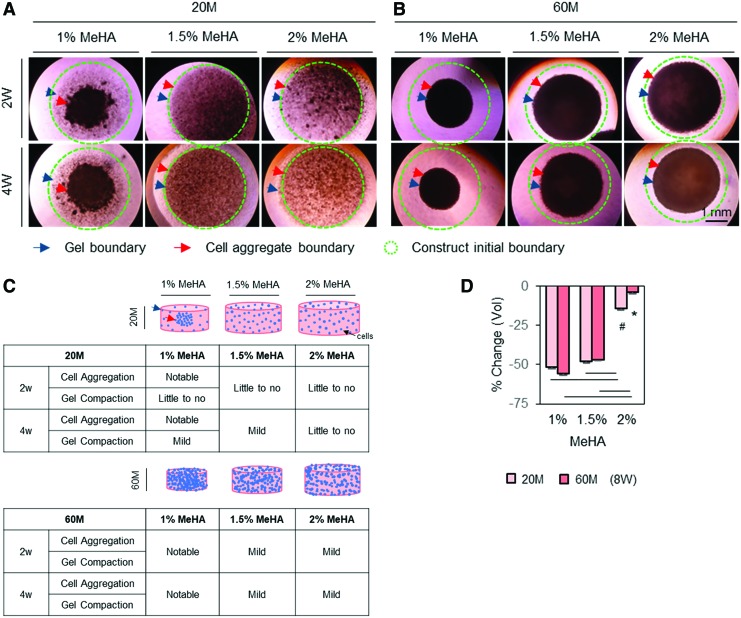
Given the donor-dependent response of hMSCs to MeHA hydrogel regarding chondrogenesis and volumetric changes, next, cell–matrix interaction and matrix accumulation in response to modulation of cell and gel macromer density were assessed to improve the functional properties of hMSC-laden constructs further. As shown in Figure 3, a central circular boundary outlined by a dashed circle (green) indicated an initial outer boundary of cell-laden construct, and dark particles or aggregate in the dashed circle indicated cells and/or aggregate. Blue arrows showed changes in outer construct boundary, indicating volumetric changes in the constructs, whereas red arrows showed the outer boundary of the cell aggregate, indicating the distribution of cells. By visual assessment from these macroscopic images, constructs with low seeding (20 million cells/mL) and macromer (1% MeHA) density showed a marked aggregation of cells and/or aggregates near the center, while the gel boundary remained unchanged (Fig. 3A). However, with an increase in macromer density (1.5% or 2% MeHA), cell aggregation was minimized. Likewise, when the cell density was increased threefold (60 million cells/mL), constructs with high cell (60 million cells/mL) and low macromer (1% MeHA) density showed a marked increase in compaction, as indicated by the outer boundary of constructs and volumetric changes (Fig. 3B). With an increase in macromer density (1.5% or 2% MeHA), the volumetric changes were reduced (Fig. 3B). Schematics summarize these changes in cell distribution and volumetric changes associated with modulation of cell and macromer densities (Fig. 3C). After 8 weeks of culture in CM+, cell-laden construct at 2% MeHA maintained the initial construct geometry (4–14%), while those at 1% or 1.5% MeHA contracted by 47–56% in volume (Fig. 3D).
FIG. 3.
Changes in cell distribution and construct volume associated with manipulation of cell and gel macromer density in MSC-laden MeHA hydrogel. To improve functional properties of hMSC-laden MeHA hydrogel further while stabilizing construct volume, cell (20 and 60 million cells/mL) and macromer (1%, 1.5% and 2% MeHA) density was manipulated using one young/healthy donor (21M). Top view images were captured under a light microscope, with cell distribution indicated by dark spots. An initial construct outer boundary at day 0 is indicated by a dashed circle (green), and cell (red) and construct (blue) outer boundary are indicated by arrows. (A–C) By visual inspection, constructs with low seeding (20 million cells/mL) and macromer (1% MeHA) density showed a marked aggregation of cells (red arrow) near the center, but gel boundary remained unchanged (blue arrow). With an increase in macromer density (1.5% or 2% MeHA), cell aggregation was mitigated (A). However, when cell density was increased by threefold (60 million cells/mL), compaction of constructs became more notable at low macromer density (B). Schematics summarizing cellular response as a function of macromer density (C). Dimensional changes (% change in volume) at 8 weeks showed that increased macromer density (2% MeHA) maintained cell distribution and construct dimensions (D) (light bars = 20 million cells/mL; dark bars = 60 million cells/mL; n = 4/group, #20M groups (1%, 1.5% and 2%, respectively; *60M groups; p < 0.05; scale bar = 1 mm). Color images available online at www.liebertpub.com/tea
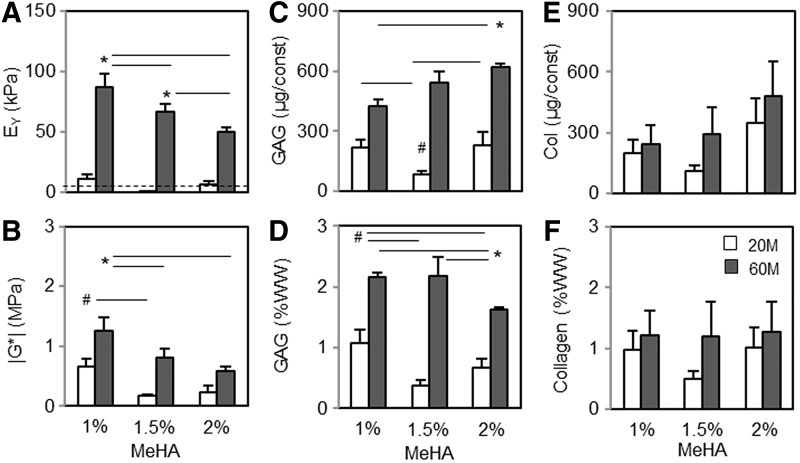
Following these macroscopic observations, the functional properties of constructs were evaluated after 8 weeks of culture in CM+ (Fig. 4). Consistent with previous work,12 the increase in cell density markedly enhanced the mechanical properties and biochemical content (Fig. 4A–D). Equilibrium (EY; kPa) and dynamic (|G*|; MPa) modulus of constructs at 60 million cells were highest in 1% MeHA (87 kPa and 1.25 MPa, respectively), but significantly decreased with an increase in macromer density (50–66 kPa and 0.6–0.8 MPa, respectively; p < 0.05; Fig. 4A and B). Conversely, GAG content per construct (μg/construct) increased with an increase in macromer density (422 and 625 μg/construct for 1% and 2% MeHA, respectively; p < 0.05; Fig. 4C). However, GAG content per WW was greatest in 1% MeHA (2.1 and 1.6 %WW for 1% and 2% MeHA, respectively; p < 0.05) due to the decrease in construct contraction with an increase in macromer density (Fig. 4D). The collagen content per construct also slightly increased with an increase in macromer density (240 and 480 μg/construct for 1% and 2% MeHA, respectively; Fig. 4E), but the content per %WW basis was similar for all groups (Fig. 4F).
FIG. 4.
Functional properties of hMSC-laden MeHA constructs at varying cell and macromer densities at 8 weeks. (A) Equilibrium modulus (EY; kPa). (B) Dynamic modulus (|G*|; MPa). (C and D) GAG content (μg/construct and %WW). (E and F) Collagen content (μg/construct and %WW). n = 4/group; white bars = 20M/mL; black bars = 60M/mL; #20M groups (1%, 1.5% and 2%, respectively). *60M groups; p < 0.05. WW, wet weight.
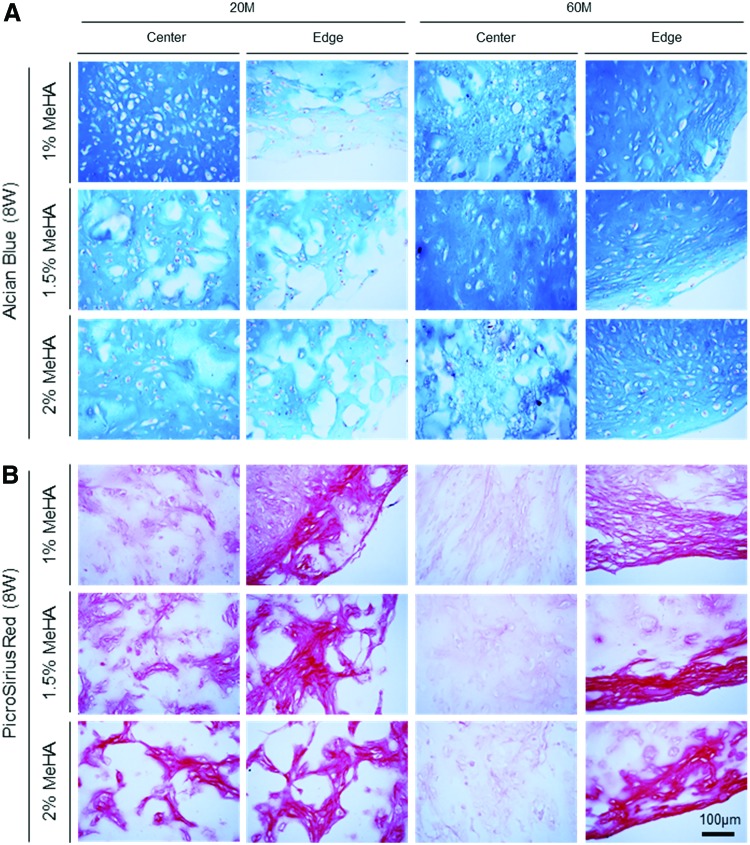
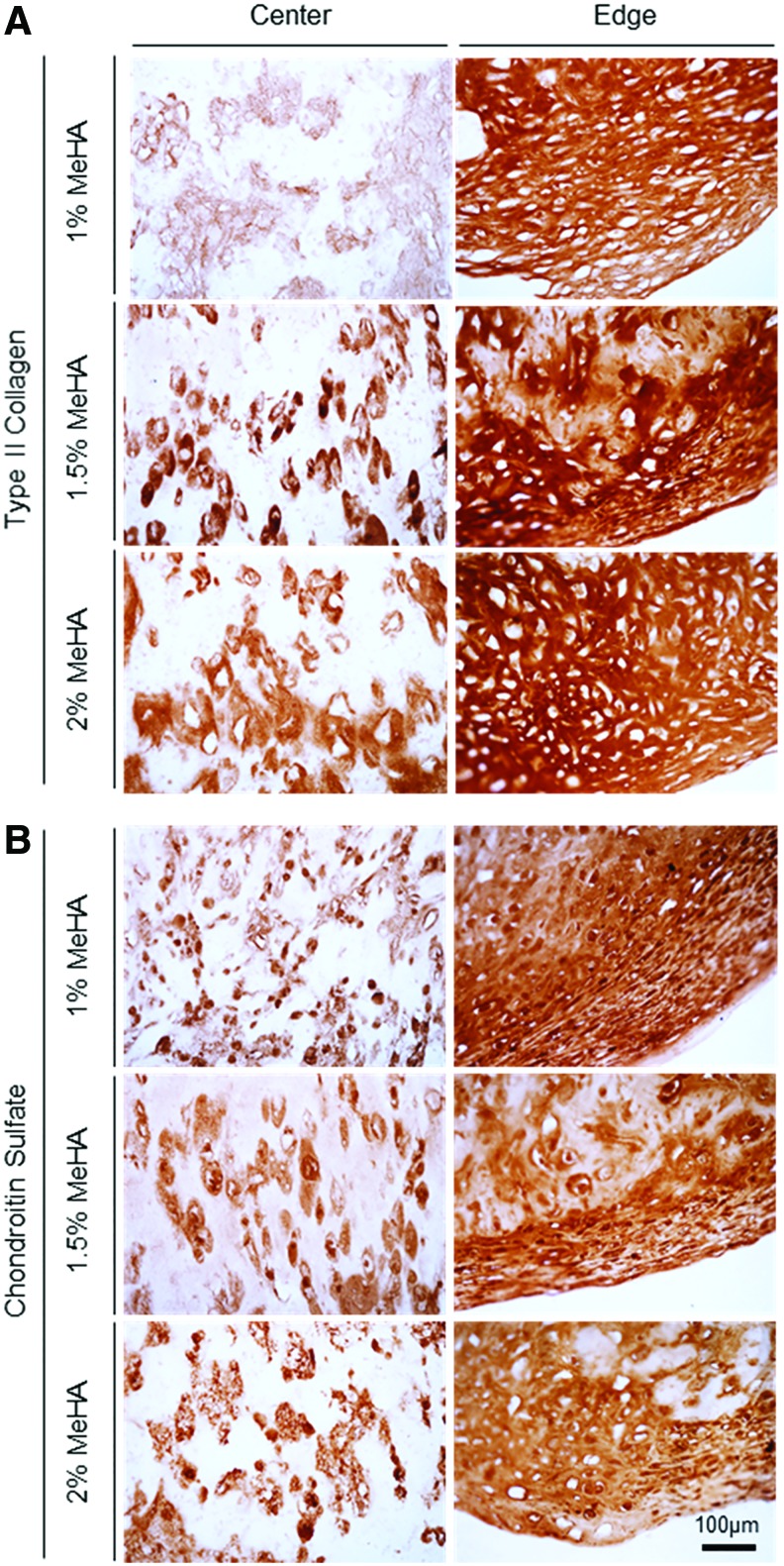
For histological analysis, matrix intensity and distribution were associated with distribution of cells within the constructs. For constructs with low cell (20 million cells/mL) and macromer (1% MeHA) density, Alcian blue staining showed a dense matrix rich in PG near the center compared to the edge, whereas other constructs with higher macromer (1.5% and 2% MeHA) density showed PG deposition with an even distribution (Fig. 5A and Supplementary Fig. S4). With an increase in cell density (60 million cells/mL), matrix accumulation was notably enhanced through the constructs for all groups. Likewise, collagen in constructs (20 million cells/mL) showed dense noncontiguous foci that were distributed across the entire cross-section (Fig. 5B). Collagen in constructs at 60 million cells/mL showed dense staining near the edge region, with little matrix in the core region (right panel). Immunohistochemistry of high-density constructs (60 million cells/mL) showed that type II collagen and CS were highly concentrated in 1% MeHA constructs, and staining density and connectivity of these foci decreased with increasing macromer concentration (Fig. 6 and Supplementary Fig. S5). Overall, there was little type I collagen produced under any condition.
FIG. 5.
Histological analysis of hMSC-laden MeHA constructs at varying cell and macromer density. (A) Alcian blue staining of proteoglycans and (B) Picrosirius red staining of collagen at 8 weeks in hMSC-laden MeHA constructs at 20 and 60 million cells/mL densities (scale bar = 100 μm). Color images available online at www.liebertpub.com/tea
FIG. 6.
Immunohistochemistry of hMSC-laden MeHA construct formed at varying macromer densities. (A) Type II collagen and (B) chondroitin sulfate at 60 million cells/mL cell density at 8 weeks (scale bar = 100 μm). Color images available online at www.liebertpub.com/tea
Discussion
This study evaluated the chondrogenic potential of hMSCs from multiple donors in 3D MeHA hydrogel and pellet culture. When encapsulated in MeHA hydrogels and induced to undergo chondrogenesis, hMSCs from different donors showed a differential response to the 3D microenvironment. Of seven donors, two young/healthy and one aged/arthritic donor maintained construct geometry with the least amount of contraction and accumulated extracellular matrix to increase mechanical properties. Conversely, the remaining four aged/arthritic donors severely contracted or rapidly dissolved the MeHA hydrogel, precluding any further mechanical analysis. These data show that hMSCs from different donors react to the same microenvironment in a different way. Indeed, two of these donors self-aggregated in MeHA hydrogels, resulting in the formation of a pellet-like mass with little PG production. The remaining two aged/arthritic donors failed to form cartilage matrix in 1% MeHA hydrogels and pellet culture. The differential matrix production in MeHA hydrogels compared to pellet culture suggests a microenvironmental influence on the differentiation and maturation process. Some of these differences might be compounded by age-related changes in hMSCs, specifically acting to accelerate compaction and decrease chondrogenic capacity.35 Similar findings have been noted in the bovine system, where adult bovine MSCs laden in MeHA hydrogels showed marked contraction and produced the least matrix and lowest properties compared to fetal and juvenile MSCs.35 The data may also suggest that the disease status (and perhaps endogenous inflammatory state) of the donors with osteoarthritis might impact outcomes by accelerating hydrogel dissolution via the secretion of degradative enzymes (e.g., hyaluronidase or matrix metalloproteinase), compromising initial construct integrity.
For clinical application, an engineered cartilage construct should possess mechanical and biochemical properties comparable to native levels and maintain a stable 3D configuration when implanted into a defect. The present data suggest that not every donor will yield such an outcome. It was found that for some donors, MeHA hydrogels with low macromer density yielded constructs with greater mechanical properties, but these had severely contracted, resulting in a construct that no longer had the appropriate geometry, making them unsuitable for filling the defect with the intended construct dimension.
To address this issue of contraction and further enhance the functional properties of hMSC-laden hydrogels, next, cell and macromer density was manipulated to explore cellular responses and functional matrix production. It was shown that MSC-laden hydrogel constructs seeded at low cell and gel macromer density showed marked volumetric changes along with cell aggregation toward the center of the construct, with dense matrix formation compared to the peripheral region. However, casting the same number of cells (20 million cells/mL) with the same donor in high macromer density prevented gel compaction and resulted in an even distribution of cells. While matrix distribution was more uniform, overall matrix accumulation was relatively low. Conversely, with an increase in cell density, overall matrix production markedly improved, as did construct compaction, especially for the constructs with low macromer density. Due to the increased matrix production and compaction, mechanical properties were greater in constructs with high cell and low macromer density compared to those with both high cell and high macromer density. This demonstrates that by simultaneously increasing cell and macromer density, one can promote matrix production and dimensional stability.
Interestingly, while increasing MeHA macromer density decreased some mechanical properties, overall matrix production measured on a per construct (μg/construct) (GAG and collagen production) was similar or even greater in higher density gels. Previous studies have shown that MeHA hydrogels present a biologic interface with which MSCs can interact. Thus, a high macromer density may provide a greater interaction of cell surface receptors (e.g., CD44) with the material.27 Recent works by Bian et al., Kwon et al., and Vega et al. have also shown that adding physical cell adhesion moieties to the gel can improve chondrogenesis.11,36,37 These studies showed that cells do not adhere strongly to the base HA gel in the absence of such engineered cell-hydrogel moieties. However, conjugation of cadherin peptides onto HA hydrogels improved chondrogenic differentiation of MSCs and cartilage matrix production compared to those without such moieties. Further, an increase in cell density markedly improved mechanical properties (similar to the native level) when using bovine MSCs.12 Based on these results, there appears to be a trade-off between the benefit in functional properties and the risk in structural instability when growing MeHA-based constructs with hMSCs.
To take advantage of this phenomenon, one possible approach would be to cast constructs larger than the defect to be filled by preculturing these constructs with at high cell and low HA density, and allow the constructs to contract during maturation. These matured constructs with improved functional properties could then be trimmed to match the size of the defect to be treated. However, contraction might reoccur when matrix is degraded by activation of pro-inflammatory signals.38 Alternatively, reducing skeletal tension by treating hMSC-laden constructs with the Rho/ROCK pathways inhibitor Y27632 during chondrogenic induction might improve dimensional stability. The Rho/ROCK pathway is a major regulator of cytoskeletal tension and plays important roles in stem-cell activities involved in cell–cell interactions and chondrogenesis.39,40 For instance, Woods et al. showed that Rho/ROCK pathway activation inhibited chondrogeneis of limb bud chondrocytes. Treatment of high cell and low MeHA macromer density constructs with Rho/ROCK inhibitors may block intercellular self-aggregation while optimizing matrix diffusivity of newly formed matrix through the construct, yielding improved mechanical properties.
In the best of the authors' knowledge, this is the first study to demonstrate donor-dependent response of hMSCs in a 3D hydrogel construct, and cellular response in 3D is shown to be different than in pellet culture. However, this study still has limitations, and future works will be required to understand the donor-dependent response of hMSCs in this 3D microenvironment better and also to optimize patient-specific chondrogenesis. This study used seven donors of different age, sex, and/or disease status in the first study, but did not use the same donors for the second study. Using same hMSC donors for both studies would be ideal, but the number of cells available in the after expansion precluded using them for both studies. Also, to optimize functional properties at varying cell and macromer density, only one young/healthy donor was evaluated. While previous results and those from the present study confirmed changes in mechanical properties and construct geometry associated with cell and macromer density, it would be more compelling not only to use multiple donors but also to optimize chondrogenesis for each one using aged or arthritic donors. To address these issues, recent studies have introduced co-culture systems, where two different cell types are mixed together, and have demonstrated that co-culturing with chondrocytes improved hMSC chondrogenesis while reducing hMSCs hypertrophy.41,42 Further, the influence of age and/or disease status on chondrogenesis of hMSCs is not yet fully understood15,16 and requires further study. To verify the impact and contribution of these individual factors further, future work will assess chondrogenesis of hMSCs using old/healthy hMSC donors in the same microenvironment.
Conclusions
This study demonstrates donor-to-donor variations of hMSCs in their chondrogenic differentiation in 3D culture. The findings reveal marked differences across donors that will need to be addressed for any autologous therapy to be successful. Indeed, understanding and characterizing this donor-dependent hMSCs response will enable prediction of donor-specific response, via screening assays, and will direct the optimization of their chondrogenic potential for autologous clinical applications. Future studies will investigate additional donors, especially from old/aged populations with varying degeneration status, to establish the most effective approach to patient-specific articular cartilage repair using autologous MSCs.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01 EB008722 and P30 AR050950), the Department of Veterans' Affairs (I01 RX000700), and the National Science Foundation (I.E.E. and A.H.H.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Langer R., and Vacanti J.P. Tissue engineering. Science 260, 920, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Cravero J.D., Carlson C.S., Im H.J., Yammani R.R., Long D., and Loeser R.F. Increased expression of the Akt/PKB inhibitor TRB3 in osteoarthritic chondrocytes inhibits insulin-like growth factor 1-mediated cell survival and proteoglycan synthesis. Arthritis Rheum 60, 492, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dore S., Pelletier J.P., DiBattista J.A., Tardif G., Brazeau P., and Martel-Pelletier J. Human osteoarthritic chondrocytes possess an increased number of insulin-like growth factor 1 binding sites but are unresponsive to its stimulation. Possible role of IGF-1-binding proteins. Arthritis Rheum 37, 253, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Yang K.G., Saris D.B., Geuze R.E., et al. . Altered in vitro chondrogenic properties of chondrocytes harvested from unaffected cartilage in osteoarthritic joints. Osteoarthritis Cartilage 14, 561, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Mackay A.M., Beck S.C., Murphy J.M., Barry F.P., Chichester C.O., and Pittenger M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4, 415, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., and Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238, 265, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Pittenger M.F., Mackay A.M., Beck S.C., et al. . Multilineage potential of adult human mesenchymal stem cells. Science 284, 143, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Caplan A.I. Mesenchymal stem cells. J Orthop Res 9, 641, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Johnstone B., Alini M., Cucchiarini M., et al. . Tissue engineering for articular cartilage repair—the state of the art. Eur Cells Mater 25, 248, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Rai V., Dilisio M.F., Dietz N.E., and Agrawal D.K. Recent strategies in cartilage repair: a systemic review of the scaffold development and tissue engineering. J Biomed Mater Res A 105, 2343, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Vega S.L., Kwon M.Y., and Burdick J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur Cells Mater 33, 59, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson I.E., Kestle S.R., Zellars K.H., et al. . High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater 8, 3027, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byers B.A., Mauck R.L., Chiang I.E., and Tuan R.S. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A 14, 1821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salinas C.N., Cole B.B., Kasko A.M., and Anseth K.S. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng 13, 1025, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Murphy J.M., Dixon K., Beck S., Fabian D., Feldman A., and Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum 46, 704, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Scharstuhl A., Schewe B., Benz K., Gaissmaier C., Buhring H.J., and Stoop R. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells 25, 3244, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Payne K.A., Didiano D.M., and Chu C.R. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage 18, 705, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddappa R., Fernandes H., Liu J., van Blitterswijk C., and de Boer J. The response of human mesenchymal stem cells to osteogenic signals and its impact on bone tissue engineering. Curr Stem Cell Res Ther 2, 209, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Siddappa R., Licht R., van Blitterswijk C., and de Boer J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res 25, 1029, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Portalska K.J., Groen N., Krenning G., et al. . The effect of donor variation and senescence on endothelial differentiation of human mesenchymal stromal cells. Tissue Eng Part A 19, 2318, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Shintani N., and Hunziker E.B. Differential effects of dexamethasone on the chondrogenesis of mesenchymal stromal cells: influence of microenvironment, tissue origin and growth factor. Eur Cells Mater 22, 302, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Burdick J.A., Chung C., Jia X., Randolph M.A., and Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 6, 386, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim I.L., Mauck R.L., and Burdick J.A. Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials 32, 8771, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fakhari A., and Berkland C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater 9, 7081, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung C., and Burdick J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A 15, 243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knudson W., and Loeser R.F. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell Mol Life Sci 59, 36, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson I.E., Huang A.H., Sengupta S., Kestle S., Burdick J.A., and Mauck R.L. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage 17, 1639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauck R.L., Soltz M.A., Wang C.C., et al. . Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 122, 252, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Park S., Hung C.T., and Ateshian G.A. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthritis Cartilage 12, 65, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Soltz M.A., and Ateshian G.A. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech 31, 927, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Farndale R.W., Buttle D.J., and Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883, 173, 1986 [DOI] [PubMed] [Google Scholar]
- 32.Stegemann H., and Stalder K. Determination of hydroxyproline. Clin Chim Acta 18, 267, 1967 [DOI] [PubMed] [Google Scholar]
- 33.Neuman R.E., and Logan M.A. The determination of hydroxyproline. J Biol Chem 184, 299, 1950 [PubMed] [Google Scholar]
- 34.Kim M., Farrell M.J., Steinberg D.R., Burdick J.A., and Mauck R.L. Enhanced nutrient transport improves the depth-dependent properties of tri-layered engineered cartilage constructs with zonal co-culture of chondrocytes and MSCs. Acta Biomater 58, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erickson I.E., van Veen S.C., Sengupta S., Kestle S.R., and Mauck R.L. Cartilage matrix formation by bovine mesenchymal stem cells in three-dimensional culture is age-dependent. Clin Orthop Relat Res 469, 2744, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bian L., Guvendiren M., Mauck R.L., and Burdick J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A 110, 10117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon M.Y., Vega S.L., Gramlich W.M., Kim M., Mauck R.L., and Burdick J.A. Dose and timing of N-cadherin mimetic peptides regulate MSC chondrogenesis within hydrogels. Adv Healthc Mater 7, e1701199, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M., Garrity S.T., Dodge G.R., Steinberg D.R., and Mauck R.L. Role of dexamethasone in the long-term functional maturation of MSC-laden hyaluronic acid hydrogels for cartilage tissue engineering. J Orthop Res 36, 1717–1727, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woods A., Wang G., and Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem 280, 11626, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Amano M., Nakayama M., and Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 67, 545, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aung A., Gupta G., Majid G., and Varghese S. Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheum 63, 148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bian L., Zhai D.Y., Mauck R.L., and Burdick J.A. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A 17, 1137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.